Nephrotoxicity of New Antibiotics: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Data Sources and Eligibility Criteria
2.2. Search Strategy
2.3. Screening and Data Collection Process
2.4. Risk of Bias Assessment
2.5. Data Synthesis
2.6. Adherence to PRISMA Guidelines
3. Results
3.1. Search Results
3.2. β-Lactams and β-Lactam/β-Lactamase Inhibitor Combination Antibiotics
3.3. Fluoroquinolones
3.4. Other Drugs
3.5. Risk of Bias Assessment
4. Discussion
4.1. Summary and Interpretation of the Results
4.2. Strengths and Limitations
4.3. Scarcity of Post-Marketing Published Studies on Safety
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADR | Adverse drug reaction |
| AKI | Acute kidney injury |
| CDSCO | Central Drugs Standard Control Organization |
| CKD | Chronic kidney disease |
| EMA | European Medicines Agency |
| FAERS | FDA Adverse Event Reporting System |
| FDA | Food and Drug Administration |
| KDIGO | Kidney disease|improving global outcomes |
| NMPA | National Medical Products Administration |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| PMDA | Pharmaceuticals and Medical Devices Agency |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCTs | Randomized controlled trials |
References
- Jones, A.W. Early Drug Discovery and the Rise of Pharmaceutical Chemistry. Drug Test. Anal. 2011, 3, 337–344. [Google Scholar] [CrossRef]
- Mohs, R.C.; Greig, N.H. Drug Discovery and Development: Role of Basic Biological Research. Alzheimers Dement. Transl. Res. Clin. Interv. 2017, 3, 651–657. [Google Scholar] [CrossRef]
- Liebler, D.C.; Guengerich, F.P. Elucidating Mechanisms of Drug-Induced Toxicity. Nat. Rev. Drug Discov. 2005, 4, 410–420. [Google Scholar] [CrossRef]
- Campbell, R.E.; Chen, C.H.; Edelstein, C.L. Overview of Antibiotic-Induced Nephrotoxicity. Kidney Int. Rep. 2023, 8, 2211–2225. [Google Scholar] [CrossRef]
- Perazella, M.A. Pharmacology behind Common Drug Nephrotoxicities. Clin. J. Am. Soc. Nephrol. 2018, 13, 1897–1908. [Google Scholar] [CrossRef]
- Connor, S.; Roberts, R.A.; Tong, W. Drug-Induced Kidney Injury: Challenges and Opportunities. Toxicol. Res. 2024, 13, tfae119. [Google Scholar] [CrossRef]
- Appel, G.B. Aminoglycoside Nephrotoxicity. Am. J. Med. 1990, 88, 16S–20S; discussion 38S–42S. [Google Scholar] [CrossRef]
- Sanchez-Alamo, B.; Cases-Corona, C.; Fernandez-Juarez, G. Facing the Challenge of Drug-Induced Acute Interstitial Nephritis. Nephron 2023, 147, 78–90. [Google Scholar] [CrossRef]
- Morales-Alvarez, M.C. Nephrotoxicity of Antimicrobials and Antibiotics. Adv. Chronic Kidney Dis. 2020, 27, 31–37. [Google Scholar] [CrossRef]
- Morrison, L.; Zembower, T.R. Antimicrobial Resistance. Gastrointest. Endosc. Clin. N. Am. 2020, 30, 619–635. [Google Scholar] [CrossRef]
- Patek, T.M.; Teng, C.; Kennedy, K.E.; Alvarez, C.A.; Frei, C.R. Comparing Acute Kidney Injury Reports Among Antibiotics: A Pharmacovigilance Study of the FDA Adverse Event Reporting System (FAERS). Drug Saf. 2020, 43, 17–22. [Google Scholar] [CrossRef]
- Roughead, E.E.; Kerr, M.; Moffat, A.; Kassie, G.M.; Pratt, N. Medicine-Induced Acute Kidney Injury Findings from Spontaneous Reporting Systems, Sequence Symmetry Analysis and a Case-Control Study with a Focus on Medicines Used in Primary Care. Drug Saf. 2022, 45, 1413–1421. [Google Scholar] [CrossRef]
- Baptista, A.; Marreiros, A.; Macedo, A.; Coelho, A. Drug-Associated Acute Kidney Disease: Data from a World Pharmacovigilance Database. Cureus 2024, 16, e63636. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Keam, S.J. Cefepime/Enmetazobactam: First Approval. Drugs 2024, 84, 737–744. [Google Scholar] [CrossRef]
- Bassetti, M.; Ariyasu, M.; Binkowitz, B.; Nagata, T.D.; Echols, R.M.; Matsunaga, Y.; Toyoizumi, K.; Doi, Y. Designing A Pathogen-Focused Study to Address the High Unmet Medical Need Represented by Carbapenem-Resistant Gram-Negative Pathogens—The International, Multicenter, Randomized, Open-Label, Phase 3 CREDIBLE-CR Study. Infect. Drug Resist. 2019, 12, 3607–3623. [Google Scholar] [CrossRef]
- Lin, H.; Zhu, C.; Liu, S.; Bi, Y.; Hu, J.; Ju, M. Post-Market Safety Profile of Cefiderocol: A Real-World Pharmacovigilance Exploratory Analysis Based on U.S. FDA Adverse Event Reporting System (FAERS). BMC Pharmacol. Toxicol. 2025, 26, 58. [Google Scholar] [CrossRef]
- Mazzitelli, M.; Gregori, D.; Sasset, L.; Trevenzoli, M.; Scaglione, V.; Lo Menzo, S.; Marinello, S.; Mengato, D.; Venturini, F.; Tiberio, I.; et al. Cefiderocol-Based versus Colistin-Based Regimens for Severe Carbapenem-Resistant Acinetobacter Baumannii Infections: A Propensity Score-Weighted, Retrospective Cohort Study during the First Two Years of the COVID-19 Pandemic. Microorganisms 2023, 11, 984. [Google Scholar] [CrossRef]
- Risco-Risco, C.; Henriquez-Camacho, C.; Herrera-Rueda, M.; Barberán, J.; Andaluz-Ojeda, D. Cefiderocol Versus Best Available Therapy in the Treatment of Critically Ill Patients with Severe Infections Due to Resistant Gram-Negative Bacteria: A Systematic Review and Meta-Analysis. Antibiotics 2024, 13, 1048. [Google Scholar] [CrossRef]
- Viscardi, S.; Topola, E.; Sobieraj, J.; Duda-Madej, A. Novel Siderophore Cephalosporin and Combinations of Cephalosporins with β-Lactamase Inhibitors as an Advancement in Treatment of Ventilator-Associated Pneumonia. Antibiotics 2024, 13, 445. [Google Scholar] [CrossRef]
- Weaver, K.; Stallworth, K.; Blakely, K.K. Cefiderocol for Infections Caused by Multidrug-Resistant Gram-Negative Bacteria. Nurs. Womens Health 2020, 24, 377–382. [Google Scholar] [CrossRef]
- Wu, J.Y.; Srinivas, P.; Pogue, J.M. Cefiderocol: A Novel Agent for the Management of Multidrug-Resistant Gram-Negative Organisms. Infect. Dis. Ther. 2020, 9, 17–40. [Google Scholar] [CrossRef]
- Deitchman, A.N.; De Jong, D.; Barbour, A.M.; Derendorf, H. Ceftobiprole Medocaril (BAL-5788) for the Treatment of Complicated Skin Infections. Expert Rev. Anti Infect. Ther. 2016, 14, 997–1006. [Google Scholar] [CrossRef]
- Deresinski, S.C. The Efficacy and Safety of Ceftobiprole in the Treatment of Complicated Skin and Skin Structure Infections: Evidence from 2 Clinical Trials. Diagn. Microbiol. Infect. Dis. 2008, 61, 103–109. [Google Scholar] [CrossRef]
- Falcó, V.; Burgos, J.; Almirante, B. Ceftobiprole Medocaril for the Treatment of Community-Acquired Pneumonia. Expert Opin. Pharmacother. 2018, 19, 1503–1509. [Google Scholar] [CrossRef]
- Huang, H.; Gao, L.; Engelhardt, M.; Saulay, M.; Hamed, K. A Post Hoc Analysis of Two Phase III Trials Showing the Efficacy and Tolerability of Ceftobiprole in East Asian Patients. Future Microbiol. 2021, 16, 783–796. [Google Scholar] [CrossRef]
