Comparison of Bioaugmentation and Semipermeable Cover as Strategies for Micro-Pollutant Removal in Sewage Sludge Composting
Abstract
1. Introduction
2. Materials and Methods
2.1. Composting Strategies
- Inoculum preparation of Penicillium oxalicum
- The Semipermeable Cover Membrane
2.2. Experimental Design
2.3. Physicochemical Analysis and Quantification of Viable Microorganisms
- Physicochemical Parameters
- Quantification of Viable Microorganisms
2.4. Emerging Pollutants’ Determination
2.5. Quantitative Polymerase Chain Reaction (qPCR) Analysis
2.6. Amplicon Sequencing and Bioinformatic Analysis
2.7. Germination Index Tests
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Composition
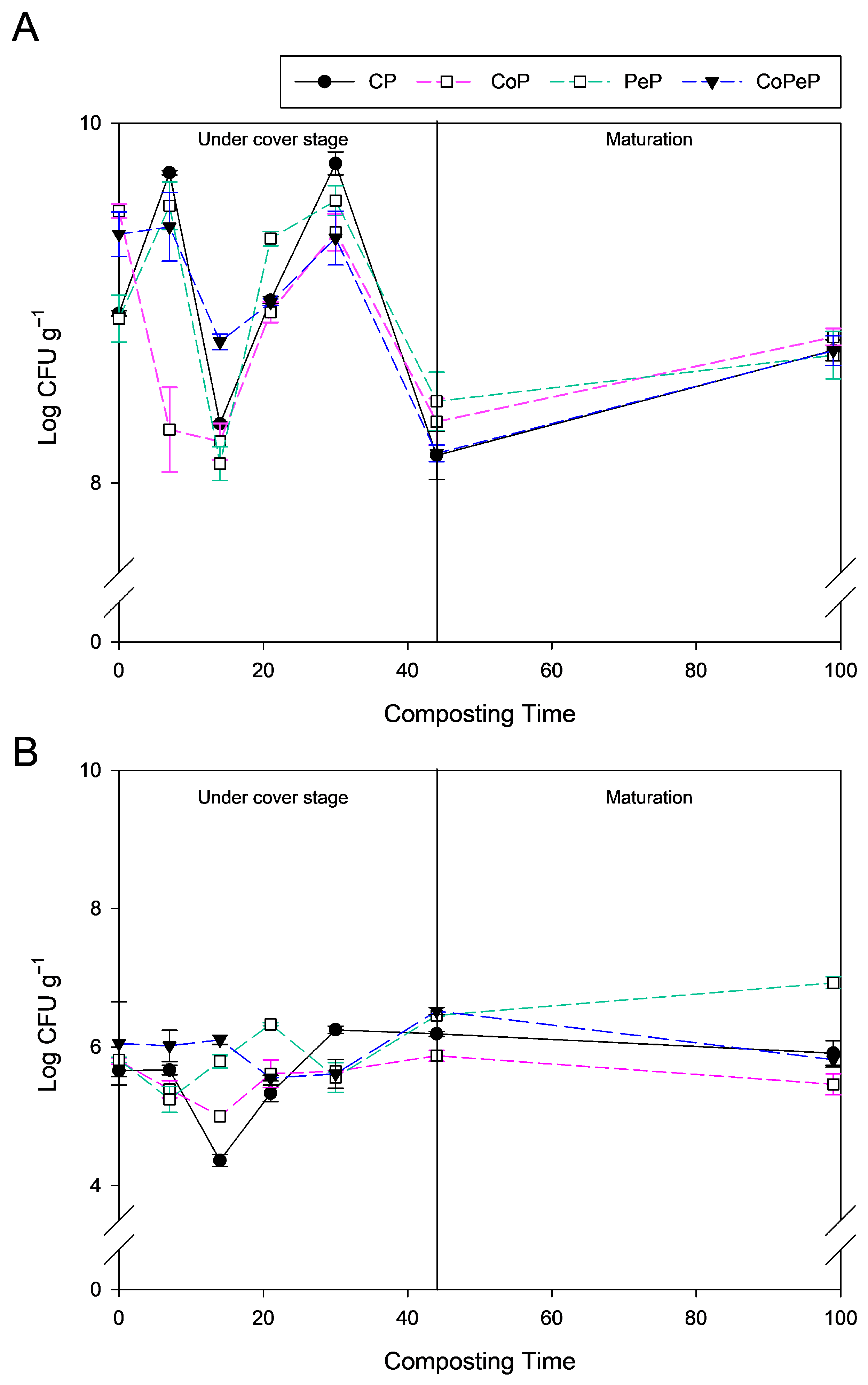
3.2. Culturable Bacteria and Fungi
3.3. Micro-Pollutant Concentration
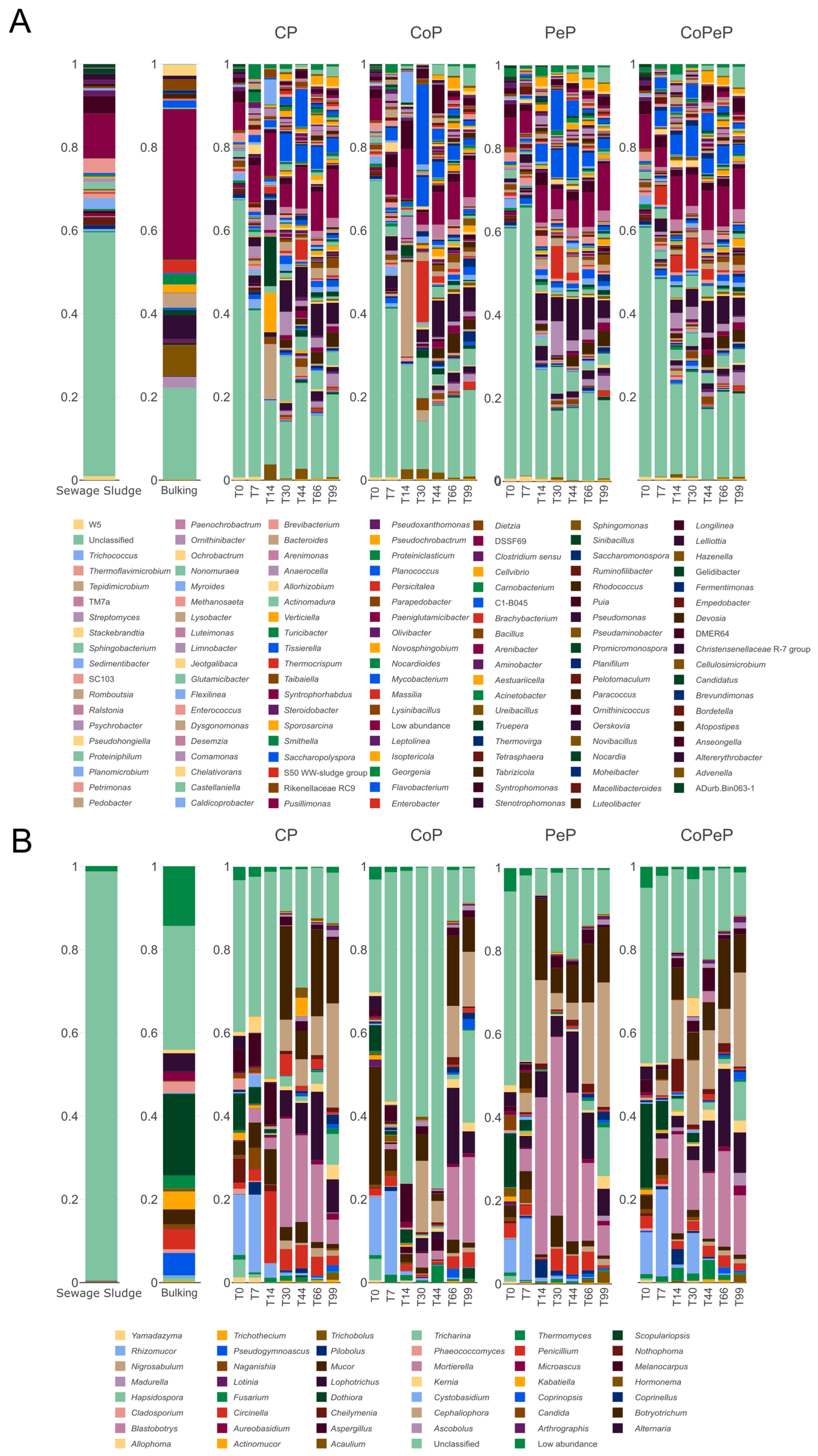
3.4. Microbial Succession During Composting
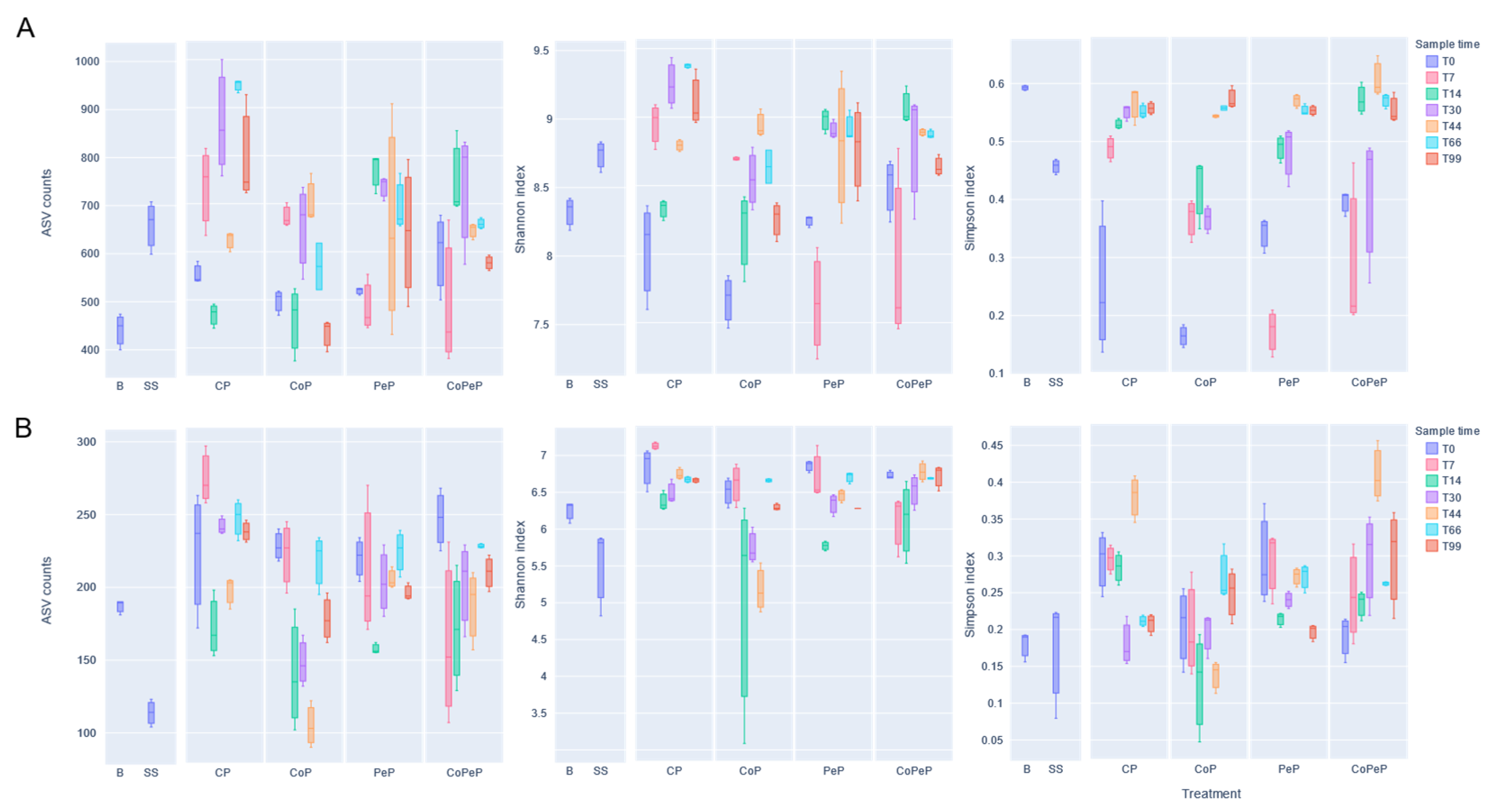
3.5. Alpha Diversity Metrics During Composting
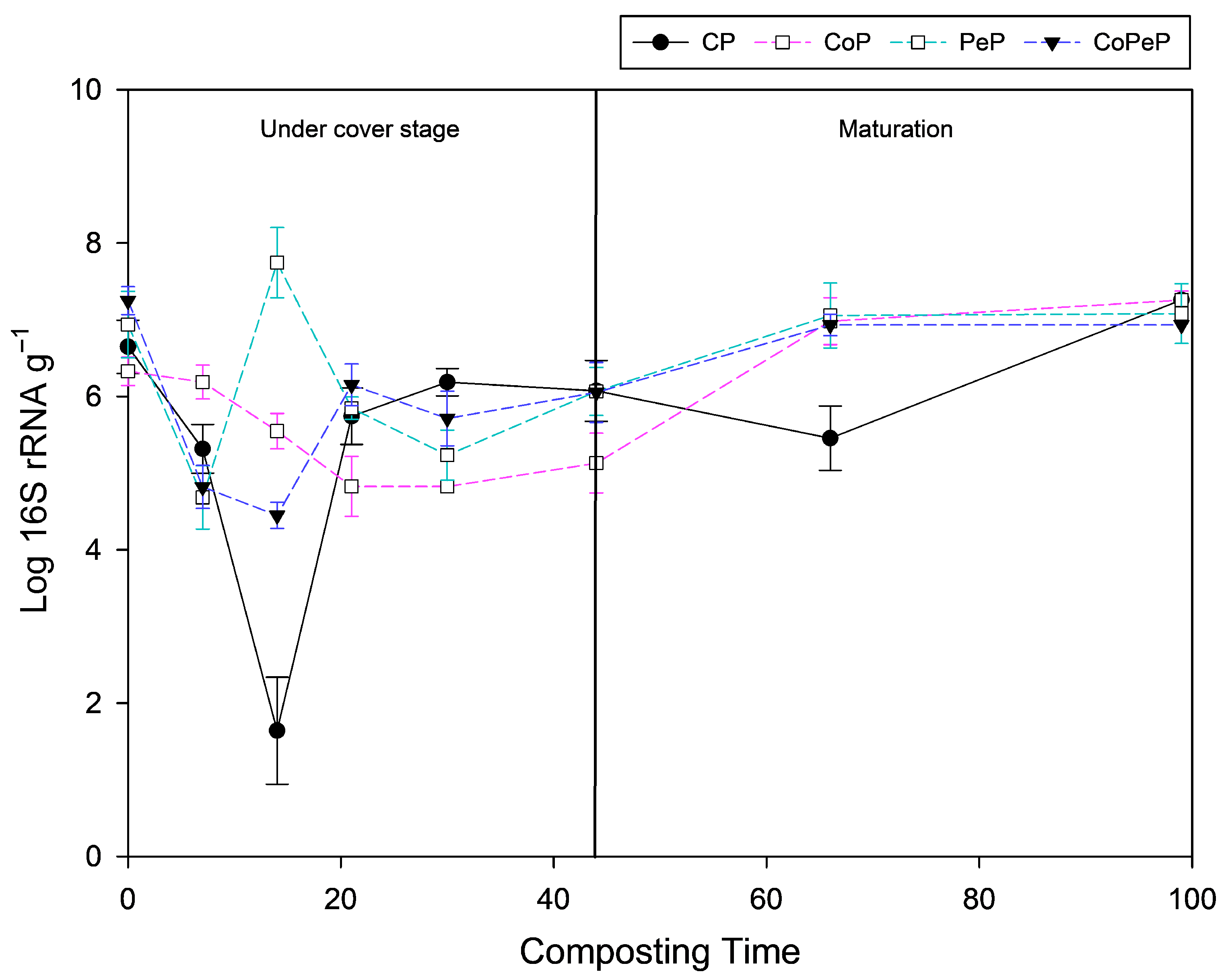
3.6. Bacterial Abundance (qPCR)
3.7. Phytotoxicity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koumoulidis, D.; Varvaris, I.; Pittaki, Z.; Hadjimitsis, D. Sewage Sludge in Agricultural Lands: The Legislative Framework in EU-28. Sustainability 2024, 16, 10946. [Google Scholar] [CrossRef]
- Rogowska, J.; Cieszynska-Semenowicz, M.; Ratajczyk, W.; Wolska, L. Micropollutants in treated wastewater. Ambio 2020, 49, 487–503. [Google Scholar] [CrossRef]
- Dubey, M.; Mohapatra, S.; Tyagi, V.K.; Suthar, S.; Kazmi, A.A. Occurrence, fate, and persistence of emerging micropollutants in sewage sludge treatment. Environ. Pollut. 2021, 273, 116515. [Google Scholar] [CrossRef]
