Nephrotoxicity and Modern Volatile Anesthetics: A Narrative Review
Abstract
1. Introduction
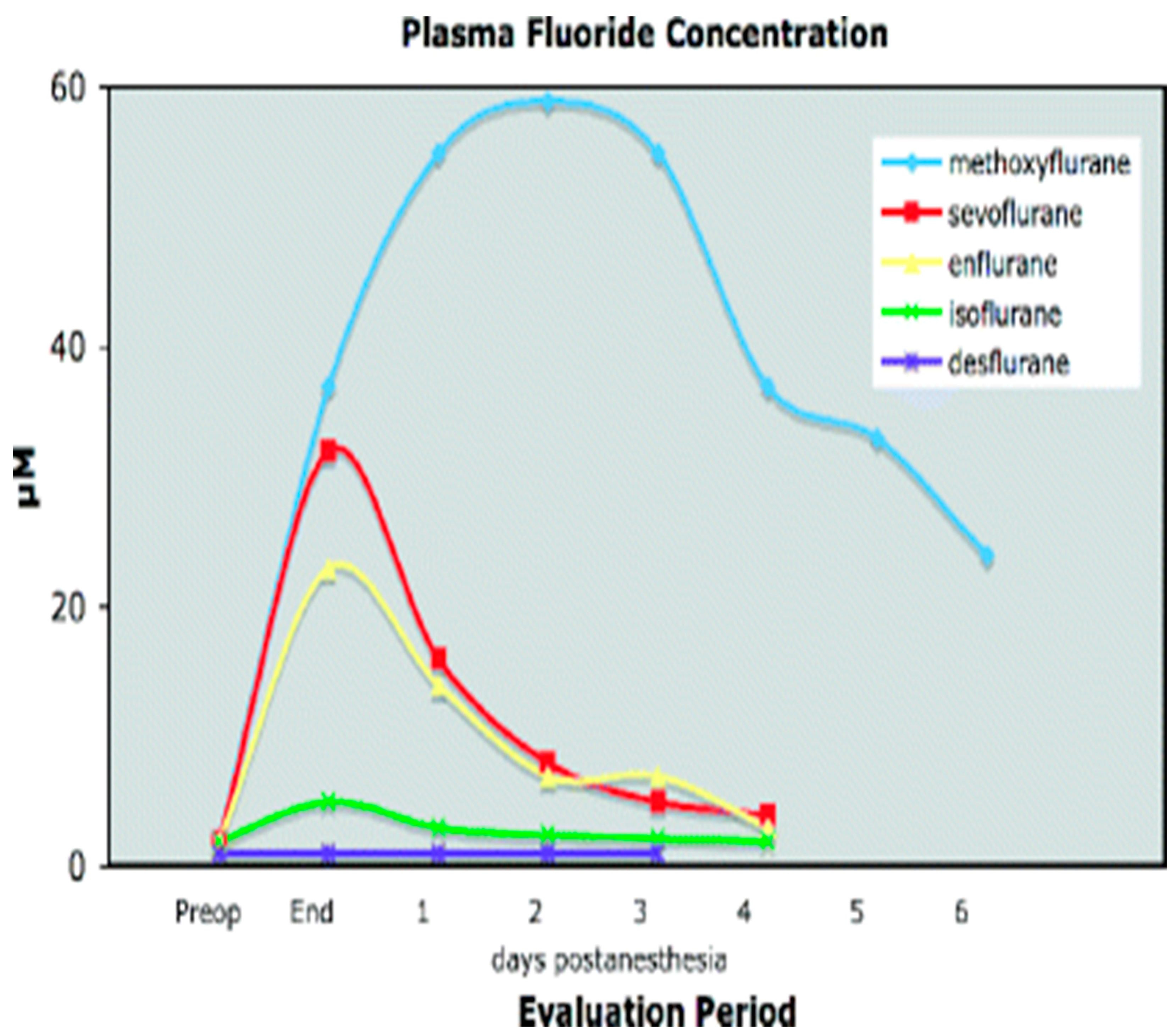
1.1. Metabolism of Volatile Anesthetics
1.2. Nephrotoxic Effects
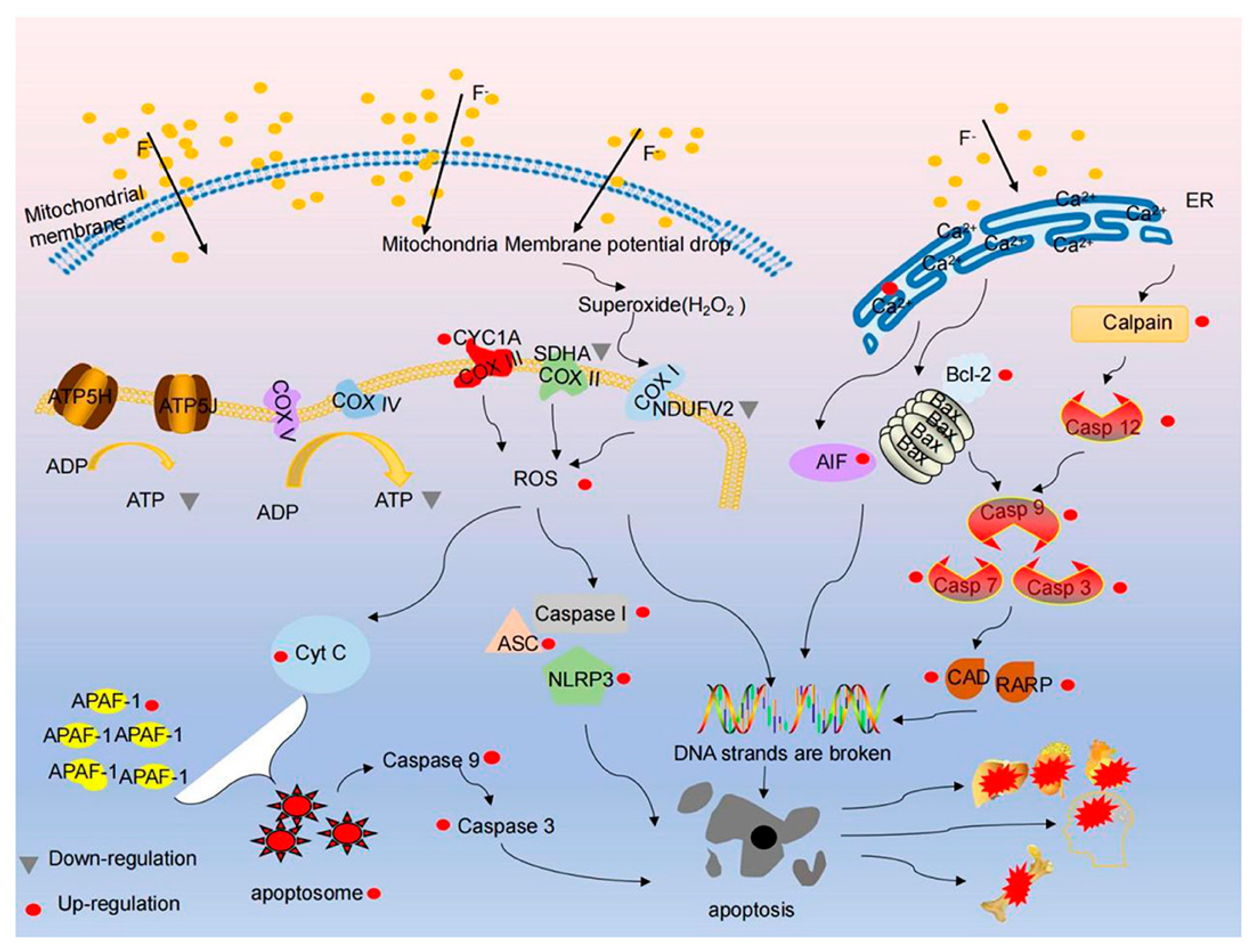
2. Hypothetical Mechanisms
2.1. Renal Sympathetic Nerve Activity and Volatile Anesthesia
- −
- Afferent arteriolar constriction (↓GFR);
- −
- Renin–angiotensin–aldosterone activation;
- −
- Direct tubular sodium reabsorption [43].