- Scheeren, T.W. Ceftobiprole Medocaril in the Treatment of Hospital-Acquired Pneumonia. Future Microbiol. 2015, 10, 1913–1928. [Google Scholar] [CrossRef]
- Welte, T.; Scheeren, T.W.; Overcash, J.S.; Saulay, M.; Engelhardt, M.; Hamed, K. Efficacy and Safety of Ceftobiprole in Patients Aged 65 Years or Older: A Post Hoc Analysis of Three Phase III Studies. Future Microbiol. 2021, 16, 543–555. [Google Scholar] [CrossRef]
- Zimmerman, J.; Giuliano, C.; Kale-Pradhan, P.B. Ceftobiprole Medocaril: A New Fifth-Generation Cephalosporin. Ann. Pharmacother. 2025, 59, 657–665. [Google Scholar] [CrossRef]
- Kaul, G.; Dasgupta, A.; Chopra, S. Contezolid in Complicated Skin and Soft Tissue Infection. Drugs Today 2022, 58, 315–326. [Google Scholar] [CrossRef]
- Yang, M.; Zhan, S.; Fu, L.; Wang, Y.; Zhang, P.; Deng, G. Prospects of Contezolid (MRX-I) against Multidrug-Resistant Tuberculosis and Extensively Drug-Resistant Tuberculosis. Drug Discov. Ther. 2022, 16, 99–101. [Google Scholar] [CrossRef]
- Yuan, H.; Wu, H.; Zhang, Y.; Huang, H.; Li, Y.; Wu, J.; Cao, G.; Yu, J.; Guo, B.; Wu, J.; et al. Clinical Pharmacology and Utility of Contezolid in Chinese Patients with Complicated Skin and Soft-Tissue Infections. Antimicrob. Agents Chemother. 2022, 66, e02430-21. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.R.; Scangarella-Oman, N.E.; Millns, H.; Flight, W.; Gatsi, S.; Jakielaszek, C.; Janmohamed, S.; Lewis, D.A. Efficacy and Safety of Gepotidacin as Treatment of Uncomplicated Urogenital Gonorrhea (EAGLE-1): Design of a Randomized, Comparator-Controlled, Phase 3 Study. Infect. Dis. Ther. 2023, 12, 2307–2320. [Google Scholar] [CrossRef] [PubMed]
- Geddes, A.M.; Stille, W. Imipenem: The First Thienamycin Antibiotic. Clin. Infect. Dis. 1985, 7, S353–S356. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.L.; Rhee, E.G.; Jumes, P.A.; Gotfried, M.H.; Zhao, T.; Mangin, E.; Bi, S.; Chavez-Eng, C.M.; Zhang, Z.; Butterton, J.R. Intrapulmonary Pharmacokinetics of Relebactam, a Novel β-Lactamase Inhibitor, Dosed in Combination with Imipenem-Cilastatin in Healthy Subjects. Antimicrob. Agents Chemother. 2018, 62, e01411-17. [Google Scholar] [CrossRef]
- Rusu, A.; Munteanu, A.-C.; Arbănași, E.-M.; Uivarosi, V. Overview of Side-Effects of Antibacterial Fluoroquinolones: New Drugs versus Old Drugs, a Step Forward in the Safety Profile? Pharmaceutics 2023, 15, 804. [Google Scholar] [CrossRef]
- Eraikhuemen, N.; Julien, D.; Kelly, A.; Lindsay, T.; Lazaridis, D. Treatment of Community-Acquired Pneumonia: A Focus on Lefamulin. Infect. Dis. Ther. 2021, 10, 149–163. [Google Scholar] [CrossRef]
- File, T.M.; Alexander, E.; Goldberg, L.; Das, A.F.; Sandrock, C.; Paukner, S.; Moran, G.J. Lefamulin Efficacy and Safety in a Pooled Phase 3 Clinical Trial Population with Community-Acquired Bacterial Pneumonia and Common Clinical Comorbidities. BMC Pulm. Med. 2021, 21, 154. [Google Scholar] [CrossRef]
- LoVecchio, F.; Schranz, J.; Alexander, E.; Mariano, D.; Meads, A.; Sandrock, C.; Moran, G.J.; Giordano, P.A. Oral 5-Day Lefamulin for Outpatient Management of Community-Acquired Bacterial Pneumonia: Post-Hoc Analysis of the Lefamulin Evaluation Against Pneumonia (LEAP) 2 Trial. J. Emerg. Med. 2021, 60, 781–792. [Google Scholar] [CrossRef]
- Paukner, S.; Mariano, D.; Das, A.F.; Moran, G.J.; Sandrock, C.; Waites, K.B.; File, T.M. Lefamulin in Patients with Community-Acquired Bacterial Pneumonia Caused by Atypical Respiratory Pathogens: Pooled Results from Two Phase 3 Trials. Antibiotics 2021, 10, 1489. [Google Scholar] [CrossRef]
- Tang, H.-J.; Wang, J.-H.; Lai, C.-C. Lefamulin vs Moxifloxacin for Community-Acquired Bacterial Pneumonia. Medicine 2020, 99, e21223. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Deng, C.; Zelenitsky, S.; Lawrence, C.K.; Adam, H.J.; Golden, A.; Berry, L.; Schweizer, F.; Zhanel, M.A.; Irfan, N.; et al. Lefamulin: A Novel Oral and Intravenous Pleuromutilin for the Treatment of Community-Acquired Bacterial Pneumonia. Drugs 2021, 81, 233–256. [Google Scholar] [CrossRef]
- Earle, W.; Bonegio, R.G.B.; Smith, D.B.; Branch-Elliman, W. Plazomicin for the Treatment of Multidrug-Resistant Klebsiella Bacteraemia in a Patient with Underlying Chronic Kidney Disease and Acute Renal Failure Requiring Renal Replacement Therapy. BMJ Case Rep. 2021, 14, e243609. [Google Scholar] [CrossRef] [PubMed]
- Komirenko, A.S.; Riddle, V.; Gibbons, J.A.; Van Wart, S.; Seroogy, J.D. A Phase 1 Study To Assess the Pharmacokinetics of Intravenous Plazomicin in Adult Subjects with Varying Degrees of Renal Function. Antimicrob. Agents Chemother. 2018, 62, e01128-18. [Google Scholar] [CrossRef] [PubMed]
- Trang, M.; Seroogy, J.D.; Van Wart, S.A.; Bhavnani, S.M.; Kim, A.; Gibbons, J.A.; Ambrose, P.G.; Rubino, C.M. Population Pharmacokinetic Analyses for Plazomicin Using Pooled Data from Phase 1, 2, and 3 Clinical Studies. Antimicrob. Agents Chemother. 2019, 63, e02329-18. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Lawson, C.D.; Zelenitsky, S.; Findlay, B.; Schweizer, F.; Adam, H.; Walkty, A.; Rubinstein, E.; Gin, A.S.; Hoban, D.J.; et al. Comparison of the Next-Generation Aminoglycoside Plazomicin to Gentamicin, Tobramycin and Amikacin. Expert Rev. Anti Infect. Ther. 2012, 10, 459–473. [Google Scholar] [CrossRef]
- Lickliter, J.D.; Lawrence, K.; O’Donnell, J.; Isaacs, R. Safety, Pharmacokinetics, and Drug-Drug Interaction Potential of Intravenous Durlobactam, a β-Lactamase Inhibitor, in Healthy Subjects. Antimicrob. Agents Chemother. 2020, 64, e00071-20. [Google Scholar] [CrossRef]
- Watkins, R.R.; Du, B.; Isaacs, R.; Altarac, D. Pathogen-Targeted Clinical Development to Address Unmet Medical Need: Design, Safety, and Efficacy of the ATTACK Trial. Clin. Infect. Dis. 2023, 76, S210–S214. [Google Scholar] [CrossRef]
- Hsueh, S.-C.; Chao, C.-M.; Wang, C.-Y.; Lai, C.-C.; Chen, C.-H. Clinical Efficacy and Safety of Cefiderocol in the Treatment of Acute Bacterial Infections: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. J. Glob. Antimicrob. Resist. 2021, 24, 376–382. [Google Scholar] [CrossRef]
- Noel, G.J. Clinical Profile of Ceftobiprole, a Novel β-Lactam Antibiotic. Clin. Microbiol. Infect. 2007, 13, 25–29. [Google Scholar] [CrossRef]
- Rizvi, M.W.; Shujatullah, F.; Malik, A.; Khan, H.M. Ceftobiprole-A Novel Cephalosporin to Combat MRSA. East. J. Med. 2013, 16, 1–8. [Google Scholar]
