Airborne SARS-CoV-2 Detection by ddPCR in Adequately Ventilated Hospital Corridors
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Points
2.2. Bioaerosol Sample Collection
2.3. RNA Isolation and Quantification
2.4. Reverse Transcription
2.5. Droplet Digital Polymerase Chain Reaction (ddPCR)
2.6. Environmental Data
2.7. Statistical Analysis
3. Results and Discussion
3.1. Airborne SARS-CoV-2 Detection in Hospital Corridors
3.2. Comparison of the Environmental Data Between Hospital Wards
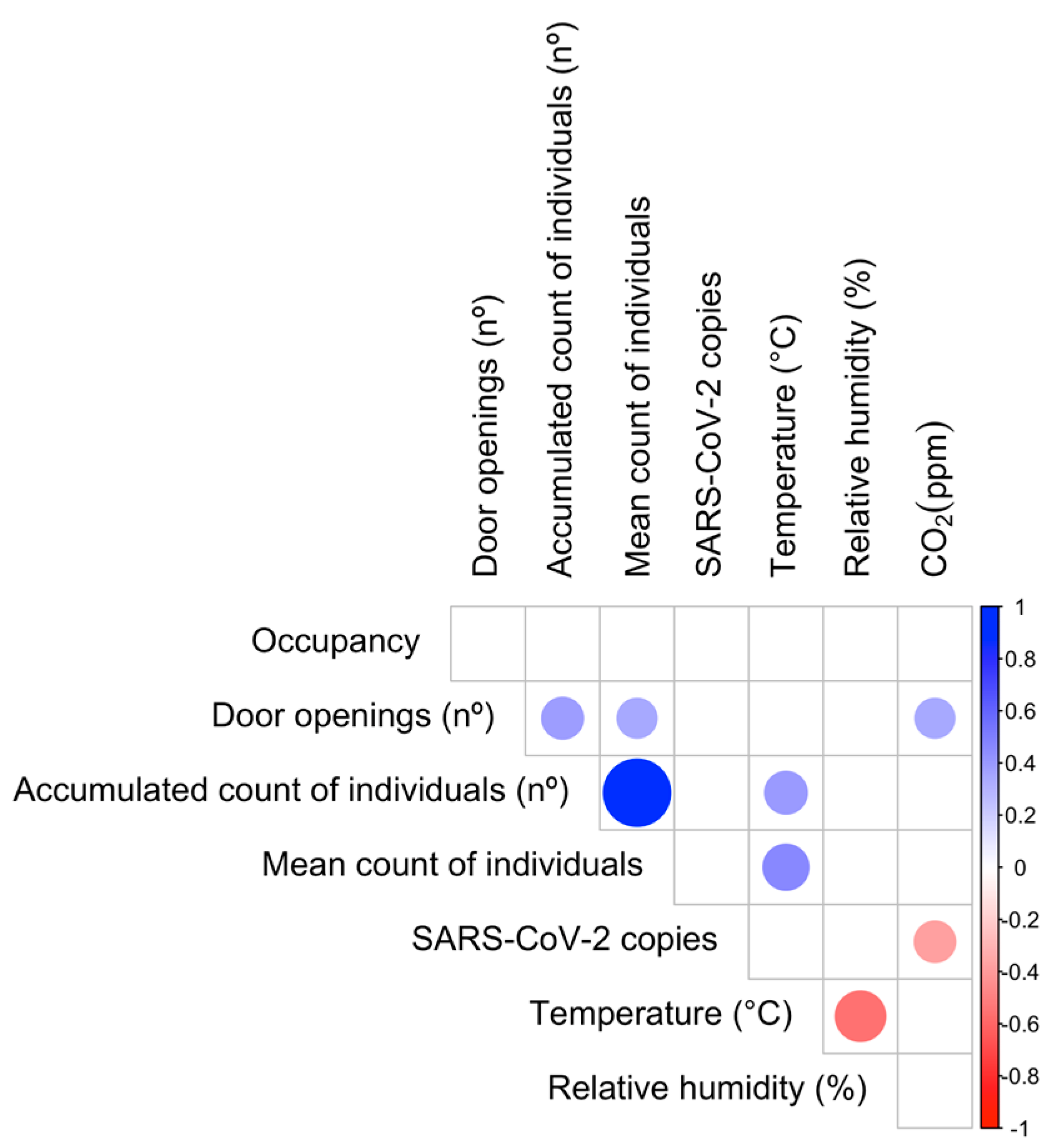
3.3. Relationship Between Airborne SARS-CoV-2 Detection and Environmental Parameters
3.4. Relationship Between Airborne SARS-CoV-2 Quantification and Environmental Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASHRAE | American Society of Heating, Refrigeration and Air-Conditioning Engineers |
| CDC | Centers for Disease Control and Prevention |
| cDNA | Complementary DNA |
| CO2 | Carbon dioxide |
| COVID-19 | Coronavirus disease 19 |
| ddPCR | Droplet digital PCR |
| DEPC | Diethyl pyrocarbonate |
| DNA | Deoxyribonucleic acid |
| dNTPs | Deoxynucleotide triphosphates |
| DTT | Dithiothreitol |
| dUTP | Deoxyuridine triphosphate |
| FAM | 6-carboxyfluorescein |
| HFOT | High-flow oxygen therapy |
| HEX | Hexachlorofluorescein |
| HUSE | Hospital Universitari Son Espases |
| IRCU | Intermediate Respiratory Care Unit |
| NDIR | Non-dispersive infrared |
| OSHA | Occupational Safety and Health Administration |
| PCR | Polymerase Chain Reaction |
| qPCR | Quantitative polymerase chain reaction |
| REHVA | Federation of European Heating and Air Conditioning Associations |
| RNA | Ribonucleic acid |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| WHO | World Health Organization |
References
- The Impact of COVID-19 on Health and Care Workers: A Closer Look at Deaths. Available online: https://iris.who.int/handle/10665/345300 (accessed on 13 May 2025).