- Hoang, S.A.; Bolan, N.; Madhubashani, A.M.P.; Vithanage, M.; Perera, V.; Wijesekara, H.; Wang, H.; Srivastava, P.; Kirkham, M.B.; Mickan, B.S.; et al. Treatment processes to eliminate potential environmental hazards and restore agronomic value of sewage sludge: A review. Environ. Pollut. 2022, 293, 118564. [Google Scholar] [CrossRef]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Angeles-de Paz, G.; León-Morcillo, R.; Guzmán, S.; Robledo-Mahón, T.; Pozo, C.; Calvo, C.; Aranda, E. Pharmaceutical active compounds in sewage sludge: Degradation improvement and conversion into an organic amendment by bioaugmentation-composting processes. Waste Manag. 2023, 168, 167–178. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003 (Text with EEA relevance). Off. J. Eur. Union 2019, 170, 1–114. [Google Scholar]
- Cui, H.-Y.; Zhao, Y.; Chen, Y.-N.; Zhang, X.; Wang, X.-Q.; Lu, Q.; Jia, L.-M.; Wei, Z.-M. Assessment of phytotoxicity grade during composting based on EEM/PARAFAC combined with projection pursuit regression. J. Hazard. Mater. 2017, 326, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Chia, W.Y.; Chew, K.W.; Le, C.F.; Lam, S.S.; Chee, C.S.C.; Ooi, M.S.L.; Show, P.L. Sustainable utilization of biowaste compost for renewable energy and soil amendments. Environ. Pollut. 2020, 267, 115662. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Lin, C.; Hoang, H.G.; Sanderson, P.; Dang, B.T.; Bui, X.T.; Nguyen, N.S.H.; Vo, D.-V.N.; Tran, H.T. Evaluate the role of biochar during the organic waste composting process: A critical review. Chemosphere 2022, 299, 134488. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.-T.; Bolan, N.S.; Lin, C.; Binh, Q.A.; Nguyen, M.