Application of a Dynamic Exposure Population Toxicokinetic Model for Perfluorooctane Sulfonic Acid (PFOS) and Extension to Perfluorodecanoic Acid (PFDA) at a North American Beef Cattle Farm with a History of Biosolids Land Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Farm Environment and Practices
2.2. Site PFAS Characterization
2.3. Biomonitoring
2.4. PFAS Analyses
2.5. Exposure Model
2.6. Toxicokinetic Model
2.7. Model Evaluation and Statistical Analyses
2.8. Farm Management Analyses
3. Results
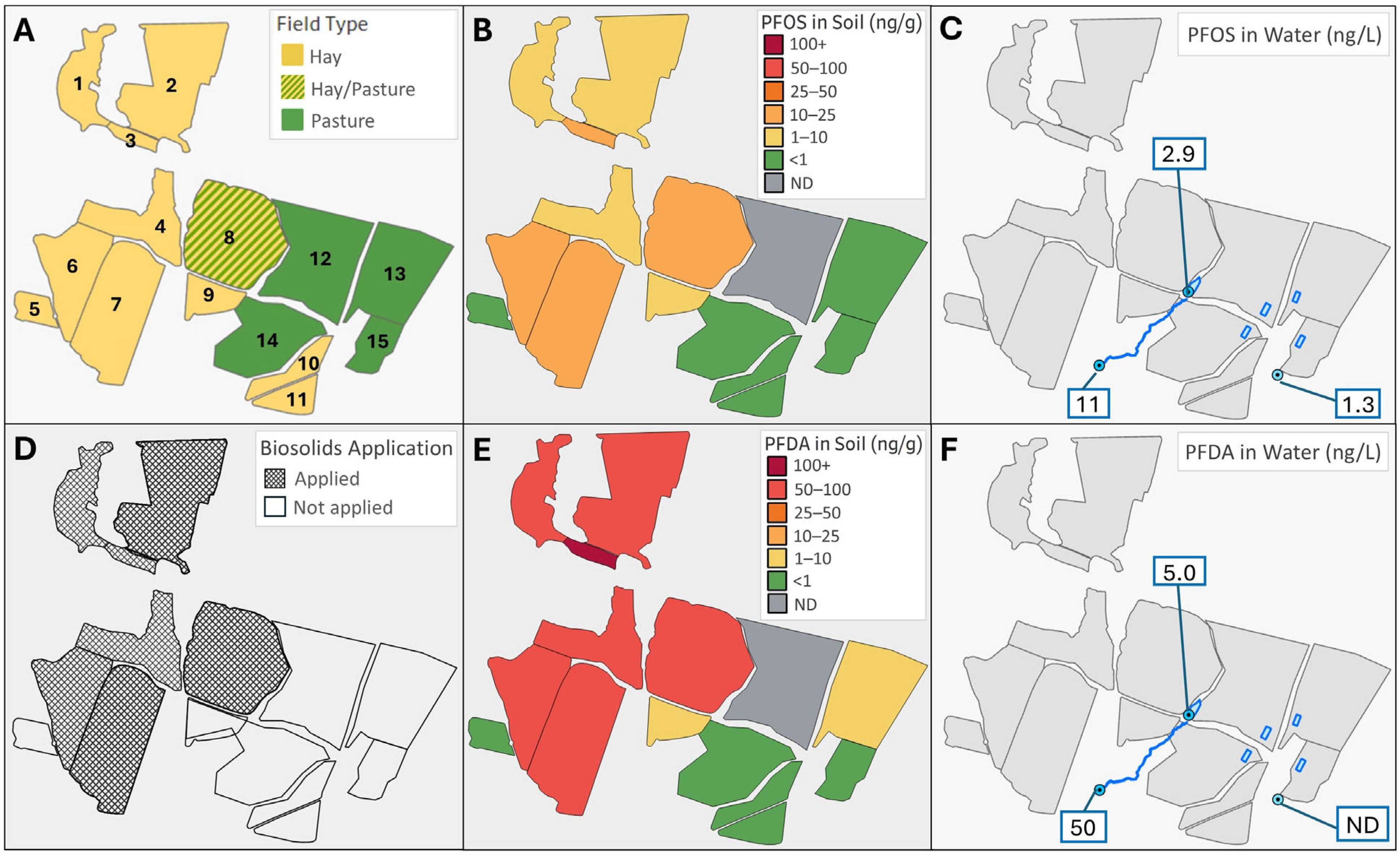
3.1. PFAS Measurements in Soil, Water, and Forage
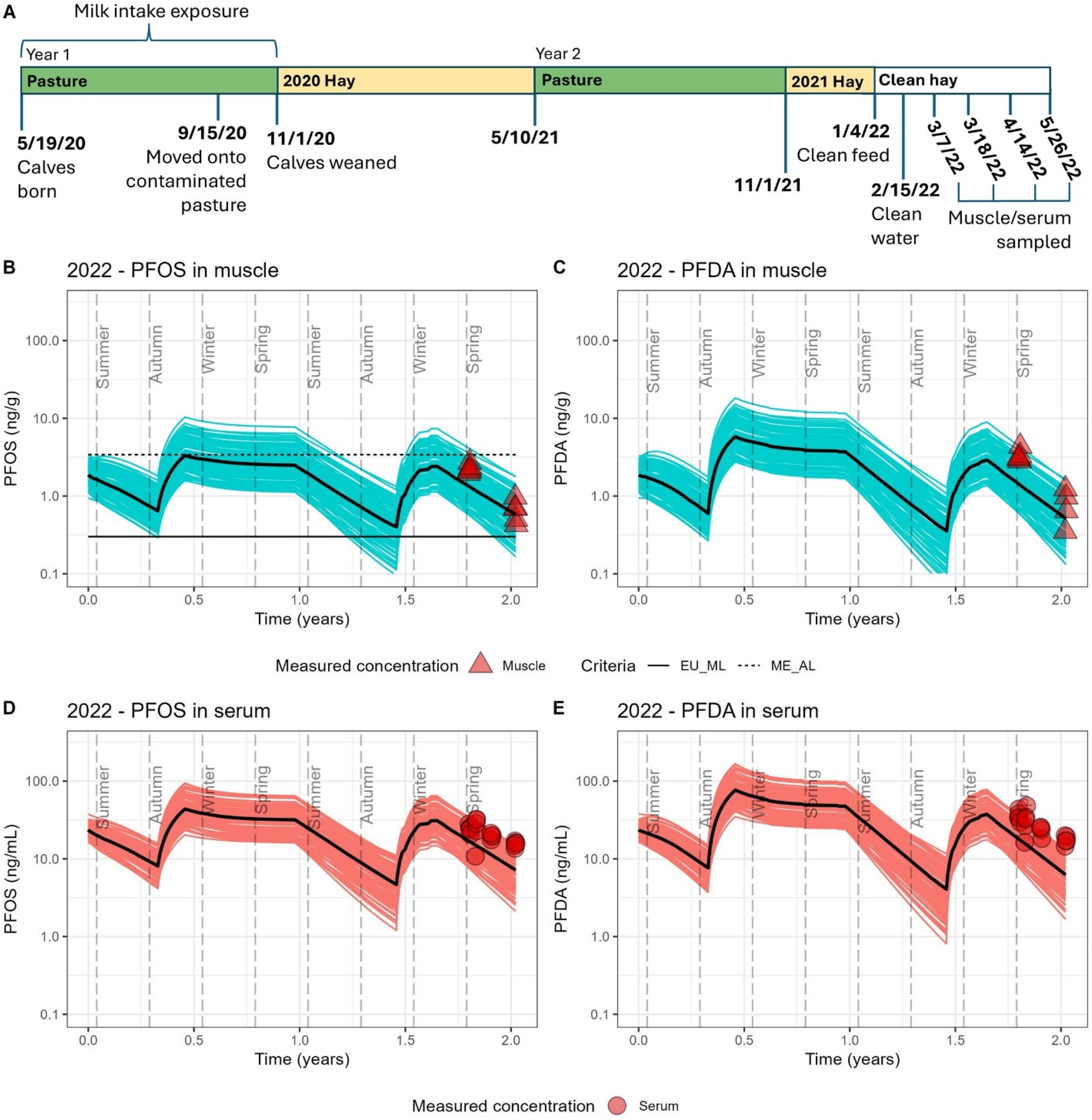
3.2. Biomonitoring of PFAS in Cattle Tissue
3.3. PFAS Exposure
3.4. Model Evaluation
3.5. Application as Farm Management Tool
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Link, G.W.; Reeves, D.M.; Cassidy, D.P.; Coffin, E.S. Per- and polyfluoroalkyl substances (PFAS) in final treated solids (Biosolids) from 190 Michigan wastewater treatment plants. J. Hazard. Mater. 2024, 463, 132734. [Google Scholar] [CrossRef] [PubMed]
