Recent Trends and Challenges on the Non-Targeted Analysis and Risk Assessment of Migrant Non-Intentionally Added Substances from Plastic Food Contact Materials
Abstract
1. Introduction
2. Literature Search and Reviewed Studies
3. Studied Polymers and Food Contact Materials
4. Sample Preparation
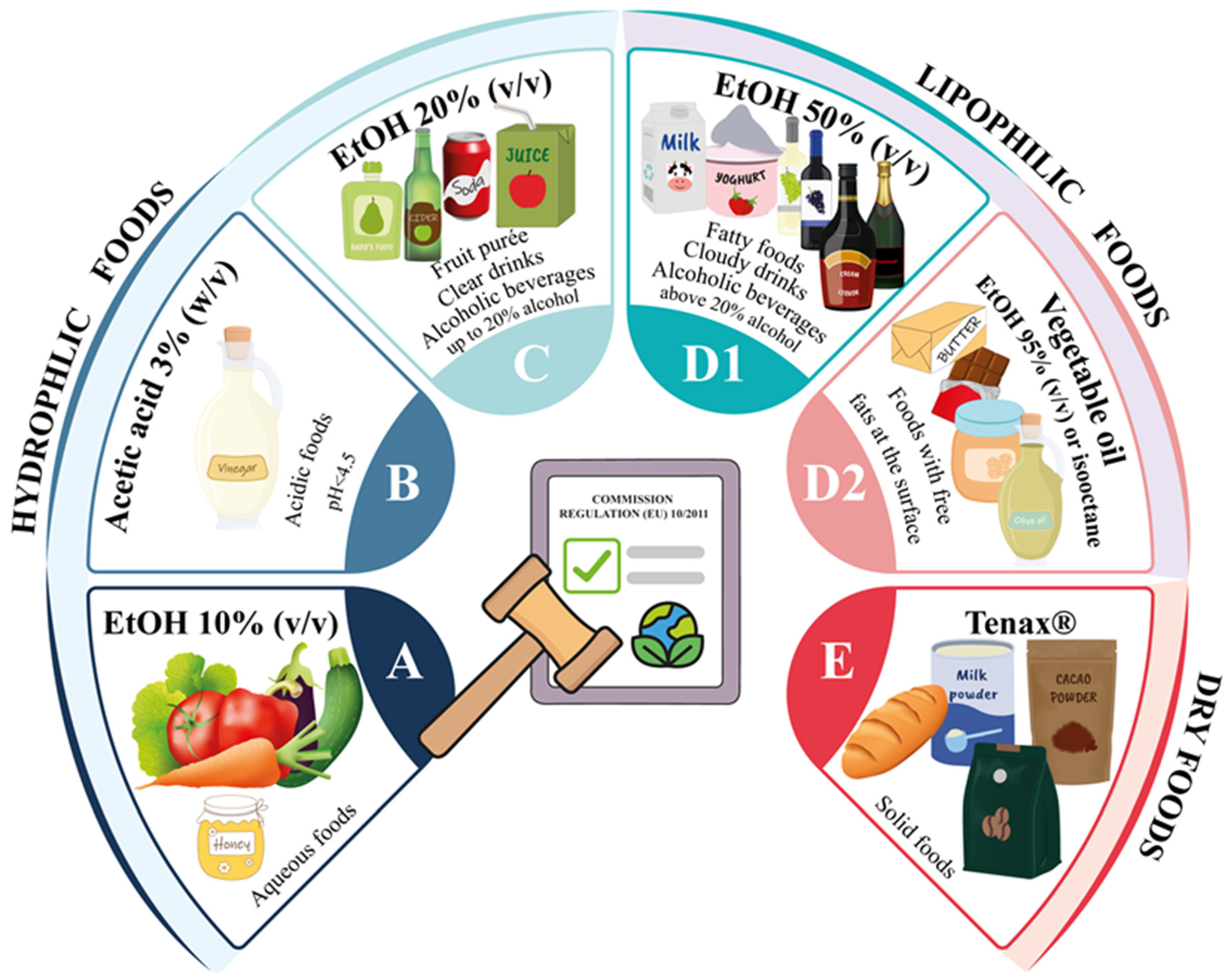
4.1. Migration Tests Using Food Simulants
4.2. Extraction Techniques
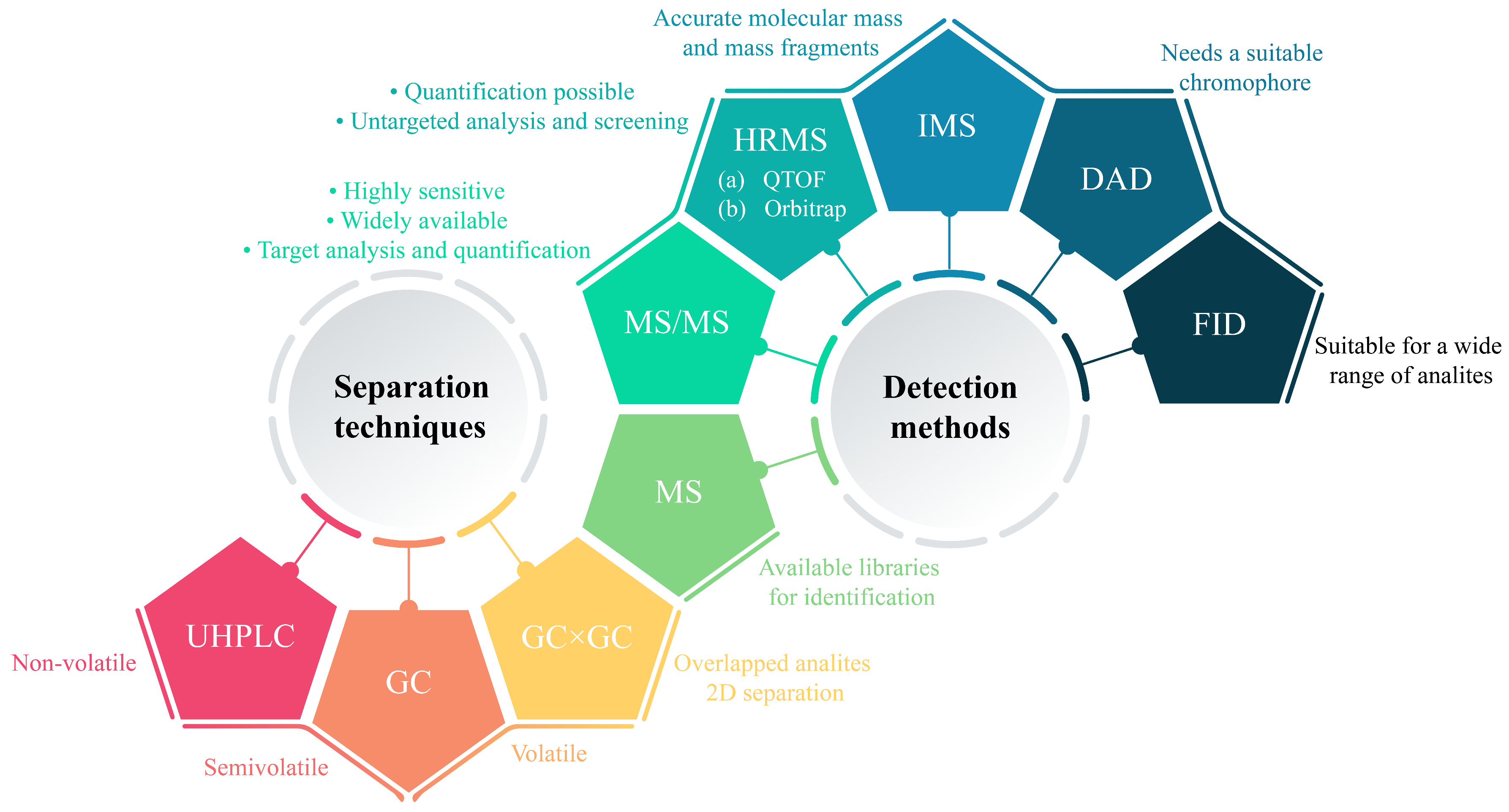
5. Instrumental Analysis
5.1. Separation Techniques
5.2. Detection Methods
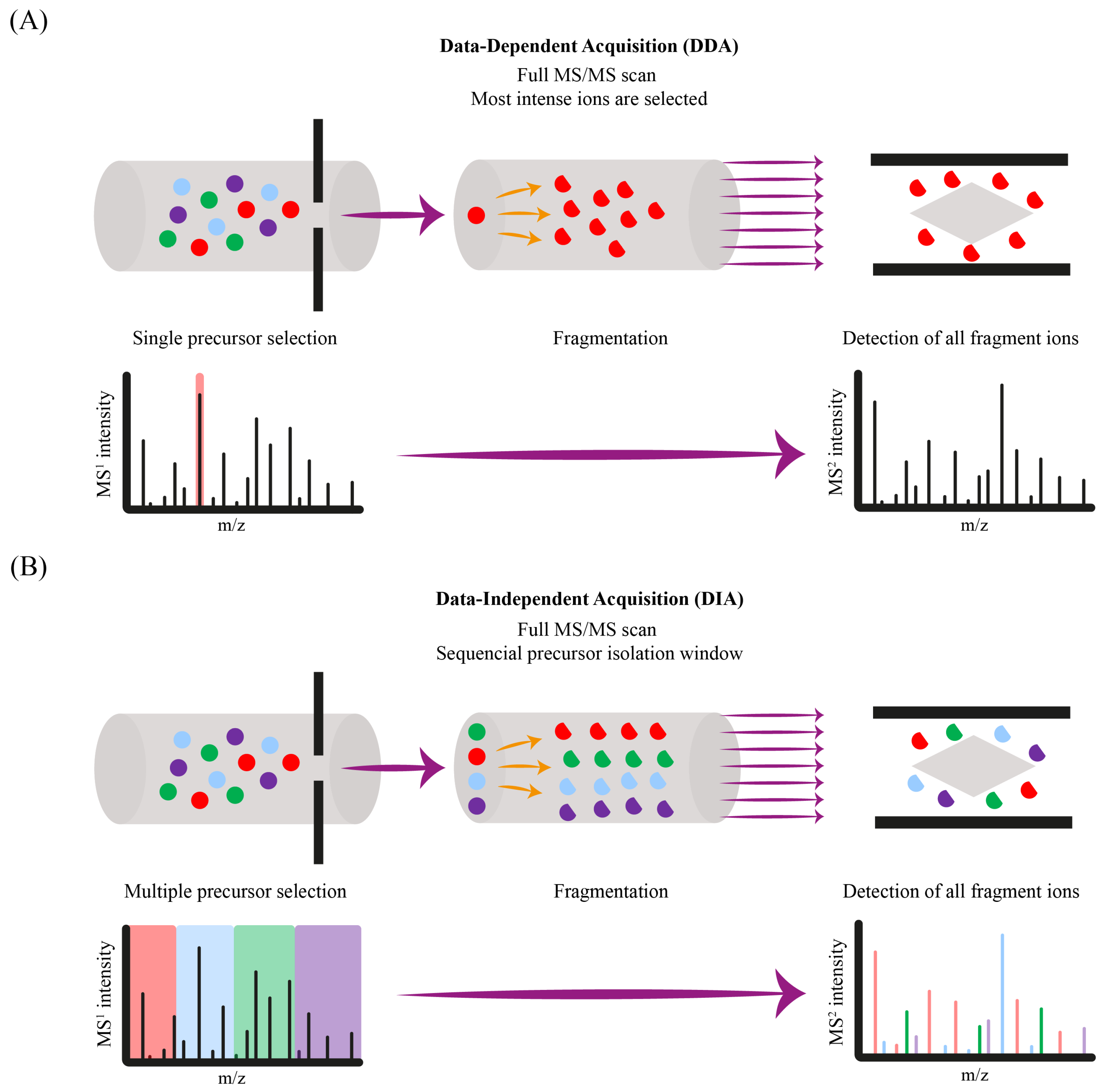
6. Data Processing and Analysis
6.1. Compound Identification Workflows
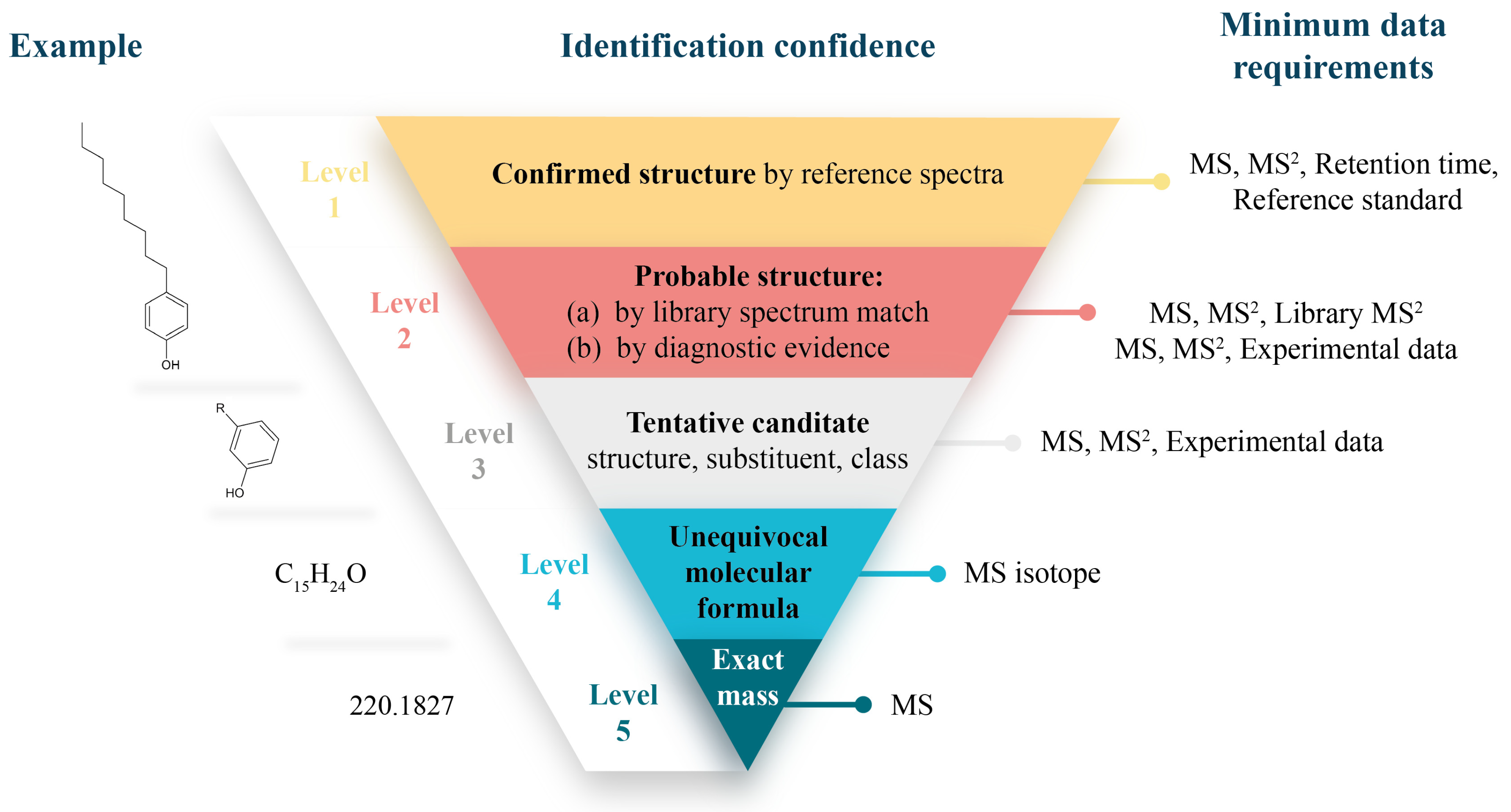
6.2. Identification Confidence and Confirmation
7. Risk Assessment
7.1. Estimated Daily Intake and Tolerable Daily Intake
7.2. Threshold of Toxicological Concern Approach
7.3. Bioassays
8. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Adipic acid |
| ABS | Acrylonitrile–butadiene–styrene |
| ACE | Acetone |
| ACN | Acetonitrile |
| AMDIS | Automated Mass spectral Deconvolution and Identification System |
| APCI | Atmospheric pressure chemical ionization |
| AZA | Azelaic acid |
| BADGE | Bisphenol A diglycidyl ether |
| BHET | Bis(2-hydroxyethyl) terephthalate |
| BHT | Butylated hydroxytoluene |
| bw | Body weight |
| CAR | Carboxen |
| CCS | Collision cross-section |
| CE | Collision energy |
| CF | Consumption factor |
| DAD | Diode array detector |
| DBP | Dibutyl phthalate |
| DCM | Dichloromethane |
| DDA | Data-dependent acquisition |
| ddMS2 | Data-dependent tandem mass spectrometry |
| DEG | Diethylene glycol |
| DIA | Data-independent acquisition |
| DMSO | Dimethyl sulfoxide |
| DVB | Divinylbenzene |
| EDI | Estimated daily intake |
| EFSA | European Food Safety Authority |
| EI | Electron ionization |
| EPA | U.S. Environmental Protection Agency |
| EPS | Expanded polystyrene |
| ESI | Electrospray ionization |
| EtOH | Ethanol |
| EVA | Ethylene–vinyl acetate |
| FCMs | Food contact materials |
| FDA | U.S. Food and Drug Administration |
| FIA | Flow injection analysis |
| FID | Flame ionization detector |
| FLD | Fluorescence detector |
| FS | Full scan |
| FWHM | Full width at half maximum |
| GC | Gas chromatography |
| GC×GC | Two-dimensional gas chromatography |
| HDPE | High-density polyethylene |
| HFIP | Hexafluoroisopropanol |
| HPLC | High-performance liquid chromatography |
| HQ | Hazard quotient |
| HRMS | High-resolution mass spectrometry |
| HS | Headspace |
| IAS | Intentionally added substances |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| IMS | Ion mobility spectrometry |
| IPA | Isophtalic acid |
| LC | Liquid chromatography |
| LDPE | Low-density polyethylene |
| LIT | Linear ion trap |
| MeOH | Methanol |
| MN | Molecular networking |
| MS | Mass spectrometry |
| MS/MS | Tandem mass spectrometry |
| NIAS | Non-intentionally added substances |
| NIST | National Institute of Standards and Technology |
| NMR | Nuclear magnetic resonance |
| NTA | Non-targeted analysis |
| PA | Polyamide |
| PBAT | Polybutylene adipate terephthalate |
| PBS | Polybutylene succinate |
| PBT | Polybutylene terephthalate |
| PC | Polycarbonate |
| PCT | Polycyclohexane-1,4-dimethylene terephthalate |