-K.; Luu, T.A.; Le, V.-G.; Pham, C.Q.; Hoang, H.-G.; Vo, D.-V.N. Succession of biochar addition for soil amendment and contaminants remediation during co-composting: A state of art review. J. Environ. Manag. 2023, 342, 118191. [Google Scholar] [CrossRef]
- Aye, S.L. Composting of rice straw with effective microorganisms (EM) and its influence on compost quality. J. Myanmar Acad. Arts Sci. 2016, 14, 317–331. [Google Scholar]
- Díaz, L.A.; Chicaiza, E.; Valle, S.; Aguiar, S.; Arias, P.; Espín, C.; Ruiz, P.; Escobar, J.A. Identificación y caracterización de microorganismos nativos de la rizósfera de la Amazonía para acelerar el proceso de compostaje de residuos orgánicos agroindustriales. In Libro de Memorias: Simposio Internacional Sobre Manejo Sostenible de Tierras y Seguridad Alimentaria–Ecuador 2017; Universidad Estatal Amazónica: Puyo, Ecuador, 2017; p. 34. [Google Scholar]
- Cao, Z.; Deng, F.; Wang, R.; Li, J.; Liu, X.; Li, D. Bioaugmentation on humification during co-composting of corn straw and biogas slurry. Bioresour. Technol. 2023, 374, 128756. [Google Scholar] [CrossRef] [PubMed]
- de Mendonça Costa, L.A.; de Mendonça Costa, M.S.S.; Damaceno, F.M.; Chiarelotto, M.; Bofinger, J.; Gazzola, W. Bioaugmentation as a strategy to improve the compost quality in the composting process of agro-industrial wastes. Environ. Technol. Innov. 2021, 22, 101478. [Google Scholar] [CrossRef]
- Wang, Q.; Li, N.; Jiang, S.; Li, G.; Yuan, J.; Li, Y.; Chang, R.; Gong, X. Composting of post-consumption food waste enhanced by bioaugmentation with microbial consortium. Sci. Total Environ. 2024, 907, 168107. [Google Scholar] [CrossRef] [PubMed]