- Cornely, O.A.; Cisneros, J.M.; Torre-Cisneros, J.; Rodríguez-Hernández, M.J.; Tallón-Aguilar, L.; Calbo, E.; Horcajada, J.P.; Queckenberg, C.; Zettelmeyer, U.; Arenz, D.; et al. Pharmacokinetics and Safety of Aztreonam/Avibactam for the Treatment of Complicated Intra-Abdominal Infections in Hospitalized Adults: Results from the REJUVENATE Study. J. Antimicrob. Chemother. 2020, 75, 618–627. [Google Scholar] [CrossRef]
- Carmeli, Y.; Cisneros, J.M.; Paul, M.; Daikos, G.L.; Wang, M.; Torre-Cisneros, J.; Singer, G.; Titov, I.; Gumenchuk, I.; Zhao, Y.; et al. Aztreonam-Avibactam versus Meropenem for the Treatment of Serious Infections Caused by Gram-Negative Bacteria (REVISIT): A Descriptive, Multinational, Open-Label, Phase 3, Randomised Trial. Lancet Infect. Dis. 2025, 25, 218–230. [Google Scholar] [CrossRef]
- Pfizer. A Prospective, Randomized, Open-Label, Comparative Study to Assess the Efficacy, Safety and Tolerability of Aztreonam-Avibactam (Atm-Avi) and Best Available Therapy for the Treatment of Serious Infections Due to Multi-Drug-Resistant Gram-Negative Bacteria Producing Metallo-Β-Lactamase (MBL); clinicaltrials.gov; National Institute of Health (NIH): Bethesda, MD, USA, 2024. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2017-004544-38/results (accessed on 1 May 2025).
- Das, S.; Fitzgerald, R.; Ullah, A.; Bula, M.; Collins, A.M.; Mitsi, E.; Reine, J.; Hill, H.; Rylance, J.; Ferreira, D.M.; et al. Intrapulmonary Pharmacokinetics of Cefepime and Enmetazobactam in Healthy Volunteers: Towards New Treatments for Nosocomial Pneumonia. Antimicrob. Agents Chemother. 2020, 65, e01468-20. [Google Scholar] [CrossRef] [PubMed]
- Kaye, K.S.; Belley, A.; Barth, P.; Lahlou, O.; Knechtle, P.; Motta, P.; Velicitat, P. Effect of Cefepime/Enmetazobactam vs Piperacillin/Tazobactam on Clinical Cure and Microbiological Eradication in Patients with Complicated Urinary Tract Infection or Acute Pyelonephritis: A Randomized Clinical Trial. JAMA 2022, 328, 1304. [Google Scholar] [CrossRef] [PubMed]
- Saisho, Y.; Katsube, T.; White, S.; Fukase, H.; Shimada, J. Pharmacokinetics, Safety, and Tolerability of Cefiderocol, a Novel Siderophore Cephalosporin for Gram-Negative Bacteria, in Healthy Subjects. Antimicrob. Agents Chemother. 2018, 62, e02163-17. [Google Scholar] [CrossRef] [PubMed]
- Portsmouth, S.; Van Veenhuyzen, D.; Echols, R.; Machida, M.; Ferreira, J.C.A.; Ariyasu, M.; Tenke, P.; Nagata, T.D. Cefiderocol versus Imipenem-Cilastatin for the Treatment of Complicated Urinary Tract Infections Caused by Gram-Negative Uropathogens: A Phase 2, Randomised, Double-Blind, Non-Inferiority Trial. Lancet Infect. Dis. 2018, 18, 1319–1328. [Google Scholar] [CrossRef]
- Wunderink, R.G.; Matsunaga, Y.; Ariyasu, M.; Clevenbergh, P.; Echols, R.; Kaye, K.S.; Kollef, M.; Menon, A.; Pogue, J.M.; Shorr, A.F.; et al. Cefiderocol versus High-Dose, Extended-Infusion Meropenem for the Treatment of Gram-Negative Nosocomial Pneumonia (APEKS-NP): A Randomised, Double-Blind, Phase 3, Non-Inferiority Trial. Lancet Infect. Dis. 2021, 21, 213–225. [Google Scholar] [CrossRef]
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D.L.; et al. Efficacy and Safety of Cefiderocol or Best Available Therapy for the Treatment of Serious Infections Caused by Carbapenem-Resistant Gram-Negative Bacteria (CREDIBLE-CR): A Randomised, Open-Label, Multicentre, Pathogen-Focused, Descriptive, Phase 3 Trial. Lancet Infect. Dis. 2021, 21, 226–240. [Google Scholar] [CrossRef]
- Bradley, J.S.; Orchiston, E.; Portsmouth, S.; Ariyasu, M.; Baba, T.; Katsube, T.; Makinde, O. Pharmacokinetics, Safety and Tolerability of Single-Dose or Multiple-Dose Cefiderocol in Hospitalized Pediatric Patients Three Months to Less Than Eighteen Years Old with Infections Treated with Standard-of-Care Antibiotics in the PEDI-CEFI Phase 2 Study. Pediatr. Infect. Dis. J. 2025, 44, 136–142. [Google Scholar] [CrossRef]
- Katsube, T.; Echols, R.; Arjona Ferreira, J.C.; Krenz, H.K.; Berg, J.K.; Galloway, C. Cefiderocol, a Siderophore Cephalosporin for Gram-Negative Bacterial Infections: Pharmacokinetics and Safety in Subjects with Renal Impairment. J. Clin. Pharmacol. 2017, 57, 584–591. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Leonildi, A.; Della Sala, L.; Vecchione, A.; Barnini, S.; Farcomeni, A.; Menichetti, F. Cefiderocol-Compared to Colistin-Based Regimens for the Treatment of Severe Infections Caused by Carbapenem-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2022, 66, e02142-21. [Google Scholar] [CrossRef] [PubMed]
- Campogiani, L.; Crea, A.M.A.; Minardi, M.L.; Ansaldo, L.; Coppola, L.; Compagno, M.; Vitale, P.; Spalliera, I.; Malagnino, V.; Teti, E.; et al. Real-Life Data on Cefiderocol Efficacy and Safety to Treat Multidrug-Resistant Acinetobacter baumannii Infections. Open Forum Infect. Dis. 2023, 10, ofad627. [Google Scholar] [CrossRef] [PubMed]
- Karruli, A.; Massa, A.; Andini, R.; Marrazzo, T.; Ruocco, G.; Zampino, R.; Durante-Mangoni, E. Clinical Efficacy and Safety of Cefiderocol for Resistant Gram-Negative Infections: A Real-Life, Single-Centre Experience. Int. J. Antimicrob. Agents 2023, 61, 106723. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.; Cornely, O.; Marcella, S.; Nguyen, S.; Gozalo, L.; Cai, B. Effectiveness and Safety of Cefiderocol in Clinical Practice for Treatment of Patients with Gram-Negative Bacterial Infections: US Interim Results of the PROVE Study. Infect. Drug Resist. 2024, 17, 4427–4443. [Google Scholar] [CrossRef]
- Oliva, A.; Liguori, L.; Covino, S.; Petrucci, F.; Cogliati-Dezza, F.; Curtolo, A.; Savelloni, G.; Comi, M.; Sacco, F.; Ceccarelli, G.; et al. Clinical Effectiveness of Cefiderocol for the Treatment of Bloodstream Infections Due to Carbapenem-Resistant Acinetobacter Baumannii during the COVID-19 Era: A Single Center, Observational Study. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 1149–1160. [Google Scholar] [CrossRef]
- Cipko, K.; Kizny Gordon, A.; Adhikari, S.; Konecny, P. Cefiderocol Treatment of Pseudomonas Aeruginosa and Extensively Drug-Resistant Acinetobacter baumannii Retained Spinal Hardware Infection Causing Reversible Acute Interstitial Nephritis. Int. J. Infect. Dis. 2021, 109, 108–111. [Google Scholar] [CrossRef]
- Sollima, A.; Rossini, F.; Lanza, P.; Pallotto, C.; Meschiari, M.; Gentile, I.; Stellini, R.; Lenzi, A.; Mulé, A.; Castagna, F.; et al. Role of Cefiderocol in Multidrug-Resistant Gram-Negative Central Nervous System Infections: Real Life Experience and State-of-the-Art. Antibiotics 2024, 13, 453. [Google Scholar] [CrossRef]
