Global Environmental Geochemistry and Molecular Speciation of Heavy Metals in Soils and Groundwater from Abandoned Smelting Sites: Analysis of the Contamination Dynamics and Remediation Alternatives in Karst Settings
Abstract
1. Introduction
2. Materials and Methods
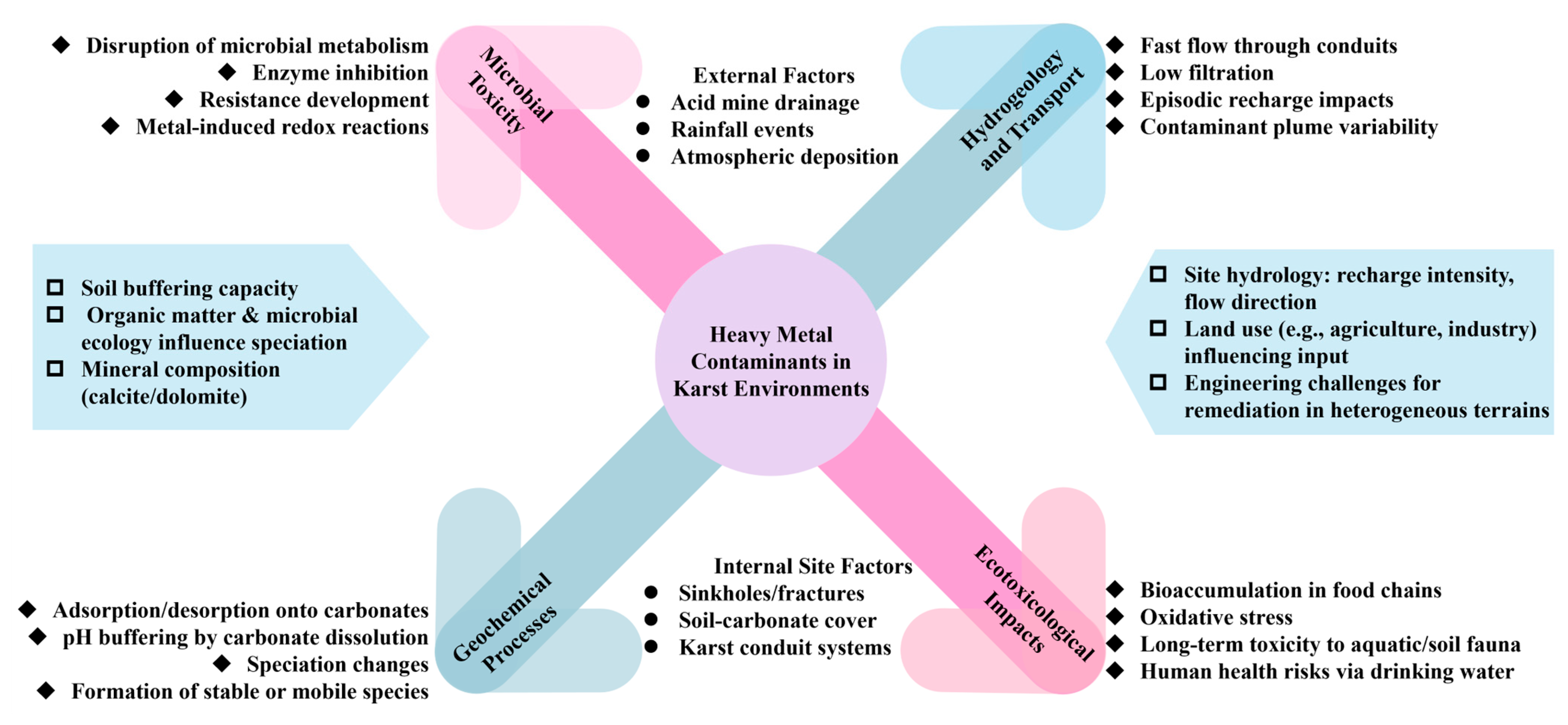
3. Vulnerability and Contamination Potential of Karst Groundwater
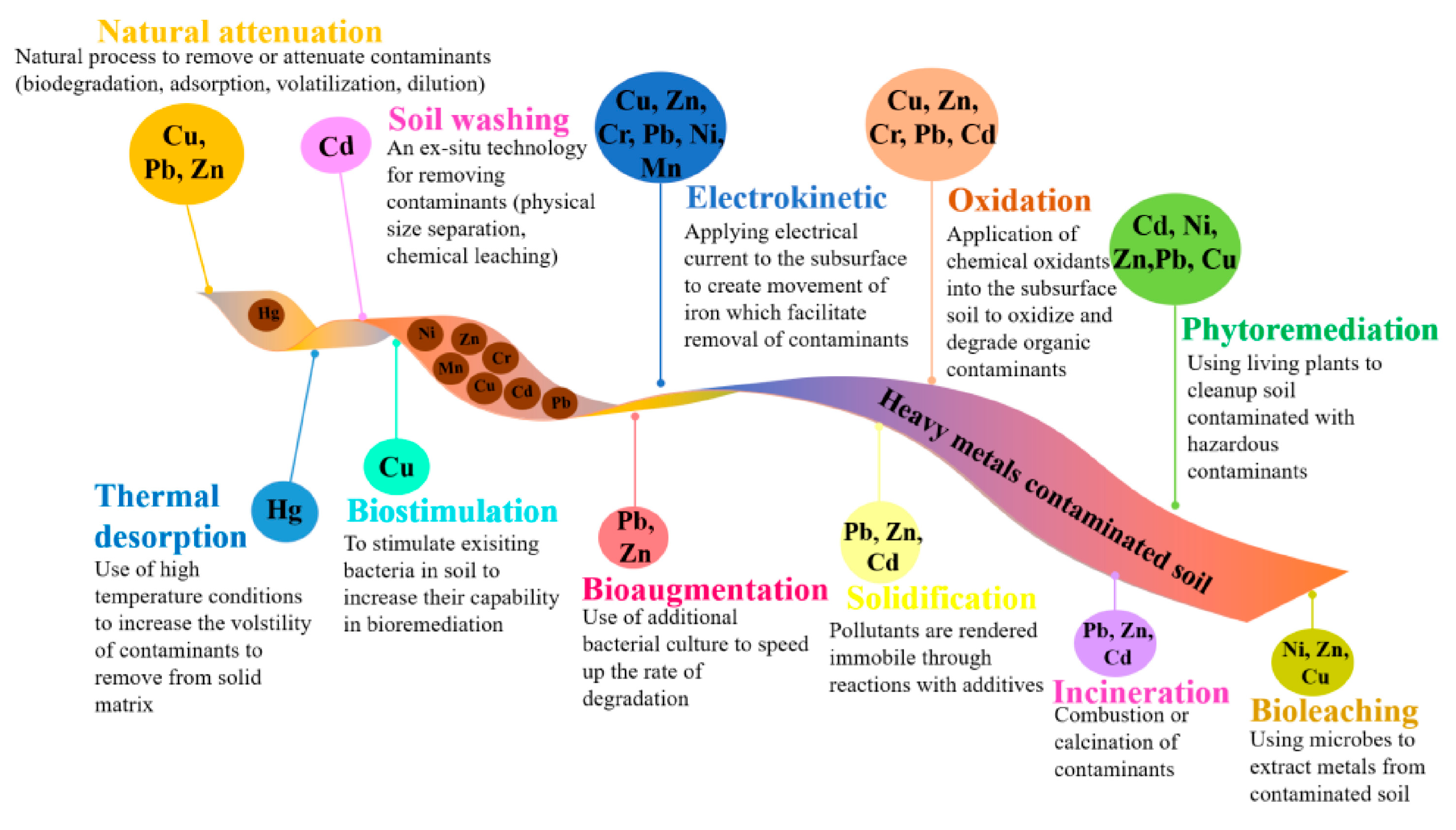
New Horizons in Karst Remediation
4. Karst Hydrogeology and the Transport of Heavy Metal
4.1. Specificities of Karst Systems: High Permeability and Varied Underground Drainage
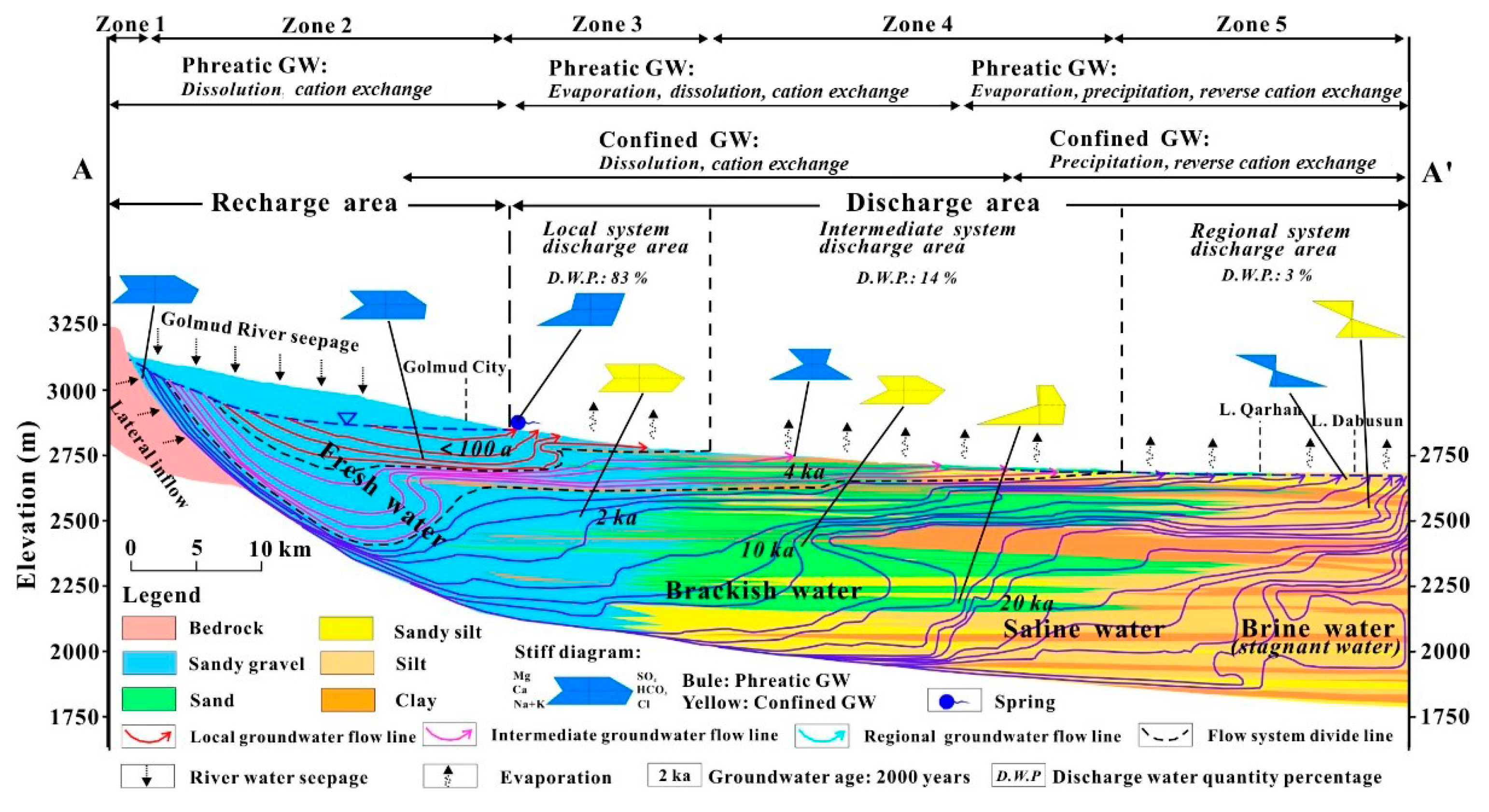
4.2. Influence of Karst Hydrogeology on Heavy Metal Distribution and Mobility in Soil and Groundwater
5. Soil–Groundwater Interactions in Karst Terrains: Heavy Metals Retention and Speciation
5.1. Retention and Transport of Heavy Metals at the Soil–Water Interface
5.2. Influence of Geochemical Processes on Heavy Metal Speciation in Karst Lands
6. Comparison of Heavy Metals Pollution in the Karst Areas on a Global Scale
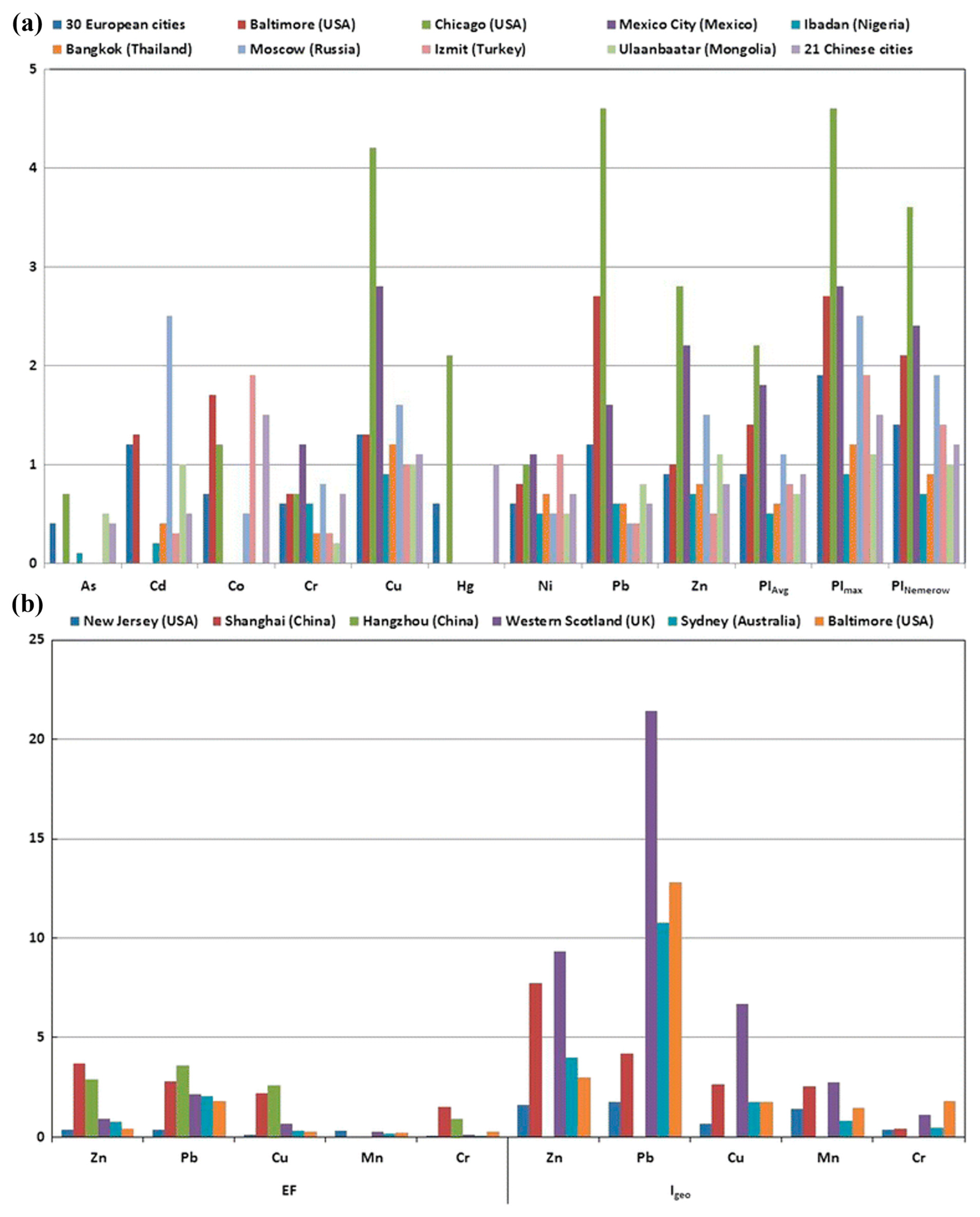
6.1. Global Karst Landscapes and Heavy Metal Contamination at Abandoned Smelting Sites
6.2. Comparative Hydrogeochemical Behavior and Metal Speciation in the Different Karsts
7. Vulnerability Analysis and Risk Assessment of Heavy Metals of Karst Water Bodies
7.1. Susceptibility of Karst Aquifers to Heavy Metal Pollution
7.2. Correlation Between Molecular Speciation and Bioavailability and Risk in Karst System
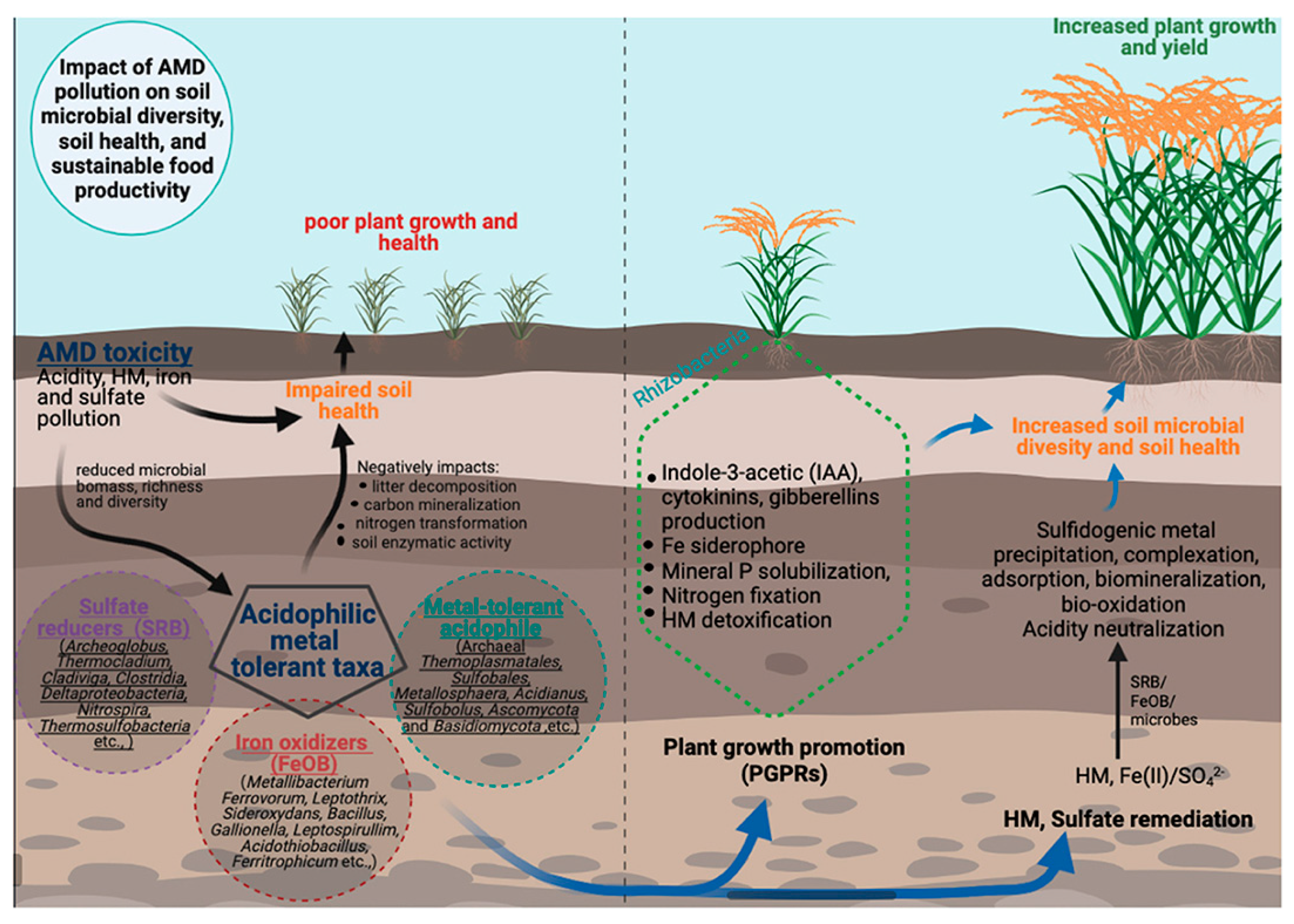
7.3. Ecotoxicological Effects of Heavy Metals in the Karst Ecosystems
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adnan, M.; Xiao, B.; Xiao, P.; Zhao, P.; Li, R.; Bibi, S. Research Progress on Heavy Metals Pollution in the Soil of Smelting Sites in China. Toxics 2022, 10, 231. [Google Scholar] [CrossRef]
- Adnan, M.; Xiao, B.; Xiao, P.; Zhao, P.; Bibi, S. Heavy Metal, Waste, COVID-19, and Rapid Industrialization in This Modern Era—Fit for Sustainable Future. Sustainability 2022, 14, 4746. [Google Scholar] [CrossRef]
- Adnan, M.; Shao, M.; Ali, M.U.; Yan, J.; Xiao, B.; An, X.; Farooq, M.; Hayat, K. Prospecting the engineered environmental carbon sinks and ensuring long-term sustainability of karst areas impacted by heavy metal. Sustain. Horiz. 2025, 14, 100126. [Google Scholar] [CrossRef]
- Liu, J.; Tang, L.; Peng, Z.; Gao, W.; Xiang, C.; Chen, W.; Jiang, J.; Guo, J.; Xue, S. The heterogeneous distribution of heavy metal(loid)s at a smelting site and its potential implication on groundwater. Sci. Total Environ. 2024, 948, 174944. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Guo, Z.; Xie, H.; Hu, Z.; Ran, H.; Li, C.; Jiang, Z. Spatial heterogeneity and source apportionment of soil metal(loid)s in an abandoned lead/zinc smelter. J. Environ. Sci. 2023, 127, 519–529. [Google Scholar] [CrossRef]
- Xue, S.; Wang, Y.; Jiang, J.; Tang, L.; Xie, Y.; Gao, W.; Tan, X.; Zeng, J. Groundwater heavy metal(loid)s risk prediction based on topsoil contamination and aquifer vulnerability at a zinc smelting site. Environ. Pollut. 2024, 341, 122939. [Google Scholar] [CrossRef]
- Xue, S.; Ke, W.; Zeng, J.; Baltazar Tabelin, C.; Xie, Y.; Tang, L.; Xiang, C.; Jiang, J. Pollution prediction for heavy metals in soil-groundwater systems at smelting sites. Chem. Eng. J. 2023, 473, 145499. [Google Scholar] [CrossRef]
- Fate and Transport of Heavy Metals in Soil, Surface Water, and Groundwater: Implications for Environmental Management. Int. J. Sci. Res. Manag. (IJSRM) 2024, 12, 202–215. [CrossRef]
- Adnan, M.; Xiao, B.; Ali, M.U.; Xiao, P.; Zhao, P.; Wang, H.; Bibi, S. Heavy metals pollution from smelting activities: A threat to soil and groundwater. Ecotoxicol. Environ. Saf. 2024, 274, 116189. [Google Scholar] [CrossRef] [PubMed]