- Ayotamuno, J.M.; Okparanma, R.N.; Araka, P.P. Bioaugmentation and composting of oil-field drill-cuttings containing polycyclic aromatic hydrocarbons (PAHs). J. Food Agric. Environ. 2009, 7, 658–664. [Google Scholar]
- Medaura, M.C.; Guivernau, M.; Moreno-Ventas, X.; Prenafeta-Boldú, F.X.; Viñas, M. Bioaugmentation of native fungi, an efficient strategy for the bioremediation of an aged industrially polluted soil with heavy hydrocarbons. Front. Microbiol. 2021, 12, 626436. [Google Scholar] [CrossRef]
- Angeles-De Paz, G.; Cubero-Cardoso, J.; Pozo, C.; Calvo, C.; Aranda, E.; Robledo-Mahón, T. Optimizing Bioaugmentation for Pharmaceutical Stabilization of Sewage Sludge: A Study on Short-Term Composting Under Real Conditions. J. Fungi 2025, 11, 67. [Google Scholar] [CrossRef]
- Robledo-Mahón, T.; Gómez-Silván, C.; Andersen, G.L.; Calvo, C.; Aranda, E. Assessment of bacterial and fungal communities in a full-scale thermophilic sewage sludge composting pile under a semipermeable cover. Bioresour. Technol. 2020, 298, 122550. [Google Scholar] [CrossRef]
- Aranda, E.; Godoy, P.; Reina, R.; Badia-Fabregat, M.; Rosell, M.; Marco-Urrea, E.; García-Romera, I. Isolation of Ascomycota fungi with capability to transform PAHs: Insights into the biodegradation mechanisms of Penicillium oxalicum. Int. Biodeterior. Biodegrad. 2017, 122, 141–150. [Google Scholar] [CrossRef]
- European Commission. Directorate-General for Environment, Support to the Evaluation of the Sewage Sludge Directive—Final Implementation Report; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- EN 13650:2001; Soil Improvers and Growing Media—Extraction of Aqua Regla Soluble Elements. ES-UNE: Madrid, Spain, 2002.