- Dobso, A.P.; Pim, S.L.; Hannah, L.; Kaufman, L.; Ahumad, J.A.; And, A.W.; Bernstein, A.; Busch, J.; Daszak, P.; Engelmann, J.; et al. Ecology and Economics for Pandemic Prevention: Investments to Prevent Tropical Deforestation and to Limit Wildlife Trade Will Protect against Future Zoonosis Outbreaks. Science 2020, 369, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Truyols-Vives, J.; Escarrer-Garau, G.; Arbona-González, L.; Toledo-Pons, N.; Sauleda-Roig, J.; Ferrer, M.D.; Fraile-Ribot, P.A.; Doménech-Sánchez, A.; García-Baldoví, H.; Sala-Llinàs, E.; et al. COVID-19 Patient Variables Associated with the Detection of Airborne SARS-CoV-2. J. Infect. Public Health 2025, 18, 102785. [Google Scholar] [CrossRef] [PubMed]
- Di Gilio, A.; Palmisani, J.; Pulimeno, M.; Cerino, F.; Cacace, M.; Miani, A.; de Gennaro, G. CO2 Concentration Monitoring inside Educational Buildings as a Strategic Tool to Reduce the Risk of SARS-CoV-2 Airborne Transmission. Environ. Res. 2021, 202, 111560. [Google Scholar] [CrossRef] [PubMed]
- Baselga, M.; Alba, J.J.; Schuhmacher, A.J. The Control of Metabolic CO2 in Public Transport as a Strategy to Reduce the Transmission of Respiratory Infectious Diseases. Int. J. Environ. Res. Public Health 2022, 19, 6605. [Google Scholar] [CrossRef]
- Peng, Z.; Jimenez, J.L. Exhaled CO2 as a COVID-19 Infection Risk Proxy for Different Indoor Environments and Activities. Environ. Sci. Technol. Lett. 2021, 8, 392–397. [Google Scholar] [CrossRef]
- Iwamura, N.; Tsutsumi, K.; Hamashoji, T.; Arita, Y.; Deguchi, T. Carbon Dioxide Levels as a Key Indicator for Managing SARS-CoV-2 Airborne Transmission Risks Across 10 Indoor Scenarios. Cureus 2024, 16, e74429. [Google Scholar] [CrossRef]
- Villanueva, F.; Notario, A.; Cabañas, B.; Martín, P.; Salgado, S.; Gabriel, M.F. Assessment of CO2 and Aerosol (PM2.5, PM10, UFP) Concentrations during the Reopening of Schools in the COVID-19 Pandemic: The Case of a Metropolitan Area in Central-Southern Spain. Environ. Res. 2021, 197, 111092. [Google Scholar] [CrossRef]
- Li, B.; Cai, W. A Novel CO2-Based Demand-Controlled Ventilation Strategy to Limit the Spread of COVID-19 in the Indoor Environment. Build. Environ. 2022, 219, 109232. [Google Scholar] [CrossRef]
- Rudnick, S.N.; Milton, D.K. Risk of Indoor Airborne Infection Transmission Estimated from Carbon Dioxide Concentration. Indoor Air 2003, 13, 237–245. [Google Scholar] [CrossRef]
- Liao, C.M.; Chang, C.F.; Liang, H.M. A Probabilistic Transmission Dynamic Model to Assess Indoor Airborne Infection Risks. Risk Anal. 2005, 25, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Iwamura, N.; Tsutsumi, K. SARS-CoV-2 Airborne Infection Probability Estimated by Using Indoor Carbon Dioxide. Environ. Sci. Pollut. Res. 2023, 30, 79227–79240. [Google Scholar] [CrossRef]
- Park, S.; Song, D. CO2 Concentration as an Indicator of Indoor Ventilation Performance to Control Airborne Transmission of SARS-CoV-2. J. Infect. Public Health 2023, 16, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Mendell, M.J.; Chen, W.; Ranasinghe, D.R.; Castorina, R.; Kumagai, K. Carbon Dioxide Guidelines for Indoor Air Quality: A Review. J. Expo. Sci. Environ. Epidemiol. 2024, 34, 555–569. [Google Scholar] [CrossRef]