- Bori, J.; Vallès, B.; Navarro, A.; Riva, M.C. Geochemistry and environmental threats of soils surrounding an abandoned mercury mine. Environ. Sci. Pollut. Res. 2016, 23, 12941–12953. [Google Scholar] [CrossRef]
- Tiwari, B.; Fatima, G.; Hadi, N.; Fedacko, J.; Magomedova, A.; Raza, A.M.; Alharis, N.; Qassam, H.; Alhmadi, H.B.; Parvez, S. Metal Toxicity: Significant Health Assessment. Kufa Med. J. 2024, 20, 213–235. [Google Scholar] [CrossRef]
- Abarikwu, S.O. Lead, Arsenic, Cadmium, Mercury: Occurrence, Toxicity and Diseases. In Pollutant Diseases, Remediation and Recycling; Springer: Cham, Switzerland, 2013; pp. 351–386. [Google Scholar]
- Rana, M.N.; Tangpong, J.; Rahman, M.M. Toxicodynamics of lead, cadmium, mercury and arsenic-induced kidney toxicity and treatment strategy: A mini review. Toxicol. Rep. 2018, 5, 704–713. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs)(arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef]
- White, W.B. Contaminant Transport in Karst Aquifers: Systematics and Mechanisms. In Karst Groundwater Contamination and Public Health: Beyond Case Studies; Springer International Publishing: Cham, Switzerland, 2018; pp. 55–81. [Google Scholar]
- Field, M. Karst Hydrology and Chemical Contamination. J. Environ. Syst. 1993, 22, 1–26. [Google Scholar] [CrossRef]
- Sinreich, M. Contaminant Attenuation in Karst Aquifers: A Paradigm Shift. In H2Karst Research in Limestone Hydrogeology; Mudry, J., Zwahlen, F., Bertrand, C., LaMoreaux, J.W., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 175–184. [Google Scholar] [CrossRef]
- Padilla, I.Y.; Vesper, D.J. Fate, Transport, and Exposure of Emerging and Legacy Contaminants in Karst Systems: State of Knowledge and Uncertainty; Springer International Publishing: Cham, Switzerland, 2018; pp. 33–49. [Google Scholar]
- Kaufmann, G.; Romanov, D.; Dreybrodt, W. Chapter 86—Modeling the evolution of karst aquifers. In Encyclopedia of Caves, 3rd ed.; White, W.B., Culver, D.C., Pipan, T., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 717–724. [Google Scholar] [CrossRef]
- Field, M.S. RISK ASSESSMENT METHODOLOGY FOR KARST AQUIFERS: (2) SOLUTE-TRANSPORT MODELING. Environ. Monit. Assess. 1997, 47, 23–37. [Google Scholar] [CrossRef]
- Kaufmann, G.; Romanov, D.; Dreybrodt, W. Modeling of Karst Aquifers. In Encyclopedia of Caves, 2nd ed.; White, W.B., Culver, D.C., Eds.; Academic Press: Amsterdam, The Netherlands, 2012; pp. 508–512. [Google Scholar] [CrossRef]
- Birk, S. Characterisation of Karst Systems by Simulating Aquifer Genesis and Spring Responses: Model Development and Application to Gypsum Karst; Tübinger Geowissenschaftliche Arbeiten (TGA): Reihe C, Hydro- Ingenieur- und Umweltgeologie; 60. 2002. Available online: http://nbn-resolving.de/urn:nbn:de:bsz:21-opus-5583 (accessed on 2 June 2025).
- D’Amore, J.; Al-Abed, S.; Scheckel, K.; Ryan, J.A. Methods for Speciation of Metals in Soils. J. Environ. Qual. 2005, 34, 1707–1745. [Google Scholar] [CrossRef]
- Pickering, I. X-Ray Absorption Spectroscopy as a Probe of Elemental Speciation; SLAC National Accelerator Lab.: Menlo Park, CA, USA, 2003. [Google Scholar] [CrossRef]
- Brown, G.; Wang, Y.; Gelabert, A.; Ha, J.; Cismasu, A.; Ona-Nguema, G.; Benzerara, K.; Miot, J.; Menguy, N.; Morin, G.; et al. Synchrotron X-ray studies of heavy metal mineral-microbe interactions. Mineral. Mag. 2008, 72, 169–173. [Google Scholar] [CrossRef]
- McNear, D.H., Jr.; Tappero, R.; Sparks, D.L. Shining Light on Metals in the Environment. Elements 2005, 1, 211–216. [Google Scholar] [CrossRef]
- Manceau, A.; Marcus, M.A.; Tamura, N. Quantitative Speciation of Heavy Metals in Soils and Sediments by Synchrotron X-ray Techniques. Rev. Mineral. Geochem. 2002, 49, 341–428. [Google Scholar] [CrossRef]