- Lazcano, R.K.; de Perre, C.; Mashtare, M.L.; Lee, L.S. Per- and polyfluoroalkyl substances in commercially available biosolid-based products: The effect of treatment processes. Water Environ. Res. 2019, 91, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Lazcano, R.K.; Choi, Y.J.; Mashtare, M.L.; Lee, L.S. Characterizing and Comparing Per- and Polyfluoroalkyl Substances in Commercially Available Biosolid and Organic Non-Biosolid-Based Products. Environ. Sci. Technol. 2020, 54, 8640–8648. [Google Scholar] [CrossRef]
- Sepulvado, J.G.; Blaine, A.C.; Hundal, L.S.; Higgins, C.P. Occurrence and Fate of Perfluorochemicals in Soil Following the Land Application of Municipal Biosolids. Environ. Sci. Technol. 2011, 45, 8106–8112. [Google Scholar] [CrossRef]
- Venkatesan, A.K.; Halden, R.U. National inventory of perfluoroalkyl substances in archived U.S. biosolids from the 2001 EPA National Sewage Sludge Survey. J. Hazard. Mater. 2013, 252–253, 413–418. [Google Scholar] [CrossRef]
- Washington, J.W.; Yoo, H.; Ellington, J.J.; Jenkins, T.M.; Libelo, E.L. Concentrations, Distribution, and Persistence of Perfluoroalkylates in Sludge-Applied Soils near Decatur, Alabama, USA. Environ. Sci. Technol. 2010, 44, 8390–8396. [Google Scholar] [CrossRef]