- ASHRAE. Position Document on INDOOR CARBON DIOXIDE; ASHRAE: Peachtree Corners, GA, USA, 2025. [Google Scholar]
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N.K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D.; et al. Aerodynamic Analysis of SARS-CoV-2 in Two Wuhan Hospitals. Nature 2020, 582, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Truyols Vives, J.; Muncunill, J.; Toledo Pons, N.; Baldoví, H.G.; Sala Llinàs, E.; Mercader Barceló, J. SARS-CoV-2 Detection in Bioaerosols Using a Liquid Impinger Collector and DdPCR. Indoor Air 2022, 32, e13002. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.A.; Koutrakis, P.; Martins, M.A.G.; Lemos, B.; Dowd, S.E.; Sunderland, E.M.; Garshick, E. Characterization of Hospital Airborne SARS-CoV-2. Respir. Res. 2021, 22, 73. [Google Scholar] [CrossRef]
- Geenen, C.; Traets, S.; Gorissen, S.; Happaerts, M.; Beuselinck, K.; Laenen, L.; Swinnen, J.; Ombelet, S.; Raymenants, J.; Keyaerts, E.; et al. Interpretation of Indoor Air Surveillance for Respiratory Infections: A Prospective Longitudinal Observational Study in a Childcare Setting. EBioMedicine 2025, 112, 105512. [Google Scholar] [CrossRef]
- Thuresson, S.; Fraenkel, C.J.; Sasinovich, S.; Medstrand, P.; Alsved, M.; Löndahl, J. One Year Weekly Size-Resolved Air Sampling of SARS-CoV-2 in Hospital Corridors and Relations to the Indoor Environment. Indoor Air 2024, 2024, 5578611. [Google Scholar] [CrossRef]
- Rufino de Sousa, N.; Steponaviciute, L.; Margerie, L.; Nissen, K.; Kjellin, M.; Reinius, B.; Salaneck, E.; Udekwu, K.I.; Rothfuchs, A.G. Detection and Isolation of Airborne SARS-CoV-2 in a Hospital Setting. Indoor Air 2022, 32, e13023. [Google Scholar] [CrossRef]
- Truyols-Vives, J.; Stiliyanov-Atanasov, K.; Sala-Llinàs, E.; Toledo-Pons, N.; Baldoví, H.G.; Mercader-Barceló, J. Efficiency and Sensitivity Optimization of a Protocol to Quantify Indoor Airborne SARS-CoV-2 Levels. J. Hosp. Infect. 2022, 130, 44–51. [Google Scholar] [CrossRef]
- Kesavan, J.; Sagripanti, J.L. Evaluation Criteria for Bioaerosol Samplers. Environ. Sci. Process Impacts 2015, 17, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Leavey, A.; Wang, Y.; O’Neil, C.; Wallace, M.A.; Burnham, C.A.D.; Boon, A.C.; Babcock, H.; Biswas, P. Comparing the Performance of 3 Bioaerosol Samplers for Influenza Virus. J. Aerosol Sci. 2018, 115, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Fabian, P.; McDevitt, J.J.; Houseman, E.A.; Milton, D.K. Airborne Influenza Virus Detection with Four Aerosol Samplers Using Molecular and Infectivity Assays: Considerations for a New Infectious Virus Aerosol Sampler. Indoor Air 2009, 19, 433–441. [Google Scholar] [CrossRef]
- Taylor, S.C.; Laperriere, G.; Germain, H. Droplet Digital PCR versus QPCR for Gene Expression Analysis with Low Abundant Targets: From Variable Nonsense to Publication Quality Data. Sci. Rep. 2017, 7, 2409. [Google Scholar] [CrossRef]
- Rashid, S.A.; Nazakat, R.; Muhamad Robat, R.; Ismail, R.; Suppiah, J.; Rajendran, K.; Raj Louis Masalamany, A.S.S.; Muhamad Hendri, N.A.; Mohamad, N.; Khairul Hasni, N.A.; et al. Droplet Digital PCR Application for the Detection of SARS-CoV-2 in Air Sample. Front. Public Health 2023, 11, 1208348. [Google Scholar] [CrossRef] [PubMed]
