Impact of Polystyrene Microplastics on Human Sperm Functionality: An In Vitro Study of Cytotoxicity, Genotoxicity and Fertility-Related Genes Expression
Highlights
- PS-MP exposure affects human sperm vitality and motility in a time-dependent way.
- PS-MPs increase oxidative stress, sperm DNA fragmentation, and ROS production.
- PS-MPs reduce sperm movement and DNA quality.
- The study suggests PS-MP exposure may disrupt sperm’s ability to fertilize oocyte.
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical
2.2. Sperm Collection and Exposure
2.3. Sperm DNA Fragmentation
2.4. RAPD-PCR and GTS
2.5. NitroBlue Tetrazolium Chloride Test (NBT)
2.6. RNA Purification and Real-Time PCR Analyses
2.7. Statistical Analysis
3. Results
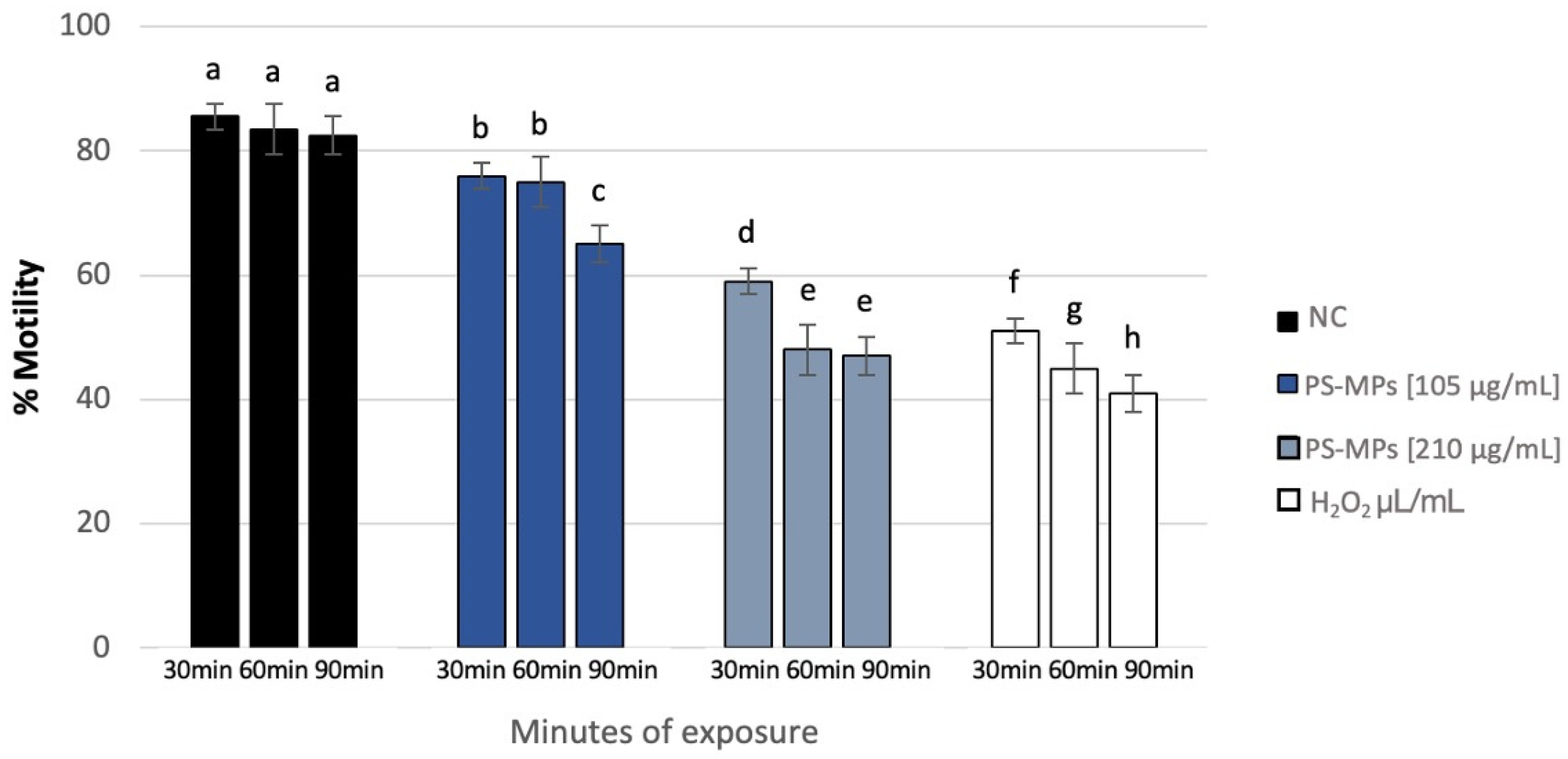
3.1. Sperm Analysis
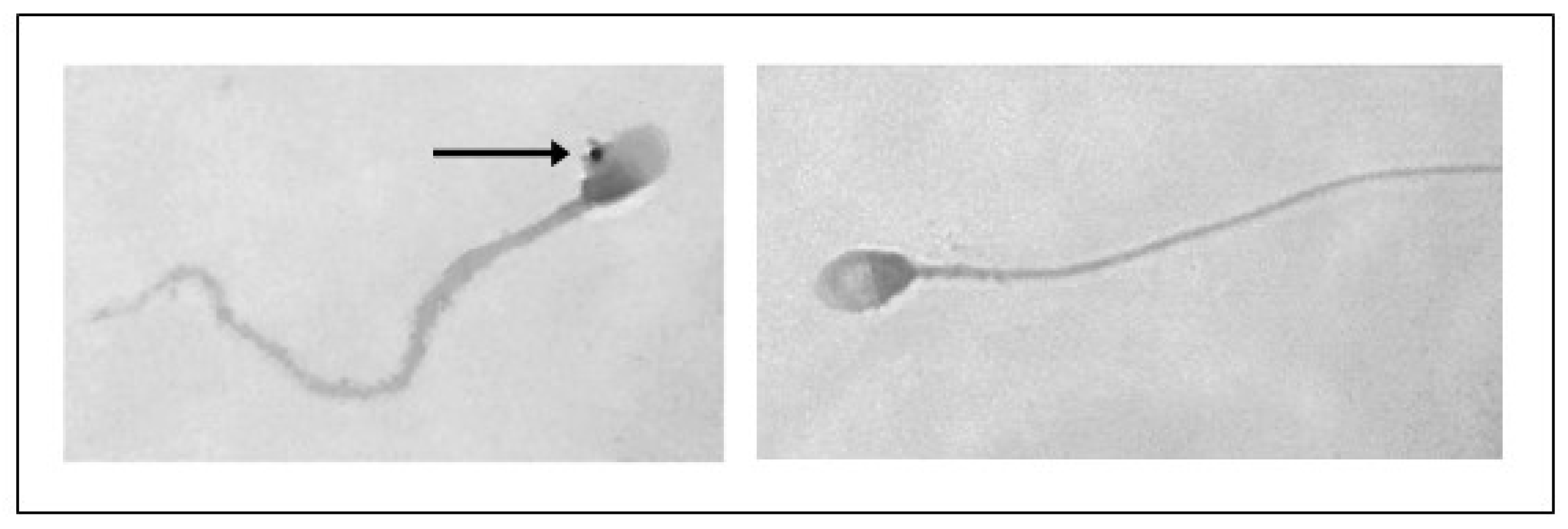
3.2. TUNEL Assay
3.3. RAPD-PCR Profile
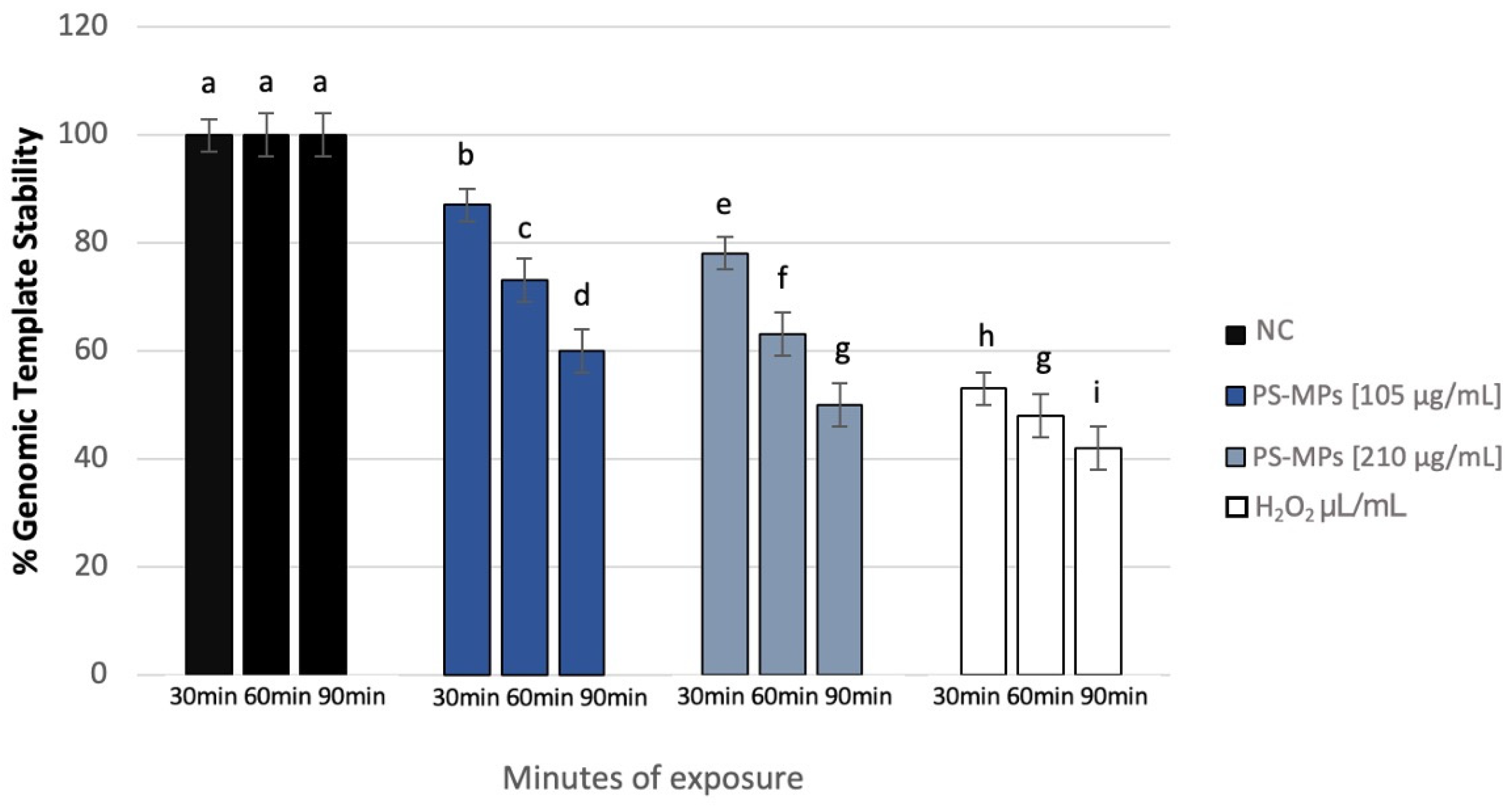
3.4. Genomic Template Stability
3.5. ROS Detection
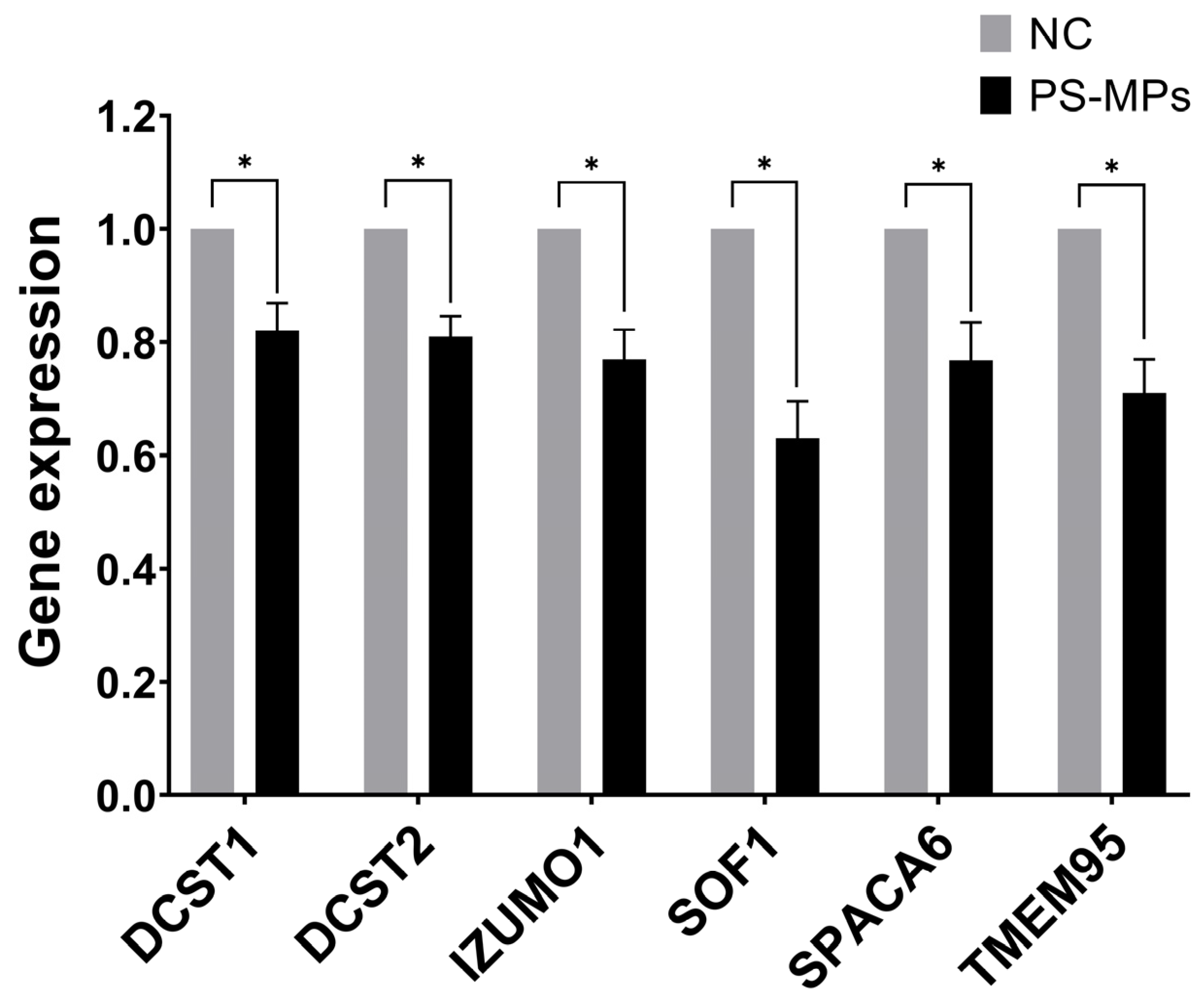
3.6. Expression of Sperm–Egg Fusion Genes
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marić, T.; Fučić, A.; Aghayanian, A. Environmental and occupational exposures associated with male infertility. Arh. Ind. Hig. Rada Toksikol. 2021, 72, 101–113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Finelli, R.; Mottola, F.; Agarwal, A. Impact of Alcohol Consumption on Male Fertility Potential: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 19, 328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rocco, L.; Durairajanayagam, D.; Mottola, F. Endocrine-Disrupting Chemicals (EDCs) and Male Infertility. In