- Manceau, A.; Lanson, B.; Schlegel, M.; Musso, M.; Hazemann, J.-L.; Chateigner, D.; Lamble, G. Quantitative Zn Speciation in Smelter Contaminated Soils By EXAFS Spectroscopy. Am. J. Sci. 2000, 300, 289–343. [Google Scholar] [CrossRef]
- Brown, G.; Foster, A.; Ostergren, J. Mineral Surfaces and Bioavailability of Heavy Metals: A Molecular-Scale Perspective. Proc. Natl. Acad. Sci. USA 1999, 96, 3388–3395. [Google Scholar] [CrossRef] [PubMed]
- Kirichkov, M.V.; Polyakov, V.A.; Shende, S.S.; Minkina, T.M.; Nevidomskaya, D.G.; Wong, M.H.; Bauer, T.V.; Shuvaeva, V.A.; Mandzhieva, S.S.; Tsitsuashvili, V.S. Application of X-ray based modern instrumental techniques to determine the heavy metals in soils, minerals and organic media. Chemosphere 2024, 349, 140782. [Google Scholar] [CrossRef] [PubMed]
- Kresic, N. Foreword: Ground Water in Karst. Ground Water 2009, 47, 319–320. [Google Scholar] [CrossRef]
- Lan, F.-N.; Zhao, Y.; Li, J.; Zhu, X.-Q. Health risk assessment of heavy metal pollution in groundwater of a karst basin, SW China. J. Groundw. Sci. Eng. 2024, 12, 49–61. [Google Scholar] [CrossRef]
- Liao, H.-W.; Jiang, Z.-C.; Zhou, H.; Qin, X.-Q.; Huang, Q.-B.; Wu, H.-Y. Heavy metal pollution and health risk assessment in karst basin around a lead-zinc mine. Huan Jing Ke Xue=Huanjing Kexue 2023, 44, 6085–6094. [Google Scholar]
- Schindel, G.M. Recommended strategies for the response to hazardous materials releases in karst. In Karst Groundwater Contamination and Public Health: Beyond Case Studies; Springer: Cham, Switzerland, 2017; pp. 255–260. [Google Scholar]
- Qin, S.; Li, X.; Huang, J.; Li, W.; Wu, P.; Li, Q.; Li, L. Inputs and transport of acid mine drainage-derived heavy metals in karst areas of Southwestern China. Environ. Pollut. 2024, 343, 123243. [Google Scholar] [CrossRef]
- Gaur, A.; Shrivastava, B. A comparative study of the methods of speciation using X-ray absorption fine structure. Acta Phys. Pol. A 2012, 121, 647–652. [Google Scholar] [CrossRef]
- Tannazi, F.; Bunker, G. Determination of chemical speciation by XAFS. Phys. Scr. 2005, 2005, 953. [Google Scholar] [CrossRef]
- Guillon, E.; Merdy, P.; Aplincourt, M. Molecular scale speciation of first-row transition elements bound to ligneous material by using X-ray absorption spectroscopy. Chem.–A Eur. J. 2003, 9, 4479–4484. [Google Scholar] [CrossRef]
- Porcaro, F.; Roudeau, S.; Carmona, A.; Ortega, R. Advances in element speciation analysis of biomedical samples using synchrotron-based techniques. TrAC Trends Anal. Chem. 2018, 104, 22–41. [Google Scholar] [CrossRef]
- Poeter, E.; Fan, Y.; Cherry, J.; Wood, W.; Mackay, D. Groundwater in Our Water Cycle—Getting to Know Earth’s Most Important Fresh Water Source; The Groundwater Project: Guelph, ON, Canada, 2020. [Google Scholar] [CrossRef]
- Bensaoula, F.; Collignon, B. Vulnerability to Pollution of Karstic Aquifers in the Tafna River Basin and Risk Mitigation Strategies (Northwest Algeria). In Groundwater in Arid and Semi-Arid Areas: Monitoring, Assessment, Modelling, and Management; Ali, S., Armanuos, A.M., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 55–80. [Google Scholar] [CrossRef]
- Campanale, C.; Losacco, D.; Triozzi, M.; Massarelli, C.; Uricchio, V.F. An Overall Perspective for the Study of Emerging Contaminants in Karst Aquifers. Resources 2022, 11, 105. [Google Scholar] [CrossRef]
- Musgrove, M.; Jurgens, B.; Opsahl, S. Karst Groundwater Vulnerability Determined by Modeled Age and Residence Time Tracers. Geophys. Res. Lett. 2023, 50, e2023GL102853. [Google Scholar] [CrossRef]