- Schmitt-Hoffmann, A.; Nyman, L.; Roos, B.; Schleimer, M.; Sauer, J.; Nashed, N.; Brown, T.; Man, A.; Weidekamm, E. Multiple-Dose Pharmacokinetics and Safety of a Novel Broad-Spectrum Cephalosporin (BAL5788) in Healthy Volunteers. Antimicrob. Agents Chemother. 2004, 48, 2576–2580. [Google Scholar] [CrossRef]
- Noel, G.J.; Bush, K.; Bagchi, P.; Ianus, J.; Strauss, R.S. A Randomized, Double-Blind Trial Comparing Ceftobiprole Medocaril with Vancomycin plus Ceftazidime for the Treatment of Patients with Complicated Skin and Skin-Structure Infections. Clin. Infect. Dis. 2008, 46, 647–655. [Google Scholar] [CrossRef]
- Noel, G.J.; Strauss, R.S.; Amsler, K.; Heep, M.; Pypstra, R.; Solomkin, J.S. Results of a Double-Blind, Randomized Trial of Ceftobiprole Treatment of Complicated Skin and Skin Structure Infections Caused by Gram-Positive Bacteria. Antimicrob. Agents Chemother. 2008, 52, 37–44. [Google Scholar] [CrossRef]
- Awad, S.S.; Rodriguez, A.H.; Chuang, Y.-C.; Marjanek, Z.; Pareigis, A.J.; Reis, G.; Scheeren, T.W.L.; Sanchez, A.S.; Zhou, X.; Saulay, M.; et al. A Phase 3 Randomized Double-Blind Comparison of Ceftobiprole Medocaril Versus Ceftazidime Plus Linezolid for the Treatment of Hospital-Acquired Pneumonia. Clin. Infect. Dis. 2014, 59, 51–61. [Google Scholar] [CrossRef]
- Bosheva, M.; Gujabidze, R.; Károly, É.; Nemeth, A.; Saulay, M.; Smart, J.I.; Hamed, K.A. A Phase 3, Randomized, Investigator-Blinded Trial Comparing Ceftobiprole with a Standard-of-Care Cephalosporin, with or Without Vancomycin, for the Treatment of Pneumonia in Pediatric Patients. Pediatr. Infect. Dis. J. 2021, 40, e222–e229. [Google Scholar] [CrossRef]
- Li, W.-Z.; Wu, H.-L.; Chen, Y.-C.; Guo, B.-N.; Liu, X.-F.; Wang, Y.; Wu, J.-F.; Zhang, J. Pharmacokinetics, Pharmacodynamics, and Safety of Single- and Multiple-Dose Intravenous Ceftobiprole in Healthy Chinese Participants. Ann. Transl. Med. 2021, 9, 936. [Google Scholar] [CrossRef] [PubMed]
- Overcash, J.S.; Kim, C.; Keech, R.; Gumenchuk, I.; Ninov, B.; Gonzalez-Rojas, Y.; Waters, M.; Simeonov, S.; Engelhardt, M.; Saulay, M.; et al. Ceftobiprole Compared with Vancomycin Plus Aztreonam in the Treatment of Acute Bacterial Skin and Skin Structure Infections: Results of a Phase 3, Randomized, Double-Blind Trial (TARGET). Clin. Infect. Dis. 2021, 73, e1507–e1517. [Google Scholar] [CrossRef] [PubMed]
- Holland, T.L.; Cosgrove, S.E.; Doernberg, S.B.; Jenkins, T.C.; Turner, N.A.; Boucher, H.W.; Pavlov, O.; Titov, I.; Kosulnykov, S.; Atanasov, B.; et al. Ceftobiprole for Treatment of Complicated Staphylococcus aureus Bacteremia. N. Engl. J. Med. 2023, 389, 1390–1401. [Google Scholar] [CrossRef] [PubMed]
- Crapis, M.; Venturini, S.; Della Siega, P.; Tonizzo, M.; Garlatti, E.; Rosa, R.D.; Basso, B.; Pontoni, E. Ceftobiprole and Pneumonia in Adults Admitted to the Emergency Department is it Time to Assess a New Therapeutic Algorithm? J. Chemother. 2021, 33, 174–179. [Google Scholar] [CrossRef]
- Arnés García, D.; Pitto-Robles, I.; Calderón Parra, J.; Calvo Salvador, M.; Herrero Rodríguez, C.; Gisbert, L.; Hidalgo-Tenorio, C. Ceft-to-Ceft Study: Real-Life Experience with Ceftaroline and Ceftobiprole in Treatment of the Principal Infectious Syndromes in a Spanish Multicenter Hospital Cohort. Antibiotics 2023, 12, 1692. [Google Scholar] [CrossRef]
- Durante-Mangoni, E.; Andini, R.; Mazza, M.C.; Sangiovanni, F.; Bertolino, L.; Ursi, M.P.; Paradiso, L.; Karruli, A.; Esposito, C.; Murino, P.; et al. Real-Life Experience with Ceftobiprole in a Tertiary-Care Hospital. J. Glob. Antimicrob. Resist. 2020, 22, 386–390. [Google Scholar] [CrossRef]
- Zampino, R.; Gallo, R.; Salemme, A.; Marrazzo, T.; Iossa, D.; Karruli, A.; Andini, R.; Esitini, D.; Moretto, S.M.; De Gregorio, F.; et al. Clinical Results with the Use of Ceftaroline and Ceftobiprole: Real-Life Experience in a Tertiary Care Hospital. Int. J. Antimicrob. Agents 2023, 62, 106883. [Google Scholar] [CrossRef]
- Membrillo De Novales, F.J.; Falcone, M.; Soriano, A.; Fernández-Hidalgo, N.; Francisci, D.; Gentile, I.; Cedar, E.; Jemmely, N.; Quevedo, J.; Estébanez, M.; et al. Safety of Ceftobiprole in Patients with Impaired Renal, Hepatic or Immune Function: A Multinational Retrospective Hospital Chart Review (RETRACE Study). Int. J. Antimicrob. Agents 2025, 65, 107450. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Kosar, J.; Baxter, M.; Dhami, R.; Borgia, S.; Irfan, N.; MacDonald, K.S.; Dow, G.; Lagacé-Wiens, P.; Dube, M.; et al. Real-Life Experience with Ceftobiprole in Canada: Results from the CLEAR (CanadianLEadership onAntimicrobialReal-Life Usage) Registry. J. Glob. Antimicrob. Resist. 2021, 24, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Ge, Y.; Hafkin, B. Single- and Multiple-Dose Study To Determine the Safety, Tolerability, Pharmacokinetics, and Food Effect of Oral MRX-I versus Linezolid in Healthy Adult Subjects. Antimicrob. Agents Chemother. 2017, 61, e02181-16. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Huang, J. Drug-Induced Nephrotoxicity: Pathogenic Mechanisms, Biomarkers and Prevention Strategies. Curr. Drug Metab. 2018, 19, 559–567. [Google Scholar] [CrossRef]
- Wu, J.; Wu, H.; Wang, Y.; Chen, Y.; Guo, B.; Cao, G.; Wu, X.; Yu, J.; Wu, J.; Zhu, D.; et al. Tolerability and Pharmacokinetics of Contezolid at Therapeutic and Supratherapeutic Doses in Healthy Chinese Subjects, and Assessment of Contezolid Dosing Regimens Based on Pharmacokinetic/Pharmacodynamic Analysis. Clin. Ther. 2019, 41, 1164–1174.e4. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, H.; Yuan, H.; Yuan, Z.; Zhang, Y. A Phase III Multicentre, Randomized, Double-Blind Trial to Evaluate the Efficacy and Safety of Oral Contezolid versus Linezolid in Adults with Complicated Skin and Soft Tissue Infections. J. Antimicrob. Chemother. 2022, 77, 1762–1769. [Google Scholar] [CrossRef]
- Yang, H.; Jin, Y.; Wang, H.; Yuan, H.; Wang, J.; Li, S.; Hu, Y.; Yang, H.; Li, X.; Liang, H.; et al. A Phase I Study of the Safety, Tolerability, and Pharmacokinetics of Contezolid Acefosamil after Intravenous and Oral Administration in Healthy Chinese Subjects. Antimicrob. Agents Chemother. 2023, 67, e00796-23. [Google Scholar] [CrossRef]
- Li, B.; Liu, Y.; Luo, J.; Cai, Y.; Chen, M.; Wang, T. Contezolid, a Novel Oxazolidinone Antibiotic, May Improve Drug-Related Thrombocytopenia in Clinical Antibacterial Treatment. Front. Pharmacol. 2023, 14, 1157437. [Google Scholar] [CrossRef]
- O’Riordan, W.; Tiffany, C.; Scangarella-Oman, N.; Perry, C.; Hossain, M.; Ashton, T.; Dumont, E. Efficacy, Safety, and Tolerability of Gepotidacin (GSK2140944) in the Treatment of Patients with Suspected or Confirmed Gram-Positive Acute Bacterial Skin and Skin Structure Infections. Antimicrob. Agents Chemother. 2017, 61, e02095-16. [Google Scholar] [CrossRef]