| PCTG | Polycyclohexylenedimethylene terephthalate glycol-modified |
| PDMS | Polydimethylsiloxane |
| PE | Polyethylene |
| PES | Polyester |
| PET | Polyethylene terephthalate |
| PETG | Polyethylene terephthalate glycol-modified |
| PI | Polyimide |
| PLA | Polylactic acid |
| PP | Polypropylene |
| PPCs | Plant fiber/plastic composites |
| PPSU | Polyphenylsulfone |
| PS | Polystyrene |
| PUR | Polyurethane |
| PVC | Polyvinyl chloride |
| P&T | Purge and trap |
| qNTA | Quantitative non-target analysis |
| QSPR | Quantitative structure-property relationship |
| QTOF | Quadrupole time-of-flight |
| RI | Retention index |
| rEPS | Recycled expanded polystyrene |
| rHDPE | Recycled high-density polyethylene |
| rLDPE | Recycled low-density polyethylene |
| rPE | Recycled polyethylene |
| rPET | Recycled polyethylene terephthalate |
| rPP | Recycled polypropylene |
| rPS | Recycled polystyrene |
| SAN | Styrene-acrylonitrile copolymer |
| SML | Specific migration limit |
| SPME | Solid-phase microextraction |
| SVOCs | Semi-volatile organic compounds |
| SWATH | Sequential windowed acquisition of all theoretical MS |
| TDI | Tolerable daily intake |
| TMBPF-DGE | Tetramethyl bisphenol F-based diglycidyl ether |
| TPU | Thermoplastic polyurethane |
| TTC | Threshold of toxicological concern |
| UHPLC | Ultra-high-performance liquid chromatography |
| UV | Ultraviolet radiation |
| VOCs | Volatile organic compounds |
| WHO | World Health Organization |
References
- Peters, R.J.B.; Groeneveld, I.; Sanchez, P.L.; Gebbink, W.; Gersen, A.; de Nijs, M.; van Leeuwen, S.P.J. Review of Analytical Approaches for the Identification of Non-Intentionally Added Substances in Paper and Board Food Contact Materials. Trends Food Sci. Technol. 2019, 85, 44–54. [Google Scholar] [CrossRef]
- Nerín, C.; Bourdoux, S.; Faust, B.; Gude, T.; Lesueur, C.; Simat, T.; Stoermer, A.; Van Hoek, E.; Oldring, P. Guidance in Selecting Analytical Techniques for Identification and Quantification of Non-Intentionally Added Substances (NIAS) in Food Contact Materials (FCMS). Food Addit. Contam. Part A 2022, 39, 620–643. [Google Scholar] [CrossRef]
- Geueke, B. FPF Dossier: Non-Intentionally Added Substances (NIAS), 2nd ed.; Zenodo: Geneva, Switzerland, 2018. [Google Scholar] [CrossRef]
- Sanchis, Y.; Yusà, V.; Coscollà, C. Analytical Strategies for Organic Food Packaging Contaminants. J. Chromatogr. A 2017, 1490, 22–46. [Google Scholar] [CrossRef] [PubMed]
- Canellas, E.; Vera, P.; Nerín, C. Ion Mobility Quadrupole Time-of-Flight Mass Spectrometry for the Identification of Non-Intentionally Added Substances in UV Varnishes Applied on Food Contact Materials. A Safety by Design Study. Talanta 2019, 205, 120103. [Google Scholar] [CrossRef]
- Aznar, M.; Ubeda, S.; Dreolin, N.; Nerín, C. Determination of Non-Volatile Components of a Biodegradable Food Packaging Material Based on Polyester and Polylactic Acid (PLA) and Its Migration to Food Simulants. J. Chromatogr. A 2019, 1583, 1–8. [Google Scholar] [CrossRef]
- European Commision. Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food. 2011. Available online: http://data.europa.eu/eli/reg/2011/10/oj (accessed on 7 April 2025).