- Suo, T.; Liu, X.; Feng, J.; Guo, M.; Hu, W.; Guo, D.; Ullah, H.; Yang, Y.; Zhang, Q.; Wang, X.; et al. DdPCR: A More Accurate Tool for SARS-CoV-2 Detection in Low Viral Load Specimens. Emerg. Microbes Infect. 2020, 9, 1259–1268. [Google Scholar] [CrossRef]
- Yun, H.; Yang, J.; Seo, J.H.; Sohn, J.R. Methodology for Sampling and Detection of Airborne Coronavirus Including SARS-CoV-2. Indoor Built Environ. 2022, 31, 1234–1241. [Google Scholar] [CrossRef]
- Wei, X.; Ma, X.; Tian, F.; Wei, Z.; Zhang, L.; Hu, K. Sampling and Analysis Methods of Air-Borne Microorganisms in Hospital Air: A Review. Biotechniques 2024, 76, 395–404. [Google Scholar] [CrossRef]
- Fears, A.C.; Klimstra, W.B.; Duprex, P.; Hartman, A.; Weaver, S.C.; Plante, K.S.; Mirchandani, D.; Plante, J.A.; Aguilar, P.V.; Fernández, D.; et al. Persistence of Severe Acute Respiratory Syndrome Coronavirus 2 in Aerosol Suspensions. Emerg. Infect. Dis. 2020, 26, 2168–2171. [Google Scholar] [CrossRef]
- Alsved, M.; Nyström, K.; Thuresson, S.; Nygren, D.; Patzi-Churqui, M.; Hussein, T.; Fraenkel, C.J.; Medstrand, P.; Löndahl, J. Infectivity of Exhaled SARS-CoV-2 Aerosols Is Sufficient to Transmit COVID-19 within Minutes. Sci. Rep. 2023, 13, 21245. [Google Scholar] [CrossRef]
- Merhi, T.; Atasi, O.; Coetsier, C.; Lalanne, B.; Roger, K. Assessing Suspension and Infectivity Times of Virus-Loaded Aerosols Involved in Airborne Transmission. Proc. Natl. Acad. Sci. USA 2022, 119, e2204593119. [Google Scholar] [CrossRef] [PubMed]
- Lednicky, J.A.; Lauzard, M.; Fan, Z.H.; Jutla, A.; Tilly, T.B.; Gangwar, M.; Usmani, M.; Shankar, S.N.; Mohamed, K.; Eiguren-Fernandez, A.; et al. Viable SARS-CoV-2 in the Air of a Hospital Room with COVID-19 Patients. Int. J. Infect. Dis. 2020, 100, 476–482. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. New Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, Y.; Zhang, A.L.; Wang, Y.; Molina, M.J. Identifying Airborne Transmission as the Dominant Route for the Spread of COVID-19. Proc. Natl. Acad. Sci. USA 2020, 117, 14857–14863. [Google Scholar] [CrossRef]
- Grimalt, J.O.; Vílchez, H.; Fraile-Ribot, P.A.; Marco, E.; Campins, A.; Orfila, J.; van Drooge, B.L.; Fanjul, F. Spread of SARS-CoV-2 in Hospital Areas. Environ. Res. 2022, 204, 112074. [Google Scholar] [CrossRef] [PubMed]
- Razzini, K.; Castrica, M.; Menchetti, L.; Maggi, L.; Negroni, L.; Orfeo, N.V.; Pizzoccheri, A.; Stocco, M.; Muttini, S.; Balzaretti, C.M. SARS-CoV-2 RNA Detection in the Air and on Surfaces in the COVID-19 Ward of a Hospital in Milan, Italy. Sci. Total Environ. 2020, 742, 140540. [Google Scholar] [CrossRef]
- Reiteration of Existing OSHA Policy on Indoor Air Quality: Office Temperature/Humidity and Environmental Tobacco Smoke|Occupational Safety and Health Administration. Available online: https://www.osha.gov/laws-regs/standardinterpretations/2003-02-24 (accessed on 20 May 2025).
- Instituto Nacional de Seguridad y Salud en el Trabajo. Calidad del Ambiente Interior en el Trabajo; Instituto Nacional de Seguridad y Salud en el Trabajo: Madrid, Spain, 2022.
- Indoor Air Quality Database—IEQ Guidelines. Available online: https://ieqguidelines.org/table (accessed on 23 May 2025).