Current and Future Advances in Male Infertility; Agarwal, A., Saleh, R., Boitrelle, F., Shah, R., Eds.; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Roepke, T.A.; Sadlier, N.C. REPRODUCTIVE TOXICOLOGY: Impact of endocrine disruptors on neurons expressing GnRH or kisspeptin and pituitary gonadotropins. Reproduction 2021, 162, F131–F145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Briffa, S.M. Looking at the Bigger Picture-Considering the Hurdles in the Struggle against Nanoplastic Pollution. Nanomaterials 2021, 11, 2536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Effects of microplastics on microalgae populations: A critical review. Sci. Total Environ. 2019, 665, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Manna, C.; Padha, S.; Verma, A.; Sharma, P.; Dhar, A.; Ghosh, A.; Bhattacharya, P. Micro(nano)plastics pollution and human health: How plastics can induce carcinogenesis to humans? Chemosphere 2022, 298, 134267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, Y.B.; He, H.R.; Zhang, J.F.; Ma, G.S. You are what you eat: Microplastics in the feces of young men living in Beijing. Sci. Total Environ. 2021, 767, 144345. [Google Scholar] [CrossRef] [PubMed]
- Lett, Z.; Hall, A.; Skidmore, S.; Alves, N.J. Environmental microplastic and nanoplastic: Exposure routes and effects on coagulation and the cardiovascular system. Environ. Pollut. 2021, 291, 118190. [Google Scholar] [CrossRef] [PubMed]
- Montano, L.; Giorgini, E.; Notarstefano, V.; Notari, T.; Ricciardi, M.; Piscopo, M.; Motta, O. Raman Microspectroscopy evidence of microplastics in human semen. Sci. Total Environ. 2023, 901, 165922. [Google Scholar] [CrossRef] [PubMed]