- European Commission. Commission Regulation (EU) 2025/351 of 21 February 2025 Amending Regulation (EU) No 10/2011 on Plastic Materials and Articles Intended to Come into Contact with Food, Amending Regulation (EU) 2022/1616 on Recycled Plastic Materials and Articles Intended to Come into Contact with Foods, and Repealing Regulation (EC) No 282/2008, and Amending Regulation (EC). 2025. Available online: http://data.europa.eu/eli/reg/2025/351/oj (accessed on 7 April 2025).
- Bignardi, C.; Cavazza, A.; Corradini, C.; Salvadeo, P. Targeted and Untargeted Data-Dependent Experiments for Characterization of Polycarbonate Food-Contact Plastics by Ultra High Performance Chromatography Coupled to Quadrupole Orbitrap Tandem Mass Spectrometry. J. Chromatogr. A 2014, 1372, 133–144. [Google Scholar] [CrossRef]
- Canellas, E.; Vera, P.; Nerín, C. UPLC–ESI-Q-TOF-MSE and GC–MS Identification and Quantification of Non-Intentionally Added Substances Coming from Biodegradable Food Packaging. Anal. Bioanal. Chem. 2015, 407, 6781–6790. [Google Scholar] [CrossRef]
- Aznar, M.; Domeño, C.; Nerín, C.; Bosetti, O. Set-off of Non Volatile Compounds from Printing Inks in Food Packaging Materials and the Role of Lacquers to Avoid Migration. Dye. Pigment. 2015, 114, 85–92. [Google Scholar] [CrossRef]
- Rusko, J.; Perkons, I.; Rasinger, J.D.; Bartkevics, V. Non-Target and Suspected-Target Screening for Potentially Hazardous Chemicals in Food Contact Materials: Investigation of Paper Straws. Food Addit. Contam. Part A 2020, 37, 649–664. [Google Scholar] [CrossRef]
- Carrero-Carralero, C.; Escobar-Arnanz, J.; Ros, M.; Jiménez-Falcao, S.; Sanz, M.L.; Ramos, L. An Untargeted Evaluation of the Volatile and Semi-Volatile Compounds Migrating into Food Simulants from Polypropylene Food Containers by Comprehensive Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry. Talanta 2019, 195, 800–806. [Google Scholar] [CrossRef]
- Paseiro-Cerrato, R.; MacMahon, S.; Ridge, C.D.; Noonan, G.O.; Begley, T.H. Identification of Unknown Compounds from Polyester Cans Coatings That May Potentially Migrate into Food or Food Simulants. J. Chromatogr. A 2016, 1444, 106–113. [Google Scholar] [CrossRef]
- Martínez-Bueno, M.J.; Cimmino, S.; Silvestre, C.; Tadeo, J.L.; Garcia-Valcárcel, A.I.; Fernández-Alba, A.R.; Hernando, M.D. Characterization of Non-Intentionally Added Substances (NIAS) and Zinc Oxide Nanoparticle Release from Evaluation of New Antimicrobial Food Contact Materials by Both LC-QTOF-MS, GC-QTOF-MS and ICP-MS. Anal. Methods 2016, 8, 7209–7216. [Google Scholar] [CrossRef]
- Bignardi, C.; Cavazza, A.; Laganà, C.; Salvadeo, P.; Corradini, C. Release of Non-Intentionally Added Substances (NIAS) from Food Contact Polycarbonate: Effect of Ageing. Food Control 2017, 71, 329–335. [Google Scholar] [CrossRef]
- Kühne, F.; Kappenstein, O.; Straβgütl, S.; Weese, F.; Weyer, J.; Pfaff, K.; Luch, A. N-Nitrosamines Migrating from Food Contact Materials into Food Simulants: Analysis and Quantification by Means of HPLC-APCI-MS/MS. Food Addit. Contam. Part A 2018, 35, 793–806. [Google Scholar] [CrossRef]
- Chen, Z.-F.; Lin, Q.-B.; Su, Q.-Z.; Zhong, H.-N.; Nerin, C. Identification of Recycled Polyethylene and Virgin Polyethylene Based on Untargeted Migrants. Food Packag. Shelf Life 2021, 30, 100762. [Google Scholar] [CrossRef]
- Martínez-Bueno, M.J.; Gómez Ramos, M.J.; Bauer, A.; Fernández-Alba, A.R. An Overview of Non-Targeted Screening Strategies Based on High Resolution Accurate Mass Spectrometry for the Identification of Migrants Coming from Plastic Food Packaging Materials. TrAC Trends Anal. Chem. 2019, 110, 191–203. [Google Scholar] [CrossRef]
- Bauer, A.; Jesús, F.; Gómez Ramos, M.J.; Lozano, A.; Fernández-Alba, A.R. Identification of Unexpected Chemical Contaminants in Baby Food Coming from Plastic Packaging Migration by High Resolution Accurate Mass Spectrometry. Food Chem. 2019, 295, 274–288. [Google Scholar] [CrossRef]
- Brenz, F.; Linke, S.; Simat, T. Linear and Cyclic Oligomers in Polybutylene Terephthalate for Food Contact Materials. Food Addit. Contam. Part A 2018, 35, 583–598. [Google Scholar] [CrossRef]
- Brenz, F.; Linke, S.; Simat, T.J. Linear and Cyclic Oligomers in PET, Glycol-Modified PET and TritanTM Used for Food Contact Materials. Food Addit. Contam. Part A 2021, 38, 160–179. [Google Scholar] [CrossRef]
- Cariou, R.; Rivière, M.; Hutinet, S.; Tebbaa, A.; Dubreuil, D.; Mathé-Allainmat, M.; Lebreton, J.; Le Bizec, B.; Tessier, A.; Dervilly, G. Thorough Investigation of Non-Volatile Substances Extractible from Inner Coatings of Metallic Cans and Their Occurrence in the Canned Vegetables. J. Hazard. Mater. 2022, 435, 129026. [Google Scholar] [CrossRef]
- Cincotta, F.; Verzera, A.; Tripodi, G.; Condurso, C. Non-Intentionally Added Substances in PET Bottled Mineral Water during the Shelf-Life. Eur. Food Res. Technol. 2018, 244, 433–439. [Google Scholar] [CrossRef]
- Díaz-Galiano, F.J.; Gómez-Ramos, M.J.; Beraza, I.; Murcia-Morales, M.; Fernández-Alba, A.R. Cooking Food in Microwavable Plastic Containers: In Situ Formation of a New Chemical Substance and Increased Migration of Polypropylene Polymers. Food Chem. 2023, 417, 135852. [Google Scholar] [CrossRef]
- Eckardt, M.; Greb, A.; Simat, T.J. Polyphenylsulfone (PPSU) for Baby Bottles: A Comprehensive Assessment on Polymer-Related Non-Intentionally Added Substances (NIAS). Food Addit. Contam. Part A 2018, 35, 1421–1437. [Google Scholar] [CrossRef]
- Galmán Graíño, S.; Sendón, R.; López Hernández, J.; Rodríguez-Bernaldo de Quirós, A. GC-MS Screening Analysis for the Identification of Potential Migrants in Plastic and Paper-Based Candy Wrappers. Polymers 2018, 10, 802. [Google Scholar] [CrossRef]
- García Ibarra, V.; Rodríguez Bernaldo de Quirós, A.; Paseiro Losada, P.; Sendón, R. Identification of Intentionally and Non-Intentionally Added Substances in Plastic Packaging Materials and Their Migration into Food Products. Anal. Bioanal. Chem. 2018, 410, 3789–3803. [Google Scholar] [CrossRef]
- García Ibarra, V.; Rodríguez Bernaldo de Quirós, A.; Paseiro Losada, P.; Sendón, R. Non-Target Analysis of Intentionally and Non Intentionally Added Substances from Plastic Packaging Materials and Their Migration into Food Simulants. Food Packag. Shelf Life 2019, 21, 100325. [Google Scholar] [CrossRef]
- Gómez Ramos, M.J.; Lozano, A.; Fernández-Alba, A.R. High-Resolution Mass Spectrometry with Data Independent Acquisition for the Comprehensive Non-Targeted Analysis of Migrating Chemicals Coming from Multilayer Plastic Packaging Materials Used for Fruit Purée and Juice. Talanta 2019, 191, 180–192. [Google Scholar] [CrossRef]
- Habchi, B.; Kassouf, A.; Padellec, Y.; Rathahao-Paris, E.; Alves, S.; Rutledge, D.N.; Maalouly, J.; Ducruet, V. An Untargeted Evaluation of Food Contact Materials by Flow Injection Analysis-Mass Spectrometry (FIA-MS) Combined with Independent Components Analysis (ICA). Anal. Chim. Acta 2018, 1022, 81–88. [Google Scholar] [CrossRef]
- Hao, T.-Y.; Xu, X.; Lin, Q.-B.; Wu, S.-L.; Wu, X.-F.; Hu, J.-L.; Zhong, H.-N.; Dong, B.; Chen, Z.-F.; Ye, Z.-K.; et al. Rapid Discrimination of Recycled and Virgin Poly(Ethylene Terephthalate) Based on Non-Targeted Screening of Semi-Volatile Organic Compounds Using a Novel Method of DSI/GC×GC-Q-TOF-MS Coupled with Various Chemometrics. Food Packag. Shelf Life 2022, 34, 100978. [Google Scholar] [CrossRef]
- Hu, Y.; Du, Z.; Sun, X.; Ma, X.; Song, J.; Sui, H.; Debrah, A.A. Non-Targeted Analysis and Risk Assessment of Non-Volatile Compounds in Polyamide Food Contact Materials. Food Chem. 2021, 345, 128625. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, Y.J.; Koo, Y.J.; Pack, E.C.; Lim, K.M.; Choi, D.W. Migration of Monomers, Plastic Additives, and Non-Intentionally Added Substances from Food Utensils Made of Melamine–Formaldehyde Resin Following Ultraviolet Sterilization. Food Control 2021, 125, 107981. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, K.Y.; Jung, J.S.; Sin, H.S.; Lee, H.G.; Jang, D.Y.; Lee, S.H.; Lim, K.M.; Choi, D. Comparison of Migration and Cumulative Risk Assessment of Antioxidants, Antioxidant Degradation Products, and Other Non-Intentionally Added Substances from Plastic Food Contact Materials. Food Packag. Shelf Life 2023, 35, 101037. [Google Scholar] [CrossRef]
- Kirchkeszner, C.; Petrovics, N.; Nyiri, Z.; Sámuel Szabó, B.; Eke, Z. Role of Gas Chromatography–Single Quadrupole Mass Spectrometry in the Identification of Compounds Migrating from Polypropylene-Based Food Contact Plastics. Microchem. J. 2022, 181, 107772. [Google Scholar] [CrossRef]
- Kubicova, M.; Puchta, E.; Säger, S.; Hug, C.; Hofmann, S.; Simat, T.J. Styrene-Acrylonitrile-Copolymer and Acrylonitrile-Butadiene-Styrene-Copolymer: A Study on Extractable and Migratable Oligomers. Food Addit. Contam. Part A 2022, 39, 397–414. [Google Scholar] [CrossRef]
- Lestido-Cardama, A.; Sendón, R.; Rodríguez Bernaldo de Quirós, A. Tentative Identification of BADGE Derivatives in Epoxy Type Coatings in a Model Sample: A Beverage Can. J. Coat. Technol. Res. 2022, 19, 1893–1900. [Google Scholar] [CrossRef]