- Rosemarin, A.; Macura, B.; Carolus, J.; Barquet, K.; Ek, F.; Järnberg, L.; Lorick, D.; Johannesdottir, S.; Pedersen, S.M.; Koskiaho, J.; et al. Circular nutrient solutions for agriculture and wastewater—A review of technologies and practices. Curr. Opin. Environ. Sustain. 2020, 45, 78–91. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Barbarick, K.A. The Clean Water Act and biosolids: A 45-year chronological review of biosolids land application research in Colorado. J. Environ. Qual. 2022, 51, 780–796. [Google Scholar] [CrossRef] [PubMed]
- Pepper, I.L.; Brusseau, M.L.; Prevatt, F.J.; Escobar, B.A. Incidence of Pfas in soil following long-term application of class B biosolids. Sci. Total Environ. 2021, 793, 148449. [Google Scholar] [CrossRef]
- Oviedo-Vargas, D.; Anton, J.; Coleman-Kammula, S.; Qin, X. Quantification of PFAS in soils treated with biosolids in ten northeastern US farms. Sci. Rep. 2025, 15, 5582. [Google Scholar] [CrossRef]
- Maine DEP. Status of Maine’s PFAS Soil and Groundwater Investigation at Sludge and Septage Land Application Sites; Maine Department of Environmental Protection: Augusta, ME, USA, 2025.
