NOX2/NLRP3-Inflammasome-Dependent Microglia Activation Promotes As(III)-Induced Learning and Memory Impairments in Developmental Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. The Animal Model and Treatment
2.2. Cell Culture and Treatment
2.3. Hematoxylin–Eosin (HE) Staining
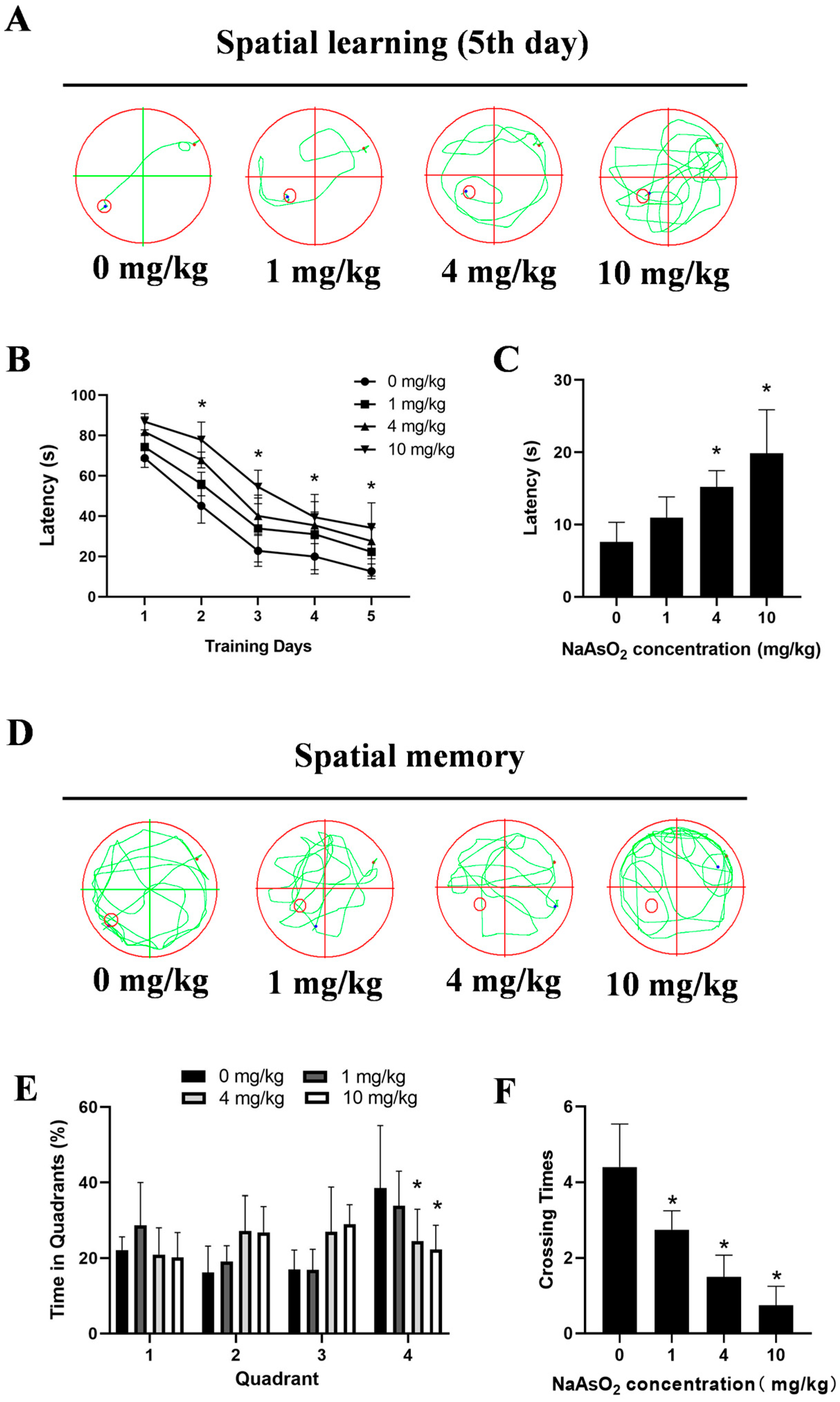
2.4. The Morris Water Maze (MWM) Test
2.5. Western Blot
2.6. Immunofluorescence Staining
2.7. Real-Time qPCR
2.8. NOX2 siRNA Transfection
2.9. Statistical Analysis
3. Results
3.1. Gestational/Lactational NaAsO2 Exposure Induces Learning and Memory Impairments in Offspring Rats