- Li, H.; Wu, X.; Wu, S.; Chen, L.; Kou, X.; Zeng, Y.; Li, D.; Lin, Q.; Zhong, H.; Hao, T.; et al. Machine Learning Directed Discrimination of Virgin and Recycled Poly(Ethylene Terephthalate) Based on Non-Targeted Analysis of Volatile Organic Compounds. J. Hazard. Mater. 2022, 436, 129116. [Google Scholar] [CrossRef]
- Mallen, T.R.; Abston, K.D.; Parizek, N.J.; Negley, J.; Shores, K.S.; Canatsey, R.D.; Dubail, S.; Maier, M.S.; Maffini, M.V. Characterization of a New Polymeric Food Contact Coating with Emphasis on the Chemical Analysis and Safety Assessment of Non-Intentionally Added Substances (NIAS). Food Chem. Toxicol. 2023, 173, 113635. [Google Scholar] [CrossRef]
- Miralles, P.; López, A.; Dualde, P.; Coscollà, C.; Yusà, V. Liquid Chromatography-Orbitrap Tribrid High-resolution Mass Spectrometry Using Data Dependent-tandem Mass Spectrometry with Triple Stage Fragmentation as a Screening Tool to Perform Identification and Risk Assessment of Unknown Substances in Food Contact Epoxy Resin. J. Sep. Sci. 2021, 44, 3020–3030. [Google Scholar] [CrossRef]
- Miralles, P.; Yusà, V.; Pineda, A.; Coscollà, C. A Fast and Automated Strategy for the Identification and Risk Assessment of Unknown Substances (IAS/NIAS) in Plastic Food Contact Materials by GC-Q-Orbitrap HRMS: Recycled LDPE as a Proof-of-Concept. Toxics 2021, 9, 283. [Google Scholar] [CrossRef]
- da Silva Oliveira, W.; Ubeda, S.; Nerín, C.; Padula, M.; Teixeira Godoy, H. Identification of Non-Volatile Migrants from Baby Bottles by UPLC-Q-TOF-MS. Food Res. Int. 2019, 123, 529–537. [Google Scholar] [CrossRef]
- Omer, E.; Cariou, R.; Remaud, G.; Guitton, Y.; Germon, H.; Hill, P.; Dervilly-Pinel, G.; Le Bizec, B. Elucidation of Non-Intentionally Added Substances Migrating from Polyester-Polyurethane Lacquers Using Automated LC-HRMS Data Processing. Anal. Bioanal. Chem. 2018, 410, 5391–5403. [Google Scholar] [CrossRef]
- Omer, E.; Bichon, E.; Hutinet, S.; Royer, A.-L.; Monteau, F.; Germon, H.; Hill, P.; Remaud, G.; Dervilly-Pinel, G.; Cariou, R.; et al. Toward the Characterisation of Non-Intentionally Added Substances Migrating from Polyester-Polyurethane Lacquers by Comprehensive Gas Chromatography-Mass Spectrometry Technologies. J. Chromatogr. A 2019, 1601, 327–334. [Google Scholar] [CrossRef]
- Omer, E.; Bakiri, A.; Hammel, Y.-A.; Sanders, M.J.; Koster, S.; Ciclet, O. Deciphering the Complexity of the Chemicals in Food Packaging Materials Using Molecular Networks. Food Chem. 2025, 462, 140853. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.; Dreolin, N.; Aznar, M.; Nerín, C.; Hancock, P. Determination of Volatile Non Intentionally Added Substances Coming from a Starch-Based Biopolymer Intended for Food Contact by Different Gas Chromatography-Mass Spectrometry Approaches. J. Chromatogr. A 2019, 1599, 215–222. [Google Scholar] [CrossRef]
- Paiva, R.; Wrona, M.; Nerín, C.; Bertochi Veroneze, I.; Gavril, G.-L.; Andrea Cruz, S. Importance of Profile of Volatile and Off-Odors Compounds from Different Recycled Polypropylene Used for Food Applications. Food Chem. 2021, 350, 129250. [Google Scholar] [CrossRef]
- Portesi, C.; Visentin, D.; Durbiano, F.; Abete, M.C.; Rizzi, M.; Maurino, V.; Rossi, A.M. Development of a Rapid Micro-Raman Spectroscopy Approach for Detection of NIAS in LDPE Pellets and Extruded Films for Food Packaging Applications. Polym. Test. 2019, 80, 106098. [Google Scholar] [CrossRef]
- Radusin, T.; Nilsen, J.; Larsen, S.; Annfinsen, S.; Waag, C.; Eikeland, M.S.; Pettersen, M.K.; Fredriksen, S.B. Use of Recycled Materials as Mid Layer in Three Layered Structures-New Possibility in Design for Recycling. J. Clean. Prod. 2020, 259, 120876. [Google Scholar] [CrossRef]
- Rung, C.; Welle, F.; Gruner, A.; Springer, A.; Steinmetz, Z.; Munoz, K. Identification and Evaluation of (Non-)Intentionally Added Substances in Post-Consumer Recyclates and Their Toxicological Classification. Recycling 2023, 8, 24. [Google Scholar] [CrossRef]
- Sapozhnikova, Y.; Nuñez, A.; Johnston, J. Screening of Chemicals Migrating from Plastic Food Contact Materials for Oven and Microwave Applications by Liquid and Gas Chromatography—Orbitrap Mass Spectrometry. J. Chromatogr. A 2021, 1651, 462261. [Google Scholar] [CrossRef]
- Song, X.-C.; Wrona, M.; Nerin, C.; Lin, Q.-B.; Zhong, H.-N. Volatile Non-Intentionally Added Substances (NIAS) Identified in Recycled Expanded Polystyrene Containers and Their Migration into Food Simulants. Food Packag. Shelf Life 2019, 20, 100318. [Google Scholar] [CrossRef]
- Su, Q.-Z.; Vera, P.; Van de Wiele, C.; Nerín, C.; Lin, Q.-B.; Zhong, H.-N. Non-Target Screening of (Semi-)Volatiles in Food-Grade Polymers by Comparison of Atmospheric Pressure Gas Chromatography Quadrupole Time-of-Flight and Electron Ionization Mass Spectrometry. Talanta 2019, 202, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Terrasse, J.; Martin, M.; Dubail, S.; Dole, P.; Casabianca, H. Non-Targeted Screening of Extracts from Polyester-Phenolic Can Coatings: Identification of New Aldehyde Molecules from Resole-Based Resins. Talanta 2022, 243, 123351. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Lin, L.; Bayen, S. Optimization of the Post-Acquisition Data Processing for the Non-Targeted Screening of Trace Leachable Residues from Reusable Plastic Bottles by High Performance Liquid Chromatography Coupled to Hybrid Quadrupole Time of Flight Mass Spectrometry. Talanta 2019, 193, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Tisler, S.; Christensen, J.H. Non-Target Screening for the Identification of Migrating Compounds from Reusable Plastic Bottles into Drinking Water. J. Hazard. Mater. 2022, 429, 128331. [Google Scholar] [CrossRef] [PubMed]
- Tisler, S.; Kristiansen, N.; Christensen, J.H. Chemical Migration from Reusable Plastic Bottles: Silicone, Polyethylene, and Polypropylene Show Highest Hazard Potential in LC-HRMS Analysis. J. Hazard. Mater. 2024, 480, 136391. [Google Scholar] [CrossRef]
- Ubeda, S.; Aznar, M.; Nerín, C. Determination of Oligomers in Virgin and Recycled Polyethylene Terephthalate (PET) Samples by UPLC-MS-QTOF. Anal. Bioanal. Chem. 2018, 410, 2377–2384. [Google Scholar] [CrossRef]
- Ubeda, S.; Aznar, M.; Alfaro, P.; Nerín, C. Migration of Oligomers from a Food Contact Biopolymer Based on Polylactic Acid (PLA) and Polyester. Anal. Bioanal. Chem. 2019, 411, 3521–3532. [Google Scholar] [CrossRef]
- Ubeda, S.; Aznar, M.; Nerín, C. Determination of Volatile Compounds and Their Sensory Impact in a Biopolymer Based on Polylactic Acid (PLA) and Polyester. Food Chem. 2019, 294, 171–178. [Google Scholar] [CrossRef]
- Thoden van Velzen, E.U.; Brouwer, M.T.; Stärker, C.; Welle, F. Effect of Recycled Content and RPET Quality on the Properties of PET Bottles, Part II: Migration. Packag. Technol. Sci. 2020, 33, 359–371. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Nerín, C. Identification of Non Volatile Migrant Compounds and NIAS in Polypropylene Films Used as Food Packaging Characterized by UPLC-MS/QTOF. Talanta 2018, 188, 750–762. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Barknowitz, G.; Goshawk, J.; Nerín, C. Ion-Mobility Quadrupole Time-of-Flight Mass Spectrometry: A Novel Technique Applied to Migration of Nonintentionally Added Substances from Polyethylene Films Intended for Use as Food Packaging. Anal. Chem. 2019, 91, 12741–12751. [Google Scholar] [CrossRef] [PubMed]
- Vera, P.; Canellas, E.; Nerín, C. Compounds Responsible for Off-Odors in Several Samples Composed by Polypropylene, Polyethylene, Paper and Cardboard Used as Food Packaging Materials. Food Chem. 2020, 309, 125792. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Nerín, C.; Dreolin, N.; Goshawk, J. The Migration of NIAS from Ethylene-Vinyl Acetate Corks and Their Identification Using Gas Chromatography Mass Spectrometry and Liquid Chromatography Ion Mobility Quadrupole Time-of-Flight Mass Spectrometry. Food Chem. 2022, 366, 130592. [Google Scholar] [CrossRef]
- Yusà, V.; López, A.; Dualde, P.; Pardo, O.; Fochi, I.; Pineda, A.; Coscolla, C. Analysis of Unknowns in Recycled LDPE Plastic by LC-Orbitrap Tribrid HRMS Using MS3 with an Intelligent Data Acquisition Mode. Microchem. J. 2020, 158, 105256. [Google Scholar] [CrossRef]
- Yusà, V.; López, A.; Dualde, P.; Pardo, O.; Fochi, I.; Miralles, P.; Coscollá, C. Identification of 24 Unknown Substances (NIAS/IAS) from Food Contact Polycarbonate by LC-Orbitrap Tribrid HRMS-DDMS3: Safety Assessment. Int. J. Anal. Chem. 2021, 2021, 6654611. [Google Scholar] [CrossRef]
- Zhang, H.; Su, Q.-Z.; Shang, G.-Q.; Weng, Y.-X.; Zhu, L. Elucidation of Non-Intentionally Added Substances from Plant Fiber/Plastic Composites by UPLC-QTOF/MS. Foods 2023, 12, 678. [Google Scholar] [CrossRef] [PubMed]
- Mzmine. Untargeted LC-MS Workflow—Mzmine Documentation. 2024. Available online: https://mzmine.github.io/mzmine_documentation/workflows/lcmsworkflow/lcms-workflow.html (accessed on 14 May 2025).