- Taylor, S.N.; Morris, D.H.; Avery, A.K.; Workowski, K.A.; Batteiger, B.E.; Tiffany, C.A.; Perry, C.R.; Raychaudhuri, A.; Scangarella-Oman, N.E.; Hossain, M.; et al. Gepotidacin for the Treatment of Uncomplicated Urogenital Gonorrhea: A Phase 2, Randomized, Dose-Ranging, Single-Oral Dose Evaluation. Clin. Infect. Dis. 2018, 67, 504–512. [Google Scholar] [CrossRef]
- Hossain, M.; Tiffany, C.; Raychaudhuri, A.; Nguyen, D.; Tai, G.; Alcorn, H.; Preston, R.A.; Marbury, T.; Dumont, E. Pharmacokinetics of Gepotidacin in Renal Impairment. Clin. Pharmacol. Drug Dev. 2020, 9, 560–572. [Google Scholar] [CrossRef]
- Overcash, J.S.; Tiffany, C.A.; Scangarella-Oman, N.E.; Perry, C.R.; Tao, Y.; Hossain, M.; Barth, A.; Dumont, E.F. Phase 2a Pharmacokinetic, Safety, and Exploratory Efficacy Evaluation of Oral Gepotidacin (GSK2140944) in Female Participants with Uncomplicated Urinary Tract Infection (Acute Uncomplicated Cystitis). Antimicrob. Agents Chemother. 2020, 64, e00199-20. [Google Scholar] [CrossRef]
- Tiffany, C.; Dumont, E.F.; Hossain, M.; Srinivasan, M.; Swift, B. Pharmacokinetics, Safety, and Tolerability of Gepotidacin Administered as Single or Repeat Ascending Doses, in Healthy Adults and Elderly Subjects. Clin. Transl. Sci. 2022, 15, 2251–2264. [Google Scholar] [CrossRef] [PubMed]
- Barth, A.; Hossain, M.; Perry, C.R.; Gross, A.S.; Ogura, H.; Shabbir, S.; Thomas, S.; Dumont, E.F.; Brimhall, D.B.; Srinivasan, M.; et al. Pharmacokinetic, Safety, and Tolerability Evaluations of Gepotidacin (GSK2140944) in Healthy Japanese Participants. Clin. Pharmacol. Drug Dev. 2023, 12, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.; Perry, C.R.; Hooton, T.M.; Scangarella-Oman, N.E.; Millns, H.; Powell, M.; Jarvis, E.; Dennison, J.; Sheets, A.; Butler, D.; et al. Oral Gepotidacin versus Nitrofurantoin in Patients with Uncomplicated Urinary Tract Infection (EAGLE-2 and EAGLE-3): Two Randomised, Controlled, Double-Blind, Double-Dummy, Phase 3, Non-Inferiority Trials. Lancet 2024, 403, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.D.C.; Wilson, J.; Workowski, K.A.; Taylor, S.N.; Lewis, D.A.; Gatsi, S.; Flight, W.; Scangarella-Oman, N.E.; Jakielaszek, C.; Lythgoe, D.; et al. Oral Gepotidacin for the Treatment of Uncomplicated Urogenital Gonorrhoea (EAGLE-1): A Phase 3 Randomised, Open-Label, Non-Inferiority, Multicentre Study. Lancet 2025, 405, 1608–1620. [Google Scholar] [CrossRef]
- Sims, M.; Mariyanovski, V.; McLeroth, P.; Akers, W.; Lee, Y.-C.; Brown, M.L.; Du, J.; Pedley, A.; Kartsonis, N.A.; Paschke, A. Prospective, Randomized, Double-Blind, Phase 2 Dose-Ranging Study Comparing Efficacy and Safety of Imipenem/Cilastatin plus Relebactam with Imipenem/Cilastatin Alone in Patients with Complicated Urinary Tract Infections. J. Antimicrob. Chemother. 2017, 72, 2616–2626. [Google Scholar] [CrossRef]
- Rhee, E.G.; Rizk, M.L.; Calder, N.; Nefliu, M.; Warrington, S.J.; Schwartz, M.S.; Mangin, E.; Boundy, K.; Bhagunde, P.; Colon-Gonzalez, F.; et al. Pharmacokinetics, Safety, and Tolerability of Single and Multiple Doses of Relebactam, a β-Lactamase Inhibitor, in Combination with Imipenem and Cilastatin in Healthy Participants. Antimicrob. Agents Chemother. 2018, 62, e00280-18. [Google Scholar] [CrossRef]
- Bhagunde, P.; Colon-Gonzalez, F.; Liu, Y.; Wu, J.; Xu, S.S.; Garrett, G.; Jumes, P.; Lasseter, K.; Marbury, T.; Rizk, M.L.; et al. Impact of Renal Impairment and Human Organic Anion Transporter Inhibition on Pharmacokinetics, Safety and Tolerability of Relebactam Combined with Imipenem and Cilastatin. Br. J. Clin. Pharmacol. 2020, 86, 944–957. [Google Scholar] [CrossRef]
- Motsch, J.; Murta De Oliveira, C.; Stus, V.; Köksal, I.; Lyulko, O.; Boucher, H.W.; Kaye, K.S.; File, T.M.; Brown, M.L.; Khan, I.; et al. RESTORE-IMI 1: A Multicenter, Randomized, Double-Blind Trial Comparing Efficacy and Safety of Imipenem/Relebactam vs Colistin Plus Imipenem in Patients with Imipenem-Nonsusceptible Bacterial Infections. Clin. Infect. Dis. 2020, 70, 1799–1808. [Google Scholar] [CrossRef]
- Titov, I.; Wunderink, R.G.; Roquilly, A.; Rodríguez Gonzalez, D.; David-Wang, A.; Boucher, H.W.; Kaye, K.S.; Losada, M.C.; Du, J.; Tipping, R.; et al. A Randomized, Double-Blind, Multicenter Trial Comparing Efficacy and Safety of Imipenem/Cilastatin/Relebactam Versus Piperacillin/Tazobactam in Adults with Hospital-Acquired or Ventilator-Associated Bacterial Pneumonia (RESTORE-IMI 2 Study). Clin. Infect. Dis. 2021, 73, e4539–e4548. [Google Scholar] [CrossRef]
- Roberts, J.A.; Nicolau, D.P.; Martin-Loeches, I.; Deryke, C.A.; Losada, M.C.; Du, J.; Patel, M.; Rizk, M.L.; Paschke, A.; Chen, L.F. Imipenem/Cilastatin/Relebactam Efficacy, Safety and Probability of Target Attainment in Adults with Hospital-Acquired or Ventilator-Associated Bacterial Pneumonia among Patients with Baseline Renal Impairment, Normal Renal Function, and Augmented Renal Clearance. JAC-Antimicrob. Resist. 2023, 5, dlad011. [Google Scholar] [CrossRef]
- Caniff, K.E.; Rebold, N.; Xhemali, X.; Tran, N.; Eubank, T.A.; Garey, K.W.; Guo, Y.; Chang, M.; Barber, K.E.; Krekel, T.; et al. Real-World Applications of Imipenem-Cilastatin-Relebactam: Insights From a Multicenter Observational Cohort Study. Open Forum Infect. Dis. 2025, 12, ofaf112. [Google Scholar] [CrossRef] [PubMed]
- Machuca, I.; Dominguez, A.; Amaya, R.; Arjona, C.; Gracia-Ahufinger, I.; Carralon, M.; Giron, R.; Gea, I.; De Benito, N.; Martin, A.; et al. Real-World Experience of Imipenem—Relebactam Treatment as Salvage Therapy in Difficult-to-Treat Pseudomonas Aeruginosa Infections (IMRECOR Study). Infect. Dis. Ther. 2025, 14, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Totsuka, K.; Sesoko, S.; Fukase, H.; Ikushima, I.; Odajima, M.; Niwayama, Y. Pharmacokinetic Study of Lascufloxacin in Non-Elderly Healthy Men and Elderly Men. J. Infect. Chemother. 2020, 26, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Takazono, T.; Hosogaya, N.; Fukushima, K.; Morio, R.; Irifune, S.; Miyamura, T.; Harada, Y.; Nagayoshi, Y.; Kondo, A.; Mihara, T.; et al. Efficacy and Safety of Lascufloxacin for Nursing- and Healthcare-Associated Pneumonia: A Single-Arm, Open-Label Clinical Trial. J. Infect. Chemother. 2024, 30, 597–602. [Google Scholar] [CrossRef]
- Iwanaga, N.; Hosogaya, N.; Takazono, T.; Tsukamoto, Y.; Morio, R.; Irifune, S.; Miyamura, T.; Harada, Y.; Nagayoshi, Y.; Kondo, A.; et al. Efficacy and Safety of Intravenous-to-Oral Lascufloxacin Switch Therapy in Community-Onset Pneumonia: A Single-Arm, Open-Label Clinical Trial. Cureus 2025, 17, e80404. [Google Scholar] [CrossRef]