2.2. New Volatile Agents in Transplantation
2.3. Volatile Anesthetics in Non-Cardiac Surgery
2.4. Volatile Anesthetics in Cardiac Surgery
2.5. Comparative Nephrotoxicity: Desflurane vs. Sevoflurane vs. Isoflurane
2.6. Prevention of Volatile Anesthetic-Induced Acute Kidney Injury
2.7. Volatile Anesthesia and Renal Autoregulation
3. Clinical Reality: European Medicines Agency Data
4. Reversibility of Nephrotoxic Effects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pringle, H.; Mannsell, R.C.B.; Pringle, S. Clinieal effeets of ether anaesthesia on renal aetivity. Br. Med. J. 1905, 2, 542–545. [Google Scholar] [CrossRef]
- Fukazawa, K.; Lee, H.T. Volatile anesthetics and AKI: Risks, mechanisms, and a potential therapeutic window. J. Am. Soc. Nephrol. 2014, 25, 884–892. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bovill, J.G. Inhalation anaesthesia: From diethyl ether to xenon. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 121–142. [Google Scholar] [CrossRef] [PubMed]
- Ong Sio, L.C.L.; Dela Cruz, R.G.C.; Bautista, A.F. Sevoflurane and renal function: A meta-analysis of randomized trials. Med. Gas. Res. 2017, 7, 186–193. [Google Scholar]
- Haynes, R.C., Jr. Agents affecting calcification. In Goodman and Gilman’s. The pharmacological Basis of Therapeutics; Gilman, A.F., RaH, T.W., Nies, A.S., Taylor, P., Eds.; Pergamon Press: New York, NY, USA, 1990; p. 1518. [Google Scholar]
- Roman, R.J.; Carter, J.R.; North, W.C.; Kauker, M.L. Renal tubular site of action of fluoride in Fischer 344 rats. Anesthesiology 1977, 46, 260–264. [Google Scholar] [CrossRef]
- Van dyke, R.; Chenowith, M.; Poznak, A.Y. Metabolism of volatile anesthetics: I. Conversion in vivo of several anesthetics to 14CO2 and chloride. Biochem. Pharmacol. 1964, 13, 1239–1248. [Google Scholar] [CrossRef]
- Subcommittee on the National Halothane Study of the Committee on Anesthesia; National Academy of Sciences-National Research Council. Summary of the national halothane study: Possible association between halothane anesthesia and postoperative necrosis. JAMA 1966, 197, 775–783. [Google Scholar] [CrossRef]
- Mazze, R.I.; Trudell, J.R.; Cousins, M.J. Methoxyflurane metabolism and renal dysfunction: C1inical correlation in man. Anesthesiology 1971, 35, 247–252. [Google Scholar] [CrossRef]
- Nebert, D.W.; Adesnik, M.; Coon, M.J. The P450 gene super-family: Recommended nomenclature. DNA 1987, 6, 1–7. [Google Scholar] [CrossRef]
- Conney, A.H. Pharmacological implications of microsomal enzyme induction. Pharmacol. Rev. 1967, 19, 317–348. [Google Scholar] [CrossRef]
- Waxman, D.J. Interactions of hepatic cytochromes P-450 with steroid hormones: Regioselectively and stereospecificity of steroid metabolism and hormonal regulation of rat P-450 enzyme expression. Biochem. Pharmacol. 1988, 37, 71–79. [Google Scholar] [CrossRef]
- Pantuck, E.J.; Pantuck, C.B.; Conney, A.H. Effect of streptozotocin-induced diabetes in the rat on the metaboJism of fluorinated volatile anesthetics. Anesthesiology 1987, 66, 24–28. [Google Scholar] [CrossRef]
- Jarnberg, P.O. Renal toxicity of anesthetic agents. In Clinical Nephrotoxins; De Broe, M.E., Porter, G.A., Bennett, W.M., Verpooten, G.A., Eds.; Springer: Dordrecht, The Netherlands, 1998. [Google Scholar] [CrossRef]