- Kowalczyk, J.; Ehlers, S.; Oberhausen, A.; Tischer, M.; Fürst, P.; Schafft, H.; Lahrssen-Wiederholt, M. Absorption, Distribution, and Milk Secretion of the Perfluoroalkyl Acids PFBS, PFHxS, PFOS, and PFOA by Dairy Cows Fed Naturally Contaminated Feed. J. Agric. Food Chem. 2013, 61, 2903–2912. [Google Scholar] [CrossRef] [PubMed]
- Vestergren, R.; Orata, F.; Berger, U.; Cousins, I.T. Bioaccumulation of perfluoroalkyl acids in dairy cows in a naturally contaminated environment. Environ. Sci. Pollut. Res. 2013, 20, 7959–7969. [Google Scholar] [CrossRef] [PubMed]
- Simones, T.L.; Evans, C.; Goossen, C.P.; Kersbergen, R.; Mallory, E.B.; Genualdi, S.; Young, W.; Smith, A.E. Uptake of Per- and Polyfluoroalkyl Substances in Mixed Forages on Biosolid-Amended Farm Fields. J. Agric. Food Chem. 2024, 72, 23108–23117. [Google Scholar] [CrossRef]
- Death, C.; Bell, C.; Champness, D.; Milne, C.; Reichman, S.; Hagen, T. Per- and polyfluoroalkyl substances (PFAS) in livestock and game species: A review. Sci. Total Environ. 2021, 774, 144795. [Google Scholar] [CrossRef]
- Mikkonen, A.T.; Martin, J.; Upton, R.N.; Barker, A.O.; Brumley, C.M.; Taylor, M.P.; Mackenzie, L.; Roberts, M.S. Spatio-temporal trends in livestock exposure to per- and polyfluoroalkyl substances (PFAS) inform risk assessment and management measures. Environ. Res. 2023, 225, 115518. [Google Scholar] [CrossRef] [PubMed]
- Bräunig, J.; Baduel, C.; Heffernan, A.; Rotander, A.; Donaldson, E.; Mueller, J.F. Fate and redistribution of perfluoroalkyl acids through AFFF-impacted groundwater. Sci. Total Environ. 2017, 596–597, 360–368. [Google Scholar] [CrossRef]
- EFSA. Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J. 2020, 18, e06223. [Google Scholar] [CrossRef]
- U.S. FDA. Analytical Results of Testing Food for PFAS from Environmental Contamination. Available online: https://www.fda.gov/food/environmental-contaminants-food/analytical-results-testing-food-pfas-environmental-contamination (accessed on 20 March 2025).
- FSANZ. 27th Australian Total Diet Study Per- and Poly-Fluoroalkyl Substances; Food Standards Australia New Zealand: Wellington, New Zealand, 2021. [Google Scholar]
- Ghaznavi, S.M.; Zimmerman, C.; Shea, M.E.; MacRae, J.D.; Peckenham, J.M.; Noblet, C.L.; Apul, O.G.; Kopec, A.D. Management of per- and polyfluoroalkyl substances (PFAS)-laden wastewater sludge in Maine: Perspectives on a wicked problem. Biointerphases 2023, 18. [Google Scholar] [CrossRef]
- Richterová, D.; Govarts, E.; Fábelová, L.; Rausová, K.; Rodriguez Martin, L.; Gilles, L.; Remy, S.; Colles, A.; Rambaud, L.; Riou, M.; et al. PFAS levels and determinants of variability in exposure in European teenagers—Results from the HBM4EU aligned studies (2014–2021). Int. J. Hyg. Environ. Health 2023, 247, 114057. [Google Scholar] [CrossRef]
- U.S. FDA. Questions and Answers on PFAS in Food. Available online: https://www.fda.gov/food/process-contaminants-food/questions-and-answers-pfas-food (accessed on 20 March 2025).
- Michigan DHHS. Evaluation of PFOS in Frozen Wholesale Beef from Cattle Exposed to PFOS Through Contaminated Crops and Soil Due to Biosolids Applications at a Farm in Livingston County, Michigan; Michigan Department of Health and Human Services: Lansing, MI, USA, 2023.
- New Mexico Environment Department. Depopulation and Removal Plan with Narrative to Application for DIPP Cow Buy-Out Indemnity Benefits for Highland Dairy Cow Herd; New Mexico Environment Department: Santa Fe, NM, USA, 2022.
- Maine Department of Agriculture Conservation and Forestry. Letter from Nancy McBrady, Bureau Director, Maine Department of Agriculture Conservation and Forestry to a Central Maine Farmer on Voluntary Surrender of All Live Beef Cattle on the Farm; Maine Department of Agriculture, Conservation and Forestry: Augusta, ME, USA, 2021.
- Maine CDC. Derivation of PFOS Soil Screening Levels for a Soil-to-Fodder-to-Cow’s Milk Agronomic Pathway; Maine Center for Disease Control & Prevention: Augusta, ME, USA, 2020.