- SW-846 Test Method 7473: Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry. 2007. Available online: https://www.epa.gov/hw-sw846/sw-846-test-method-7473-mercury-solids-and-solutions-thermal-decomposition-amalgamation (accessed on 22 July 2025).
- Montemurro, N.; Joedicke, J.; Pérez, S. Development and Application of a QuEChERS Method with Liquid Chromatography-Quadrupole Time of Flight-Mass Spectrometry for the Determination of 50 Wastewater-Borne Pollutants in Earthworms Exposed through Treated Wastewater. Chemosphere 2021, 263, 128222. [Google Scholar] [CrossRef]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Zucconi, F.; Pera, A.; Forte, M.; DeBertolli, M. Evaluating toxicity of immature compost. Biocycle 1981, 22, 54–57. [Google Scholar]
- Gell, K.; van Groenigen, J.; Cayuela, M.L. Residues of bioenergy production chains as soil amendments: Immediate and temporal phytotoxicity. J. Hazard. Mater. 2011, 186, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Tambone, F.; Scaglia, B.; D’Imporzano, G.; Schievano, A.; Orzi, V.; Salati, S.; Adani, F. Assessing amendment and fertilizing properties of digestates from anaerobic digestion through a comparative study with digested sludge and compost. Chemosphere 2010, 81, 577–583. [Google Scholar] [CrossRef]
- Di Maria, F.; Sordi, A.; Cirulli, G.; Gigliotti, G.; Massaccesi, L.; Cucina, M. Co-treatment of fruit and vegetable waste in sludge digesters. An analysis of the relationship among bio-methane generation, process stability and digestate phytotoxicity. Waste Manag. 2014, 34, 1603–1608. [Google Scholar] [CrossRef]
- Pérez, A.; Céspedes, C.; Núñez, P. Caracterización física-química y biológica de enmiendas orgánicas aplicadas en la producción de cultivos en República Dominicana. Rev. Cienc. Suelo Nutr. Veg. 2008, 8, 10–29. [Google Scholar]
- Escobar, M.; Ji, J.; Wang, Y.; Feng, M.; Bao, C.; Ma, J.; Cui, S.; Zang, S.; Zhang, J.; Zhang, W. Effect of thermal treatment of illite on the bioavailability of copper and zinc in the aerobic composting of pig manure with corn straw. Front. Microbiol. 2024, 15, 1411251. [Google Scholar] [CrossRef]
- Smolders, E.; Oorts, K.; Lombi, E.; Schoeters, I.; Ma, Y.; Zrna, S.; McLaughlin, M.J. The availability of copper in soils historically amended with sewage sludge, manure, and compost. J. Environ. Qual. 2012, 41, 506–514. [Google Scholar] [CrossRef]
- Robledo-Mahón, T.; Martín, M.A.; Gutiérrez, M.C.; Toledo, M.; González, I.; Aranda, E.; Chica, A.F.; Calvo, C. Sewage sludge composting under semi-permeable film at full-scale: Evaluation of odour emissions and relationships between microbiological activities and physico-chemical variables. Environ. Res. 2019, 177, 108624. [Google Scholar] [CrossRef]
- González, I.; Robledo-Mahón, T.; Silva-Castro, G.A.; Rodríguez-Calvo, A.; Gutiérrez, M.C.; Martín, M.Á.; Chica, A.F.; Calvo, C. Evolution of the composting process with semi-permeable film technology at industrial scale. J. Clean. Prod. 2016, 115, 245–254. [Google Scholar] [CrossRef]