- Raines, K.S.; Doniach, S.; Bhanot, G. The Transmission of SARS-CoV-2 Is Likely Comodulated by Temperature and by Relative Humidity. PLoS ONE 2021, 16, e0255212. [Google Scholar] [CrossRef]
- Verheyen, C.A.; Bourouiba, L. Associations between Indoor Relative Humidity and Global COVID-19 Outcomes. J. R. Soc. Interface 2022, 19, 20210865. [Google Scholar] [CrossRef]
- Dabisch, P.; Schuit, M.; Herzog, A.; Beck, K.; Wood, S.; Krause, M.; Miller, D.; Weaver, W.; Freeburger, D.; Hooper, I.; et al. The Influence of Temperature, Humidity, and Simulated Sunlight on the Infectivity of SARS-CoV-2 in Aerosols. Aerosol Sci. Technol. 2021, 55, 142–153. [Google Scholar] [CrossRef]
- Zhao, C.; Song, B.; Zhang, C.; Gao, R.; He, L. The Promotion Effect of Human Activity Intensity on Displacement Ventilation Efficiency by the Enhancement Indoor Thermal Stratification. Case Stud. Therm. Eng. 2024, 61, 104928. [Google Scholar] [CrossRef]
- Haddrell, A.; Oswin, H.; Otero-Fernandez, M.; Robinson, J.F.; Cogan, T.; Alexander, R.; Mann, J.F.S.; Hill, D.; Finn, A.; Davidson, A.D.; et al. Ambient Carbon Dioxide Concentration Correlates with SARS-CoV-2 Aerostability and Infection Risk. Nat. Commun. 2024, 15, 3487. [Google Scholar] [CrossRef] [PubMed]
- Donskey, C.J. High Technology and Low Technology Measures to Reduce Risk of SARS-CoV-2 Transmission. Am. J. Infect. Control 2023, 51, A126–A133. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.T.; Alleman, L.Y.; Coddeville, P.; Galloo, J.C. Indoor Particle Dynamics in Schools: Determination of Air Exchange Rate, Size-Resolved Particle Deposition Rate and Penetration Factor in Real-Life Conditions. Indoor Built Environ. 2017, 26, 1335–1350. [Google Scholar] [CrossRef]
- Howard-Reed, C.; Wallace, L.A.; Emmerich, S.J. Effect of Ventilation Systems and Air Filters on Decay Rates of Particles Produced by Indoor Sources in an Occupied Townhouse. Atmos. Environ. 2003, 37, 5295–5306. [Google Scholar] [CrossRef]
- Thatcher, T.L.; Lai, A.C.K.; Moreno-Jackson, R.; Sextro, R.G.; Nazaroff, W.W. Effects of Room Furnishings and Air Speed on Particle Deposition Rates Indoors. Atmos. Environ. 2002, 36, 1811–1819. [Google Scholar] [CrossRef]