- Shimada, D.; Seki, M. Effectiveness of Drip Infusion of Lascufloxacin, a Novel Fluoroquinolone Antibiotic, for Patients with Pneumonia Including Chronic Lung Disease Exacerbations and Lung Abscesses. Infect. Drug Resist. 2024, 17, 911–918. [Google Scholar] [CrossRef]
- Prince, W.T.; Ivezic-Schoenfeld, Z.; Lell, C.; Tack, K.J.; Novak, R.; Obermayr, F.; Talbot, G.H. Phase II Clinical Study of BC-3781, a Pleuromutilin Antibiotic, in Treatment of Patients with Acute Bacterial Skin and Skin Structure Infections. Antimicrob. Agents Chemother. 2013, 57, 2087–2094. [Google Scholar] [CrossRef]
- Alexander, E.; Goldberg, L.; Das, A.F.; Moran, G.J.; Sandrock, C.; Gasink, L.B.; Spera, P.; Sweeney, C.; Paukner, S.; Wicha, W.W.; et al. Oral Lefamulin vs Moxifloxacin for Early Clinical Response Among Adults with Community-Acquired Bacterial Pneumonia: The LEAP 2 Randomized Clinical Trial. JAMA 2019, 322, 1661. [Google Scholar] [CrossRef]
- File, T.M.; Goldberg, L.; Das, A.; Sweeney, C.; Saviski, J.; Gelone, S.P.; Seltzer, E.; Paukner, S.; Wicha, W.W.; Talbot, G.H.; et al. Efficacy and Safety of Intravenous-to-Oral Lefamulin, a Pleuromutilin Antibiotic, for the Treatment of Community-Acquired Bacterial Pneumonia: The Phase III Lefamulin Evaluation Against Pneumonia (LEAP 1) Trial. Clin. Infect. Dis. 2019, 69, 1856–1867. [Google Scholar] [CrossRef]
- Wicha, W.W.; Prince, W.T.; Lell, C.; Heilmayer, W.; Gelone, S.P. Pharmacokinetics and Tolerability of Lefamulin Following Intravenous and Oral Dosing. J. Antimicrob. Chemother. 2019, 74, iii19–iii26. [Google Scholar] [CrossRef]
- Wicha, W.W.; Marbury, T.C.; Dowell, J.A.; Crandon, J.L.; Leister, C.; Ermer, J.; Gelone, S.P. Pharmacokinetics and Safety of Lefamulin After Single Intravenous Dose Administration in Subjects with Impaired Renal Function and Those Requiring Hemodialysis. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2021, 41, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wei, Q.; Bian, X.; Yang, X.; Yu, J.; Wang, J.; Yang, H.; Cao, G.; Wu, X.; Zhang, J. Pharmacokinetic, Pharmacokinetic/Pharmacodynamic, and Safety Investigations of Lefamulin in Healthy Chinese Subjects. Antibiotics 2023, 12, 1391. [Google Scholar] [CrossRef] [PubMed]
- Mehta, Y.; Sutar, A.R.; Zirpe, K.; Kothari, J.N.; Alapati, C.; Pathak, M.; Nagvekar, V.C.; Mehta, K.D.; Debnath, K. Prescription-Event Monitoring Study on Safety and Efficacy of Levonadifloxacin (Oral and I.V.) in Management of Bacterial Infections: Findings of Real-World Observational Study. Int. J. Appl. Basic Med. Res. 2022, 12, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.D.; Sharma, J.B.; Anand, A.; Reddy, N.P.K.; Kadam, P.; Debnath, K.; Bhapkar, S.; Thampi, B.M. Real-World Evidence of Efficacy and Safety of Levonadifloxacin (Oral and IV) in the Management of Acute Bacterial Skin and Skin Structure Infections (ABSSSI): Findings of a Retrospective, Multi-Center Study. Cureus 2022, 14, e24299. [Google Scholar] [CrossRef]
- Saseedharan, S.; Zirpe, K.; Mehta, Y.; Dubey, D.; Sutar, A.; Debnath, K.; Newale, S. Efficacy and Safety of Oral and IV Levonadifloxacin Therapy in Management of Bacterial Infections: Findings of a Prospective, Observational, Multi-Center, Post-Marketing Surveillance Study. Cureus 2024, 16, e55178. [Google Scholar] [CrossRef]
- Telkhade, A.; Patel, C.; Rathode, R.K.; Singh, B.; Deshpande, S.; More, B.; Deshpande, S. A Prospective, Observational, Open-Label, Non-Comparative, Multi-Center Study for the Evaluation of Efficacy and Safety of Levonadifloxacin (Intravenous and Oral) in Patients with Community-Acquired Bacterial Pneumonia. Cureus 2024, 16, e76315. [Google Scholar] [CrossRef]
- Cass, R.T.; Brooks, C.D.; Havrilla, N.A.; Tack, K.J.; Borin, M.T.; Young, D.; Bruss, J.B. Pharmacokinetics and Safety of Single and Multiple Doses of ACHN-490 Injection Administered Intravenously in Healthy Subjects. Antimicrob. Agents Chemother. 2011, 55, 5874–5880. [Google Scholar] [CrossRef]
- Connolly, L.E.; Riddle, V.; Cebrik, D.; Armstrong, E.S.; Miller, L.G. A Multicenter, Randomized, Double-Blind, Phase 2 Study of the Efficacy and Safety of Plazomicin Compared with Levofloxacin in the Treatment of Complicated Urinary Tract Infection and Acute Pyelonephritis. Antimicrob. Agents Chemother. 2018, 62, e01989-17. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.E.; Cloutier, D.J.; Komirenko, A.S.; Cebrik, D.S.; Krause, K.M.; Keepers, T.R.; Connolly, L.E.; Miller, L.G.; Friedland, I.; Dwyer, J.P. Once-Daily Plazomicin for Complicated Urinary Tract Infections. N. Engl. J. Med. 2019, 380, 729–740. [Google Scholar] [CrossRef]
- Gall, J.; Choi, T.; Riddle, V.; Van Wart, S.; Gibbons, J.A.; Seroogy, J. A Phase 1 Study of Intravenous Plazomicin in Healthy Adults to Assess Potential Effects on the QT/QTc Interval, Safety, and Pharmacokinetics. Clin. Pharmacol. Drug Dev. 2019, 8, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.; Preston, R.A.; Mamikonyan, G.; Stone, E.; Isaacs, R. Pharmacokinetics, Safety, and Tolerability of Intravenous Durlobactam and Sulbactam in Subjects with Renal Impairment and Healthy Matched Control Subjects. Antimicrob. Agents Chemother. 2019, 63, e00794-19. [Google Scholar] [CrossRef] [PubMed]
- Kaye, K.S.; Shorr, A.F.; Wunderink, R.G.; Du, B.; Poirier, G.E.; Rana, K.; Miller, A.; Lewis, D.; O’Donnell, J.; Chen, L.; et al. Efficacy and Safety of Sulbactam-Durlobactam versus Colistin for the Treatment of Patients with Serious Infections Caused by Acinetobacter Baumannii-Calcoaceticus Complex: A Multicentre, Randomised, Active-Controlled, Phase 3, Non-Inferiority Clinical Trial (ATTACK). Lancet Infect. Dis. 2023, 23, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Y.; Zhang, J.; Zhang, Y.; Yu, J.; Cao, G.; Chen, Y.; Guo, B.; Shi, Y.; Huang, J.; et al. Short-Term Safety, Tolerability, and Pharmacokinetics of MRX-I, an Oxazolidinone Antibacterial Agent, in Healthy Chinese Subjects. Clin. Ther. 2018, 40, 322–332.e5. [Google Scholar] [CrossRef]
- Falagas, M.E.; Vouloumanou, E.K.; Sgouros, K.; Athanasiou, S.; Peppas, G.; Siempos, I.I. Patients Included in Randomised Controlled Trials Do Not Represent Those Seen in Clinical Practice: Focus on Antimicrobial Agents. Int. J. Antimicrob. Agents 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Stahlmann, R. Clinical Toxicological Aspects of Fluoroquinolones. Toxicol. Lett. 2002, 127, 269–277. [Google Scholar] [CrossRef]