- van Asselt, E.D.; Kowalczyk, J.; van Eijkeren, J.C.H.; Zeilmaker, M.J.; Ehlers, S.; Fürst, P.; Lahrssen-Wiederholt, M.; van der Fels-Klerx, H.J. Transfer of perfluorooctane sulfonic acid (PFOS) from contaminated feed to dairy milk. Food Chem. 2013, 141, 1489–1495. [Google Scholar] [CrossRef]
- Drew, R.; Hagen, T.G.; Champness, D.; Sellier, A. Half-lives of several polyfluoroalkyl substances (PFAS) in cattle serum and tissues. Food Addit. Contam. Part A 2021, 39, 320–340. [Google Scholar] [CrossRef]
- Mikkonen, A.T.; Martin, J.; Upton, R.N.; Moenning, J.-L.; Numata, J.; Taylor, M.P.; Roberts, M.S.; Mackenzie, L. Dynamic exposure and body burden models for per- and polyfluoroalkyl substances (PFAS) enable management of food safety risks in cattle. Environ. Int. 2023, 180, 108218. [Google Scholar] [CrossRef]
- Maine DEP. Memorandum to Licensed Facilities That Land Apply, Compost, or Process Sludge in Maine; Maine Department of Environmental Protection: Augusta, ME, USA, 2019.
- Maine DEP. SOP-RWM-DR-001 Water Sample Collection From Water Supply Wells; Maine Department of Environmental Protection: Augusta, ME, USA, 2021.
- Maine DEP. SOP-RWM-DR004 Surface Water and Sediment Sampling; Maine Department of Environmental Protection: Augusta, ME, USA, 2021.
- Genualdi, S.; Young, W.; DeJager, L.; Begley, T. Method Development and Validation of Per- and Polyfluoroalkyl Substances in Foods from FDA’s Total Diet Study Program. J. Agric. Food Chem. 2021, 69, 5599–5606. [Google Scholar] [CrossRef]
- U.S. FDA. Foods Program Compendium of Analytical Laboratory Methods. Determination of 16 Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) in Food Using Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) 2021; U.S. FDA: Silver Spring, MD, USA, 2024.
- USDA Food Safety and Inspection Service. CLG-PFAS 2.04 Effective: 02/28/23 Screening, Determination, and Confirmation of PFAS by UHPLC-MS-MS; USDA Food Safety and Inspection Service: Washington, DC, USA, 2023.
- Weyrauch, K.; Ochoa, C.; Matsuda, R.; Duverna, R.; Lenov, I. A Survey of the Levels of 16 Per- and Polyfluoroalkyl Substances in Meat, Chicken, and Siluriformes Fish, 2019 to 2023. Food Prot. Trends 2025, 45, 154. Available online: https://www.foodprotection.org/publications/food-protection-trends/archive/2025-05-a-survey-of-the-levels-of-16-per-and-polyfluoroalkyl-substances-in-meat-chicken-and-silurifo (accessed on 19 June 2025).
- NOAA National Centers for Environmental information. Climate Data Online. Available online: https://www.ncei.noaa.gov/cdo-web/ (accessed on 26 August 2024).
- Stahl, T.; Heyn, J.; Thiele, H.; Hüther, J.; Failing, K.; Georgii, S.; Brunn, H. Carryover of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) from Soil to Plants. Arch. Environ. Contam. Toxicol. 2009, 57, 289–298. [Google Scholar] [CrossRef]
- U.S. EPA. Preliminary Remediation Goals for Radionuclides (PRG). Available online: https://epa-prgs.ornl.gov/radionuclides/ (accessed on 29 October 2024).
- Wood, P.D.P. Algebraic Model of the Lactation Curve in Cattle. Nature 1967, 216, 164–165. [Google Scholar] [CrossRef]
- George, P.D. A Deterministic Model of Net Nutrient Requirements for the Beef Cow; Cornell University: Ithaca, NY, USA, 1984. [Google Scholar]
- Fox, D.G.; Sniffen, C.J.; O’Connor, J.D. Adjusting Nutrient Requirements of Beef Cattle for Animal and Environmental Variations. J. Anim. Sci. 1988, 66, 1475–1495. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Fox, D.G. Predicting milk and forage intake of nursing calves. J. Anim. Sci. 2009, 87, 3380–3391. [Google Scholar] [CrossRef]
- PennState Extension. Whole Milk Considerations. Available online: https://extension.psu.edu/whole-milk-considerations (accessed on 10 May 2024).