- Reberski, J.L.; Terzić, J.; Maurice, L.D.; Lapworth, D.J. Emerging organic contaminants in karst groundwater: A global level assessment. J. Hydrol. 2022, 604, 127242. [Google Scholar] [CrossRef]
- Musgrove, M.; Katz, B.G.; Fahlquist, L.S.; Crandall, C.A.; Lindgren, R.J. Factors affecting public-supply well vulnerability in two karst aquifers. Groundwater 2014, 52, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Leins, T.; Scheller, M.; Çallı, K.Ö.; Ravbar, N.; Mayaud, C.; Petrič, M.; Liu, Y.; Hartmann, A. A new process-based approach for defining karst aquifer vulnerability to contamination risks under global changes. Sci. Total Environ. 2025, 966, 178561. [Google Scholar] [CrossRef]
- Marín, A.I.; Andreo, B. Vulnerability to Contamination of Karst Aquifers. In Karst Aquifers—Characterization and Engineering; Stevanović, Z., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 251–266. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, B.; Zhang, W.; Zou, J.; Jia, R.; Yang, Y. Hydrochemical Characteristics and Evolution under the Influence of Multiple Anthropogenic Activities in Karst Aquifers, Northern China. Water 2024, 16, 1656. [Google Scholar] [CrossRef]
- Shan, Q.; Tian, X.; Xie, H.; Gong, Z.; Lin, Y.; Dang, Z.; Jun, L.; Zou, S.; Zhu, T. Hydrogeochemical characteristics, driving factors, and health risk assessment of karst groundwater in Southwest Hubei Province, China. Water Environ. Res. Res. Publ. Water Environ. Fed. 2024, 96, e11069. [Google Scholar] [CrossRef]
- Zhang, Q.; Hah, G.-L. Speciation Characteristics and Risk Assessment of Soil Heavy Metals from Puding Karst Critical Zone, Guizhou Province. Huan Jing Ke Xue=Huanjing Kexue/[Bian Ji Zhongguo Ke Xue Yuan Huan Jing Ke Xue Wei Yuan Hui "Huan Jing Ke Xue" Bian Ji Wei Yuan hui.] 2022, 43, 3269–3277. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, C.; Pei, J. Research on the Vulnerability Assessment of Karst Groundwater. Groundwater 2008, 30, 14–18. [Google Scholar] [CrossRef]
- Field, M.S. Investigating and Remediating Contaminated Karst Aquifers; Springer International Publishing: Cham, Switzerland, 2018; pp. 101–115. [Google Scholar]
- Priya, A.K.; Muthiah, M.; Ali, S.S.; Kornaros, M. Heavy Metals Removal from Contaminated Soil by Phytoremediation. Encyclopedia. 2023. Available online: https://encyclopedia.pub/entry/47036 (accessed on 2 June 2025).
- Randrianarivelo, M.; Zhou, W.; Barsa, M. Remedial investigations of karst aquifers: A case study at former Marietta Air Force Station, Lancaster County, Pennsylvania. Carbonates Evaporites 2019, 34, 233–247. [Google Scholar] [CrossRef]
- Byl, T.; Bradley, M.; Thomas, L.K.; Painter, R. Bioremediation Potential in Karst Aquifers of Tennessee and Kentucky; Springer International Publishing: Cham, Switzerland, 2018; pp. 97–100. [Google Scholar]
- Kumar, H.; Sahoo, P.K.; Mittal, S. Chapter 23—Sustainable remediation of heavy metals: A review of current status and its future prospects. In New Trends in Removal of Heavy Metals from Industrial Wastewater; Shah, M.P., Rodriguez Couto, S., Kumar, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 571–610. [Google Scholar] [CrossRef]
- Parmar, S.; Singh, V. Phytoremediation approaches for heavy metal pollution: A review. J. Plant Sci. Res. 2015, 2, 135. [Google Scholar]
- Swetha, N.; Rajasekar, B.; Hudge, B.V.; Mishra, P.; Harshitha, D.N. Phytoremediation of heavy metal contaminated soils using various flower and ornamentals. Int. J. Plant Soil Sci. 2023, 35, 747–752. [Google Scholar] [CrossRef]
- Shakeel, M.; Yaseen, T. An insight into phytoremediation of heavy metals from soil assisted by ancient fungi from glomeromycota-arbuscular mycorrhizal fungi. Sci. Technol. Dev. 2015, 34, 215–220. [Google Scholar] [CrossRef][Green Version]