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-Independent MS/MS Deconvolution for Comprehensive Metabolome Analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Pence, H.E.; Williams, A. ChemSpider: An Online Chemical Information Resource. J. Chem. Educ. 2010, 87, 1123–1124. [Google Scholar] [CrossRef]
- European Commision. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed. SANTE/11813/2017. 2018. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/SANTE_11813_2017-fin.pdf (accessed on 7 April 2025).
- Smith, C.A.; Maille, G.O.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. METLIN. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A Public Repository for Sharing Mass Spectral Data for Life Sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef]
- Allen, F.; Pon, A.; Wilson, M.; Greiner, R.; Wishart, D. CFM-ID: A Web Server for Annotation, Spectrum Prediction and Metabolite Identification from Tandem Mass Spectra. Nucleic Acids Res. 2014, 42, W94–W99. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Nothias, L.-F.; Wang, M.; Kim, D.H.; Dorrestein, P.C.; Kang, K.B.; Yoo, H.H. Tandem Mass Spectrometry Molecular Networking as a Powerful and Efficient Tool for Drug Metabolism Studies. Anal. Chem. 2022, 94, 1456–1464. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Parenty, A.; Moreau, X.; Campagne, J.-M. Macrolactonizations in the Total Synthesis of Natural Products. Chem. Rev. 2006, 106, 911–939. [Google Scholar] [CrossRef]
- EFSA Scientific Committee. Scientific Opinion on Exploring Options for Providing Advice about Possible Human Health Risks Based on the Concept of Threshold of Toxicological Concern (TTC). EFSA J. 2012, 10, 2750. [Google Scholar] [CrossRef]
- Food Contact Additives (FCA). Risk Assessment of Non-Listed Substances (NLS) and Non-Intentionally Added Substances (NIAS) under the Requirements of Article 3 of the Framework Regulation (EC) 1935/2004. 2020. Available online: https://fca.cefic.org/wp-content/uploads/2021/02/FCA_Risk_Assessment_Guidelines_v30-1.pdf (accessed on 7 April 2025).
- World Health Organization (WHO). Principles and Methods for the Risk Assessment of Chemicals in Food. 2020. Available online: https://www.who.int/publications/i/item/9789241572408 (accessed on 7 April 2025).
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) Related to Use of Formaldehyde as a Preservative during the Manufacture and Preparation of Food Additives. EFSA J. 2007, 5, 415. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific Opinion on Melamine in Food and Feed. EFSA J. 2010, 8, 1573. [Google Scholar] [CrossRef]
- US Food and Drug Administration (FDA). Guidance for Industry: Preparation of Premarket Submissions for Food Contact Substances (Chemistry Recommendations). 2007. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-preparation-premarket-submissions-food-contact-substances-chemistry (accessed on 7 April 2025).
- US Food and Drug Administration (FDA). Advances in Dose Addition for Chemical Mixtures: A White Paper. 2023. Available online: https://assessments.epa.gov/risk/document/&deid%3D359745 (accessed on 12 June 2025).
- Cramer, G.M.; Ford, R.A.; Hall, R.L. Estimation of Toxic Hazard—A Decision Tree Approach. Food Cosmet. Toxicol. 1976, 16, 255–276. [Google Scholar] [CrossRef]
- EFSA Scientific Committee; More, S.J.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Hougaard Bennekou, S.; Koutsoumanis, K.P.; Machera, K.; et al. Guidance on the Use of the Threshold of Toxicological Concern Approach in Food Safety Assessment. EFSA J. 2019, 17, e05708. [Google Scholar] [CrossRef]
- Patlewicz, G.; Jeliazkova, N.; Safford, R.J.; Worth, A.P.; Aleksiev, B. An Evaluation of the Implementation of the Cramer Classification Scheme in the Toxtree Software. SAR QSAR Environ. Res. 2008, 19, 495–524. [Google Scholar] [CrossRef] [PubMed]

| FCMs (Polymers) | Sample Preparation | Analytical Technique | Instrumental Conditions | Mass Spectrometry | Data Processing | Identified Compounds | Risk Assessment | Ref. |
|---|---|---|---|---|---|---|---|---|
| Printed films (PP) and UV varnishes | (1) Extraction: EtOH 95%, room temp, 1 h (2) Migration: D2 (EtOH 95%), 60 °C, 10 days. | (1) GC-MS (2) UHPLC-IMS-QTOF-MS | (1) HP5-MS (30 m × 0.25 mm, 0.25 µm); 40 °C (2 min), 10 °C/min to 300 °C (2 min); 1 µL SL, 1 mL/min. (2) UPLC™ BEH C18 (2.1 × 100 mm, 1.7 µm), 0.3 mL/min, 5 µL inj., 35 °C, H2O:MeOH (0.1% formic), gradient, 13 min | (1) EI, FS (2) ESI(+/−), MSE | Manual: Chemdraw, MassFragment, NIST MS, Chemspider, and MassLynx. Analytical standards or technical mixtures | (20) Photoinitiators for UV curing, ester monomers, and reaction products (20 compounds) | TTC (Cramer) and ToxTree | [5] |
| Pellets and films (PLA and biodegradable fossil-based PES) | (1) Total dissolution/precipitation: DCM/EtOH. (2) Migration: A, B, D2 (EtOH 95%), 60 °C, 10 days | UHPLC-QTOF-MS | UPLC BEH C18 (2.1 × 100 mm, 1.7 µm), 0.4 mL/min, 10 µL inj., 40 °C, H2O:MeOH (0.1% formic), gradient, 10 min | ESI(+), MSE | Automatic: MassLynx, Chemspider, SciFinder, and MassFragment. | (37) AA, PA, BD cyclic oligomers, and PLA oligomers | - | [6] |
| Food storage containers (PP) | Migration: H2O, A, B, D2 (isooctane), 40 °C, 10 days, rotary evaporator and reconstitution (ACE) | GC × GC-HRMS (TOF) | Column 1: 8% phenyl (equiv.) polycarborane siloxane, 30 m × 0.25 mm, 0.25 µm; 45 °C (2.5 min), 20 °C/min 190 °C, 3 °C/min 300 °C (30 min). Column 2: 50% phenyl 50% methyl-polysilphenylene siloxane, 1.7 m × 0.1 mm, 0.1 µm; 75 °C (2.5 min), 20 °C/min 210 °C, 3 °C/min 300 °C (30 min); 1 µL SL, He at 30 psi | EI, 70 eV, FS 75–700 m/z | Automatic: ChromaToF, libraries, and the scientific literature. Confirmation with standards. | (107) Antioxidants, degradation products, contaminants, and 23 unknowns | - | [13] |
| (PE and rPE) | Migration: D2 (EtOH 95%), 60 °C, 10 days | GC-MS | HP-5MS (30 m × 0.25 mm, 0.25 µm), 1 µL SL 250 °C, He 1 mL/min; 50 °C, 10 °C/min 200 °C, 5 °C/min 250 °C (10 min), 5 °C/min 300 °C (6 min) | EI, 70 eV, 230 °C, FS 50–550 m/z | Automatic: MS-DIAL and NIST MS library. Multivariate analysis: SIMCA and SPSS. | (80) 10 hydrocarbons, 29 esters, 3 aldehydes, 9 alcohols, 2 ethers, 4 acids, 4 benzene derivatives, 4 ketones, 3 amides, 2 piperazine derivatives, and 10 unknowns | TTC (Cramer) | [18] |
| Baby food squeezes (PET and PE) | Migration: B, C, 40 °C, 10 days | UHPLC-QTOF-MS | Zorbax Eclipse Plus C8 (2.1 × 100 mm, 1.8 µm), 0.3 mL/min, 5 µL inj., 35 °C, H2O:MeOH (ammonium formate/formic), gradient, 20 min | ESI(+), DIA-MS2 using SWATH | Automatic: SciexOS, the literature, in silico MS2, and in-house databases (237 IAS/NIAS). Analytical standards. | (42) 3 IAS, 35 polyester oligomers (29 cyclic and 6 linear), and 4 NIAS (plasticizers) | TTC (Cramer) | [20] |
| Pellets and slotted spoon (PBT) | (1) Extraction: DCM, ACN, DMSO, EtOH 20%, 60 °C, 1 h, evaporation and reconstitution (2) Total dissolution/precipitation: DCM/2-propanol (3) Migration: H2O, 100 °C, 2 h | HPLC-DAD/MS | Multospher® 120 RP C18 (3 × 250 mm, 5 µm), 0.4 mL/min, 10 µL inj., 60 °C, H2O (0.015% formic): 2-propanol, gradient, >85 min | ESI(+/−), FS | Manual processing and identification. Quantification with BHET. | (27) cyclic and linear oligomers | TTC (Cramer) | [21] |
| Pellets and bottles (PET, PETG, and Tritan) | (1) Extraction: DCM, ACN, DMSO, EtOH 20%, 60 °C, 1 h, evaporation and reconstitution. (2) Total dissolution/precipitation: DCM/2-propanol | HPLC-DAD/MS | Multospher® 120 RP C18 (3 × 250 mm, 5 µm), 0.4 mL/min, 10 µL inj., 60 °C, H2O (0.015% formic): 2-propanol, gradient, >85 min | ESI(+/−), FS | Manual processing and identification. Quantification with BHET. | (100) linear and cyclic oligomers | TTC (Cramer) and ToxTree | [22] |
| Inner coatings from metallic cans (PES) | Extraction: 2 cm2 coating with 2 mL CAN, 40 °C, 24 h | UHPLC-HRMS (Q-Orbitrap) | Hypersil Gold C18 (2.1 × 100 mm, 1.9 µm), 0.4 mL/min, 10 µL inj., 40 °C, H2O:ACN (both 10 mM ammonium acetate), gradient, 20 min | H-ESI(+), FS, targeted-MS2 | Automatic: The R environment, in-house databases, and the scientific literature. Response factors of internal standards. | (125) 84 oligoesters, epoxidized soybean oil, BADGE, benzoguanamine derivatives, and phenol-formaldehyde oligomers | - | [23] |