- Kappelt, N.; Russell, H.S.; Kwiatkowski, S.; Afshari, A.; Johnson, M.S. Correlation of Respiratory Aerosols and Metabolic Carbon Dioxide. Sustainability 2021, 13, 12203. [Google Scholar] [CrossRef]
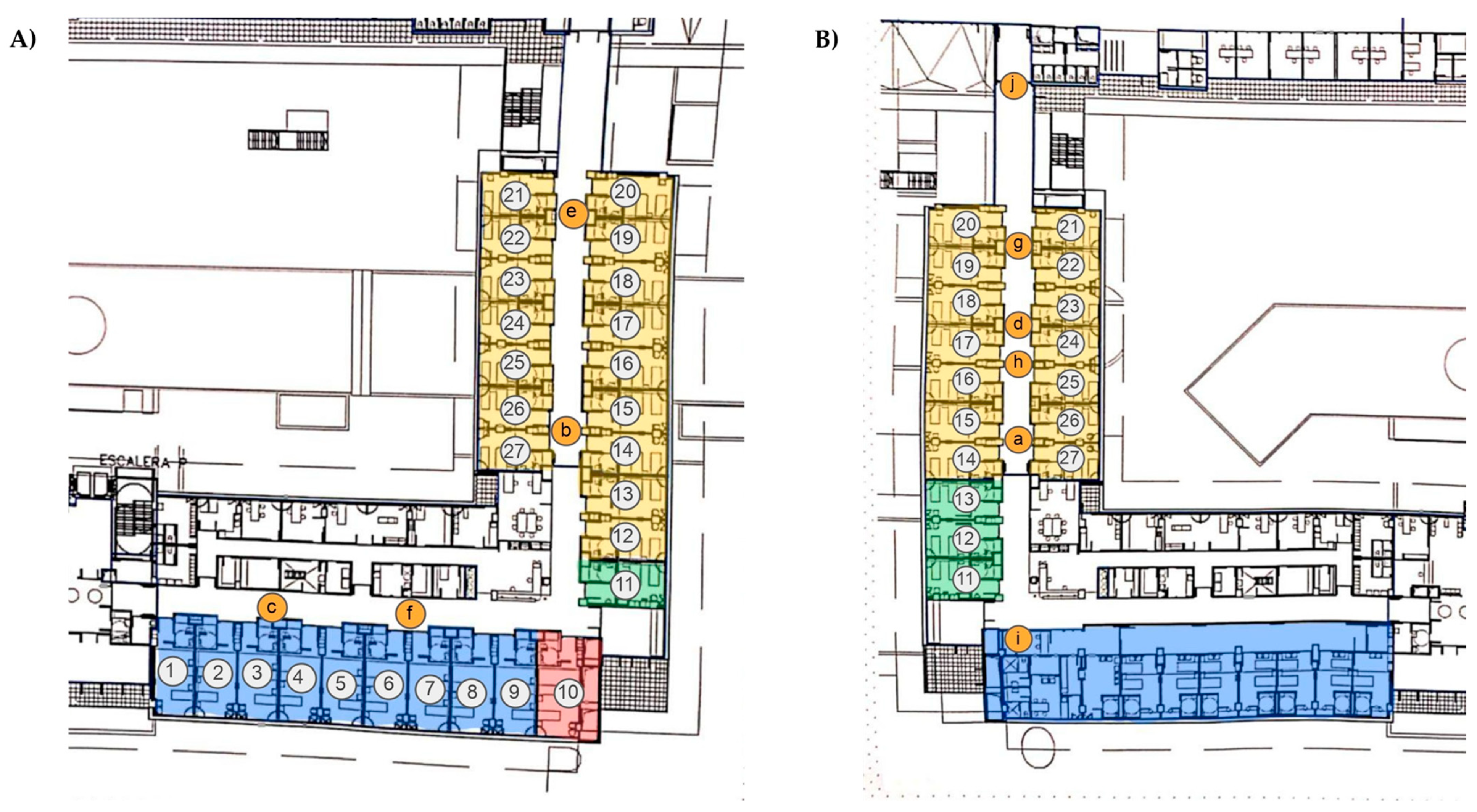
| Sample | Date | Ward | Sampling Point | Occupied/Total Rooms | People Counting Mean | Door Openings | Temperature (°C) | Relative Humidity (%) | CO2 (ppm) | SARS-CoV-2 Copies/m3 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 25 Jan 2022 | 3O | A | 6/17 | 3.6 ± 1.6 | 27 | 24 ± 1.4 | 30.5 ± 2.7 | 596.7 ± 39.6 | 12.4 |
| 2 | 3.1 ± 1.4 | 14 | 25.8 ± 0.4 | 27.7 ± 0.5 | 625.2 ± 39.7 | 14.2 | ||||
| 3 | 4.3 ± 1.4 | 23 | 25.9 ± 0.3 | 27.6 ± 0.5 | 578.4 ± 21.6 | 0 | ||||
| 4 | 2.8 ± 1.1 | 29 | 26 ± 0.0 | 26.8 ± 0.5 | 575 ± 19.7 | 0 | ||||
| 5 | 26 Jan 2022 | 2N | B | 25/26 | 4 ± 1.9 | 41 | 25.2 ± 1.0 | 26.9 ± 1.7 | 604.1 ± 25.8 | 0 |
| 6 | 2.7 ± 1.6 | 36 | 26.8 ± 0.4 | 24.9 ± 0.5 | 605.6 ± 20.0 | 0 | ||||
| 7 | 4.5 ± 1.3 | 64 | 25.3 ± 0.5 | 27.3 ± 0.5 | 602.3 ± 24.8 | 0 | ||||
| 8 | 4 ± 1.8 | 36 | 26.8 ± 0.4 | 26.1 ± 0.9 | 651.2 ± 28.6 | 12.4 | ||||
| 9 | 27 Jan 2022 | 2N | C | 25/26 | 2.9 ± 1 | 87 | 23.8 ± 0.9 | 26.7 ± 1.4 | 671.5 ± 23.1 | 0 |
| 10 | 3.5 ± 1.6 | 72 | 25 ± 0.0 | 26.2 ± 0.4 | 739.7 ± 49.1 | 0 | ||||
| 11 | 1.7 ± 1.1 | 21 | 25 ± 0.0 | 25.3 ± 0.5 | 636.5 ± 12.0 | 0 | ||||
| 12 | 2.3 ± 0.9 | 65 | 25 ± 0.0 | 25.5 ± 0.7 | 650.8 ± 18.2 | 0 | ||||
| 13 | 31 Jan 2022 | 3O | D | 7/17 | 3.1 ± 1.1 | 69 | 23.7 ± 0.9 | 27.7 ± 1.49 | 548.6 ± 13.6 | 23.1 |
| 14 | 2.3 ± 1.2 | 41 | 23.6 ± 0.5 | 23.2 ± 1.2 | 474.6 ±26.4 | 10.7 | ||||
| 15 | 2.3 ± 0.6 | 35 | 24.9 ± 0.9 | 19.3 ± 1.2 | 520.1 ± 29.9 | 0 | ||||
| 16 | 2.8 ± 0.8 | 28 | 26 ± 0.0 | 17.6 ± 0.5 | 587.3 ± 27.9 | 10.7 | ||||
| 17 | 1 Feb 2022 | 2N | E | 25/26 | 3 ± 1.4 | 200 | 23.7 ± 0.9 | 30.2 ± 1.2 | 540.3 ± 41.3 | 44.4 |
| 18 | 1.7 ± 0.5 | 39 | 25 ± 0.0 | 27.7 ± 0.5 | 562.2 ± 10.6 | 12.4 | ||||
| 19 | 2.7 ± 1.1 | 44 | 25.2 ± 0.4 | 27.4 ± 0.5 | 563.2 ± 3.0 | 0 | ||||
| 20 | 2.1 ± 0.8 | 32 | 24.6 ± 0.5 | 27.8 ± 0.5 | 551.3 ± 9.7 | 51.6 | ||||
| 21 | 2 Feb 2022 | 2N | F | 26/26 | 2.7 ± 1.3 | 43 | 24.4 ± 0.9 | 27.5 ± 1.7 | 611.8 ± 30.5 | 16 |