- Mandell, L.; Tillotson, G. Safety of Fluoroquinolones: An Update. Can. J. Infect. Dis. J. Can. Mal. Infect. 2002, 13, 54–61. [Google Scholar] [CrossRef]
- Stahlmann, R.; Lode, H.M. Risks Associated with the Therapeutic Use of Fluoroquinolones. Expert Opin. Drug Saf. 2013, 12, 497–505. [Google Scholar] [CrossRef]
- Falagas, M.E.; Rafailidis, P.I.; Kasiakou, S.K.; Hatzopoulou, P.; Michalopoulos, A. Effectiveness and Nephrotoxicity of Colistin Monotherapy vs. Colistin-Meropenem Combination Therapy for Multidrug-Resistant Gram-Negative Bacterial Infections. Clin. Microbiol. Infect. 2006, 12, 1227–1230. [Google Scholar] [CrossRef]
- Kelesidis, T.; Falagas, M.E. The Safety of Polymyxin Antibiotics. Expert Opin. Drug Saf. 2015, 14, 1687–1701. [Google Scholar] [CrossRef] [PubMed]
- Pagkalis, S.; Mantadakis, E.; Mavros, M.N.; Ammari, C.; Falagas, M.E. Pharmacological Considerations for the Proper Clinical Use of Aminoglycosides. Drugs 2011, 71, 2277–2294. [Google Scholar] [CrossRef] [PubMed]
- Mavros, M.N.; Polyzos, K.A.; Rafailidis, P.I.; Falagas, M.E. Once versus Multiple Daily Dosing of Aminoglycosides for Patients with Febrile Neutropenia: A Systematic Review and Meta-Analysis. J. Antimicrob. Chemother. 2011, 66, 251–259. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K. Toxicity of Polymyxins: A Systematic Review of the Evidence from Old and Recent Studies. Crit. Care 2006, 10, R27. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K.; Kofteridis, D.P.; Roditakis, G.; Samonis, G. Effectiveness and Nephrotoxicity of Intravenous Colistin for Treatment of Patients with Infections Due to Polymyxin-Only-Susceptible (POS) Gram-Negative Bacteria. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 596–599. [Google Scholar] [CrossRef]
- Griffin, B.R.; Faubel, S.; Edelstein, C.L. Biomarkers of Drug-Induced Kidney Toxicity. Ther. Drug Monit. 2019, 41, 213–226. [Google Scholar] [CrossRef]
- Beger, R.D.; Sun, J.; Schnackenberg, L.K. Metabolomics Approaches for Discovering Biomarkers of Drug-Induced Hepatotoxicity and Nephrotoxicity. Toxicol. Appl. Pharmacol. 2010, 243, 154–166. [Google Scholar] [CrossRef] [PubMed]

| Drug (Brand Name) | Year of Approval, Country/Region | Chemical Structure | Class | Indications |
|---|---|---|---|---|
| Gepotidacin (Blujepa) | FDA 2025 | 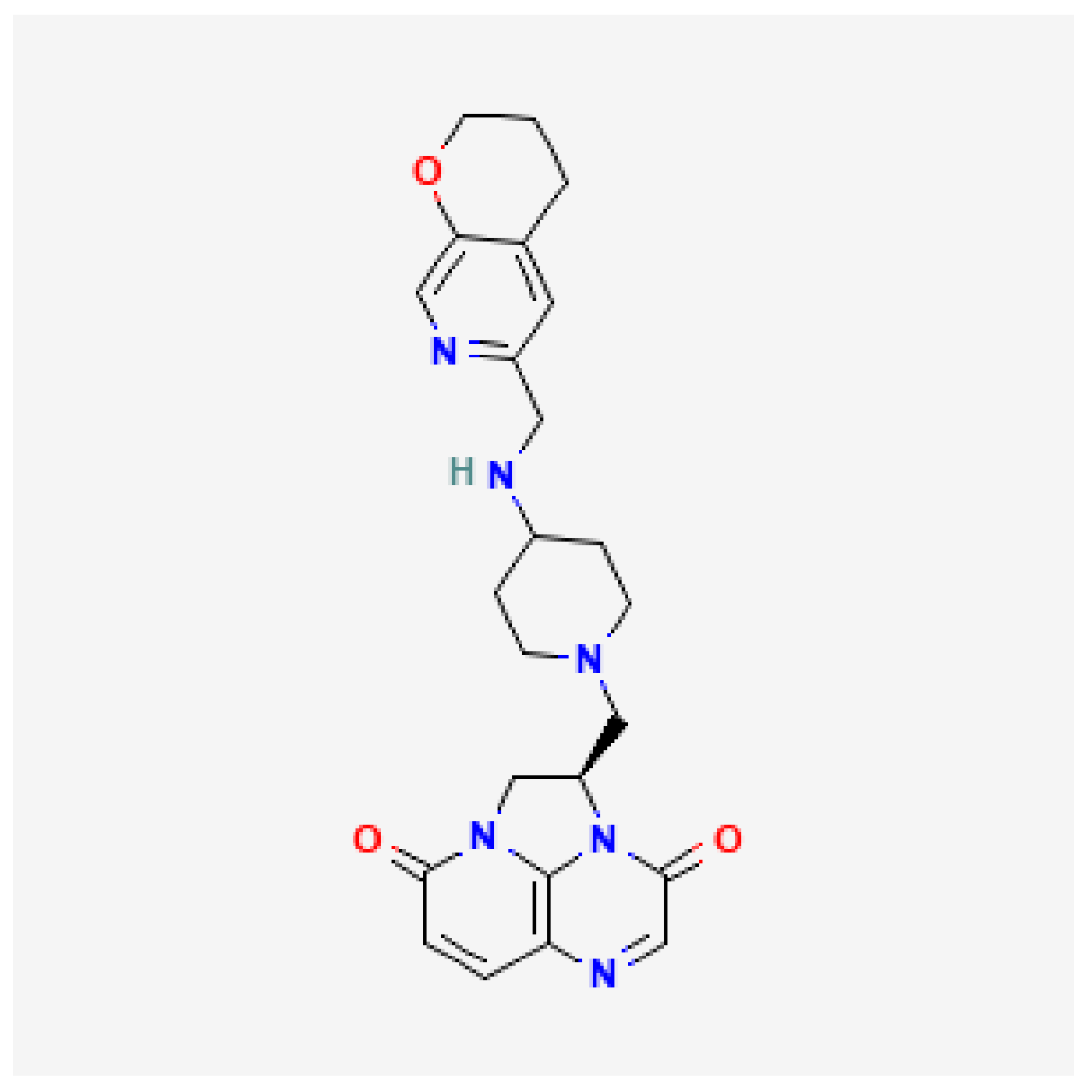 | DNA topoisomerase II and IV inhibitor | Uncomplicated UTIs |
| Aztreonam/avibactam (Emblaveo) | FDA 2025, EMA 2024, UK 2024 | 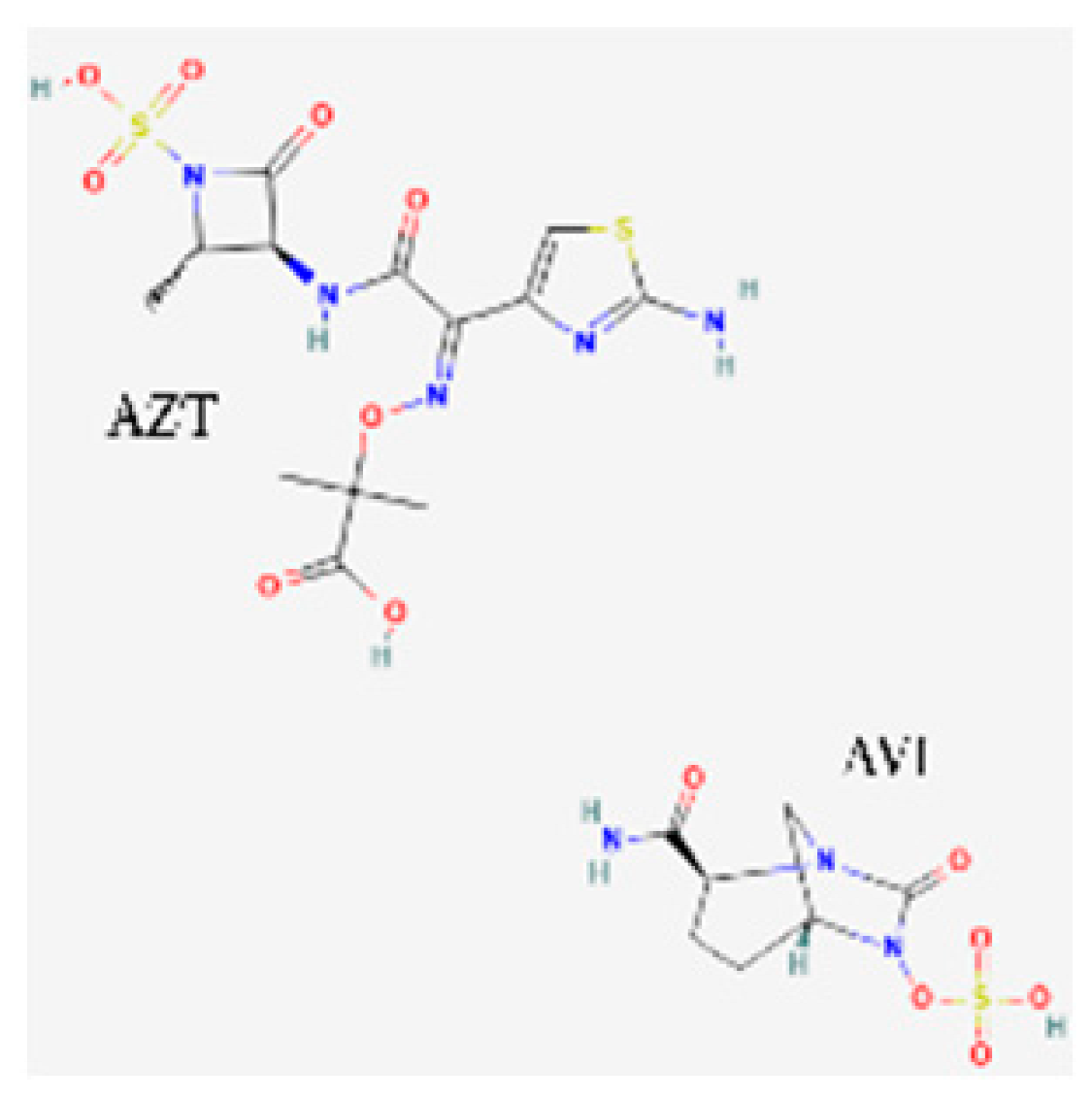 | BL/BLI | cIAIs, HAP, VAP, cUTIs |
| Cefepime/enmetazobactam (Exblifep) | EMA 2024, FDA 2024 | 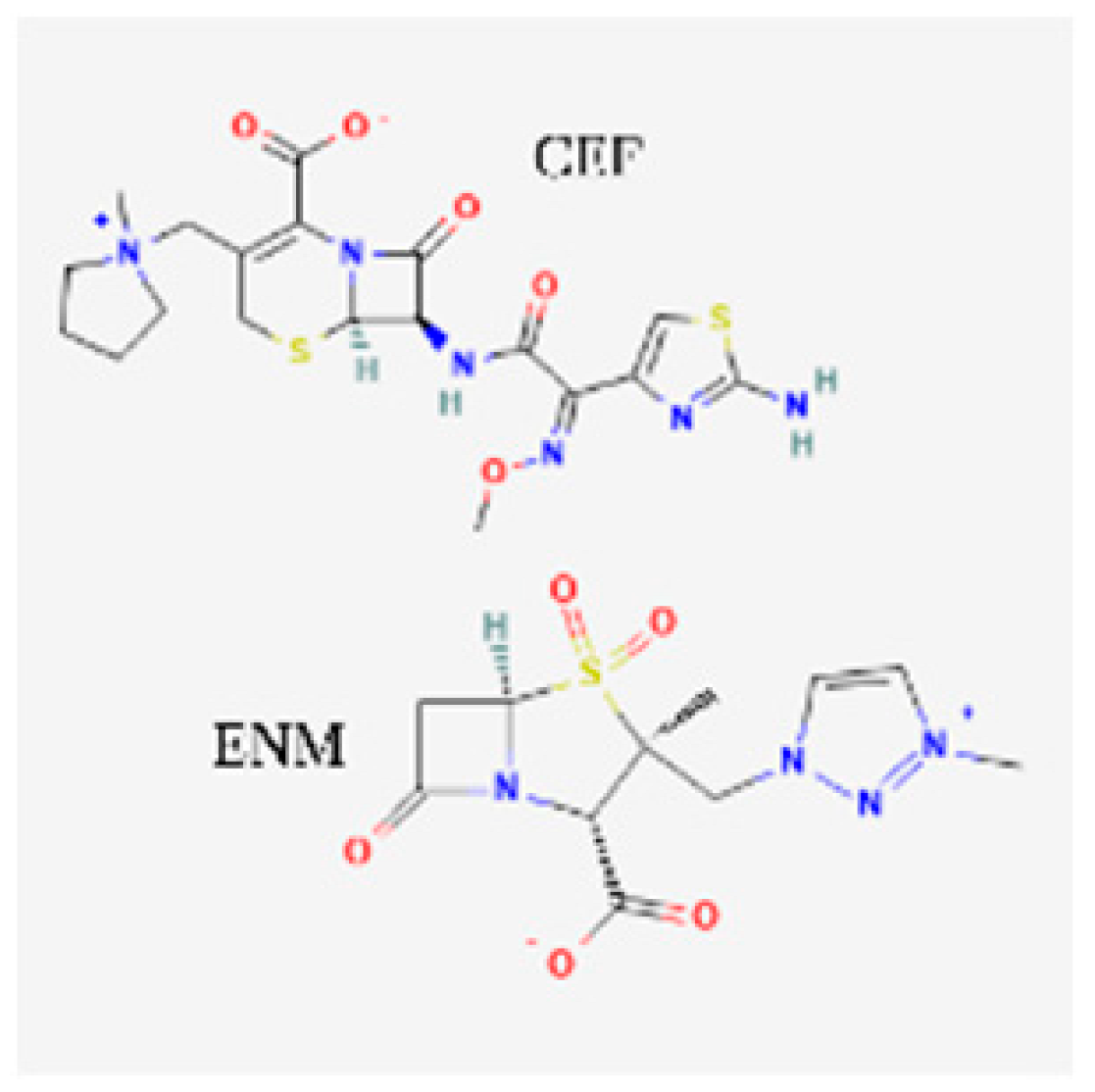 | BL/BLI | cUTIs, HAP, VAP |
| Ceftobiprole (Zevtera) | FDA 2024 | 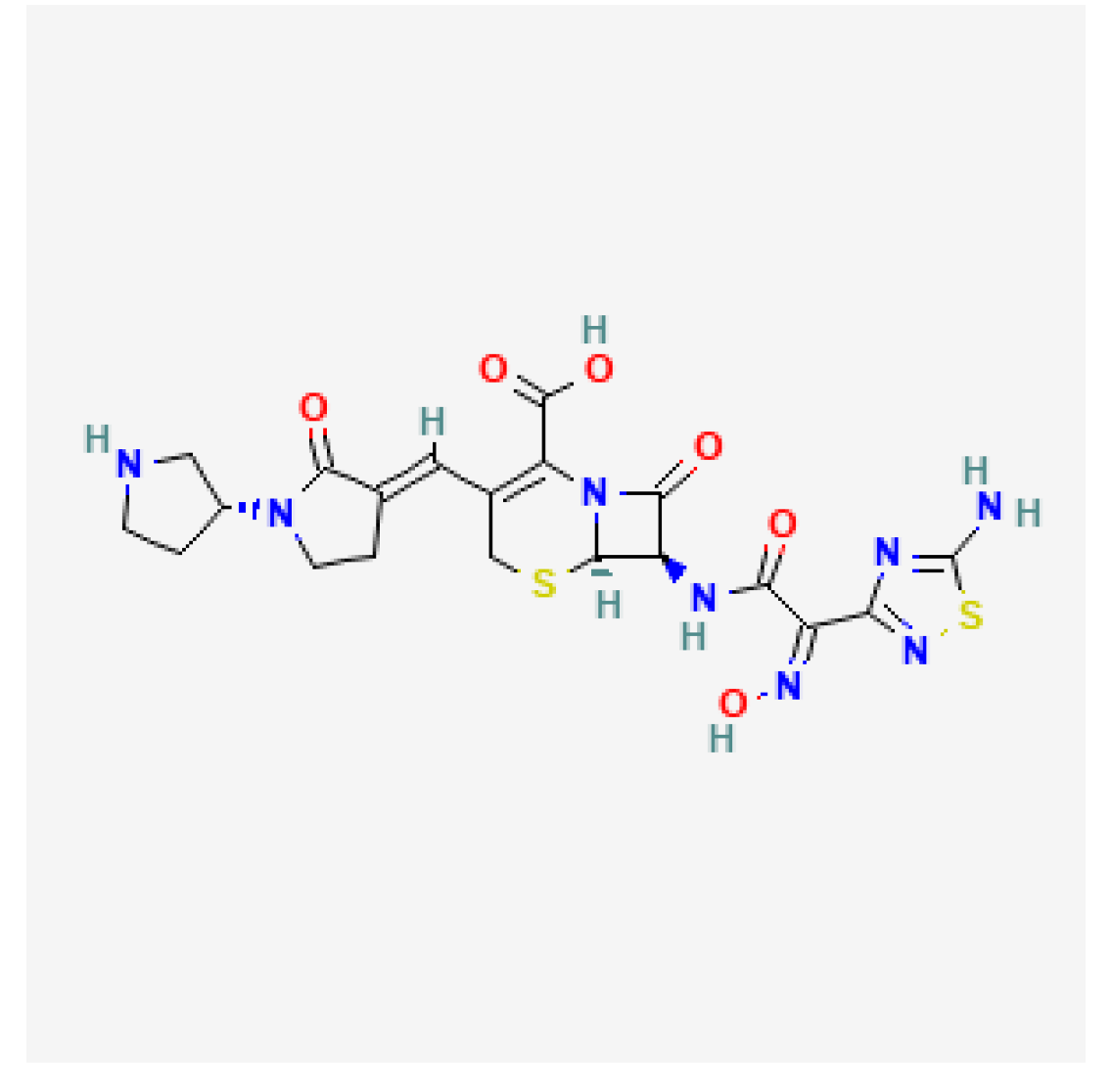 | 5th gen cephalosporin | HAP, VAP, CAP, IE, ABSSSI |
| Levonadifloxacin (Emrok) | NMPA 2024 | 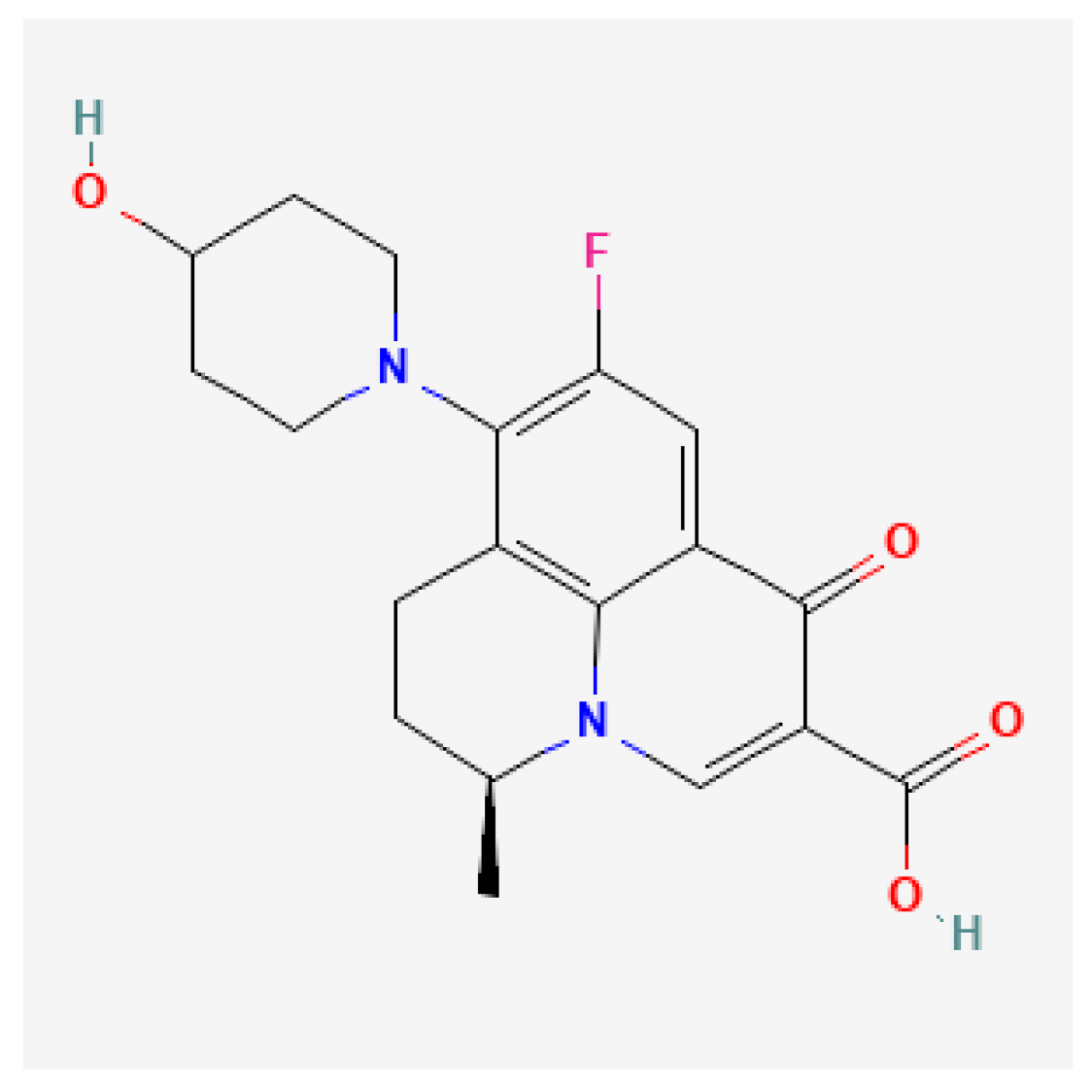 | Fluoroquinolone | ABSSSI |
| Sulbactam/durlobactam (Xacduro) | FDA 2023 |  | BL/BLI | HAP, VAP |
| Contezolid (Youxitai) | NMPA 2021 | 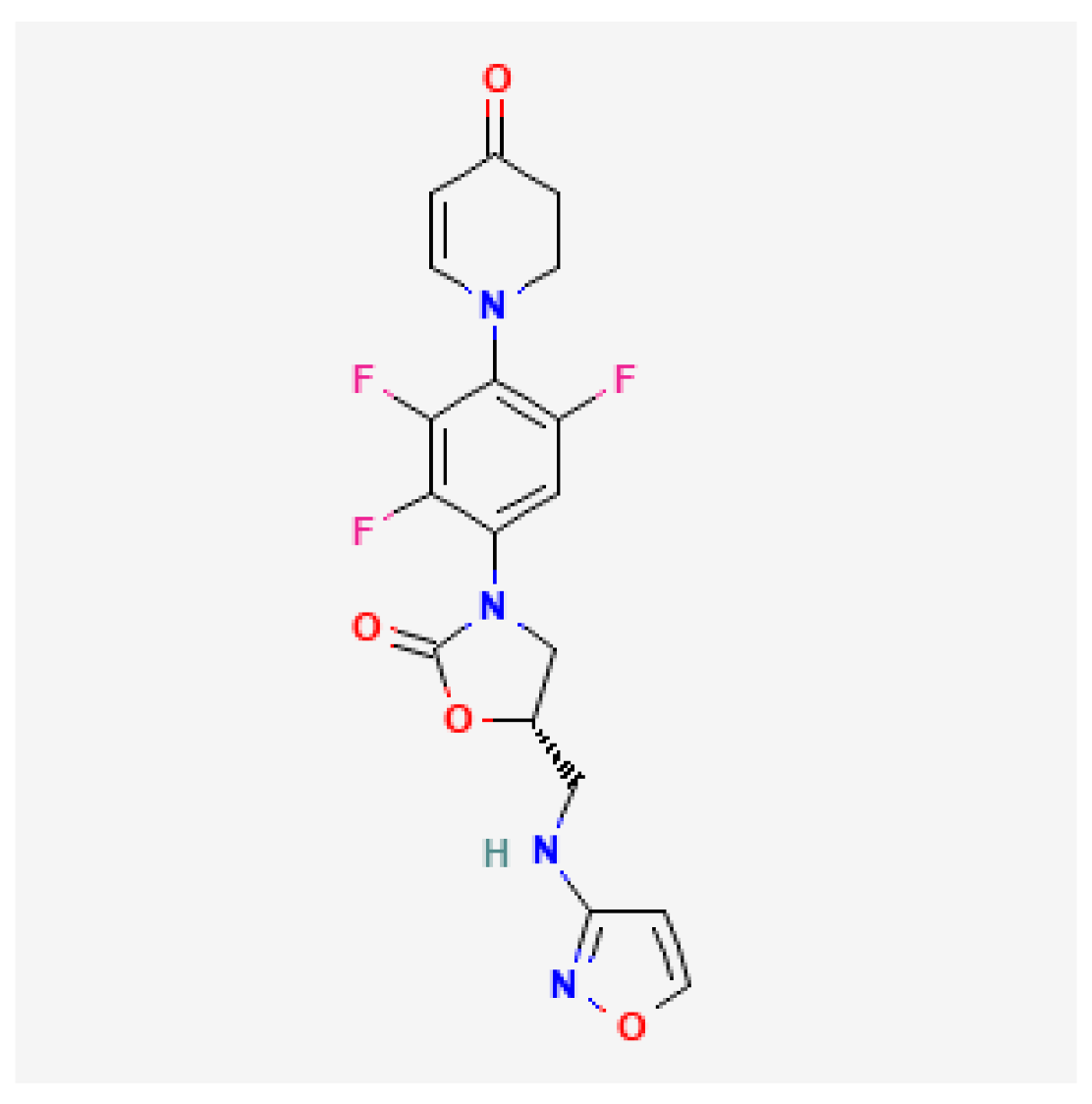 | Oxazolidinone | cSSTI |
| Cefiderocol (Fetcroja, Fetroja *) | EMA 2020, FDA 2020 | 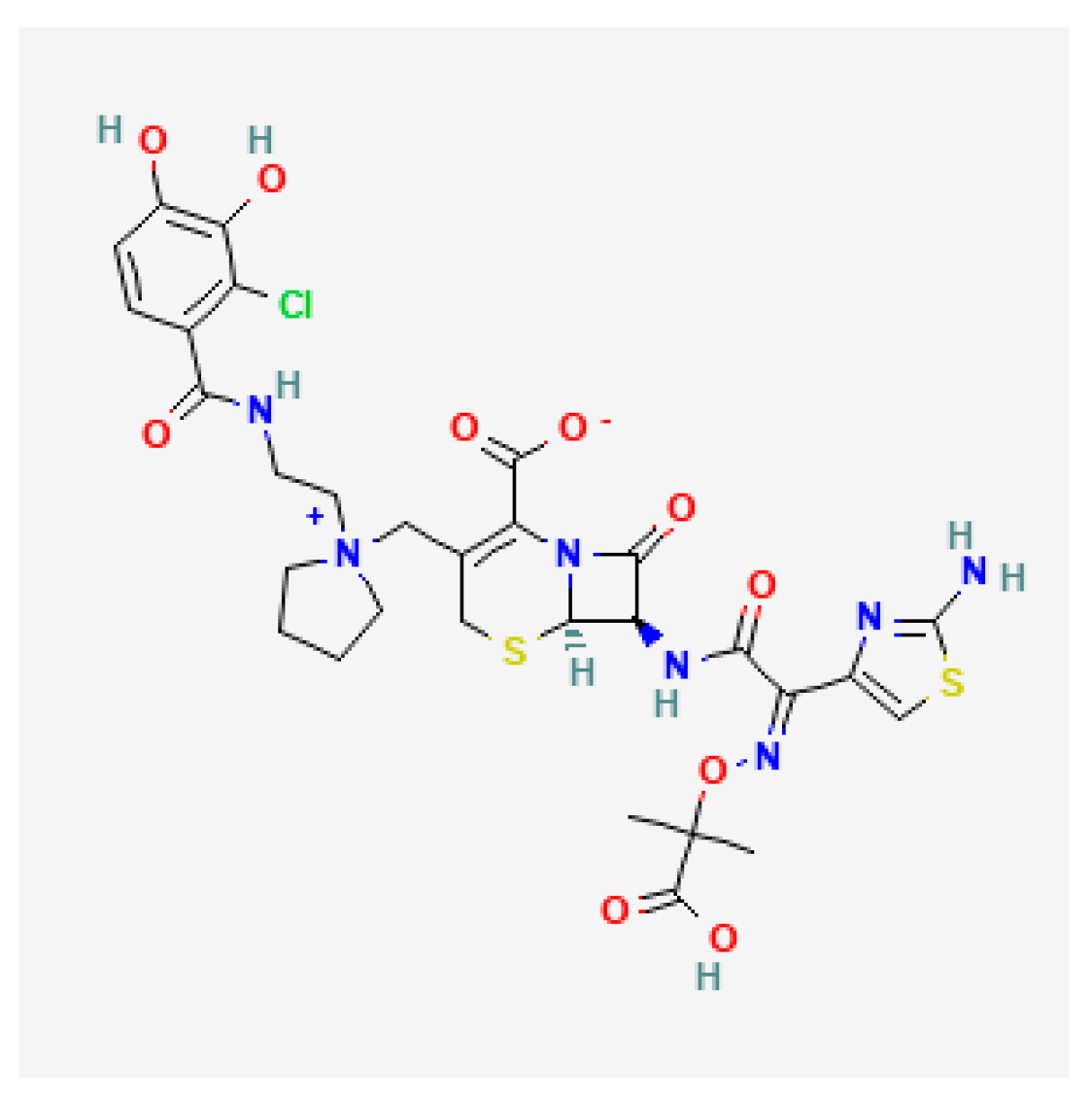 | Siderophore cephalosporin | cUTI, HAP, VABP |
| Imipenem/cilastatin/relebactam (Recarbrio) | EMA 2020, FDA 2019 | 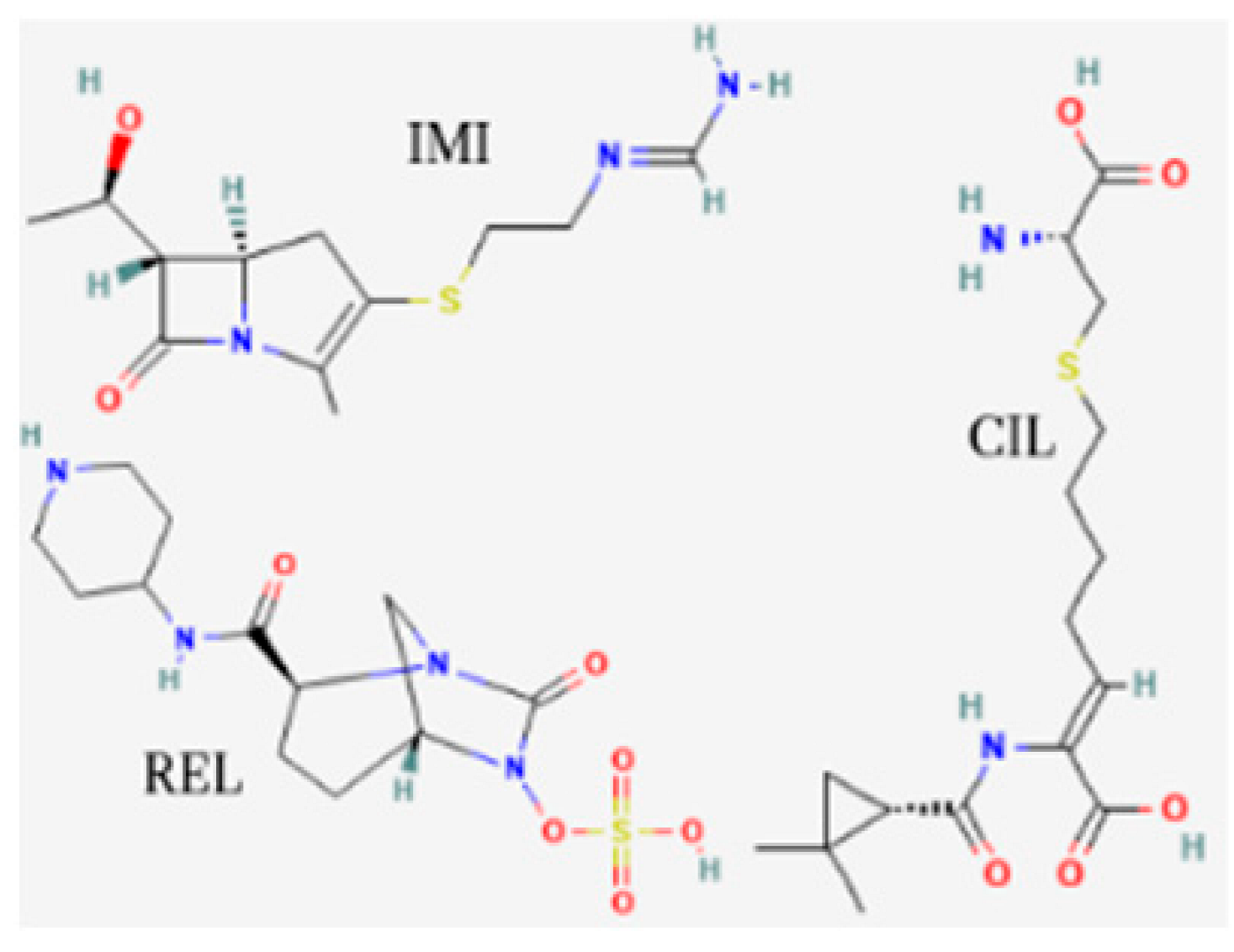 | BL/BLI | HABP, VABP, cUTI, cIAI |
| Lefamulin (Xenleta) | EMA 2020, FDA 2019 |  | Pleuromutilin, protein synthesis inhibitor | CAP, ABSSSI |
| Lascufloxacin (Lasvic) | Japan 2019 |  | Fluoroquinolone | CAP, RTIs |
| Plazomicin (Zemdri) | FDA 2018 |  | Aminoglycoside | cUTIs |
| Author, Year | Ref. | Study Type | Specific Infection or Healthy Subjects | Nephrotoxicity Reported n/N (%) | Manifestation of Nephrotoxicity | Pharmacovigilance and Prescribing Label Data on Nephrotoxicity |
|---|---|---|---|---|---|---|
| Aztreonam/avibactam | VigiBase: NR FAERS: NR EudraVigilance: NR EMA: Uncommon FDA: NL | |||||
| Cornely, 2020 | [52] | Phase 2a trial | cIAIs | 0/34 (0) | ||
| Carmeli, 2025 | [53] | Phase 3 trial | Gram (−) infections | 0/275 (0) | ||
| ASSEMBLE study a | [54] | Phase 3 trial | Gram (−) infections | 0/12 (0) | ||
| Cefepime/enmetazobactam | VigiBase: NR FAERS: NR EudraVigilance: NR EMA: Uncommon FDA: Less than 1% | |||||
| Das, 2020 | [55] | Phase 1 trial | Healthy subjects | 0/20 (0) | ||
| Kaye, 2022 | [56] | Phase 3 trial | cUTIs or acute pyelonephritis | 22/516 (4.3) | Kidney and urinary adverse events, not explicitly specified. | |
| Cefiderocol | VigiBase: 40/500 (8%) reports FAERS: 29 AKI, 9 renal impairment, 3 blood creatinine increase, 2 renal failure, 2 GFR decrease, and 2 renal tubular disorder out of 522 reports EudraVigilance: 30/197 (15.2%) reports EMA: NK FDA: Reported in ≤4% of patients in clinical trials | |||||
| Saisho, 2018 | [57] | Phase 1 trial | Healthy subjects | 0/16 (0) | ||
| Portsmouth, 2018 | [58] | Phase 2 trial | cUTIs | 0/300 (0) | ||
| Wunderink, 2020 | [59] | Phase 3 trial | Gram (−) HAP | 0/148 (0) | ||
| Bassetti, 2020 | [60] | Phase 3 trial | CR Gram (−) infections | 0/101 (0) | ||
| Bradley, 2025 | [61] | Phase 2 trial | Pediatric infections | 2/53 (3.7) | Defined as renal impairment in 2/29 (6.9%) patients receiving multiple doses of cefiderocol. | |
| Katsube, 2017 | [62] | Prospective cohort study | Gram (−) infections in patients with baseline renal impairment | 0/38 (0) | ||
| Falcone, 2022 | [63] | Retrospective cohort study | CRAB infections | 0/47 (0) | ||
| Campogiani, 2023 | [64] | Retrospective cohort study | MDR A. baumannii infections | 0/11 (0) | ||
| Karruli, 2023 | [65] | Retrospective cohort study | MDR Gram (−) infections | 0/28 (0) | ||
| Clancy, 2024 | [66] | Retrospective cohort study | Gram (−) infections | 1/244 (0.4) | Acute interstitial nephritis | |
| Oliva, 2024 | [67] | Retrospective cohort study | CRAB infections | 0/50 (0) | ||
| Cipko, 2021 | [68] | Case report | P. aeruginosa and XDR A. baumannii retained spinal hardware infection | Acute interstitial nephritis | ||
| Sollima, 2020 | [69] | Case series | MDR Gram (−) CNS infections | 0/5 (0) | ||
| Ceftobiprole | VigiBase: 6/140 (3%) reports FAERS: 1/94 (1.1%) reports FDA: Reported in ≥2% of patients in clinical trials | |||||
| Schmitt-Hoffman, 2004 | [70] | Phase 1 trial | Healthy subjects | 0/16 (0) | ||
| Noel, 2008 | [71] | Phase 3 trial | cSSSIs | 1/348 (0.3) | Two-fold increase in creatinine upper normal limit in laboratory tests. | |
| Noel, 2008 | [72] | Phase 3 trial | cSSSIs | 10/543 (1.9) | Renal related adverse events, not explicitly specified. | |