| Bottled mineral water (PET) | Extraction: HS-SPME DVB/CAR/PDMS fiber, 60 °C, 15 min, constant stirring | GC-MS (IT) | Desorption: 3 min, 260 °C SL, CP-Wax 52 CB (60 m × 0.25 mm, 0.25 µm); 60 °C (5 min), 5 °C/min 240 °C (20 min); He 10 psi | EI, FS 30–300 m/z | Manual: NIST library, RI, and the literature data. Aldehydes standards and standard addition calibration. | (26) 8 aldehydes (C8-C15), 9 unsaturated aldehydes (C7-C11), 5 hydrocarbons (C11-C19), 1 ketone, 1 terpene, 1 phthalate, and 1 aromatic hydrocarbon | - | [24] |
| Microwavable food containers (PET, PP) | Migration: A, 40 °C, 10 days | UHPLC-HRMS (Q-Orbitrap) | AccucoreTM C8 (2.1 × 100 mm, 2.6 µm), 0.35 mL/min, 30 °C, H2O:MeOH (ammonium formate/formic), gradient, 17 min | H-ESI(+), FS 100–1000 m/z/ddMS2 | Automatic: Compound Discoverer, databases. MassFrontier. Analytical standards | (16) 1 maltose derivative of photoinitiator HMPP and 15 PPGs | - | [25] |
| Baby feeding bottles (PPSU) | (1) Total dissolution/precipitation. (2) Migration: D1, 70 °C, 2 h | Multitechnique (1H-NMR, SEC-DAD, HPLC-DAD/MS/AD/FLD, and GC-MS) | - | ESI(+), EI | Confirmation and quantification with reference standards. | Monomers, linear and cyclic oligomers and derivatives, and volatile and non-volatile migrants. | SML (R. 10/2011) TTC (Cramer) and ToxTree | [26] |
| Plastic candy wrappers | Extraction: ACN, 70 °C, 6 h, evaporation and reconstitution | GC-MS | ZB-5MS (30 m × 0.25 mm, 0.25 µm), 1 µL SL 300 °C, He (1 mL/min); 40 °C (2 min), 9 °C/min 300 °C (3 min) | EI,70 eV FS 35–500 m/z | Manual: NIST MS and Wiley libraries. Reference standards. | (23) n-alkanes (C21-C29), BHT, degradation products, plasticizers | TTC (Cramer), ToxTree | [27] |
| Corn and potato snacks, cookies, cakes packaging (PP) | Extraction: ACN, 70 °C, 24 h, and evaporation | GC-MS | ZB-5MS (30 m × 0.25 mm, 0.25 µm), 1 µL split 1:10 300 °C, He (1 mL/min); 120 °C (2.5 min), 9 °C/min 200 °C (2 min), 9 °C/min 300 °C (1 min), 20 °C/min 320 °C (7 min) | EI 70 eV, FS 40–400 m/z | Manual: NIST MS, Wiley libraries. Reference standards. | (>40) alkanes, aldehydes, alcohols, phthalates, citrates, adipates, phosphates, phenolic compounds, diisocyanates, and fatty acids. | SML (R. 10/2011) TTC (Cramer) and ToxTree | [28] |
| Films (PE, PET, PA, PP, and EVA) | (1) P&T: He 40 mL/min, 60 °C, 20 min (Vocarb 3000 Trap, Supelco, Bellefonte, PA, USA) (2) Extraction: ACN, 70 °C, 24 h. (3) Migration: E, 60 °C, 10 days, desorption with ACE; D2 (isooctane), 20 °C, 2 days | (1) P&T-GC-MS (2) GC-MS | (1) P&T: ZB-624 (30 m × 0.25 mm, 1.4 µm); 35 °C (4 min), 5 °C/min 210 °C (5 min); He 1 mL/min. (2) ZB-5MS (30 m × 0.25 mm, 0.25 µm), 1 µL SL 300 °C, He (1 mL/min); 50 °C (3 min), 8 °C/min 300 °C (3 min) | (1) EI 70 eV, FS 20–400 m/z (2) 40–500 m/z | Manual: NIST MS, Wiley libraries. Reference standards. | (>30) volatiles: alkanes, aldehydes, alkenes, alcohols, and aromatics (50) semi-volatiles: citrates, phthalates, adipates, phosphates, alkanes, aldehydes, and carboxylic acids | SML (R. 10/2011) TTC (Cramer) and ToxTree | [29] |
| Packaging for fruit purée and juice (PE and PA) | Migration: B, C, 40 °C, 10 days | UHPLC-QTOF-MS | Zorbax Eclipse Plus C8 (2.1 × 100 mm, 1.8 µm), 0.3 mL/min, 5 µL inj., 35 °C, H2O:MeOH (ammonium formate/formic), gradient, 20 min | ESI(+), DIA-MS2 (SWATH), | Automatic: SciexOS. Manual identification: ChemSpider, Metlin, Massbank, in silico fragmentation, and the scientific literature. Caprolactam reference standards. | (26) NIAS: caprolactam, bis(2-methoxyethyl) adipate, additives, and cyclic oligomers. | EU REACH-ECHA TTC (Cramer) and ToxTree | [30] |
| Pellets, containers, and films (PE) | Extraction: DCM, Soxhlet for 16 h, evaporation and reconstitution (ACN) | (1) Flow injection analysis (FIA)-MS. (2) FIA-HRMS (Q-Orbitrap) | FIA: 10 µL inj., flow 0.2 mL/min, elution solvent H2O:ACN 35:65 v/v, 0.1% formic acid (+) or 0.1% ammonia (-), 5 min | (1) ESI(+/−); FS 100–1200 m/z. (2) FS/MS2 50–1500 m/z | Automatic: Matlab-Independent component analysis. Confirmation with Irganox 1010, Irganox 1076, Tinuvin 770, and Irgafos 168 standards. | (8) Irganox 1076, Irganox 1010, Irgafos 168, alkylbenzene sulfonates, phthalic anhydride, DBP, and DEHP. | - | [31] |
| Bottles, powders, and pellets (PET, rPET) | Total dissolution/precipitation: HFIP/MeOH | Direct sample introduction/GC × GC-HRMS (Q-TOF) | Column 1: DB-5MS, Column 2: DB-17MS (1 m × 0.25 mm × 0.25 µm), He 30 psi, 0.1 µL inj. 250 °C; 40 °C (5 min), 8 °C/min 300 °C (8 min) | EI 70 eV, FS 50–500 m/z | Manual: NIST MS library, and PubChem. Multivariate analysis: SIMCA, SPSS, and Matlab. | (368) 267 SVOCs, 41 (rPET), and 60 (vPET). | - | [32] |
| Kitchenwares (PA) | (1) Total dissolution/precipitation: HFIP/MeOH. (2) Migration: A, B, D2 (EtOH 95%), 70 °C, 2 h | UHPLC-QTOF-MS | BEH C18 (2.1 × 100 mm, 1.7 µm), 0.3 mL/min, 2 µL inj., 30 °C, H2O:MeOH (0.1% formic), gradient, 8 min | ESI(+/−); MSE, 50–1200 m/z | Automatic: UNIFI and a home-made database. Reference standards. | (64) phthalates, N-butyltri-n-hexyl citrate, antioxidants, slip agents, fatty acids TPP, PA, and PEG oligomers. | SML (R. 10/2011) TTC (Cramer) and ToxTree | [33] |
| Kitchenwares (melamine–formaldehyde resin) | Migration: D2 (EtOH 95%), 70 °C, 2 h | HPLC-QTOF-MS | Nova-Pak C18 (2 × 150 mm, 3 µm), 0.3 mL/min, 20 µL inj., 35 °C, H2O (0.1% formic):MeOH, gradient, 40 min | ESI(−), FS 100–600 m/z | Chemspider, PubChem, MassBank, and mzCloud. Melamine and formaldehyde standards. | Plasticizers, slip agents, surface-active agents, raw materials, processing aids, coating agents, and UV-protecting agents. | EDI and TDI (EFSA) | [34] |
| Films and rubbers (PP, PE, melamine resin, PC, PET, PS, PCT, ABS, TPU, PLA, acrylic resin, PBT, Fluororesin, PVC, PA, and PI) | Migration: n-heptane, D2 (EtOH 95%, isooctane), H2O, C, 4% acetic acid, 70 °C, 2 h | (1) UHPLC-QTOF-MS (2) GC-MS | 1) Zorbax Eclipse Plus C18 (2.1 × 50 mm, 1.8 µm), 0.5 mL/min, 1 µL inj., 40 °C, H2O (0.1% formic): MeOH, gradient, 14 min 2) DB-5MS UI (30 m × 0.25 mm × 0.25 µm), 1 µm split 1:10, 280 °C, He (1 mL/min); 40 °C (3 min), 5 °C/min 300 °C (5 min) | (1) ESI(+/−); FS 100–1300 m/z (2) EI, 70 eV, FS 40–600 m/z | Automatic: MassHunter and the NIST MS library. Quantification with reference standards. | (1) (69) antioxidants, cyclic oligoesters, plasticizers, surfactants, emulsifiers, and flavor components. (2) (22) 1,3-ditert-butylbenzene, 2,4-di-tert-butylphenol, antioxidants phenylethyl alcohol, solvents, hydrocarbons, and styrene dimers. | Toxicity reference values (ECHA, NIH, and FDA) or TTC (Cramer) | [35] |
| Single-use and reusable containers (PP) | Migration: D2 (isooctane), 60 °C, 10 days | (1) GC-MS, (2) GC-TOF | 1) DB-5MS UI (30 m × 0.25 mm × 0.25 µm), 1 µL SL 300 °C, He (2 mL/min); 40 °C (1 min), 25 °C/min 320 °C (10 min). 2) RTX-5MS (15 m × 0.25 mm × 0.25 µm), 1 µL split 1:50 250 °C, He (2 mL/min); 50 °C (1.5 min), 30 °C/min 320 °C (3 min) | EI, 70 eV, FS 50–700 m/z | Automatic: AMDIS and NIST MS. Confirmation with reference standards. | (69) 24 n-alkanes and 45 volatile and semi-volatile compounds. | - | [36] |
| Kitchenwares, disposable glasses, and reusable cups (SAN and ABS) | (1) Extraction: MeOH, ultrasounds, 50 °C, 30 min. (2) Migration: H2O, A, B, C, D1, 70/100 °C, 2 h | Multitechnique (HPLC-DAD/MS, HPLC-UV/Chemiluminescent nitrogen detector, HPLC-DAD/FLD, and GC-MS) | - | ESI(+), EI | Manual: The NIST MS library and the scientific literature. Confirmation with reference standards. | (7) 6 oligomers of styrene and acrylonitrile and primary aromatic amine. | TTC (Cramer) and ToxTree | [37] |
| Beverage can (Epoxy coating) | Extraction: ACN, 70 °C, 1 day | HPLC-MS/MS | Phenosphere ODS C18 (3.2 × 150 mm, 3 µm), 0.5 mL/min, 1 µL inj., 40 °C, H2O:ACN/MeOH, gradient, 28 min | APCI(+/−); FS 100–1000 m/z | Manual: A home-made database (BADGE derivatives and epoxy resin-related). Bisphenol-related standards. | (10) BADGE derivatives. | TTC (Cramer) and ToxTree | [38] |
| Pellets and bottles (PET and rPET) | Extraction: HS-SPME PDMS/DVB/Carbon WR,110 °C, 30 min; desorption: 250 °C, 2 min | HS-SPME-GC×GC-QTOF-MS | Column 1: HP-5MS (30 m × 0.25 mm × 0.25 µm). Column 2: DB-17MS (1 m × 0.25 mm × 0.25 µm), He 1.2 mL/min; 40 °C (5 min), 8 °C/min 260 °C (8 min) | EI 70 eV, FS 35–500 m/z | Automatic: MassHunter, the NIST MS library, RI, and ClassyFire | (1247) VOCs: hydrocarbons, benzenoids, organic oxygen compounds, lipids, and lipid-like compounds. | - | [39] |