- Orhan, H.; Sahin, A.; Sahin, G.; Aypar, U.; Vermeulen, N.P. Urinary lipid and protein oxidation products upon halothane, isoflurane, or sevoflurane anesthesia in humans: Potential biomarkers for a subclinical nephrotoxicity. Biomarkers 2013, 18, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Honore, P.M.; De Bels, D.; Barreto Gutierrez, L.; Redant, S.; Gallerani, A.; Boer, W. Sevoflurane and nephrogenic diabetes insipidus on the rise: Copeptin to the rescue? Crit. Care 2019, 23, 304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- L’Heudé, M.; Poignant, S.; Elaroussi, D.; Espitalier, F.; Ferrandière, M.; Laffon, M. Nephrogenic diabetes insipidus associated with prolonged sedation with sevoflurane in the intensive care unit. Br. J. Anaesth. 2019, 122, e73–e75. [Google Scholar] [CrossRef] [PubMed]
- Cousins, M.; Mazze, R. Methoxyflurane nephrotoxicity. JAMA 1973, 225, 1611–1616. [Google Scholar] [CrossRef]
- Eger, E.I., 2nd; Koblin, D.D.; Bowland, T.; Ionescu, P.; Laster, M.J.; Fang, Z.; Gong, D.; Sonner, J.; Weiskopf, R.B. Nephrotoxicity of sevoflurane versus desflurane anesthesia in volunteers. Anesth. Analg. 1997, 84, 160–168. [Google Scholar] [CrossRef]
- Keller, K.A.; Callan, C.; Prokocimer, P.; Delgado-Herrera, L.; Friedman, M.B.; Hoffman, G.M.; Wooding, W.L.; Cusick, P.K.; Krasula, R.W. Inhalation toxicity study of a haloalkene degradant of sevoflurane, compound A (PIFE), in Sprague-Dawley rats. Anesthesiology 1995, 83, 1220–1232. [Google Scholar] [CrossRef]
- Rush, G.F.; Willis, L.R. Renal tubular effects of sodium fluoride. J. Pharmacol. Exp. Ther. 1982, 223, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.S., Jr.; Smith, F.A.; Gardner, O.E.; O’Brien, J.A.; Hodge, H.C. Renal clearance of fluoride. Proc. Soc. Exp. Biol. 1956, 92, 879–883. [Google Scholar] [CrossRef]
- Carlson, C.H.; Armstrong, W.O.; Singer, L. Distribution and excretion of radiofluoride in the human. Proc. Soc. Exp. Biol. 1960, 104, 235–239. [Google Scholar] [CrossRef]
- Hosking, D.J.; Chamberlain, M.J. Studies in man with 18F. Clin. Sci. 1972, 42, 153–161. [Google Scholar] [CrossRef]
- Ekstrand, J.; Ehrnebo, M.; Boreus, L.O. Fluoride bioavailability after intravenous and oral administration: Importance of renal clearance and urine flow. Clin. Pharmacol. Ther. 1978, 23, 329–337. [Google Scholar] [CrossRef]
- Whitford, G.M.; Pashely, D.H.; Stringer, G.I. Fluoride renal clearance: A pH-dependent event. Am. J. Physiol. 1976, 230, 527–532. [Google Scholar] [CrossRef]
- Ekstrand, J.; Ehrnebo, M.; Whitford, G.M.; Jarnberg, P.-O. Fluoride pharmacokinetics during acid-base changes in man. Eur. J. Clin. Pharmacol. 1980, 18, 189–194. [Google Scholar] [CrossRef]
- Jarnberg, P.-O.; Ekstrand, J.; Irestedt, L. Renal fluoride excretion and plasma fluoride levels during and after enflurane anesthesia are dependent on urinary pH. Anesthesiology 1981, 54, 48–52. [Google Scholar] [CrossRef]
- Barzel, U.S.; Jowsey, J. The effect of chronic acid and alkali administration on bone turnover in adult rats. Clin. Sci. 1969, 36, 517–524. [Google Scholar]
- Frink, E.J., Jr.; Ghantous, H.; Malan, T.P. Plasma inorganic fluoride with sevoflurane anesthesia: Correlation with indices of hepatic and renal function. Anesth. Analg. 1992, 74, 231–235. [Google Scholar] [CrossRef]
- Cook, T.L.; Beppu, W.J.; Hitt, B.A. A comparison of renal effects and metaboJism of sevoflurane and methoxyflurane in enzyme induced rats. Anesth. Ana1g. 1975, 54, 829–835. [Google Scholar]