| Awad, 2014 | [73] | Phase 3 trial | HAP | 0/386 (0) | ||
| Bosheva, 2021 | [74] | Phase 3 trial | Pneumonia in pediatric patients | 0/94 (0) | ||
| Li, 2021 | [75] | Phase 1 trial | Healthy subjects | 0/12 (0) | ||
| Overcash, 2021 | [76] | Phase 3 trial | ABSSIs | 0/334 (0) | ||
| Holland, 2023 | [77] | Phase 3 trial | S. aureus bacteremia | 0/191 (0) | ||
| Crapis, 2021 | [78] | Retrospective cohort study | Severe CAP or HAP | 0/48 (0) | ||
| Arnés García, 2023 | [79] | Retrospective cohort study | Various infections | 0/227 (0) | ||
| Durante-Mangoni, 2020 | [80] | Retrospective cohort study | Various infections | 0/29 (0) | ||
| Zampino, 2023 | [81] | Retrospective cohort study | Various infections | 0/63 | ||
| Membrillo De Novales, 2025 | [82] | Retrospective cohort study | Various infections | High dose: 9/46 [19.6%, 2/46 (4.3%) drug-related]; Recommended dose: 26/350 (7.4%, unspecified) | AKI | |
| Zhanel, 2021 | [83] | Cross-sectional, survey-based registry study | Various infections | 0/38 b | ||
| Contezolid | VigiBase: No data NMPA: No data | |||||
| Eckburg, 2017 | [84] | Phase 1 trial | Healthy subjects | 0/33 (0) | ||
| Wu, 2018 | [85] | Phase 1 trial | Healthy subjects | 0/84 (0) | ||
| Wu, 2019 | [86] | Phase 1 trial | Healthy subjects | 0/68 (0) | ||
| Zhao, 2022 | [87] | Phase 3 trial | cSSTIs | 0/354 (0) | ||
| Yang, 2023 | [88] | Phase 1 trial | Healthy subjects | 0/55 (0) | ||
| Li, 2023 | [89] | Case series | Various infections | 0/3 (0) | ||
| Gepotidacin | VigiBase: No data FAERS: No data FDA: NL | |||||
| O′Riordan, 2017 | [90] | Phase 2 trial | ABSSSIs | 0/122 (0) | ||
| Taylor, 2018 | [91] | Phase 2 trial | Uncomplicated urogenital gonorrhea | 0/105 (0) | ||
| Hossain, 2020 | [92] | Phase 1 trial | Healthy subjects and patients with CKD | 0/32 | ||
| Overcash, 2020 | [93] | Phase 2a trial | Acute uncomplicated cystitis | 0/22 (0) | ||
| Tiffany, 2022 | [94] | Phase 1 trial | Healthy adults and elderly subjects | Repeated ascending oral doses in subjects aged 18–60 years: 48/72 (66.7) Repeated dosing in subjects aged ≥ 65 years: 4/29 (13.8) | Proteinuria | |
| Barth, 2023 | [95] | Phase 1 trial | Healthy subjects | 0/36 (0) | ||
| Wagenlehner, 2024 | [96] | Phase 3 trial | Uncomplicated UTIs | 0/1580 (0) | ||
| Ross, 2025 | [97] | Phase 3 trial | Uncomplicated urogenital gonorrhea | 0/309 (0) | ||
| Imipenem/cilastatin/relebactam | VigiBase: 5/63 (4%) reports FAERS: 1 tubulointerstitial nephritis and 1 blood creatinine increase out of 82 reports EudraVigilance: 4/56 (7.1%) reports EMA: listed as uncommon (blood creatinine increase) and rare (acute renal failure, oliguria/anuria, polyuria, urine discoloration) FDA: reported in <1% of patients in a clinical trial | |||||
| Sims, 2017 | [98] | Phase 2 trial | cUTIs | 1/99 (1) | Proteinuria | |
| Rhee, 2018 | [99] | Phase 1 trial | Healthy subjects | 0/90 (0) | ||
| Bhagunde, 2020 | [100] | Phase 1 trial | CKD patients and healthy subjects | 0/63 (0) | ||
| Motsch, 2020 | [101] | Phase 3 trial | IR bacterial infections | 3/29 (10.3) | AKI | |
| Titov, 2020 | [102] | Phase 3 trial | HAP, VAP | 0/266 (0) | ||
| Roberts, 2023 | [103] | Phase 3 trial | HAP, VAP in patients with CKD | 0/266 (0) | ||
| Caniff, 2025 | [104] | Retrospective cohort study | Gram (−) infections | 1/106 (0.9) | AKI | |
| Machuca, 2024 | [105] | Case series | DTT, P. aeruginosa infections | 0/14 (0) | ||
| Lascufloxacin | VigiBase: 6/63 (8%) reports PMDA: No data | |||||
| Totsuka, 2019 | [106] | Phase 1 trial | Healthy subjects | 2/77 (2.6) | Isolated serum creatinine increased | |
| Takazono, 2024 | [107] | Phase 3 trial | Nursing home- and healthcare-associated pneumonia | 0/71 (0) | ||
| Iwanaga, 2025 | [108] | Phase 3 trial | CAP | 1/114 (0.9) | AKI | |
| Shimada, 2024 | [109] | Retrospective cohort study | LRTIs | 0/55 (0) | ||
| Lefamulin | VigiBase: NR FAERS: NR EudraVigilance: NR EMA: NL FDA: NL | |||||
| Prince, 2013 | [110] | Phase 2 trial | ABSSSIs | 0/207 (0) | ||
| Alexander, 2019 | [111] | Phase 3 trial | CAP | 0/368 (0) | ||
| File, 2019 | [112] | Phase 3 trial | CAP | 0/273 (0) | ||
| Wicha, 2019 | [113] | Phase 1 and 2 trials | Healthy subjects and ABSSSIs | 0/207 (0) | ||
| Wicha, 2021 | [114] | Phase 1 trial | Subjects with CKD | 0/23 (0) | ||
| Hu, 2023 | [115] | Phase 1 trial | Healthy subjects | 2/20 (5) | AKI | |
| Levonadifloxacin | VigiBase: NR CDSCO: NL | |||||
| Mehta, 2022 | [116] | Retrospective cohort study | ABSSSIs | 0/227 (0) | ||
| Mehta, 2022 | [117] | Retrospective | Various bacterial infections | 0/1229 (0) | ||
| Saseedharan, 2024 | [118] | Prospective | Various bacterial infections | 0/1266 (0) | ||
| Telkhade, 2024 | [119] | Prospective | CAP | 0/92 (0) | ||
| Plazomicin | VigiBase: 3/11 (15%) reports FAERS: 5 blood creatinine increase and 2 AKI out of 11 reports FDA: Nephrotoxicity warning | |||||
| Cass, 2011 | [120] | Phase 1 trial | Healthy subjects | 0/32 (0) | ||
| Connoly, 2018 | [121] | Phase 2 trial | cUTIs and acute pyelonephritis | 7/96 (2.1) | One patient with AKI, one with mild azotemia, and five with a 0.5 mg/dL increase in creatinine from baseline. | |
| Wagenlehner, 2019 | [122] | Phase 3 trial | cUTIs | 32/303 (10.5) | 11/303 (3.6%) patients with deterioration of renal function, 21/303 (6.9%) patients had an increase in serum creatinine ≥ 0.5 mg/dL. | |
| Gall, 2019 | [123] | Phase 1 trial | Healthy subjects | 0/111 (0) | ||
| Sulbactam/durlobactam | VigiBase: NR FAERS: NR FDA: reported in 6% of patients in a clinical trial | |||||
| O’Donnell, 2019 | [124] | Phase 1 trial | Patients with CKD and healthy controls | 0/34 (0) | ||
| Kaye, 2023 | [125] | Phase 3 trial | A. baumannii infections | 12/91 (13.2) | AKI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stathopoulos, P.; Romanos, L.T.; Loutradis, C.; Falagas, M.E. Nephrotoxicity of New Antibiotics: A Systematic Review. Toxics 2025, 13, 606. https://doi.org/10.3390/toxics13070606
Stathopoulos P, Romanos LT, Loutradis C, Falagas ME. Nephrotoxicity of New Antibiotics: A Systematic Review. Toxics. 2025; 13(7):606. https://doi.org/10.3390/toxics13070606
Chicago/Turabian StyleStathopoulos, Panagiotis, Laura T. Romanos, Charalampos Loutradis, and Matthew E. Falagas. 2025. "Nephrotoxicity of New Antibiotics: A Systematic Review" Toxics 13, no. 7: 606. https://doi.org/10.3390/toxics13070606
APA StyleStathopoulos, P., Romanos, L. T., Loutradis, C., & Falagas, M. E. (2025). Nephrotoxicity of New Antibiotics: A Systematic Review. Toxics, 13(7), 606. https://doi.org/10.3390/toxics13070606