- Zhu, G.; Zhao, J.; Chen, Q.; Guo, Q.; Cheng, D.; Bijaya, G.; Li, W. The comparative potential of four compositae plants for phytoremediation of karst lead/zinc mine tailings contaminated soil. Bioresources 2022, 17, 2997. [Google Scholar] [CrossRef]
- Sinha, R.K.; Herat, S.; Tandon, P. A review of phytoremediation as a cost-effective, ecologically sustainable and socially acceptable bioengineering technlogy. In National Environment Conference 2003; Environmental Engineering Society, Queensland Chapter: Brisbane, QLD, Australia, 2003; pp. 436–441. [Google Scholar]
- Shao, M.; Liu, Z.; Zeng, S.; Sun, H.; He, H.; Adnan, M.; Yan, J.; Shi, L.; Han, Y.; Lai, C.; et al. Carbon sinks associated with biological carbon pump in karst surface waters: Progress, challenges, and prospects. Environ. Res. 2025, 267, 120712. [Google Scholar] [CrossRef]
- Kalhor, K.; Ghasemizadeh, R.; Rajic, L.; Alshawabkeh, A. Assessment of groundwater quality and remediation in karst aquifers: A review. Groundw. Sustain. Dev. 2019, 8, 104–121. [Google Scholar] [CrossRef]
- Fryar, A.E. Chapter 2—Groundwater of carbonate aquifers. In Global Groundwater; Mukherjee, A., Scanlon, B.R., Aureli, A., Langan, S., Guo, H., McKenzie, A.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 23–34. [Google Scholar] [CrossRef]
- White, W.B. Hydrogeology of Karst Aquifers. In Encyclopedia of Caves, 2nd ed.; White, W.B., Culver, D.C., Eds.; Academic Press: Amsterdam, The Netherlands, 2012; pp. 383–391. [Google Scholar] [CrossRef]
- Ravbar, N. Variability of groundwater flow and transport processes in karst under different hydrologic conditions. Acta Carsologica 2013, 42, 327–338. [Google Scholar] [CrossRef]
- Ponta, G.M.L. Karst Hydrogeology. In Cave and Karst Systems of Romania; Ponta, G.M.L., Onac, B.P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 41–45. [Google Scholar] [CrossRef]
- Ghosh, S.; Selvakumar, G.; Kennedy Ajilda, A.A.; Webster, T.J. Chapter 10—Microbial biosorbents for heavy metal removal. In New Trends in Removal of Heavy Metals from Industrial Wastewater; Shah, M.P., Rodriguez Couto, S., Kumar, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 213–262. [Google Scholar] [CrossRef]
- Adesiyan, I.; Bisi-Johnson, M.; Aladesanmi, O.; Okoh, A.; Ogunfowokan, A. Concentrations and Human Health Risk of Heavy Metals in Rivers in Southwest Nigeria. J. Health Pollut. 2018, 8, 180907. [Google Scholar] [CrossRef]
- Zhang, W.; Xin, C.; Yu, S. A Review of Heavy Metal Migration and Its Influencing Factors in Karst Groundwater, Northern and Southern China. Water 2023, 15, 3690. [Google Scholar] [CrossRef]
- Mahler, B.J.; Personné, J.-C.; Lynch, F.L.; Van Metre, P.C. Sediment and Sediment-Associated Contaminant Transport Through Karst; Springer: Boston, MA, USA, 2004; pp. 23–46. [Google Scholar]
- Mahler, B.J.; Personne, J.-C.; Lynch, F.L.; Van Metre, P.C. Sediment And Sediment-Associated Contaminant Transport Through Karst. In Studies of Cave Sediments: Physical and Chemical Records of Paleoclimate; Sasowsky, I.D., Mylroie, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 23–46. [Google Scholar] [CrossRef]
- Polk, J.S.; Vanderhoff, S.; Groves, C.; Miller, B.; Bolster, C. Complex Epikarst Hydrologeology and Contaminant Transport In A South-Central Kentucky Karst Landscape. In Proceedings of the 16th International Congress of Speleology, Brno, Czech Republic, 21–28 July 2013; p. 110. [Google Scholar]
- Zhao, H.; Zhou, H.; Huang, K.; Pan, Y.; Peng, Y.; He, X.; Wang, S.; Wan, J. Epikarst Controls of Runoff Composition in Subterranean Stream After Rainstorm Events. Hydrol. Process. 2024, 38, e15305. [Google Scholar] [CrossRef]