- Hanaki, C.; Fujii, K.; Morio, M.; Tashima, T. Decomposition of sevoflurane by sodalime. Hiroshima J. Med. Sci. 1987, 36, 61–65. [Google Scholar]
- Macdonald, T.L. Chernical mechanisms of halocarbon metabolism. CRC Crit. Rev. Toxicol. 1983, 11, 85–97. [Google Scholar] [CrossRef]
- Premuzic, V.; Stambolija, V.; Lozic, M.; Kovacevic, J.; Prelevic, V.; Peklic, M.; Scap, M.; Sekulic, A.; Basic-Jukic, N.; Mihaljevic, S.; et al. The effect of different anesthetics on the incidence of AKI and AKD after neurosurgical procedures. PLoS ONE 2024, 19, e0315295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davison, E.; Affleck, A.; Daratha, K.B. Intraoperative Hypotension and Acute Kidney Injury in Non-cardiac Surgery at a Large Tertiary Care Medical Center. AANA J. 2022, 90, 58–63. [Google Scholar] [PubMed]
- Hallqvist, L.; Granath, F.; Huldt, E.; Bell, M. Intraoperative hypotension is associated with acute kidney injury in noncardiac surgery. Eur. J. Anaesthesiol. 2018, 35, 273–279. [Google Scholar] [CrossRef]
- Yildirim, M.; Kucuk, H.F.; Demir, T.; Yakupoglu, S.; Yavuz, A.; Ari, E. Early Allograft Function in Renal Transplant Recipients: Is it Affected by Volatile Anesthetics? Transplant. Proc. 2015, 47, 1352–1355. [Google Scholar] [CrossRef] [PubMed]
- Frink, E.J., Jr.; Malan, T.; Atlas, M.; Dominguez, L.M.; DiNardo, J.A.; Brown, B.R. Clinical Comparison of Sevoflurane and Isoflurane in Healthy Patients. Anesth. Analg. 1992, 74, 241–245. [Google Scholar] [CrossRef]
- Bedford, R.F.; Ives, H.E. The renal safety of sevoflurane. Anesth. Analg. 2000, 90, 505–508. [Google Scholar] [CrossRef]
- Ray, E.C.; Abdel-Kader, K.; Bircher, N.; Rondon-Berrios, H. Case report: Proximal tubule impairment following volatile anesthetic exposure. Physiol. Rep. 2015, 3, e12560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Myles, P.S.; McIlroy, D.R.; Bellomo, R.; Wallace, S. Importance of intraoperative oliguria during major abdominal surgery: Findings of the Restrictive versus Liberal Fluid Therapy in Major Abdominal Surgery trial. Br. J. Anaesth. 2019, 122, 726–733. [Google Scholar] [CrossRef]
- Iguchi, N.; Kosaka, J.; Booth, L.C.; Iguchi, Y.; Evans, R.G.; Bellomo, R.; May, C.N.; Lankadeva, Y.R. Renal perfusion, oxygenation, and sympathetic nerve activity during volatile or intravenous general anaesthesia in sheep. Br. J. Anaesth. 2019, 122, 342–349. [Google Scholar] [CrossRef]
- Osborn, J.; Tyshynsky, R.; Vulchanova, L. Function of renal nerves in kidney physiology and pathophysiology. Annu. Rev. Physiol. 2021, 83, 429–450. [Google Scholar] [CrossRef]
- Hart, E.C.; Head, G.A.; Carter, J.R.; Wallin, B.G.; May, C.N.; Hamza, S.M.; Hall, J.E.; Charkoudian, N.; Osborn, J.W. Recording sympathetic nerve activity in conscious humans and other mammals: Guidelines and the road to standardization. Am. J. Physiol. Heart Circulat Physiol. 2017, 312, H1031–H1051. [Google Scholar] [CrossRef]
- Taavo, M.; Rundgren, M.; Frykholm, P.; Larsson, A.; Franzén, S.; Vargmar, K.; Valarcher, J.F.; DiBona, G.F.; Frithiof, R. Role of renal sympathetic nerve activity in volatile aneshesia’s effect on renal excretory function. Function 2021, 2, zqab042. [Google Scholar] [CrossRef]