- Olicón-Hernández, D.R.; Gómez-Silván, C.; Pozo, C.; Andersen, G.L.; González-Lopez, J.; Aranda, E. Penicillium oxalicum XD-3.1 removes pharmaceutical compounds from hospital wastewater and outcompetes native bacterial and fungal communities in fluidised batch bioreactors. Int. Biodeterior. Biodegrad. 2021, 158, 105179. [Google Scholar] [CrossRef]
- Angeles-de Paz, G.; Ledezma-Villanueva, A.; Robledo-Mahón, T.; Pozo, C.; Calvo, C.; Aranda, E.; Purswani, J. Assembled mixed co-cultures for emerging pollutant removal using native microorganisms from sewage sludge. Chemosphere 2023, 313, 137472. [Google Scholar] [CrossRef]
- Seo, J.; Lee, Y.-G.; Kim, S.-D.; Cha, C.-J.; Ahn, J.-H.; Hur, H.-G. Biodegradation of the insecticide N, N-diethyl-m-toluamide by fungi: Identification and toxicity of metabolites. Arch. Environ. Contam. Toxicol. 2005, 48, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Han, S.; Lin, Y. Chapter 8—Role of microbes and microbial dynamics during composting. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Awasthi, M., Zhang, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 169–220. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, W.; Liu, G.; Cui, B.; Zhang, L.; Wuyun, D.; Wang, Q.; Wang, G.; Li, G.; Yuan, J. Effect of a semi-permeable membrane covered composting on greenhouse gas emissions and bacterial community succession: A comparative study with biomass materials covering. J. Clean. Prod. 2024, 434, 140146. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Z.; Jiang, J.; Zhu, F.; Guo, X.; Jia, P.; Li, H.; Liu, Z.; Huang, S.; Zhang, Y.; et al. Enhancement of nitrogen on core taxa recruitment by Penicillium oxalicum stimulated microbially-driven soil formation in bauxite residue. J. Hazard. Mater. 2024, 473, 134647. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, L.; Zhu, F.; Wang, Y.; Jiang, J.; Chen, K.; Xue, S. Stable Aggregate Formation and Microbial Diversity Resilience in Soil Formation of Bauxite Residue: Roles of Extracellular Polymeric Substances Secreted by Penicillium oxalicum. ACS ES T Eng. 2023, 3, 1758–1769. [Google Scholar] [CrossRef]
- Angeles-de Paz, G.; León-Morcillo, R.; Štovícek, A.; Sagova-Mareckova, M.; Robledo-Mahón, T.; Calvo, C.; Aranda, E. Dynamic population changes during a bioaugmented sewage sludge composting process: Improvement of pharmaceutical active compounds degradation and conversion into an organic soil amendment. J. Environ. Chem. Eng. 2024, 12, 112937. [Google Scholar] [CrossRef]
- Long, B.; Liao, L.; Jia, F.; Luo, Y.; He, J.; Zhang, W.; Shi, J. Oxalic acid enhances bioremediation of Cr(VI) contaminated soil using Penicillium oxalicum SL2. Chemosphere 2023, 311, 136973. [Google Scholar] [CrossRef]
- Ruiz-Villalba, A.; Ruijter, J.M.; van den Hoff, M.J. Use and misuse of Cq in qPCR data analysis and reporting. Life 2021, 11, 496. [Google Scholar] [CrossRef]
- Finore, I.; Feola, A.; Russo, L.; Cattaneo, A.; Di Donato, P.; Nicolaus, B.; Poli, A.; Romano, I. Thermophilic bacteria and their thermozymes in composting processes: A review. Chem. Biol. Technol. Agric. 2023, 10, 7. [Google Scholar] [CrossRef]