| 22 | 2.6 ± 0.7 | 51 | 25.8 ± 0.4 | 24.8 ± 0.6 | 599 ± 41.9 | 51.6 | ||||
| 23 | 3.1 ± 0.7 | 36 | 27 ± 0.9 | 21.1 ± 1.4 | 547 ± 54.9 | 14.2 | ||||
| 24 | 2.7 ± 0.7 | 19 | 25.42 ± 0.7 | 21.8 ± 0.6 | 523.6 ± 55.4 | 16 | ||||
| 25 | 3 Feb 2022 | 3O | G | 8/17 | 1.2 ± 0.6 | 28 | 23.4 ± 0.8 | 25.5 ± 1.3 | 569 ± 19.5 | 48 |
| 26 | 1.1 ± 0.3 | 14 | 25 ± 0.0 | 24.8 ± 0.8 | 571.7 ± 24.2 | 32 | ||||
| 27 | 1.9 ± 1.4 | 9 | 25 ± 0.0 | 27.5 ± 0.5 | 582.5 ± 26.3 | 0 | ||||
| 28 | 1.8 ± 0.8 | 28 | 25.7 ± 0.5 | 27.9 ± 0.3 | 590.6 ± 14.5 | 0 | ||||
| 29 | 7 Feb 2022 | 3O | H | 12/17 | 3.2 ± 0.8 | 57 | 24.2 ± 0.8 | 29.8 ± 1.2 | 592.8 ± 44.0 | 0 |
| 30 | 2 ± 0.6 | 23 | 25 ± 0.0 | 28.6 ± 0.7 | 587.7 ± 27.1 | 0 | ||||
| 31 | 3.4 ± 1.2 | 65 | 25.2 ± 0.4 | 28 ± 0.0 | 588.1 ± 35.8 | 12.4 | ||||
| 32 | 2.8 ± 1.8 | 58 | 25.7 ± 0.5 | 27.5 ± 0.5 | 627.1 ± 47.4 | 14.2 | ||||
| 33 | 8 Feb 2022 | 3O | I | 12/17 | 3.5 ± 1 | 1 | 24.2 ± 0.7 | 28 ± 1.5 | 512.3 ± 24.6 | 39.1 |
| 34 | 3.4 ± 1.3 | 6 | 25.5 ± 0.5 | 25.6 ± 0.5 | 542.5 ± 11.1 | 23.1 | ||||
| 35 | 3.5 ± 1 | 14 | 25.7 ± 0.5 | 25.1 ± 0.3 | 544.8 ± 23.5 | 12.4 | ||||
| 36 | 3.1 ± 1.6 | 0 | 25.3 ± 0.5 | 24.3 ± 0.5 | 557 ± 39.7 | 53.3 | ||||
| 37 | 9 Feb 2022 | 3O | J | 17/17 | 1 ± 0.0 | 18 | 22.4 ± 0.7 | 33.2 ± 0.4 | 539.9 ± 20.4 | 30.2 |
| 38 | 1 ± 0.0 | 13 | 23 ± 0.0 | 31.9 ± 0.3 | 547.8 ± 10.2 | 0 | ||||
| 39 | 1.1 ± 0.3 | 9 | 23.4 ± 0.5 | 31.3 ± 0.5 | 611.6 ± 11.7 | 16 | ||||
| 40 | 1 ± 0.0 | 14 | 24 ± 0.0 | 31 ± 0.4 | 576.6 ± 36.6 | 28.4 |
| [All] N = 40 | 2N N = 16 | 3O N = 24 | p-Overall | |
|---|---|---|---|---|
| Occupancy | 14.5 [8.00; 25.0] | 25.0 [25.0; 25.2] | 10.0 [7.00; 12.0] | <0.001 *** |
| Door openings (nº) | 30.5 [14.0; 45.8] | 42.0 [36.0; 64.2] | 23.0 [3.25; 30.5] | 0.001 ** |
| Total count of individuals (nº) | 32.2 (11.8) | 35.2 (10.2) | 30.2 (12.5) | 0.172 |
| Mean count of individuals | 2.62 (0.94) | 2.87 (0.81) | 2.46 (1.00) | 0.166 |
| SARS-CoV-2 detection | 0.469 | |||
| NO | 16 (40.0%) | 8 (50.0%) | 8 (33.3%) | |
| YES | 24 (60.0%) | 8 (50.0%) | 16 (66.7%) | |
| SARS-CoV-2 copies/ m3 | 12.44 [0.00; 23.11] | 6.22 [0.00; 16] | 12.44 [0.00; 24.44] | 0.493 |
| Temperature (°C) | 24.9 (1.06) | 25.2 (0.98) | 24.7 (1.06) | 0.094 |