- Vethaak, A.D.; Legler, J. Microplastics and human health. Science 2021, 371, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Sternschuss, G.; Ostergard, D.R.; Patel, H. Post-implantation alterations of polypropylene in the human. J. Urol. 2012, 188, 27–32, Erratum in J. Urol. 2012, 188, 1052. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.D.; Chen, C.W.; Chen, Y.C.; Chen, H.H.; Lee, J.S.; Lin, C.H. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J. Hazard. Mater. 2020, 385, 121575. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Cheng, J. Exposure to polystyrene microplastics impairs gonads of zebrafish (Danio rerio). Chemosphere 2021, 263, 128161. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Ma, T.; Sha, X.; Liu, Z.; Zhou, Y.; Meng, X.; Chen, Y.; Han, X.; Ding, J. Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater. 2021, 401, 123430. [Google Scholar] [CrossRef] [PubMed]
- Mottola, F.; Carannante, M.; Barretta, A.; Palmieri, I.; Rocco, L. Reproductive cytotoxic and genotoxic impact of polystyrene microplastic on Paracentrotus lividus spermatozoa. Curr. Res. Toxicol. 2024, 6, 100173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gallo, A.; Boni, R.; Tosti, E. Gamete quality in a multistressor environment. Environ. Int. 2020, 138, 105627. [Google Scholar] [CrossRef] [PubMed]
- Shams, M.; Alam, I.; Chowdhury, I. Aggregation and stability of nanoscale plastics in aquatic environment. Water Res. 2020, 171, 115401. [Google Scholar] [CrossRef] [PubMed]