- Ghasemizadeh, R.; Hellweger, F.; Butscher, C.; Padilla, I.; Vesper, D.; Field, M.; Alshawabkeh, A. Groundwater flow and transport modeling of karst aquifers, with particular reference to the North Coast Limestone aquifer system of Puerto Rico. Hydrogeol. J. 2012, 20, 1441. [Google Scholar] [CrossRef]
- Huang, C.; Elliott, H.; Ashmead, R. Interfacial reactions and the fate of heavy metals in soil-water systems. J. Water Pollut. Control Fed. 1977, 49, 745–756. [Google Scholar]
- Vesper, D.J. Contamination of Cave Waters by Heavy Metals. In Encyclopedia of Caves, 2nd ed.; White, W.B., Culver, D.C., Eds.; Academic Press: Amsterdam, The Netherlands, 2012; pp. 161–166. [Google Scholar] [CrossRef]
- Şenilă, M.; Levei, E.A.; Şenilă, L.R.; Oprea, G.M.; Roman, C.M. Mercury in soil and perennial plants in a mining-affected urban area from Northwestern Romania. J. Environ. Sci. Health Part A 2012, 47, 614–621. [Google Scholar] [CrossRef]
- Jiang, J.; Junlin, C.; Xiaoduo, O.; Haohao, L.; Wang, S. Prediction of heavy metal contamination in soil-groundwater systems at contaminated sites. Environ. Technol. 2024, 46, 3011–3023. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Sarkar, B.; Basak, B.; Mandal, S.; Biswas, B.; Srivastava, P. Soil mineralogical perspective on immobilization/mobilization of heavy metals. In Adaptive Soil Management: From Theory to Practices; Springer: Singapore, 2017; pp. 89–102. [Google Scholar]
- Li, X.; Schindler, M.; Zhou, J.; Samaradiwakara, S.; Wu, L. Interaction between metal (loid) s and soil mineral-organic matter associations. Crit. Rev. Environ. Sci. Technol. 2025, 55, 624–647. [Google Scholar] [CrossRef]
- Kretzschmar, R.; Schafer, T. Metal retention and transport on colloidal particles in the environment. Elements 2005, 1, 205–210. [Google Scholar] [CrossRef]
- Löv, Å. New Insights into Solubility Control Mechanisms and the Role of Particle-and Colloid-Facilitated Transport of Metals in Contaminated Soils; Acta Universitatis Agriculturae Sueciae: Uppsala, Sweden, 2018. [Google Scholar]
- Sparks, D.L. Kinetics and mechanisms of chemical reactions at the soil mineral/water interface. In Soil Physical Chemistry; CRC press: Boca Raton, FL, USA, 2018; pp. 135–192. [Google Scholar]
- Savenko, A.V. Experimental modeling of the immobilization of heavy metals at the carbonate adsorption–precipitation geochemical barrier. Geochem. Int. 2016, 54, 719–731. [Google Scholar] [CrossRef]
- Salminen, J.; Kobylin, P. Carbon Dioxide-Metal Carbonate Systems in Chemical Processes and Environmental Applications. ECS Meet. Abstr. 2006, MA2005-02, 1158. [Google Scholar] [CrossRef]
- Sun, J.; Tang, C.; Wu, P.; Liu, C.; Zhang, R. Migration of Cu, Zn, Cd and As in epikarst water affected by acid mine drainage at a coalfield basin, Xingren, Southwest China. Environ. Earth Sci. 2013, 69, 2623–2632. [Google Scholar] [CrossRef]
- Cidu, R.; Biddau, R.; Spano, T. Temporal Variations in Water Chemistry at Abandoned Underground Mines Hosted in a Carbonate Environment. Mine Water Environ. 2005, 24, 77–87. [Google Scholar] [CrossRef]
- Lee, M.-K.; Saunders, J. Effects of pH on Metals Precipitation and Sorption: Field Bioremediation and Geochemical Modeling Approaches. Vadose Zone J. 2003, 2, 177–185. [Google Scholar] [CrossRef]
- Özler, H.M. Carbonate weathering and connate seawater influencing karst groundwaters in the Gevas–Gurpinar–Güzelsu basins, Turkey. Environ. Earth Sci. 2010, 61, 323–340. [Google Scholar] [CrossRef]
- Li Vigni, L.; Daskalopoulou, K.; Calabrese, S.; Cardellini, C.; Kyriakopoulos, K.; Ionescu, A.; Brugnone, F.; Parello, F.; D’Alessandro, W. Geochemical Characterization of Groundwater Quality in Hellenic Karst Systems; Bulletin of the Geological Society of Greece: Athens, Greece, 2019; pp. 324–325. Available online: https://hdl.handle.net/10447/389577 (accessed on 2 June 2025).