| Coatings for metal cans (Epoxy coating) | (1) Extraction: ACN, room temp, 24 h (2) Migration: C, D1, 21 °C, 1 h | HPLC-TOF-MS | Atlantis dC18 (150 × 2.1 mm, 3 µm), 30 °C | ESI(+/−); FS 100–1100 m/z | Automatic: MassHunter, Mass Profiler Professional, and the V70 NIAS database (TMBPF-DGE oligomers). Semi-quantification with BADGE. | (66) 16 identified TMBPF-DGE oligomers and TMBPF-DGE + hydroquinone | Prioritization and hazard assessment using bioassays | [40] |
| Food metal cans (Epoxy coating) | Migration: A, 60 °C, 10 days | UHPLC-HRMS (Q-Orbitrap-LIT) | Hypersil Gold C18 (2.1 × 100 mm, 1.9 µm), 0.3 mL/min, 5 µL inj., H2O:MeOH (0.1% formic), gradient, 30 min | H-ESI(+), FS 100–900 m/z /MS2/MS3 LIT. AcquireX DeepScan mode | Automatic: Compound Discoverer, mzCloud, ChemSpider, and MassLists. Reference standards and semi-quantification (average response factor). | (263) 22 IAS/241 NIAS: polymer additives | SML (R. 10/2011) TTC (Cramer) and ToxTree | [41] |
| Film (rLDPE) | Extraction: ACE, 40 °C, 1 h | GC-HRMS (Q-Orbitrap) | TG-5MS (30 m × 0.25 mm, 0.25 µm), 1 µL SL 280 °C, He (1.2 mL/min); 40 °C (5 min), 5 °C/min 315 °C (10 min) | EI 70 eV, FS 40–500 m/z | Automatic: Compound Discoverer, the NIST MS library, RI, and GC Orbitrap databases. Reference standards and semi-quantification (average response factor). | (83) 12 IAS/71 NIAS: additives, metabolites, industrial compounds, and PE oligomers | SML (R. 10/2011) TTC (Cramer) and ToxTree | [42] |
| Baby bottles (PP, Tritan, and silicone) | Migration: D1, 70 °C, 2 h | UHPLC-QTOF-MS | UPLC BEH C18 (2.1 × 100 mm, 1.7 µm), 0.3 mL/min, 10 µL inj., 40 °C, H2O:MeOH (0.1% formic), gradient, 10 min | ESI(+/−); 50–1000 m/z, MSE | Automatic: MassLynx. Manual: ChemSpider, SciFinder, and MassFragment. Reference standards and external calibration. | (27) 2.2′-(tridecylimino)bis-ethanol and derivatives, clarifying agents, glycerol derivatives, erucamide, and N-acetylvaline | SML (R. 10/2011) TTC (Cramer) and ToxTree | [43] |
| Metallic FCMs (PES-PUR coatings) | Extraction: ACN, 40 °C, 24 h, evaporation and reconstitution | UHPLC-HRMS (Q-Orbitrap) | Hypersil Gold C18 (2.1 × 100 mm, 1.9 µm), 0.4 mL/min, 10 µL inj., 40 °C, H2O:ACN (10 mM ammonium acetate), gradient, 20 min | H-ESI(+,−); FS 155–1200 m/z, targeted-MS2 (22 precursors) | Automatic: the R environment and a home-made database of predicted oligomers. | (58) 28 predicted oligomers and 26 unpredicted monomers and oligomers | SML (R. 10/2011) | [44] |
| Metallic FCMs (PES-PUR coatings) | Extraction: ACN, 40 °C, 24 h, evaporation and reconstitution | Multitechnique (GC-EI-MS, GC-Q-Orbitrap, GC-APCI-TOF-HRMS, and GC × GC-EI-TOF-MS) | - | - | Manual: The NIST MS library. | isophorone diisocyanate, 4,4-diphenylmethane diisocyanate, and cyclic oligoesters | - | [45] |
| Pellets (Bioplastics) | Extraction: n-Hexane:ACE (1:1), room temp, overnight, evaporation and reconstitution | UHPLC-HRMS (Q-Orbitrap) | Acquity BEH C8 (2.1 × 100 mm, 1.7 µm), 0.4 mL/min, 5 µL inj., 40 °C, H2O (formic/formate):MeOH, gradient, 33 min | H-ESI(+); FS 80–1200 m/z, DDA-MS2 | Manual: The Xcalibur Qual Browser. Automatic: Progenesis QI and MN using Python and Cytoscape. | (96) PLA, PBAT, and PBS oligomers | - | [46] |
| Powder, pellets, and retail samples (PLA and starch biopolymers) | (1) Extraction: MeOH, 40 °C, 1 h, evaporation. (2) Migration: A, B, D2 (EtOH 95%), 70 °C, 6 h | (1) GC-EI-MS; (2) APGC-QTOF-MS | HP-5MS (30 m × 0.25 mm, 0.25 µm), 1 µL SL 250 °C, He 1 mL/min; 50 °C (5 min), 10 °C/min 300 °C (5 min) | (1) EI 70 eV, FS 50–450 m/z; (2) APCI(+), 50–650 m/z, MSE | Automatic: MSD ChemStation, the NIST MS library, and UNIFI. Reference standards and external calibration. | (21) 14 identified antioxidants, lubricants, fatty acids, alcohols, slip agents, plasticizers, glucitol, and mono-2-ethyloxoexyl adipate | TTC (Cramer) | [47] |
| Pristine, contaminated, washed, and/or recycled pellets (rPP) | Extraction: HS-SPME (DVB/CAR/PDMS), 120 °C, 30 min | HS-SPME-GC-Olfactometry (O)-MS | HP-5MS (30 m × 0.25 mm, 0.25 µm), SPME desorption 250 °C, 2 min; He 1 mL/min; 40 °C (5 min), 10 °C/min 300 °C (2 min). Olfactometry with 3 trained panelist | EI, FS 45–350 m/z | Manual: The NIST MS Library, the scientific literature, and the FlavorDB, Pherobase, Flavornet databases. | (45) IAS: toluene, benzophenone, tetracosane; NIAS: glycerin, alkanes, and alkenes | - | [48] |
| Pellets and films (LDPE) | - | Raman spectroscopy; Micro-Raman imaging; ICP-MS (Ca and Ti) | - | - | Identification and quantification using of Ca and Ti by ICP-MS. | CaCO3, CaSO4, PS, and TiO2 | TTC (Cramer) | [49] |
| Multilayer films (LDPE and rLDPE) | (1) Extraction: Soxhlet, CHCl3, 60 °C, 5 h. (2) Migration: D2 (EtOH 95%), 20–60 °C, 10 days | HPLC-DAD; GC-MS | (1) HPLC: ZORBAX Eclipse XDB-C18, 10 µL, 1 mL/min. (2) GC: ZB-5MSPlus (Phenomenex, Torrance, CA, USA) (30 m × 0.25 mm × 0.25 mm), split (2:1 ratio), 60 °C to 300 °C at 10 °C/min | - | Confirmation and quantification using commercial standards. | Antioxidants, 2,4-di-tert-butylphenol (arvin 4), arvin 8, DEHP, DEHTP, and oligomers | SML (R. 10/2011) | [50] |
| Recyclates (rHDPE, rLDPE, rPE, rPET, rPP, and rPS) | Extraction: DCM, 40 °C, 3 days (polyolefins, PET); ACE, 60 °C, 3 days (PS) | Multitechnique (GC-FID; GC-MS; HS-GC-MS; HPLC-MS) | - | EI, FS 40–800 m/z; ESI, FS 500–1200 m/z | Manual: The NIST MS Library and retention time comparison with internal standards. Quantification of Irgafos 168, oxidized Irgafos 168, Irganox 245, Irganox 1010, Irganox 1076, Irganox 1330, and limonene | (205) 175 tentatively identified and 30 unknowns: alkanes, plasticizers, thermal stabilizers, flame retardants, antioxidants, and light and heat stabilizers. | TTC (Cramer) and (Non)-Genotoxic Carcinogenicity Alert | [51] |
| Microwave trays and bags and oven bags | Migration: Microwave, D2 (95% EtOH), 102 °C, 5 min; Oven, D2 (95% EtOH), 60 °C, 6 h | (1) GC-HRMS (Q-Orbitrap) (2) UHPLC-HRMS (Q-Orbitrap) | (1) GC: TG-5SilMS (30 m, 0.25 mm, 0.25 µm), 1 µL SL, He 1 mL/min, 50 °C (2 min), 20 °C/min 150 °C, 6 °C/min 320 °C (10 min); (2) LC: C18 (100 × 1 mm × 1.7 µm), inj. 2 µL, 45 °C, 60 µL/min, H2O:ACN gradient 35 min | (1) GC: EI 70 eV, FS 50–750 m/z (2) LC: H-ESI(+/−); FS/ddMS2 150–800 m/z | Automatic: Compound Discoverer, the NIST MS Library, GC-Orbitrap libraries; mzCloud, the Extractables and Leachables HRAM database, and ChemSpider. | (74) 65 IAS and 9 NIAS | - | [52] |
| Containers (expanded PS and rEPS) | (1) Extraction: HS-SPME, 30–50 μm DVB/CAR/PDMS, 100 °C (qual)/85 °C (quant), 15 min; desorption: 250 °C, 2 min. (2) Migration: A, B, 60 °C, 10 days | HS-SPME-GC-MS; SPME-GC-MS | HP-5MS (30 m × 0.25 mm, 0.25 µm), SL 250 °C, He 1 mL/min; 50 °C (5 min), 10 °C/min 300 °C (5 min) | EI, FS 45–400 m/z | Statistical analysis: SIMCA. Validation with reference standards of ethylbenzene, o-xylene, styrene, and 1,4-diphenylbutane. | (99) hydrocarbons, aldehydes, ketones, alcohols, esters, benzene derivatives, aromatics, styrene dimmers, and additives | SML (R. 10/2011) TTC (Cramer) and ToxTree | [53] |
| Sheets (PP) | Extraction: DCM, ultrasounds, 1 h, evaporation | (1) EI-GC-MS (2) APCI-QTOF-MS | (1) GC-MS: HP-5MS (30 m × 0.25 mm, 0.25 µm), 2 µL SL 250 °C, He 2.4 mL/min; 50 °C (3 min), 10 °C/min 300 °C (12 min) (2) APCI-QTOF-MS: SPB 5 (30 m × 0.25 mm, 0.25 µm), 2 µL SL 250 °C, He 3.5 mL/min; 50 °C (3 min), 10 °C/min 300 °C (12 min) | (1) GC-MS: EI, FS 40–700 m/z (2) QTOF-MS: 40–700 m/z, MSE | Manual: Masslynx and AMDIS from NIST. Confirmation with analytical standards. | (27) antioxidants, lubricants, catalysts, and transformation products | - | [54] |
| Can coatings (PES-phenolic resole-based resins) | Extraction: ACN, 40 °C, 24 h | (1) GC-MS (2) GC-HRMS (Q-Orbitrap) | (1) GC-MS: HP-5MS (30 m × 0.25 mm, 0.25 µm), 1 µL SL 280 °C; He 1 mL/min; 80 °C (2 min), 10 °C/min 270 °C (20 min), 5 °C/min 320 °C (15 min) (2) GC-HRMS: TG-5SilMS (30 m × 0.25 mm, 0.25 µm), 1 µL SL 280 °C; He 1 mL/min; 80 °C (4 min), 10 °C/min 320 °C (15 min) | (1) GC-MS: EI 70 eV, FS 50–750 m/z (2) GC-HRMS: EI 10 and 70 eV, FS 50–750 m/z | Manual: NIST MS and Wiley libraries and a home-made oligomers database. Semi-quantification of phenol-based molecules and PES oligomers. Diethyl terephthalate as the internal standard. | Cyclic PES oligomers and aldehydes | - | [55] |