- Frithiof, R.; Soehnlein, O.; Eriksson, S.; Fenhammar, J.; Hjelmqvist, H.; Lindbom, L.; Rundgren, M. The effects of isoflurane anesthesia andmechanical ventilation on renal function during endotoxemia. Acta Anaesthesiol. Scand. 2011, 55, 401–410. [Google Scholar] [CrossRef]
- Garcia, C.; Julier, K.; Bestmann, L.; Zollinger, A.; von Segesser, L.K.; Pasch, T.; Spahn, D.R.; Zaugg, M. Preconditioning with sevofluranedecreases PECAM-1 expression and improves one-year cardiovas-cular outcome in coronary artery bypass graft surgery. Br. J. Anaesth. 2005, 94, 159–165. [Google Scholar] [CrossRef]
- Sindhvananda, W.; Phisaiphun, K.; Prapongsena, P. No renal protection from volatile-anesthetic preconditioning in open heart surgery. J. Anesth. 2013, 27, 48–55. [Google Scholar] [CrossRef]
- Dharmalingam, S.K.; Amirtharaj, G.J.; Ramachandran, A.; Korula, M. Volatile anesthetic preconditioning modulates oxidative stress and ni-tric oxide in patients undergoing coronary artery bypass grafting. Ann. Card. Anaesth. 2021, 24, 319–326. [Google Scholar] [CrossRef]
- Meldrum, D.J.; Griffiths, R.; Kenna, J.G. Gallstones and isoflurane hepatitis. Anaesthesia 1998, 53, 905–909. [Google Scholar] [CrossRef]
- Ihtiyar, E.; Algin, C.; Haciolu, A.; Isiksoy, S. Fatal isoflurane hepatotoxicity without re-exposure. Indian J. Gastroenterol. 2006, 25, 41–42. [Google Scholar]
- Peiris, L.J.; Agrawal, A.; Morris, J.E.; Basnyat, P.S. Isoflurane hepatitis-induced liver failure: A case report. J. Clin. Anesth. 2012, 24, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuijs-Moeke, G.J.; Nieuwenhuijs, V.B.; Seelen, M.A.J.; Berger, S.P.; van den Heuvel, M.C.; Burgerhof, J.G.M.; Ottens, P.J.; Ploeg, R.J.; Leuvenink, H.G.D.; Struys, M.M.R.F. Propofol-based anaesthesia versus sevoflurane-based anaesthesia for living donor kidney transplantation: Results of the VAPOR-1 randomized controlled trial. Br. J. Anaesth. 2017, 118, 720–732. [Google Scholar] [CrossRef]
- Lee, H.-J.; Bae, J.; Kwon, Y.; Jang, H.S.; Yoo, S.; Jeong, C.W.; Kim, J.-T.; Kim, W.H. General anesthetic agents and renal function after nephrectomy. J. Clin. Med. 2019, 8, 1530. [Google Scholar] [CrossRef]
- Bang, J.Y.; Lee, J.; Oh, J.; Song, J.G.; Hwang, G.S. The influence of propofol and sevoflurane on acute kidney injury after colorectal surgery: A retrospective cohort study. Anesth. Analg. 2016, 123, 363–370. [Google Scholar] [CrossRef]
- Kim, B.R.; Yoon, S.; Song, G.Y.; Lee, S.; Bahk, J.H.; Nam, K. The impact of total intravenous anesthesia versus inhalation anesthesia on acute kidney injury after major abdominal surgery: A propensity score analysis. J. Anesth. 2021, 35, 112–121. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Park, J.; Lee, S.-H.; Oh, A.-R.; Lee, J.-H.; Min, J.J. Effects of volatile versus total intravenous anesthesia on occurrence of myocardial injury after non-cardiac surgery. J. Clin. Med. 2019, 8, 1999. [Google Scholar] [CrossRef]
- Ammar, A.S.; Mahmoud, K.M. Comparative effect of propofol versus sevoflurane on renal ischemia/reperfusion injury after elective open abdominal aortic aneurysm repair. Saudi J. Anaesth. 2016, 10, 301–307. [Google Scholar]
- Yoo, Y.C.; Shim, J.K.; Song, Y.; Yang, S.Y.; Kwak, Y.L. Anesthetics influence the incidence of acute kidney injury following valvular heart surgery. Kidney Int. 2014, 86, 414–422. [Google Scholar] [CrossRef]