- Mottola, F.; Palmieri, I.; Carannante, M.; Barretta, A.; Roychoudhury, S.; Rocco, L. Oxidative Stress Biomarkers in Male Infertility: Established Methodologies and Future Perspectives. Genes 2024, 15, 539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banikazemi, Z.; Farshadi, M.; Rajabi, A.; Homayoonfal, M.; Sharifi, N.; Sharafati Chaleshtori, R. Nanoplastics: Focus on the role of microRNAs and long non-coding RNAs. Chemosphere 2022, 308 Pt 1, 136299. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, R.C.; Cardoso, R.M.; Domingues, E.L.B.C.; Gonçalves, R.V.; Lima, G.D.A.; Novaes, R.D. The emerging risk of microplastics and nanoplastics on the microstructure and function of reproductive organs in mammals: A systematic review of preclinical evidence. Life Sci. 2022, 295, 120404. [Google Scholar] [CrossRef] [PubMed]
- Noda, T.; Lu, Y.; Fujihara, Y.; Oura, S.; Koyano, T.; Kobayashi, S.; Matzuk, M.M.; Ikawa, M. Sperm proteins SOF1, TMEM95, and SPACA6 are required for sperm-oocyte fusion in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 11493–11502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Inoue, N.; Ikawa, M.; Isotani, A.; Okabe, M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 2005, 434, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Doe, B.; Goulding, D.; Wright, G.J. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature 2014, 508, 483–487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Inoue, N.; Hamada, D.; Kamikubo, H.; Hirata, K.; Kataoka, M.; Yamamoto, M.; Ikawa, M.; Okabe, M.; Hagihara, Y. Molecular dissection of IZUMO1, a sperm protein essential for sperm-egg fusion. Development 2013, 140, 3221–3229. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Hagihara, Y.; Wright, D.; Suzuki, T.; Wada, I. Oocyte-triggered dimerization of sperm IZUMO1 promotes sperm-egg fusion in mice. Nat. Commun. 2015, 6, 8858. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Inoue, N.; Hagihara, Y.; Wada, I. Evolutionarily conserved sperm factors, DCST1 and DCST2, are required for gamete fusion. eLife 2021, 10, e66313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Noda, T.; Blaha, A.; Fujihara, Y.; Gert, K.R.; Emori, C.; Deneke, V.E.; Oura, S.; Panser, K.; Lu, Y.; Berent, S.; et al. Sperm membrane proteins DCST1 and DCST2 are required for sperm-egg interaction in mice and fish. Commun. Biol. 2022, 5, 332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Binner, M.I.; Kogan, A.; Panser, K.; Schleiffer, A.; Deneke, V.E.; Pauli, A. The Sperm Protein Spaca6 is Essential for Fertilization in Zebrafish. Front. Cell Dev. Biol. 2022, 9, 806982. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barbaux, S.; Ialy-Radio, C.; Chalbi, M.; Dybal, E.; Homps-Legrand, M.; Do Cruzeiro, M.; Vaiman, D.; Wolf, J.P.; Ziyyat, A. Sperm SPACA6 protein is required for mammalian Sperm-Egg Adhesion/Fusion. Sci. Rep. 2020, 10, 5335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lorenzetti, D.; Poirier, C.; Zhao, M.; Overbeek, P.A.; Harrison, W.; Bishop, C.E. A transgenic insertion on mouse chromosome 17 inactivates a novel immunoglobulin superfamily gene potentially involved in sperm-egg fusion. Mamm. Genome 2014, 25, 141–148. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO Press: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240030787 (accessed on 17 March 2025).