- Xiao, Y.; Shao, J.; Frape, S.; Cui, Y.; Dang, X.; Wang, S.; Ji, Y. Groundwater origin, flow regime and geochemical evolution in arid endorheic watersheds: A case study from the Qaidam Basin, Northwest China. Hydrol. Earth Syst. Sci. Discuss. 2018, 22, 4381–4400. [Google Scholar] [CrossRef]
- Omar, K.; Vilcáez, J. Transport of Ba, Sr, Cd, Pb, and as in dolomite saline aquifers injected with petroleum produced water. Geoenergy Sci. Eng. 2023, 231, 212342. [Google Scholar] [CrossRef]
- Vishnu, D.; Dhandapani, B.; Senthil Kumar, K.; Balaji, G.; Mahadevan, S. Chapter 8—Removal of heavy metals from mine waters by natural zeolites. In New Trends in Removal of Heavy Metals from Industrial Wastewater; Shah, M.P., Rodriguez Couto, S., Kumar, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 161–175. [Google Scholar] [CrossRef]
- Ansari, M.I.; Malik, A. Biosorption of nickel and cadmium by metal resistant bacterial isolates from agricultural soil irrigated with industrial wastewater. Bioresour. Technol. 2007, 98, 3149–3153. [Google Scholar] [CrossRef]
- Cristani, M.; Naccari, C.; Nostro, A.; Pizzimenti, A.; Trombetta, D.; Pizzimenti, F. Possible use of Serratia marcescens in toxic metal biosorption (removal). Environ. Sci. Pollut. Res. 2012, 19, 161–168. [Google Scholar] [CrossRef]
- Wu, H.; Wu, Q.; Wu, G.; Gu, Q.; Wei, L.; Kothe, E. Cd-Resistant Strains of B. cereus S5 with Endurance Capacity and Their Capacities for Cadmium Removal from Cadmium-Polluted Water. PLoS ONE 2016, 11, e0151479. [Google Scholar] [CrossRef]
- Ozdemir, S.; Kilinc, E.; Celik, K.S.; Okumus, V.; Soylak, M. Simultaneous preconcentrations of Co2+, Cr6+, Hg2+ and Pb2+ ions by Bacillus altitudinis immobilized nanodiamond prior to their determinations in food samples by ICP-OES. Food Chem. 2017, 215, 447–453. [Google Scholar] [CrossRef]
- Tajer-Mohammad-Ghazvini, P.; Kasra-Kermanshahi, R.; Nozad-Golikand, A.; Sadeghizadeh, M.; Ghorbanzadeh-Mashkani, S.; Dabbagh, R. Cobalt separation by Alphaproteobacterium MTB-KTN90: Magnetotactic bacteria in bioremediation. Bioprocess Biosyst. Eng. 2016, 39, 1899–1911. [Google Scholar] [CrossRef]
- Yalçın, M.S.; Özdemir, S.; Kılınç, E. Preconcentrations of Ni(II) and Co(II) by using immobilized thermophilic Geobacillus stearothermophilus SO-20 before ICP-OES determinations. Food Chem. 2018, 266, 126–132. [Google Scholar] [CrossRef]
- Zárate, A.M.; Florez, J.Z.; Angulo, E.; Varela-Prieto, L.; Infante, C.; Barrios, F.; Barraza, B.; Gallardo, D.; Valdés, J. Burkholderia tropica as a Potential Microalgal Growth-Promoting Bacterium in the Biosorption of Mercury from Aqueous Solutions. J. Microbiol. Biotechnol. 2017, 27, 1138–1149. [Google Scholar] [CrossRef]
- Mondal, P.; Majumder, C.; Mohanty, B. Treatment of arsenic contaminated water in a batch reactor by using Ralstonia eutropha MTCC 2487 and granular activated carbon. J. Hazard. Mater. 2008, 153, 588–599. [Google Scholar] [CrossRef]
- Prasad, K.S.; Ramanathan, A.L.; Paul, J.; Subramanian, V.; Prasad, R. Biosorption of arsenite (As+3) and arsenate (As+5) from aqueous solution by Arthrobacter sp biomass. Environ. Technol. 2013, 34, 2701–2708. [Google Scholar] [CrossRef]
- Podder, M.; Majumder, C. Corynebacterium glutamicum MTCC 2745 immobilized on granular activated carbon/MnFe2O4 composite: A novel biosorbent for removal of As(III) and As(V) ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 168, 159–179. [Google Scholar] [CrossRef] [PubMed]
- Asadi Haris, S.; Altowayti, W.A.H.; Ibrahim, Z.; Shahir, S. Arsenic biosorption using pretreated biomass of psychrotolerant Yersinia sp. strain SOM-12D3 isolated from Svalbard, Arctic. Environ. Sci. Pollut. Res. 2018, 25, 27959–27970. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Liu, H.; Zhao, Z.; Long, J.; Li, J.; Jiang, C.; Rao, C. Spatial Distribution and Migration of Cadmium in Contaminated Soils Associated with a Geochemical Anomaly: A Case Study in Southwestern China. Pol. J. Environ. Stud. 2019, 28, 3799–3807. [Google Scholar] [CrossRef] [PubMed]
- Mijošek, T.; Kljaković-Gašpić, Z.; Kralj, T.; Valić, D.; Redžović, Z.; Šariri, S.; Karamatić, I.; Filipović Marijić, V. Spatial and temporal variability of dissolved metal(loid)s in water of the karst ecosystem: Consequences of long-term exposure to wastewaters. Environ. Technol. Innov. 2023, 32, 103254. [Google Scholar] [CrossRef]
- Liao, H.-W.; Jiang, Z.-C.; Zhou, H.; Qin, X.-Q.; Huang, Q.-B.; Zhong, L.; Pu, Z.-G. Dissolved Heavy Metal Pollution and Assessment of a Karst Basin around a Mine, Southwest China. Int. J. Environ. Res. Public Health 2022, 19, 14293. [Google Scholar] [CrossRef]
- Li, W.; Zhu, T.; Yang, H.; Zhang, C.; Zou, X. Distribution Characteristics and Risk Assessment of Heavy Metals in Soils of the Typical Karst and Non-Karst Areas. Land 2022, 11, 1346. [Google Scholar] [CrossRef]
- Qin, W.; Han, D.; Song, X.; Liu, S. Sources and migration of heavy metals in a karst water system under the threats of an abandoned Pb–Zn mine, Southwest China. Environ. Pollut. 2021, 277, 116774. [Google Scholar] [CrossRef]
- Weissmannová, H.D.; Pavlovský, J. Indices of soil contamination by heavy metals—Methodology of calculation for pollution assessment (minireview). Environ. Monit. Assess. 2017, 189, 616. [Google Scholar] [CrossRef]
- Roy, S.; Gupta, S.K.; Prakash, J.; Habib, G.; Kumar, P. A global perspective of the current state of heavy metal contamination in road dust. Environ. Sci. Pollut. Res. 2022, 29, 33230–33251. [Google Scholar] [CrossRef]
- Van Beynen, P.; Townsend, K. A disturbance index for karst environments. Environ. Manag. 2005, 36, 101–116. [Google Scholar] [CrossRef]
- Mazzei, M.; Parise, M. On the implementation of environmental indices in karst. In Karst Groundwater Contamination and Public Health: Beyond Case Studies; Springer: Cham, Switzerland, 2017; pp. 245–247. [Google Scholar]
- Ding, R.; Wei, D.; Wu, Y.; Liao, Z.; Lu, Y.; Chen, Z.; Gao, H.; Xu, H.; Hu, H. Profound regional disparities shaping the ecological risk in surface waters: A case study on cadmium across China. J. Hazard. Mater. 2024, 465, 133450. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.A.; Martin, J.M.; Michelangeli, M.; Thoré, E.S.; Sandoval-Herrera, N.; McCallum, E.S.; Szabo, D.; Callahan, D.L.; Clark, T.D.; Bertram, M.G. Advancing the spatiotemporal dimension of wildlife–pollution interactions. Environ. Sci. Technol. Lett. 2025, 12, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Fritz, S.A.; Bininda-Emonds, O.R.; Purvis, A. Geographical variation in predictors of mammalian extinction risk: Big is bad, but only in the tropics. Ecol. Lett. 2009, 12, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Munyai, R.; Ogola, H.J.O.; Modise, D.M. Microbial Community Diversity Dynamics in Acid Mine Drainage and Acid Mine Drainage-Polluted Soils: Implication on Mining Water Irrigation Agricultural Sustainability. Front. Sustain. Food Syst. 2021, 5, 701870. [Google Scholar] [CrossRef]
- Xu, D.; Wang, Y.; Zhang, R.; Guo, J.; Zhang, W.; Yu, K. Distribution, speciation, environmental risk, and source identification of heavy metals in surface sediments from the karst aquatic environment of the Lijiang River, Southwest China. Environ. Sci. Pollut. Res. 2016, 23, 9122–9133. [Google Scholar] [CrossRef]