- National Research Council. Nutrient Requirements of Beef Cattle: Seventh Revised Edition: Update 2000; The National Academies Press: Washington, DC, USA, 2000; p. 248. [Google Scholar]
- Maine CDC. Action levels for PFOS in beef for use in determining whether beef at a farm is adulterated. In Memorandum to the Department of Agriculture, Conservation and Forestry; Maine Center for Disease Control & Prevention: Augusta, ME, USA, 2020. [Google Scholar]
- European Union. COMMISSION REGULATION (EU) 2022/2388 of 7 December 2022 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Perfluoroalkyl Substances in Certain Foodstuffs; European Union: Brussels, Belgium, 2022. [Google Scholar]
- USDA. 5 Area Weekly Weighted Average Direct Slaughter Cattle (January 2023–December 2024). Available online: https://mymarketnews.ams.usda.gov/viewReport/2477 (accessed on 13 January 2025).
- University of Maine Cooperative Extension. Bulletin #1071, What to Expect When Buying a Freezer Beef. Available online: https://extension.umaine.edu/publications/1071e/ (accessed on 13 January 2025).
- Federal Reserve Economic Data. Table Data—Average Price: Ground Beef, 100% Beef (Cost per Pound/453.6 Grams) in U.S. City Average. Available online: https://fred.stlouisfed.org/data/APU0000703112 (accessed on 7 February 2025).
- USDA National Agricultural Statistics Service. 2024 State Agriculture Overview Maine. Available online: https://www.nass.usda.gov/Quick_Stats/Ag_Overview/stateOverview.php?state=MAINE (accessed on 13 January 2025).
- USDA National Agricultural Statistics Service. Prices Received: Cattle Prices Received by Month, US. Available online: https://www.nass.usda.gov/Charts_and_Maps/Agricultural_Prices/priceca.php (accessed on 13 January 2025).
- Lupton, S.J.; Dearfield, K.L.; Johnston, J.J.; Wagner, S.; Huwe, J.K. Perfluorooctane Sulfonate Plasma Half-Life Determination and Long-Term Tissue Distribution in Beef Cattle (Bos taurus). J. Agric. Food Chem. 2015, 63, 10988–10994. [Google Scholar] [CrossRef]
- U.S. EPA. Draft Sewage Sludge Risk Assessment for Perfluorooctanoic Acid (PFOA) CASRN 335-67-1 and Perfluorooctane Sulfonic Acid (PFOS) CASRN 1763-23-1; U.S. EPA: Washington, DC, USA, 2025.
- Johnston, J.J.; Ebel, E.D.; Williams, M.S.; Esteban, E.; Lupton, S.J.; Scholljegerdes, E.J.; Ivey, S.L.; Powell, M.R.; Smith, D.J. Blood-Based Ante-Mortem Method for Estimating PFOS in Beef from Contaminated Dairy Cattle. ACS Agric. Sci. Technol. 2023, 3, 835–844. [Google Scholar] [CrossRef]
- U.S. EPA. IRIS Toxicological Review of Perfluorodecanoic Acid (PFDA) and Related Salts; U.S. EPA: Washington, DC, USA, 2024.
- Chou, W.-C.; Tell, L.A.; Baynes, R.E.; Davis, J.L.; Cheng, Y.-H.; Maunsell, F.P.; Riviere, J.E.; Lin, Z. Development and application of an interactive generic physiologically based pharmacokinetic (igPBPK) model for adult beef cattle and lactating dairy cows to estimate tissue distribution and edible tissue and milk withdrawal intervals for per- and polyfluoroalkyl substances (PFAS). Food Chem. Toxicol. 2023, 181, 114062. [Google Scholar] [CrossRef]
- German Federal Institute for Risk Assessment. From the Trough to the Plate—Digitally Calculated. Available online: https://www.bfr.bund.de/en/press_information/2023/22/from_the_trough_to_the_plate___digitally_calculated-313418.html (accessed on 3 January 2024).
- Johnson, J.D.; Gibson, S.J.; Ober, R.E. Cholestyramine-enhanced fecal elimination of carbon-14 in rats after administration of ammonium [14C]perfluorooctanoate or potassium [14C]perfluorooctanesulfonate. Fundam. Appl. Toxicol. 1984, 4, 972–976. [Google Scholar] [CrossRef]
- Ramos, P.; Ashworth, D.J. Per- and poly-fluoroalkyl substances in agricultural contexts and mitigation of their impacts using biochar: A review. Sci. Total Environ. 2024, 927, 172275. [Google Scholar] [CrossRef]
- U.S. EPA. Method 1633, Revision A Analysis of Per- and Polyfluoroalkyl Substances (PFAS) in Aqueous, Solid, Biosolids, and Tissue Samples by LC-MS/MS; U.S. EPA: Washington, DC, USA, 2024.