- Lin, M.H.; Morshedi, M.; Srisombut, C.; Nassar, A.; Oehninger, S. Plasma membrane integrity of cryopreserved human sperm: An investigation of the results of the hypoosmotic swelling test, the water test, and eosin-Y staining. Fertil. Steril. 1998, 70, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hou, B.; Wang, Z.; Yang, Y. Polystyrene microplastics induce mitochondrial damage in mouse GC-2 cells. Ecotoxicol. Environ. Saf. 2022, 237, 113520. [Google Scholar] [CrossRef] [PubMed]
- Grillo, G.; Falvo, S.; Latino, D.; Chieffi Baccari, G.; Venditti, M.; Di Fiore, M.M.; Minucci, S.; Santillo, A. Polystyrene microplastics impair the functions of cultured mouse Leydig (TM3) and Sertoli (TM4) cells by inducing mitochondrial-endoplasmic reticulum damage. Ecotoxicol. Environ. Saf. 2024, 274, 116202. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Sun, K.; Wang, S.; Gong, D. Polystyrene microplastics induce apoptosis and necroptosis in swine testis cells via ROS/MAPK/HIF1α pathway. Environ. Toxicol. 2022, 37, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, M.; Bao, T.T.; Lan, H. Long-term exposure to polystyrene microplastics triggers premature testicular aging. Part. Fibre Toxicol. 2023, 20, 35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aves, A.R.; Revell, L.E.; Gaw, S.; Ruffell, H.; Schuddeboom, A.; Wotherspoon, N.E.; LaRue, M.; McDonald, A.J. First Evidence of Microplastics in Antarctic Snow. Cryosphere 2022, 16, 2127–2145. [Google Scholar] [CrossRef]
- Bergmann, M.; Collard, F.; Fabres, J.; Gabrielsen, G.W.; Provencher, J.F.; Rochman, C.M.; van Sebille, E.; Tekman, M.B. Plastic Pollution in the Arctic. Nat. Rev. Earth Environ. 2022, 3, 323–337. [Google Scholar] [CrossRef]
- Napper, I.E.; Davies, B.F.R.; Clifford, H.; Elvin, S.; Koldewey, H.J.; Mayewski, P.A.; Miner, K.R.; Potocki, M.; Elmore, A.C.; Gajurel, A.P.; et al. Reaching New Heights in Plastic Pollution—Preliminary Findings of Microplastics on Mount Everest. One Earth 2020, 3, 621–630. [Google Scholar] [CrossRef]
- Peng, X.; Chen, M.; Chen, S.; Dasgupta, S.; Xu, H.; Ta, K.; Du, M.; Li, J.; Guo, Z.; Bai, S. Microplastics Contaminate the Deepest Part of the World’s Ocean. Geochem. Perspect. Lett. 2018, 9, 1–5. [Google Scholar] [CrossRef]
- Mottola, F.; Iovine, C.; Carannante, M.; Santonastaso, M.; Rocco, L. In Vitro Combination of Ascorbic and Ellagic Acids in Sperm Oxidative Damage Inhibition. Int. J. Mol. Sci. 2022, 23, 14751. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iovine, C.; Mottola, F.; Santonastaso, M.; Finelli, R.; Agarwal, A.; Rocco, L. In vitro ameliorative effects of ellagic acid on vitality, motility and DNA quality in human spermatozoa. Mol. Reprod. Dev. 2021, 88, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; He, L.; Peng, W.; Ding, L.; Tang, K.; Fang, D.; Zhang, Y. Selection of optimal reference genes for quantitative RT-PCR studies of boar spermatozoa cryopreservation. Cryobiology 2014, 68, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Giebler, M.; Greither, T.; Handke, D.; Seliger, G.; Behre, H.M. Lower Spermatozoal PIWI-LIKE 1 and 2 Transcript Levels Are Significantly Associated with Higher Fertilization Rates in IVF. Int. J. Mol. Sci. 2021, 22, 11320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krause, S.; Baranov, V.; Nel, H.A.; Drummond, J.D.; Kukkola, A.; Hoellein, T.; Sambrook Smith, G.H.; Lewandowski, J.; Bonet, B.; Packman, A.I.; et al. Gathering at the top? Environmental controls of microplastic uptake and biomagnification in freshwater food webs. Environ. Pollut. 2021, 268 Pt A, 115750. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Raps, H.; Cropper, M.; Bald, C.; Brunner, M.; Canonizado, E.M.; Charles, D.; Chiles, T.C.; Donohue, M.J.; Enck, J.; et al. The MinderooMonaco Commission on Plastics and Human Health. Ann. Glob. Health 2023, 89, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, S.W.; Ryu, K.Y. Increased clearance of non-biodegradable polystyrene nanoplastics by exocytosis through inhibition of retrograde intracellular transport. J. Hazard. Mater. 2022, 439, 129576. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Q.; Yu, H.; Yang, L.; Sun, Y.; Xu, N.; Wang, N.; Lei, Z.; Hou, J.; Jin, Y.; et al. Polystyrene microplastics induce blood-testis barrier disruption regulated by the MAPK-Nrf2 signaling pathway in rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 47921–47931. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Deng, T.; Duan, J.; Xie, J.; Yuan, J.; Chen, M. Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway. Ecotoxicol. Environ. Saf. 2020, 190, 110133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Z.; Ma, S.; Chen, R.; Wang, S.; Zhang, H.; Hua, Z.; Sun, Z. Repair mechanism of Yishen Tongluo formula on mouse sperm DNA fragmentation caused by polystyrene microplastics. Pharm. Biol. 2023, 61, 488–498. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ijaz, M.U.; Tahir, A.; Samad, A.; Anwar, H. Nobiletin ameliorates nonylphenol-induced testicular damage by improving biochemical, steroidogenic, hormonal, spermatogenic, apoptotic and histological profile. Hum. Exp. Toxicol. 2021, 40, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Wu, L.; Mou, L.; Liu, C. Polystyrene microplastics trigger testosterone decline via GPX1. Sci Total Environ. 2024, 947, 174536. [Google Scholar] [CrossRef] [PubMed]
- Mirzayans, R.; Murray, D. Do TUNEL and Other Apoptosis Assays Detect Cell Death in Preclinical Studies? Int. J. Mol. Sci. 2020, 21, 9090. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Atienzar, F.A.; Jha, A.N. The random amplified polymorphic DNA (RAPD) assay and related techniques applied to genotoxicity and carcinogenesis studies: A critical review. Mutat. Res. 2006, 613, 76–102. [Google Scholar] [CrossRef] [PubMed]
- Rocco, L.; Valentino, I.V.; Scapigliati, G.; Stingo, V. RAPD-PCR analysis for molecular characterization and genotoxic studies of a new marine fish cell line derived from Dicentrarchus labrax. Cytotechnology 2014, 66, 383–393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.; Le Goïc, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Q.; Zhu, L.; Weng, J.; Jin, Z.; Cao, Y.; Jiang, H.; Zhang, Z. Detection and characterization of microplastics in the human testis and semen. Sci. Total Environ. 2023, 877, 162713. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.G.; Aston, K.I.; Meyer, T.; Carrell, D.T. The Sperm Epigenome, Male Aging, and Potential Effects on the Embryo. Adv. Exp. Med. Biol. 2015, 868, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Dubey, I.; Khan, S.; Kushwaha, S. Developmental and reproductive toxic effects of exposure to microplastics: A review of associated signaling pathways. Front. Toxicol. 2022, 4, 901798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nowicka-Bauer, K.; Nixon, B. Molecular Changes Induced by Oxidative Stress that Impair Human Sperm Motility. Antioxidants 2020, 9, 134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abbasi, R.; Shineh, G.; Mobaraki, M.; Doughty, S.; Tayebi, L. Structural parameters of nanoparticles affecting their toxicity for biomedical applications: A review. J. Nanoparticle Res. 2023, 25, 43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toledano-Magaña, Y.; Flores-Santos, L.; Montes de Oca, G.; González-Montiel, A.; García-Ramos, J.C.; Mora, C.; Saavedra-Ávila, N.A.; Gudiño-Zayas, M.; González-Ramírez, L.C.; Laclette, J.P.; et al. Toxicological Evaluations in Macrophages and Mice Acutely and Chronically Exposed to Halloysite Clay Nanotubes Functionalized with Polystyrene. ACS Omega 2021, 6, 29882–29892. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, Y.; Li, J.; Chen, J.; Miao, X.; Li, G.; He, Q.; Xu, H.; Li, H.; Wei, Y. Cytotoxic effects of polystyrene nanoplastics with different surface functionalization on human HepG2 cells. Sci. Total Environ. 2020, 723, 138180. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Dausend, J.; Hafner, M.; Musyanovych, A.; Röcker, C.; Landfester, K.; Mailänder, V.; Nienhaus, G.U. Specific effects of surface amines on polystyrene nanoparticles in their interactions with mesenchymal stem cells. Biomacromolecules 2010, 11, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Grazia Mele, V.; Chioccarelli, T.; Diano, N.; Cappetta, D.; Ferraro, B.; Telesca, M.; Moggio, M.; Porreca, V.; De Angelis, A.; Berrino, L.; et al. Variation of sperm quality and circular RNA content in men exposed to environmental contamination with heavy metals in ‘Land of Fires’, Italy. Hum. Reprod. 2024, 39, 1628–1644. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hosken, D.J.; Hodgson, D.J. Why do sperm carry RNA? Relatedness, conflict, and control. Trends Ecol. Evol. 2014, 29, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Galeraud-Denis, I.; Lambard, S.; Carreau, S. Relationship between chromatin organization, mRNAs profile and human male gamete quality. Asian J. Androl. 2007, 9, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Ostermeier, G.C. Towards a better understanding of RNA carriage by ejaculate spermatozoa. Hum. Reprod. Update 2006, 12, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chen, X.; Wang, Z.; Wang, D. Is transcription in sperm stationary or dynamic? J. Reprod. Dev. 2017, 63, 439–443. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, G.D.; Lalancette, C.; Linnemann, A.K.; Leduc, F.; Boissonneault, G.; Krawetz, S.A. The sperm nucleus: Chromatin, RNA, and the nuclear matrix. Reproduction 2011, 141, 21–36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- St Laurent, G.; Shtokalo, D.; Tackett, M.R.; Yang, Z.; Vyatkin, Y.; Milos, P.M.; Seilheimer, B.; McCaffrey, T.A.; Kapranov, P. On the importance of small changes in RNA expression. Methods 2013, 63, 18–24. [Google Scholar] [CrossRef] [PubMed]






| SPERM PARAMETERS | SD |
|---|---|
| Semen volume (mL) | 0.5 |
| pH | 0.3 |
| Sperm concentration (×106 sperm/mL) | 15.6 |
| Viability (%) | 4.0 |
| Total Motility (%) | 3.0 |
| Normal morphology | 4.0 |
| Official Symbol | Official Full Name | PCR Primers | Gene Type | Organism |
|---|---|---|---|---|
| DCST1 | DC-STAMP domain containing 1 | 5′-AAGCTGCGTTGCTCCTATGT-3′ 5′-CATGCTTGCGGTCAAACCAA-3′ | Protein coding | Homo sapiens |
| DCST2 | DC-STAMP domain containing 2 | 5′-GCAACAGCTCCAAGAAGTGC-3′ 5′-GGTGAGAATGCTGACAGGCT-3′ | Protein coding | Homo sapiens |
| IZUMO1 | Izumo sperm–oocyte fusion 1 | 5′-TTTCACGTCACAGTGTTGCC-3′ 5′-GAGGCTTATGGACGACTCCG-3′ | Protein coding | Homo sapiens |
| SOF1 | Sperm−oocyte fusion required 1 | 5′-AAAAGTCGAAGCAGGGAGGT-3′ 5′-GCATTAGCTTTCCACAGGCG-3′ | Protein coding | Homo sapiens |
| SPACA6 | Sperm acrosome associated 6 | 5′-GGCGACCAGGCTATGTTTTC-3′ 5′-GGCATATCTCGGAAATAGGACAAG-3′ | Protein coding | Homo sapiens |
| TMEM95 | Transmembrane protein 95 | 5′-AAAGTGGCTTGTGAAGACGC-3′ 5′-ACTGTCTTTGGAGCTGGGAC-3′ | Protein coding | Homo sapiens |
| GAPDH (reference gene) | Glyceraldehyde-3-phosphate dehydrogenase | 5′-GAAGGTGAAGGTCGGAGTC-3′ 5′-GAAGATGGTGATGGGATTT-3′ | Protein coding | Homo sapiens |
| Minutes of Exposure |
Lost Bands at
105 μg/mL PS-MPs |
Lost Bands at
210 μg/mL PS-MPs |
Gained Bands at
105 μg/mL PS-MPs |
Gained Bands at
210 μg/mL PS-MPs |
|---|---|---|---|---|
| 30′ | 290 bp | 290–350 bp | - | 690 bp |
| 60′ | 500–700–1000 bp | 350–500–700–1000–1100 bp | 200 bp | - |
| 90′ | 290–400–550–1100 bp | 290–320–700–1000 bp | 350 bp | 200–300 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mottola, F.; Carannante, M.; Palmieri, I.; Ibello, L.; Montano, L.; Pezzullo, M.; Mosca, N.; Potenza, N.; Rocco, L. Impact of Polystyrene Microplastics on Human Sperm Functionality: An In Vitro Study of Cytotoxicity, Genotoxicity and Fertility-Related Genes Expression. Toxics 2025, 13, 605. https://doi.org/10.3390/toxics13070605
Mottola F, Carannante M, Palmieri I, Ibello L, Montano L, Pezzullo M, Mosca N, Potenza N, Rocco L. Impact of Polystyrene Microplastics on Human Sperm Functionality: An In Vitro Study of Cytotoxicity, Genotoxicity and Fertility-Related Genes Expression. Toxics. 2025; 13(7):605. https://doi.org/10.3390/toxics13070605
Chicago/Turabian StyleMottola, Filomena, Maria Carannante, Ilaria Palmieri, Lorenzo Ibello, Luigi Montano, Mariaceleste Pezzullo, Nicola Mosca, Nicoletta Potenza, and Lucia Rocco. 2025. "Impact of Polystyrene Microplastics on Human Sperm Functionality: An In Vitro Study of Cytotoxicity, Genotoxicity and Fertility-Related Genes Expression" Toxics 13, no. 7: 605. https://doi.org/10.3390/toxics13070605
APA StyleMottola, F., Carannante, M., Palmieri, I., Ibello, L., Montano, L., Pezzullo, M., Mosca, N., Potenza, N., & Rocco, L. (2025). Impact of Polystyrene Microplastics on Human Sperm Functionality: An In Vitro Study of Cytotoxicity, Genotoxicity and Fertility-Related Genes Expression. Toxics, 13(7), 605. https://doi.org/10.3390/toxics13070605