| Reusable bottles (Unknown, PP, and Tritan) | Migration: D1, 40 °C, 10 days | HPLC-QTOF-MS | Poroshell 120 Phenyl Hexyl (2.7 μm × 3.0 mm×100 mm) with Poroshell 120 EC-C18 (2.7 μm × 3.0 mm×10 mm) guard column; 10 µL inj., 20 °C, 0.2 mL/min; H2O:MeOH (0.1% formic); gradient, 20 min | All ions mode (MS2), 50–1700 m/z | Automatic: MassHunter Profiling and Agilent Extractables and Leachables LC/QTOF databases. Analytical standards. | Monomethyl terephthalate | - | [56] |
| Reusable bottles (PE and biodegradable PE) | Migration: drinking water stored for 24 h and SPE | UHPLC-QTOF-MS | Acquity BEH C18 (2.1 × 100 mm, 1.7 µm), 0.3 mL/min, 2 µL inj., 40 °C, H2O:ACN (0.1% formic), gradient, 24 min | ESI(+), MSE and MS2 50–1200 m/z | Automatic: UNIFI with Norman lists “database of chemicals associated with plastic (CPPdb)” and “plastic additives by ECHA, PubChem, MassBank”. | (>3500) oligomers, aromatic amines, plasticizers, antioxidants, and photoinitiators | TTC (Cramer) and ToxTree | [57] |
| Reusable bottles (Silicone, HDPE, LDPE, PP, PS, PET, PETG, and PCTG) | Migration: tap water at room temp., 48 h, evaporation and reconstitution (MeOH) | UHPLC-QTOF-MS | BEH C18 (2.1 × 100 mm, 1.7 µm), 0.3 mL/min, 1 µL inj., H2O:ACN (0.1% formic), gradient, 16 min | DIA-MSE, ESI(+/−), 50–1000 m/z | Manual: MSDial. Quantitative non-target analysis (qNTA) employing QSPR. Analytical standards of six model additives. | Phthalates, color agents, sorbitol-based nuclear clarifying agents, BADGE derivates, intermediates, plasticizer, and oligomers | TTC (Cramer) | [58] |
| Pellets, bottles (PET and rPET) | (1) Total dissolution/precipitation: HFIP, 40 °C, 24 h; MeOH, 4 °C, 1 h (2) Extraction: DCM, ultrasounds, 1 h, evaporation and reconstitution (MeOH) (3) Migration: A, B, D2 (95% EtOH), 60 °C, 10 days | UHPLC-QTOF-MS | BEH C18 (2.1 × 100 mm, 1.7 µm), 0.3 mL/min, 10 µL inj., 35 °C, H2O:MeOH (0.1% formic), gradient, 8 min | ESI(+/−), MSE | Automatic: MassLynx, MassFragment. Quantification with the oligomer AA-DEG-IPA-DEG. | PET cyclic and linear oligomers | - | [59] |
| Pellets and films (PLA and PES biopolymers) | (1) Total dissolution/precipitation: DCM, ultrasounds, 1 h; EtOH, 4 °C, 1 h. (2) Migration: A, B, D2 (95% EtOH), 60 °C, 10 days | UHPLC-QTOF-MS, UHPLC-IMS-QTOF-MS | BEH C18 (2.1 × 100 mm, 1.7 µm), 0.3 mL/min, 10 µL inj., 40 °C, H2O:MeOH (0.1% formic), gradient, 12 min | ESI(+/−), MSE | Automatic: MassFragment and ChemDraw Ultra. Quantification with the oligomer AA-DEG-IPA-DEG. | (39) PLA cyclic and linear oligomers | - | [60] |
| Pellets and films (PLA and PES biopolymers) | (1) Total dissolution/precipitation: DCM, ultrasounds, 1 h; EtOH, 4 °C, 1 h. (2) Migration: A, B, D2 (95% EtOH), 60 °C, 10 days | (1) GC-MS (2) APGC-QTOF-MS (3) GC-O-MS | (1,3) GC-(O)-MS: HP-5MS (30 m × 0.25 mm × 0.25 μm), HS-SPME, He 1 mL/min, 40 °C (5 min), 10 °C/min 300 °C (2) APGC-QTOF-MS: HP-5MS (30 m × 0.25 mm × 0.25 μm), 1 µL SL 250 °C, He 1.2 mL/min, 60 °C (5 min), 10 °C/min 300 °C (5 min) | GC-MS: EI-MS; APGC-QTOF-MS: API(+), MSE, 50–550 m/z | Automatic: MassLynx. Quantification using a cyclic ester oligomer, octanal, 1-octen-3-one, (E)-2-nonenal, sotolon, citronellal, dodecanal, and nonanal as standards. | (15) lactide, cyclopentanone, cyclic dimer, adipic acid, butanediol, palmitic acid, oleamide, glycerol 1-palmitate, glycerol 1-stearate, and erucamide | - | [61] |
| Bottles and pellets (rPET) | (1) Extraction: DCM, 40 °C, 3 days. (2) Migration: H2O, 40 °C, 10 days | HS-GC-MS, GC-MS, GC-FID | DB1 MS (30 m × 0.25 mm × 0.25 μm), various temperature programs and conditions | EI, FS 40–800 m/z | Manual: The NIST MS library and AKTS SML for migration modeling. Quantification with analytical standards. | Acetaldehyde, ethylene glycol, 2-methyl-1,3-dioxolane, limonene, acetone, butanone, furan, benzene, styrene, and oligomers | SML (R. 10/2011) TTC (Cramer) | [62] |
| Films (PP) | Migration: A, B, D2 (95% EtOH), E, 60 °C, 10 days | UHPLC-QTOF-MS | BEH C18 (2.1 × 100 mm, 1.7 µm), 0.3 mL/min, 5 µL inj., 35 °C, H2O:MeOH (0.1% formic), gradient, 15 min | ESI(+/−), MSE | Automatic: MassLynx, CromaLynx, and MassFragment. Quantification with analytical standards. | (76) Irganox 1076 and Irganox 1010 degradation products or impurities | SML (R. 10/2011) TTC (Cramer) | [63] |
| Films (HDPE and LDPE) | Migration: A, B, D1, D2 (95% EtOH), E, 60 °C, 10 days | UHPLC-IMS-QTOF-MS | BEH C18 (2.1 × 100 mm, 1.7 µm), 0.3 mL/min, 5 µL inj., 35 °C, H2O:MeOH (0.1% formic), gradient, 13 min | ESI(+/−), high definition MSE | Automatic: UNIFI and ChemSpider. Quantification with analytical standards. | (35) Irganox 1010 and Irganox 1076 degradation, breakdown, impurity, or reaction products | SML (R. 10/2011) TTC (Cramer) | [64] |
| Bags and tetrabrik (PP and PE) | Migration: E, 60 °C, 10 days | GC-O-MS | BP-20 (30 m × 0.25 mm × 0.25 μm), HS-SPME, He 1 mL/min, 40 °C (5 min), 10 °C/min 220 °C (10 min) | FS/SIM, 50–450 m/z | Manual: NIST and Wiley MS libraries. Quantification and confirmation with analytical standards. | (46) acetic, propanoic, butyric acid, octanal, nonanal, decanal, trimethylbenzenes, and terpenes | SML (R. 10/2011) TTC (Cramer) | [65] |
| Corks (EVA) | Migration: B, C, 60 °C, 10 days | (1) GC-MS (2) UHPLC-IMS-QTOF-MS | (1) GC-MS: HP-5 MS (30 m × 0.25 mm × 0.25 μm), SPME, He 1 mL/min, 50 °C (5 min), 10 °C/min 300 °C (5 min). (2) UHPLC-IMS-QTOF-MS: BEH C18 (2.1 × 100 mm, 1.7 µm), 0.3 mL/min, 5 µL inj., 40 °C, H2O:MeOH (0.1% formic), gradient, 13 min | (1) GC-MS: FS, 50–450 m/z; (2) UHPLC-IMS-QTOF-MS: 50–1000 m/z, high definition MSE | Automatic: UNIFI, Chemspider, NIST, and Wiley MS libraries. Quantification and confirmation with analytical standards. | (50) antioxidants, lubricants, cyclic oligomers, and breakdown and oxidation products | SML (R. 10/2011) TTC (Cramer) | [66] |
| Post-consumer film (rLDPE) | Migration: A, 40 °C, 10 days | UHPLC-HRMS (Q-Orbitrap-LIT) | Hypersil Gold C18 (2.1 × 100 mm, 1.9 µm), 0.3 mL/min, 5 µL inj., 40 °C, H2O:MeOH, gradient, 30 min | H-ESI(+/−), FS 100–900 m/z, MS2/MS3 | AcquireX DeepScan and Interative Processing Exclusion. Automatic: Compound Discoverer, mzCloud, and ChemSpider. | (28) additives and plasticizers | SML (R. 10/2011) TTC (Cramer) | [67] |
| Bowls (PC) | Migration: C, 100 °C, 2 h | UHPLC-HRMS (Q-Orbitrap-LIT) | Hypersil Gold C18 (2.1 × 100 mm, 1.9 µm), 0.3 mL/min, 5 µL inj., 40 °C, H2O:MeOH, gradient, 30 min | H-ESI(+/−), FS 100–900 m/z, MS2/MS3 | AcquireX DeepScan and Interative Processing Exclusion. Automatic: Compound Discoverer, mzCloud, and ChemSpider. Analytical standards. | (24) plasticizers, slip agents, antioxidants, UV stabilizers, and fragrances | SML (R. 10/2011) TTC (Cramer) | [68] |
| Bowls and cups (PPCs) | Extraction: EtOH, isooctane, 4% acetic acid, 40–60 °C, 1–2 h | UHPLC-QTOF-MS | Zorbax SB C18 (2.1 × 100 mm, 1.8 µm), 0.3 mL/min, 3 µL inj., 40 °C, H2O (0.1% formic):MeOH, gradient, 35 min | ESI(+/−), DDA-MS2, 50–1000 m/z | Automatic: MS-DIAL, MS-FINDER, NIST, MoNA, and GNPS libraries. | (115) plasticizers, pesticides, bisphenols, oligomers, and melamine derivatives | SML (R. 10/2011) TTC (Cramer) | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miralles, P.; Fuentes-Ferragud, E.; Socas-Hernández, C.; Coscollà, C. Recent Trends and Challenges on the Non-Targeted Analysis and Risk Assessment of Migrant Non-Intentionally Added Substances from Plastic Food Contact Materials. Toxics 2025, 13, 543. https://doi.org/10.3390/toxics13070543
Miralles P, Fuentes-Ferragud E, Socas-Hernández C, Coscollà C. Recent Trends and Challenges on the Non-Targeted Analysis and Risk Assessment of Migrant Non-Intentionally Added Substances from Plastic Food Contact Materials. Toxics. 2025; 13(7):543. https://doi.org/10.3390/toxics13070543
Chicago/Turabian StyleMiralles, Pablo, Esther Fuentes-Ferragud, Cristina Socas-Hernández, and Clara Coscollà. 2025. "Recent Trends and Challenges on the Non-Targeted Analysis and Risk Assessment of Migrant Non-Intentionally Added Substances from Plastic Food Contact Materials" Toxics 13, no. 7: 543. https://doi.org/10.3390/toxics13070543
APA StyleMiralles, P., Fuentes-Ferragud, E., Socas-Hernández, C., & Coscollà, C. (2025). Recent Trends and Challenges on the Non-Targeted Analysis and Risk Assessment of Migrant Non-Intentionally Added Substances from Plastic Food Contact Materials. Toxics, 13(7), 543. https://doi.org/10.3390/toxics13070543