- MDIFW and Maine CDC. PFAS in Deer and Turkeys in the Fairfield Area, Maine; Maine Center for Disease Control & Prevention: Augusta, ME, USA, 2024.
- Fries, G.F.; Marrow, G.S.; Snow, P.A. Soil Ingestion by Dairy Cattle. J. Dairy Sci. 1982, 65, 611–618. [Google Scholar] [CrossRef]
- Oklahoma State University Extension. Nutrient Requirements of Beef Cattle E-974; Oklahoma State University: Stillwater, OK, USA, 2023. [Google Scholar]


| Location | Date(s) | n a | Cattle Age (Years) | Cattle Type | Tissues Sampled | Data Application |
|---|---|---|---|---|---|---|
| Study site | 7 December 2021 | 1 | 2 | Beef | Muscle | Exposure model: 2021 scenario |
| Study site | 7 March, 18 March, 14 April, and 26 May 2022 | 5 | 2 | Beef | Muscle, Serum, Plasma | Exposure model: 2022 scenario |
| Study site | 28 June and 18 September 2023 | 6 | 1.5 | Beef | Serum | Exposure model: 2023 scenario |
| Study site | 30 April and 5 September 2024 | 8 | 1.5 | Beef | Serum | Exposure model: 2024 scenario |
| Offsite | 11 October 2022 | 5 | 10 | Beef | Muscle, Serum, Plasma | Model parameterization: muscle partition coefficient |
| Offsite | 25 November 2020 and 3 September 2021 | 19 | NR b | Beef, Dairy | Muscle, Plasma | Model parameterization: muscle partition coefficient |
| Offsite | 13 October 2022 | 3 | 2-4 | Beef | Muscle, Serum | Model parameterization: muscle partition coefficient |
| Offsite | 4 November 2022 | 12 | NR b | Beef, Dairy | Serum, Plasma | Model parameterization: serum/plasma ratio |
| Exposure Scenario | Exposure History |
|---|---|
| 2021 | This animal spent two summers on pasture and two winters on stored feed prior to slaughter in April 2021 at 2 years of age. Second-cut hay was fed during its second winter only. The primary drinking water source was untreated well water. |
| 2022 | Cattle spent their first and second summers on pasture and first winter on stored feed. For the second winter, cattle were fed stored feed for 2 months, followed by an intervention in which clean feed was purchased to allow cattle to depurate for 4 months (grab sampling of purchased feed confirmed PFOS and PFDA were not present above detection limits). First- and second-cut hay was used as feed in both winters. Cattle were provided with untreated well water until February 2022, after which all drinking water was treated. They were slaughtered at 2 years of age. |
| 2023 | Cattle spent two summers on pasture and one winter on stored feed. No second-cut hay was fed during this time. During their second summer on pasture, access to contaminated supplemental hay was provided 2–3 days prior to the second serum sampling. Their primary drinking water was treated well water. |
| 2024 | Cattle spent two summers on pasture and one winter on stored feed. No second-cut hay was fed during this time. Their primary drinking water was treated well water. |
| Analyte | Parameter | Units | Mean | SD | Reference |
|---|---|---|---|---|---|
| PFOS | DT50 | days | 74.1 | 13.4 | Drew et al. [29] |
| PM | unitless | 0.08 | 0.02 | Mikkonen et al. [30], Maine farm data | |
| Pmilk | unitless | 0.015 | 0.004 | Mikkonen et al. [30] | |
| Vd | L/kg | 0.085 | 0.01 | Mikkonen et al. [30] | |
| PFDA | DT50 | days | 60.4 | 10.4 | Drew et al. [29] |