| Relative humidity (%) | 26.7 (3.17) | 26.1 (2.26) | 27.1 (3.64) | 0.281 |
| CO2 (ppm) | 583 (47.9) | 604 (56.9) | 569 (35.6) | 0.038 * |
| [All] N = 40 | NO N = 16 | YES N = 24 | p-Overall | |
|---|---|---|---|---|
| Occupancy | 14.5 [8.00; 25.0] | 21.0 [8.00; 25.0] | 12.0 [8.00; 25.0] | 0.91 |
| Door openings (nº) | 30.5 [14.0; 45.8] | 35.5 [23.0; 58.8] | 28.0 [7.00; 41.5] | 0.203 |
| Count of individuals (nº) | 32.2 (11.8) | 33.1 (12.4) | 31.6 (11.5) | 0.707 |
| Mean count of individuals | 2.62 (0.94) | 2.70 (0.99) | 2.57 (0.93) | 0.671 |
| Ward | 0.469 | |||
| 2N | 16 (40.0%) | 8 (50.0%) | 8 (33.3%) | |
| 3O | 24 (60.0%) | 8 (50.0%) | 16 (66.7%) | |
| Temperature (°C) | 24.9 (1.06) | 25.1 (0.89) | 24.8 (1.16) | 0.46 |
| Relative humidity (%) | 26.7 (3.17) | 26.9 (2.64) | 26.6 (3.53) | 0.77 |
| CO2 (ppm) | 583 (47.9) | 604 (56.9) | 569 (35.6) | 0.037 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truyols-Vives, J.; González-López, M.; Colom-Fernández, A.; Einschütz-López, A.; Sala-Llinàs, E.; Doménech-Sánchez, A.; García-Baldoví, H.; Mercader-Barceló, J. Airborne SARS-CoV-2 Detection by ddPCR in Adequately Ventilated Hospital Corridors. Toxics 2025, 13, 583. https://doi.org/10.3390/toxics13070583
Truyols-Vives J, González-López M, Colom-Fernández A, Einschütz-López A, Sala-Llinàs E, Doménech-Sánchez A, García-Baldoví H, Mercader-Barceló J. Airborne SARS-CoV-2 Detection by ddPCR in Adequately Ventilated Hospital Corridors. Toxics. 2025; 13(7):583. https://doi.org/10.3390/toxics13070583
Chicago/Turabian StyleTruyols-Vives, Joan, Marta González-López, Antoni Colom-Fernández, Alexander Einschütz-López, Ernest Sala-Llinàs, Antonio Doménech-Sánchez, Herme García-Baldoví, and Josep Mercader-Barceló. 2025. "Airborne SARS-CoV-2 Detection by ddPCR in Adequately Ventilated Hospital Corridors" Toxics 13, no. 7: 583. https://doi.org/10.3390/toxics13070583
APA StyleTruyols-Vives, J., González-López, M., Colom-Fernández, A., Einschütz-López, A., Sala-Llinàs, E., Doménech-Sánchez, A., García-Baldoví, H., & Mercader-Barceló, J. (2025). Airborne SARS-CoV-2 Detection by ddPCR in Adequately Ventilated Hospital Corridors. Toxics, 13(7), 583. https://doi.org/10.3390/toxics13070583