- Robledo-Mahón, T.; Aranda, E.; Pesciaroli, C.; Rodríguez-Calvo, A.; Silva-Castro, G.A.; González-López, J.; Calvo, C. Effect of semi-permeable cover system on the bacterial diversity during sewage sludge composting. J. Environ. Manag. 2018, 215, 57–67. [Google Scholar] [CrossRef]
- Sosa, L.L.d.; Moreno, B.; Herrera, R.A.; Panettieri, M.; Madejón, E.; Benítez, E. Organic amendments and sampling date influences on soil bacterial community composition and their predictive functional profiles in an olive grove ecosystem. Agriculture 2021, 11, 1178. [Google Scholar] [CrossRef]
- Ryckeboer, J.; Mergaert, J.; Vaes, K.; Klammer, S.; De Clercq, D.; Coosemans, J.; Insam, H.; Swings, J. A survey of bacteria and fungi occurring during composting and self-heating processes. Ann. Microbiol. 2003, 53, 349–410. [Google Scholar]
- Guo, Y.; Zhang, J.; Deng, C.; Zhu, N. Spatial heterogeneity of bacteria: Evidence from hot composts by culture-independent analysis. Asian-Australas. J. Anim. Sci. 2012, 25, 1045. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Stehouwer, R.; Cooperband, L.; Rynk, R.; Biala, J.; Bonhotal, J.; Antler, S.; Lewandowski, T.; Nichols, H. Chapter 15—Compost characteristics and quality. In The Composting Handbook; Rynk, R., Ed.; Academic Press: New York, NY, USA, 2022; pp. 737–775. [Google Scholar] [CrossRef]

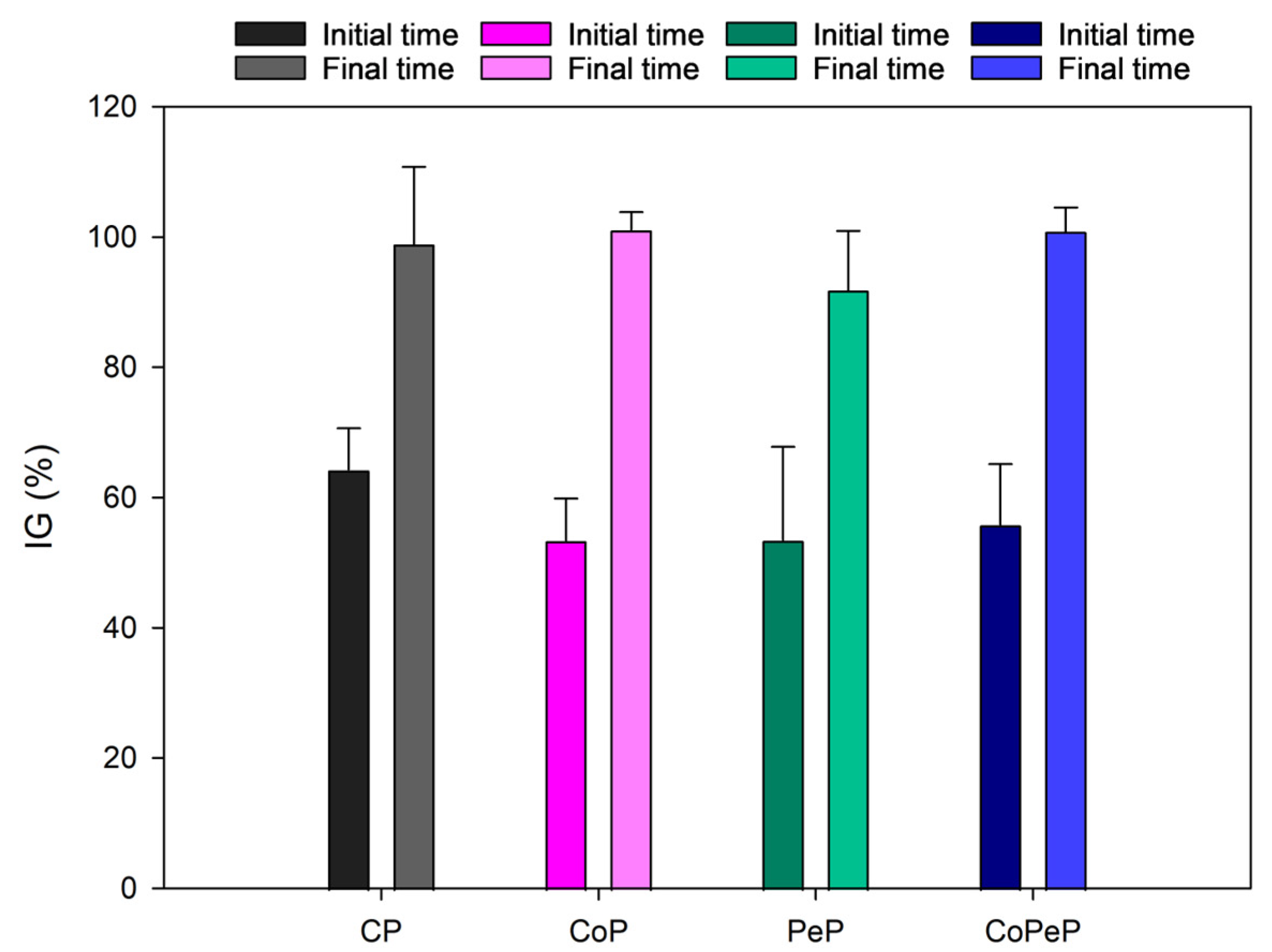
| CP | CoP | PeP | CoPeP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SM | MC | SM | MC | SM | MC | SM | MC | ||
| General parameters | pH | 7.57 | 7.37 | 7.33 | 7.30 | 7.57 | 7.10 | 7.50 | 7.20 |
| EC (dS m−1) | 0.56 ± 0.03 | 1.18 ± 0.01 | 0.56 ± 0.02 | 1.06 ± 0.01 | 0.67 ± 0.04 | 1.16 ± 0.03 | 0.61± 0.02 | 1.10 ± 0.03 | |
| M (%) | 56.20 ± 1.11 | 5.53 ± 0.34 | 55.00 ± 1.45 | 8.83 ± 0.79 | 60.63 ± 3.81 | 6.13 ± 0.38 | 59.76 ± 1.47 | 15.53 ± 0.96 | |
| DM (%) | 43.80 ± 1.11 | 94.5 ± 0.35 | 45.00 ± 1.45 | 91.16 ± 0.79 | 39.36 ± 3.81 | 93.86 ± 0.38 | 40.23 ± 1.47 | 84.46 ± 0.96 | |
| TS (%) | 44.00 ± 1.00 | 94.66 ± 0.33 | 45.33 ± 1.33 | 91.33 ± 0.88 | 39.33 ± 3.93 | 93.66 ± 0.33 | 40.33 ± 1.45 | 84.66 ± 0.88 | |
| VS (%) | 25.33 ± 0.88 | 42.33 ± 0.67 | 27.00 ± 1.00 | 33.33 ± 0.33 | 22.66 ± 2.33 | 40.66 ± 0.33 | 23.66 ± 0.88 | 34.00 ± 0.58 | |
| Macronutrients (%) | NTK (%) | 2.25 ± 0.04 | 2.34 ± 0.08 | 2.42 ± 0.05 | 2.01 ± 0.08 | 2.86 ± 0.01 | 2.35 ± 0.07 | 2.55 ± 0.04 | 2.18 ± 0.03 |
| P2O5 (%) | 2.26 ± 0.03 | 1.82 ± 0.04 | 2.40 ± 0.05 | 1.94 ± 0.06 | 3.05 ± 0.05 | 2.50 ± 0.12 | 2.58 ± 0.03 | 2.64 ± 0.10 | |
| K2O5 (%) | 0.50 ± 0.01 | 0.40 ± 0.02 | 0.50 ± 0.02 | 0.48 ± 0.02 | 0.47 ± 0.01 | 0.46 ± 0.01 | 0.51 ± 0.01 | 0.45 ± 0.01 | |
| CaO (%) | 9.30 ± 0.35 | 13.45 ± 0.88 | 9.55 ± 0.55 | 14.47 ± 0.82 | 8.77 ± 0.42 | 13.76 0.20 | 10.20 ± 0.41 | 12.21 ± 0.46 | |
| MgO (%) | 1.44 ± 0.03 | 1.66 ± 0.08 | 1.70 ± 0.05 | 1.91 ± 0.03 | 1.55 ± 0.06 | 1.62 ± 0.04 | 1.67 ± 0.03 | 1.73 ± 0.03 | |
| Organic compounds | TOM (%) | 25.60 ± 0.83 | 34.00 ± 0.78 | 26.86 ± 1.02 | 29.41 ± 0.33 | 22.80 ± 2.40 | 31.30 ± 0.42 | 23.63 ± 0.95 | 28.71 ± 0.52 |
| DOM (%) | 58.50 ± 1.50 | 50.24 ± 0.69 | 59.66 ± 0.33 | 50.63 ± 0.61 | 57.80 ± 0.70 | 51.02 ± 0.57 | 58.73 ± 0.66 | 50.05 ± 0.67 | |
| TOC (%) | 14.83 ± 0.50 | 19.72 ± 0.47 | 15.60 ± 0.60 | 17.05 ± 0.20 | 13.23 ± 1.39 | 18.15 ± 0.23 | 13.73 ± 0.54 | 16.65 ± 0.29 | |