- Erdem, A.F.; Ligaz, A.; Yuksek, M.S.; Gürsan, N.; Atalay, C. The Effects of Isoflurane, Sevoflurane and Desflurane Anesthesia on the Glyserol Model of Rhabdomyolysis-Induced Acute Renal Failure in Rats. Eurasian J. Med. 2007, 39, 33–36. [Google Scholar]
- Sivgin, V.; Kasikara, H.; Kucuk, A.; Inan, H.M.; Gok, G.; Arslan, M.; Kıran, M.M.; Ozturk, L. The Effects of Cerium Oxide on Sevoflurane Anesthesia and its Relationship to Renal Injury in Rats. Gaz. Med. J. 2023, 34, 283–287. [Google Scholar]
- Hollenberg, N.K. The renal circulation. In The Peripheral Circulations; Zelis, R., Ed.; Grune & Stratton: New York, NY, USA, 1975; p. 131. [Google Scholar]
- Groves, N.D.; Leach, K.G.; Rosen, M. Effects of halothane, enflurane and isoflurane anaesthesia on renal plasma flow. Br. J. Anaesth. 1990, 65, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Sneyd, J.R. Avoiding kidney damage in ICU sedation with sevoflurane: Use isoflurane instead. Br. J. Anaesth. 2022, 129, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Honore, P.M.; Bousbiat, I.; Perriens, E.; Blackman, S. Letter to the Editor: “Prolonged sedation with sevoflurane in comparison to intravenous sedation in critically ill patients—A randomized controlled trial”. J. Crit. Care 2023, 77, 154348. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. The role of ions, heavy metals, fluoride, and agrochemicals: Critical evaluation of potential aetiological factors of chronic kidney disease of multifactorial origin (CKDmfo/CKDu) and recommendations for its eradication. Environ. Geochem. Health 2016, 38, 639–678. [Google Scholar] [CrossRef] [PubMed]
- Yaxley, J. Anaesthesia in chronic dialysis patients: A narrative review. World J. Crit. Care Med. 2025, 14, 100503. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, S.; Zhang, G.; Luo, W.; Xu, M.; Peng, R.; Du, Z.; Liu, Y.; Bai, Z.; Xiao, X.; Qin, S. PROTAC technology: From drug development to probe technology for target deconvolution. Eur. J. Med. Chem. 2024, 276, 116725. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xiao, X. Key advances and application prospects of PROTAC technologies in the next 5 years. Future Med. Chem. 2025, 17, 987–989. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hauquiert, B.; Gonze, A.; Gennart, T.; Perriens, E.; Blackman, S.; De Lissnyder, N.; Robert, A.; Moury, J.; Nendumba, G.; Oueslati, I.; et al. Nephrotoxicity and Modern Volatile Anesthetics: A Narrative Review. Toxics 2025, 13, 514. https://doi.org/10.3390/toxics13060514
Hauquiert B, Gonze A, Gennart T, Perriens E, Blackman S, De Lissnyder N, Robert A, Moury J, Nendumba G, Oueslati I, et al. Nephrotoxicity and Modern Volatile Anesthetics: A Narrative Review. Toxics. 2025; 13(6):514. https://doi.org/10.3390/toxics13060514
Chicago/Turabian StyleHauquiert, Benedicte, Aurelien Gonze, Thibault Gennart, Emily Perriens, Sydney Blackman, Nathan De Lissnyder, Arnaud Robert, Julien Moury, Gauthier Nendumba, Ilann Oueslati, and et al. 2025. "Nephrotoxicity and Modern Volatile Anesthetics: A Narrative Review" Toxics 13, no. 6: 514. https://doi.org/10.3390/toxics13060514
APA StyleHauquiert, B., Gonze, A., Gennart, T., Perriens, E., Blackman, S., De Lissnyder, N., Robert, A., Moury, J., Nendumba, G., Oueslati, I., Gillis, P., Vornicu, O., Dincq, A.-S., Bulpa, P., Michaux, I., & Honore, P. M. (2025). Nephrotoxicity and Modern Volatile Anesthetics: A Narrative Review. Toxics, 13(6), 514. https://doi.org/10.3390/toxics13060514