| PM | unitless | 0.08 | 0.02 | Maine farm data | |
| Pmilk | unitless | 0.015 a | 0.004 | Mikkonen et al. [30] | |
| Vd | L/kg | 0.085 a | 0.01 | Mikkonen et al. [30] |
| Hay Field # a (Acres) | PFOS | PFDA | |||
|---|---|---|---|---|---|
| n b | Mean ± SD | Range | Mean ± SD | Range | |
| 2 (15) | 3 | 3.44 ± 1.47 | 1.83–4.71 | 18.64 ± 4.72 | 14.93–23.96 |
| 3 (1) | 1 | 6.89 | 24.85 | ||
| 6 (8) | 3 | 5.11 ± 2.59 | 2.13–6.82 | 18.47 ± 1.12 | 17.21–19.38 |
| 7 (13) | 8 | 8.47 ± 3.88 | 3.33–15.68 | 11.87 ± 3.18 | 8.07–16.09 |
| 8 (14) | 5 | 7.11 ± 3.82 | 0.35–9.76 | 14.21 ± 7.70 | 0.94–20.84 |
| Sample Group | n | PFAS | Tissue | Measured a | Modeled b | |||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | % Error c | ||||
| 2021 | 1 | PFOS | Muscle | 2.87 | 2.33 ± 1.12 | 0.95–6.40 | −19% | |
| PFDA | Muscle | 4.29 | 3.61± 1.73 | 1.47–9.90 | −16% | |||
| 2022 | 5 | PFOS | Muscle | 2.25 ± 0.29 | 1.95–2.71 | 1.48 ± 0.73 | 0.57–4.09 | −34% |
| PFOS | Serum | 24.6 ± 2.69 | 20.7–28.2 | 17.6 ± 6.53 | 7.49–34.3 | −29% | ||
| PFDA | Muscle | 3.30 ± 0.61 | 2.82–4.35 | 1.56 ± 0.78 | 0.58–4.32 | −53% | ||
| PFDA | Serum | 35.8 ± 5.41 | 29.6–43.9 | 18.6 ± 6.97 | 7.62–36.3 | −48% | ||
| 2023 | 6 | PFOS | Serum | 12.2 ± 2.24 | 9.84–14.3 | 20.3 ± 7.78 | 7.91–39.9 | 67% |
| PFDA | Serum | 14.6 ± 3.93 | 10.1–17.5 | 25.2 ± 9.87 | 9.25–49.5 | 73% | ||
| 2024 | 8 | PFOS | Serum | 89.1 ± 7.97 | 74.9–98.8 | 150 ± 52.9 | 70.3–295 | 68% |
| PFDA | Serum | 202 ± 18.6 | 179–235 | 256 ± 90.5 | 121–502 | 27% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astmann, B.A.; Mikkonen, A.T.; Simones, T.L.; Flanagan, M.; Pfaehler, D.; Lenov, I.; Smith, A.E. Application of a Dynamic Exposure Population Toxicokinetic Model for Perfluorooctane Sulfonic Acid (PFOS) and Extension to Perfluorodecanoic Acid (PFDA) at a North American Beef Cattle Farm with a History of Biosolids Land Application. Toxics 2025, 13, 541. https://doi.org/10.3390/toxics13070541
Astmann BA, Mikkonen AT, Simones TL, Flanagan M, Pfaehler D, Lenov I, Smith AE. Application of a Dynamic Exposure Population Toxicokinetic Model for Perfluorooctane Sulfonic Acid (PFOS) and Extension to Perfluorodecanoic Acid (PFDA) at a North American Beef Cattle Farm with a History of Biosolids Land Application. Toxics. 2025; 13(7):541. https://doi.org/10.3390/toxics13070541
Chicago/Turabian StyleAstmann, Barbara A., Antti T. Mikkonen, Thomas L. Simones, Meghan Flanagan, Duncan Pfaehler, Ivan Lenov, and Andrew E. Smith. 2025. "Application of a Dynamic Exposure Population Toxicokinetic Model for Perfluorooctane Sulfonic Acid (PFOS) and Extension to Perfluorodecanoic Acid (PFDA) at a North American Beef Cattle Farm with a History of Biosolids Land Application" Toxics 13, no. 7: 541. https://doi.org/10.3390/toxics13070541
APA StyleAstmann, B. A., Mikkonen, A. T., Simones, T. L., Flanagan, M., Pfaehler, D., Lenov, I., & Smith, A. E. (2025). Application of a Dynamic Exposure Population Toxicokinetic Model for Perfluorooctane Sulfonic Acid (PFOS) and Extension to Perfluorodecanoic Acid (PFDA) at a North American Beef Cattle Farm with a History of Biosolids Land Application. Toxics, 13(7), 541. https://doi.org/10.3390/toxics13070541