| DOC (%) | 33.90 ± 0.90 | 29.12 ± 0.40 | 34.60 ± 0.21 | 29.34 ± 0.38 | 33.53 ± 0.42 | 29.58 ± 0.31 | 34.06 ± 0.38 | 29.02 ± 0.37 | |
| MM (%) | 18.20 ± 0.90 | 33.81 ± 0.46 | 18.13 ± 0.44 | 28.80 ± 1.04 | 16.60 ± 1.40 | 30.05 ± 0.70 | 16.56 ± 0.62 | 28.50 ± 1.00 | |
| C/N | 15.03 ± 0.32 | 13.54 ± 0.32 | 14.20 ± 0.51 | 12.06 ± 0.46 | 11.76 ± 1.29 | 12.18 ± 0.28 | 13.30 ± 0.03 | 12.93 ± 0.03 | |
| Heavy metals (mg kg−1) | Zn | 525.33 ± 39.72 | 258.67 ± 8.65 | 421.33 ± 51.81 | 186.33 ± 6.33 | 822.33 ± 166.5 | 234.33 ± 6.12 | 669.00 ± 186.71 | 212.00 ± 49.52 |
| Cu | 276.00 ± 26.72 | 141.67 ± 4.41 | 208.33 ± 22.26 | 216.00 ± 19.80 | 372.67 ± 93.49 | 138.00 ± 4.93 | 283.00 ± 64.28 | 138.00± 5.56 | |
| Cr | 75.00 ± 12.42 | 52.00 ± 4.04 | 51.33 ± 7.33 | 14.33 ± 0.88 | 90.00 ± 10.26 | 14.67 ± 0.88 | 73.00 ± 24.45 | 16.00 ± 14.31 | |
| Ni | 51.33 ± 11.02 | 39.00 ± 2.12 | 38.33 ± 9.29 | 14.33 ± 1.15 | 67.67 ± 18.23 | 33.00 ± 1.41 | 33.00 ± 1.41 | 63.00 ± 25.45 | |
| Pb | 44.00 ± 2.00 | 26.00 ± 0.03 | 38.00 ± 0.44 | 22.00 ± 1.04 | 74.67 ± 19.55 | 24.67 ± 0.67 | 55.00 ± 2.08 | 24.67 ± 0.88 | |
| Hg | 0.53 ± 0.03 | 0.30 ± 0.06 | 0.47 ± 5.69 | 0.20 ± 0.03 | 0.77 ± 0.15 | 0.27 ± 0.03 | 0.67 ± 0.03 | 0.27 ± 0.03 | |
| Cd | 0.67 ± 0.03 | 0.50 ± 0.03 | 0.56 ± 0.07 | 0.50 ± 0.03 | 0.73 ± 0.43 | 0.50 ± 0.03 | 0.80 ± 0.03 | 0.50 ± 0.03 | |
| Classification RD 506/2013 | B | B | B | B | |||||
| CP | CoP | PeP | CoPeP | ||
|---|---|---|---|---|---|
| Heavy metal | Zn | 50.76 | 71.50 | 68.31 | 55.78 |
| reduction | Cu | 48.67 | 62.97 | 51.24 | 46.24 |
| % | Cr | 30.67 | 83.70 | 78.08 | 72.08 |
| Ni | 24.03 | 51.23 | 29.41 | 62.61 | |
| Pb | 40.91 | 66.96 | 55.15 | 42.11 | |
| Hg | 43.75 | 65.21 | 60.00 | 57.15 | |
| Cd | 25.00 | 31.82 | 37.50 | 11.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angeles-de Paz, G.; Díaz-Moreno, M.Á.; Trujillo-Reyes, Á.; Postigo, C.; Aranda, E.; Calvo, C.; Robledo-Mahón, T. Comparison of Bioaugmentation and Semipermeable Cover as Strategies for Micro-Pollutant Removal in Sewage Sludge Composting. Toxics 2025, 13, 620. https://doi.org/10.3390/toxics13080620
Angeles-de Paz G, Díaz-Moreno MÁ, Trujillo-Reyes Á, Postigo C, Aranda E, Calvo C, Robledo-Mahón T. Comparison of Bioaugmentation and Semipermeable Cover as Strategies for Micro-Pollutant Removal in Sewage Sludge Composting. Toxics. 2025; 13(8):620. https://doi.org/10.3390/toxics13080620
Chicago/Turabian StyleAngeles-de Paz, Gabriela, Miguel Ángel Díaz-Moreno, Ángeles Trujillo-Reyes, Cristina Postigo, Elisabet Aranda, Concepción Calvo, and Tatiana Robledo-Mahón. 2025. "Comparison of Bioaugmentation and Semipermeable Cover as Strategies for Micro-Pollutant Removal in Sewage Sludge Composting" Toxics 13, no. 8: 620. https://doi.org/10.3390/toxics13080620
APA StyleAngeles-de Paz, G., Díaz-Moreno, M. Á., Trujillo-Reyes, Á., Postigo, C., Aranda, E., Calvo, C., & Robledo-Mahón, T. (2025). Comparison of Bioaugmentation and Semipermeable Cover as Strategies for Micro-Pollutant Removal in Sewage Sludge Composting. Toxics, 13(8), 620. https://doi.org/10.3390/toxics13080620