- Liu, D.; Tian, C.; Chen, X.; Zhang, W.; Zhang, X.; Wang, Z.; Xu, D.; Chang, Y. Insights into karst groundwater hydrogeochemical characteristics and spatial evolution in the Jinan karst aquifer system, northern China. Water Supply 2023, 23, 5004–5016. [Google Scholar] [CrossRef]
- Chen, X.; Wu, P.; Liu, H.; Li, X. Source apportionment of heavy metal(loid)s in sediments of a typical karst mountain drinking-water reservoir and the associated risk assessment based on chemical speciations. Environ. Geochem. Health 2023, 45, 7585–7601. [Google Scholar] [CrossRef]
- Hamdan, I. Characterization of Groundwater Flow and Vulnerability Assessment of Karstic Aquifers—Development of a Travel Time Based Approach and Application to the Tanour and Rasoun Spring Catchment (Ajloun, NW-Jordan). Ph.D. Thesis, Göttingen University, Göttingen, Germany, 2016. [Google Scholar] [CrossRef]
- Henry, H.F.; Suk, W.A. Public health and karst groundwater contamination: From multidisciplinary research to exposure prevention. In Karst Groundwater Contamination and Public Health: Beyond Case Studies; Springer: Cham, Switzerland, 2017; pp. 7–14. [Google Scholar]
- Bo, L.; Yi-Fan, Z.; Bei-Bei, Z.; Xian-Qing, W. A risk evaluation model for karst groundwater pollution based on geographic information system and artificial neural network applications. Environ. Earth Sci. 2018, 77, 344. [Google Scholar] [CrossRef]
- Monneron-Gyurits, M.; Soubrand, M.; Joussein, E.; Courtin-Nomade, A.; Jubany, I.; Casas, S.; Bahí, N.; Faz, A.; Gabarrón, M.; Acosta, J.A. Investigating the relationship between speciation and oral/lung bioaccessibility of a highly contaminated tailing: Contribution in health risk assessment. Environ. Sci. Pollut. Res. 2020, 27, 40732–40748. [Google Scholar] [CrossRef]
- Veselic, M. Groundwater pollution control in fractured and karstified rocks. In Advanced Methods for Groundwater Pollution Control; Springer: Vienna, Austria, 1995; pp. 235–244. [Google Scholar]
- Sherlala, A.I.A.; Raman, A.A.A.; Bello, M.M.; Asghar, A. A review of the applications of organo-functionalized magnetic graphene oxide nanocomposites for heavy metal adsorption. Chemosphere 2018, 193, 1004–1017. [Google Scholar] [CrossRef]
- Reuther, R. (Ed.) Geochemical Speciation: Does it Help to Assess and Engineer the Impact of Metals? In Geochemical Approaches to Environmental Engineering of Metals; Springer: Berlin/Heidelberg, Germany, 1996; pp. 25–32. [Google Scholar] [CrossRef]
- Fytianos, K. Speciation Analysis of Heavy Metals in Natural Waters: A Review. J. AOAC Int. 2019, 84, 1763–1769. [Google Scholar] [CrossRef]
- Adamo, P.; Agrelli, D.; Zampella, M.; Caporale, A. Chemical speciation to assess bioavailability, bioaccessibility, and geochemical forms of potentially toxic metals (PTMs) in polluted soils. In Environmental Geochemistry, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 211–269. [Google Scholar] [CrossRef]
- Hao, Y.; Miao, X.; Liu, H.; Miao, D. The Variation of Heavy Metals Bioavailability in Sediments of Liujiang River Basin, SW China Associated to Their Speciations and Environmental Fluctuations, a Field Study in Typical Karstic River. Int. J. Environ. Res. Public Health 2021, 18, 3986. [Google Scholar] [CrossRef]
- Strotmann, U.; Durand, M.-J.; Thouand, G.; Eberlein, C.; Heipieper, H.J.; Gartiser, S.; Pagga, U. Microbiological toxicity tests using standardized ISO/OECD methods—Current state and outlook. Appl. Microbiol. Biotechnol. 2024, 108, 454. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Rehman, Y. Mechanisms of Toxicity of Heavy Metals and the Microbial Strategies for their Mitigation: A Review. J. Microbiol. Mol. Genet. 2024, 5, 45–63. [Google Scholar] [CrossRef]
- Hu, Z.-X.; Wu, Z.-Y.; Luo, W.-Q.; Xie, Y.-Q. Content, Sources, and Ecological Risk Assessment of Heavy Metals in Soil of Typical Karst County. Huan Jing Ke Xue=Huanjing Kexue 2024, 45, 5506–5516. [Google Scholar] [PubMed]
- Wang, S.; Fang, L.; Dapaah, M.F.; Niu, Q.; Cheng, L. Bio-remediation of heavy metal-contaminated soil by microbial-induced carbonate precipitation (MICP)—A critical review. Sustainability 2023, 15, 7622. [Google Scholar] [CrossRef]
- Helf, K.L. Mercury and methylmercury in the South Central Kentucky Karst: Its transportation, accumulation, and potential effects on vulnerable biota. In Proceedings of the 2003 National Cave and Karst Management Symposium, Gainesville, FL, USA, 13–17 October 2003. [Google Scholar]
- Allen, H.E.; Janssen, C.R. Incorporating bioavailability into criteria for metals. In Soil and Water Pollution Monitoring, Protection and Remediation; Springer: Dordrecht, The Netherlands, 2006; pp. 93–105. [Google Scholar]
- Morgan, A.J.; Kille, P.; Stürzenbaum, S.R. Microevolution and ecotoxicology of metals in invertebrates. Environ. Sci. Technol. 2007, 41, 1085–1096. [Google Scholar] [CrossRef]
- Roggatz, C.; Fletcher, N.; Benoit, D.; Algar, A.; Doroff, A.; Wright, B.; Wollenberg Valero, K.; Hardege, J. Saxitoxin and tetrodotoxin bioavailability increases in future oceans. Nat. Clim. Change 2019, 9, 840–844. [Google Scholar] [CrossRef]
- Reichardt, W. Ecotoxicity of certain heavy metals affecting bacteria-mediated biogeochemical pathways in sediments. In Sediments and Toxic Substances: Environmental Effects and Ecotoxicity; Springer: Berlin/Heidelberg, Germany, 1996; pp. 159–178. [Google Scholar]
- Cairns, J. The threshold problem in ecotoxicology. Ecotoxicology 1992, 1, 3–16. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Han, Q.; Adnan, M.; Li, M.; Wang, M.; Wang, M.; Jiang, F.; Feng, X. Global Environmental Geochemistry and Molecular Speciation of Heavy Metals in Soils and Groundwater from Abandoned Smelting Sites: Analysis of the Contamination Dynamics and Remediation Alternatives in Karst Settings. Toxics 2025, 13, 608. https://doi.org/10.3390/toxics13070608
Xu H, Han Q, Adnan M, Li M, Wang M, Wang M, Jiang F, Feng X. Global Environmental Geochemistry and Molecular Speciation of Heavy Metals in Soils and Groundwater from Abandoned Smelting Sites: Analysis of the Contamination Dynamics and Remediation Alternatives in Karst Settings. Toxics. 2025; 13(7):608. https://doi.org/10.3390/toxics13070608
Chicago/Turabian StyleXu, Hang, Qiao Han, Muhammad Adnan, Mengfei Li, Mingshi Wang, Mingya Wang, Fengcheng Jiang, and Xixi Feng. 2025. "Global Environmental Geochemistry and Molecular Speciation of Heavy Metals in Soils and Groundwater from Abandoned Smelting Sites: Analysis of the Contamination Dynamics and Remediation Alternatives in Karst Settings" Toxics 13, no. 7: 608. https://doi.org/10.3390/toxics13070608
APA StyleXu, H., Han, Q., Adnan, M., Li, M., Wang, M., Wang, M., Jiang, F., & Feng, X. (2025). Global Environmental Geochemistry and Molecular Speciation of Heavy Metals in Soils and Groundwater from Abandoned Smelting Sites: Analysis of the Contamination Dynamics and Remediation Alternatives in Karst Settings. Toxics, 13(7), 608. https://doi.org/10.3390/toxics13070608