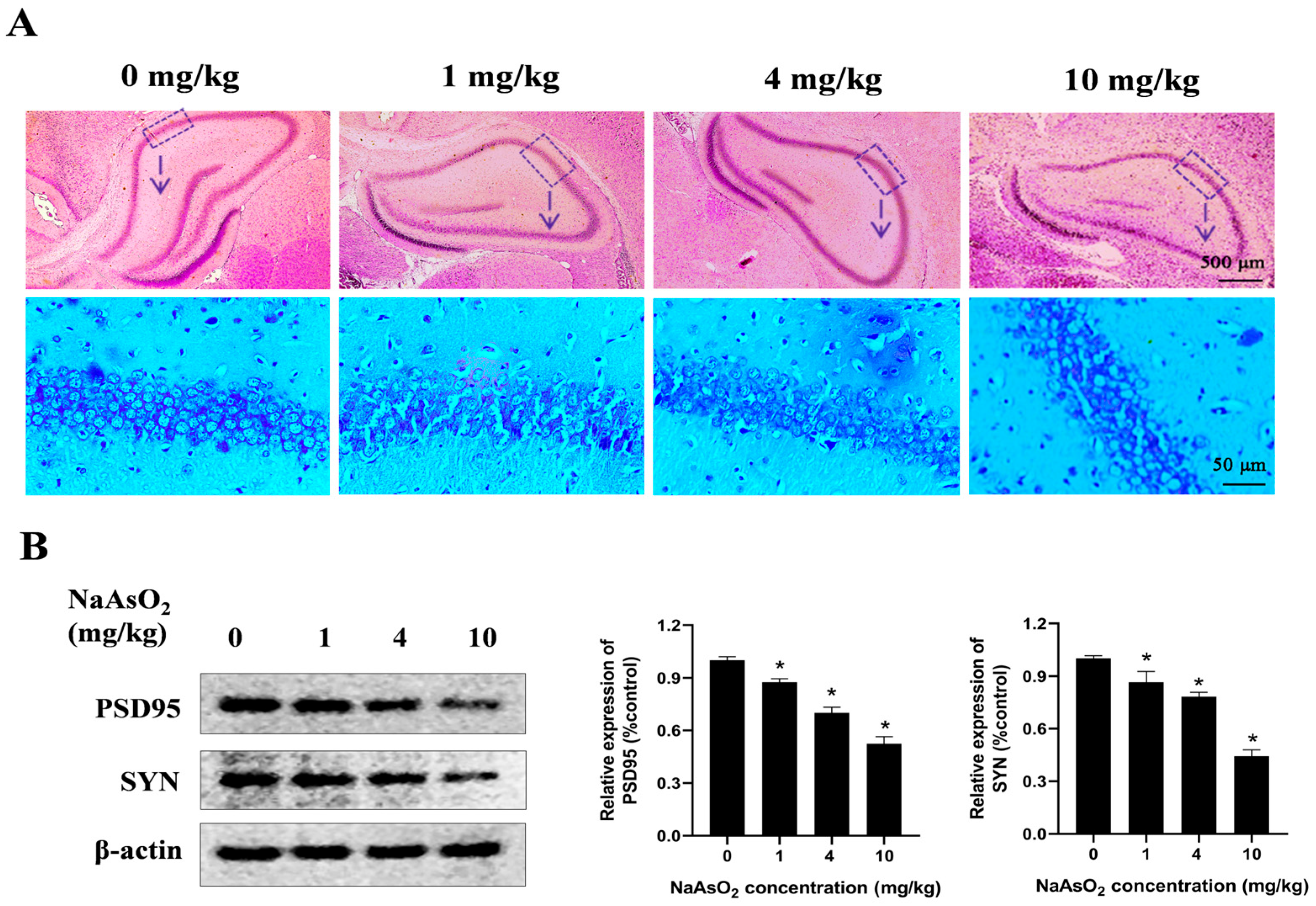
3.2. Gestational/Lactational NaAsO2 Exposure Causes Microglial Activation in the Hippocampus of Offspring Rats
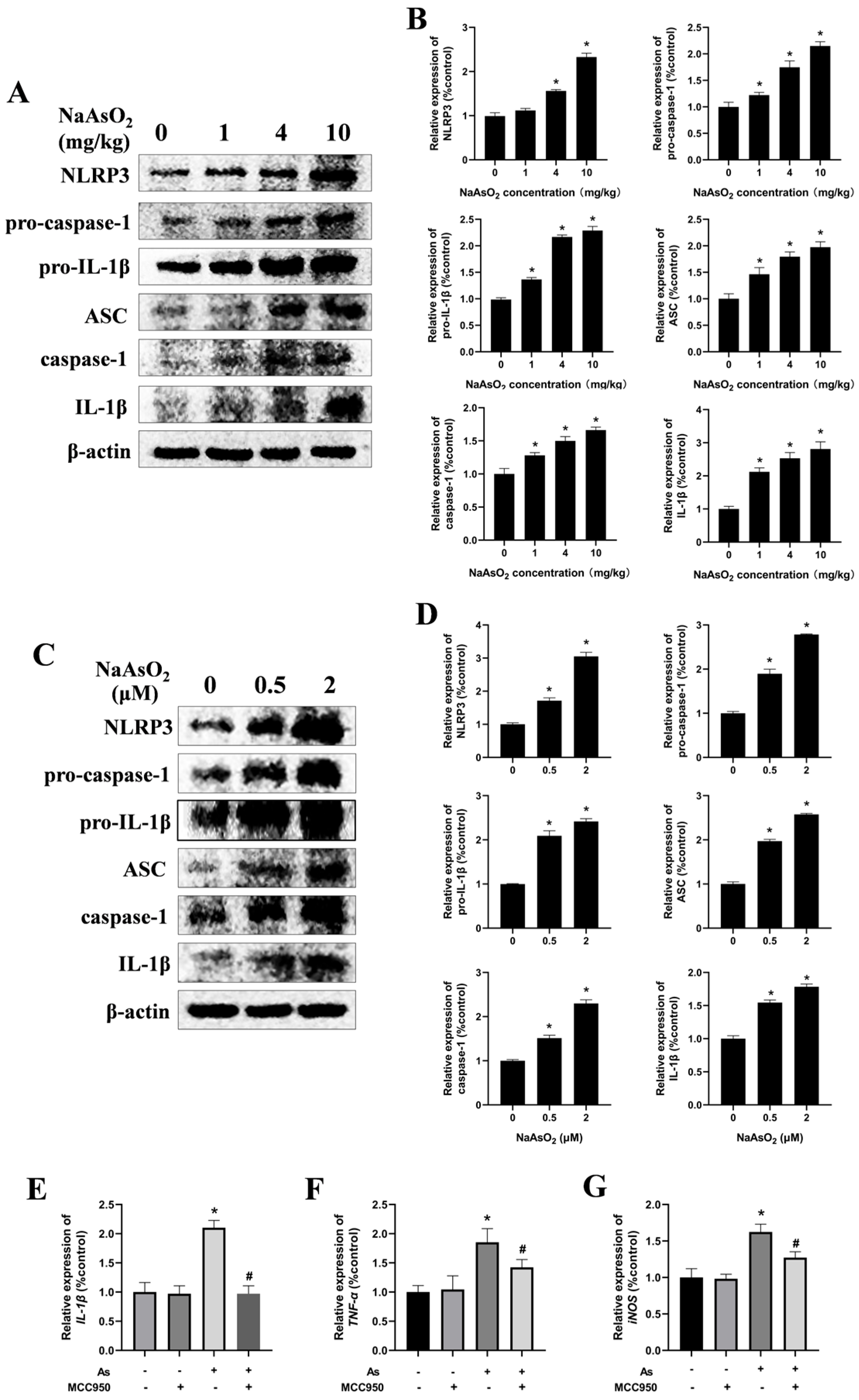
3.3. The NLRP3 Inflammasome Contributes to NaAsO2-Induced Activation of the Microglia
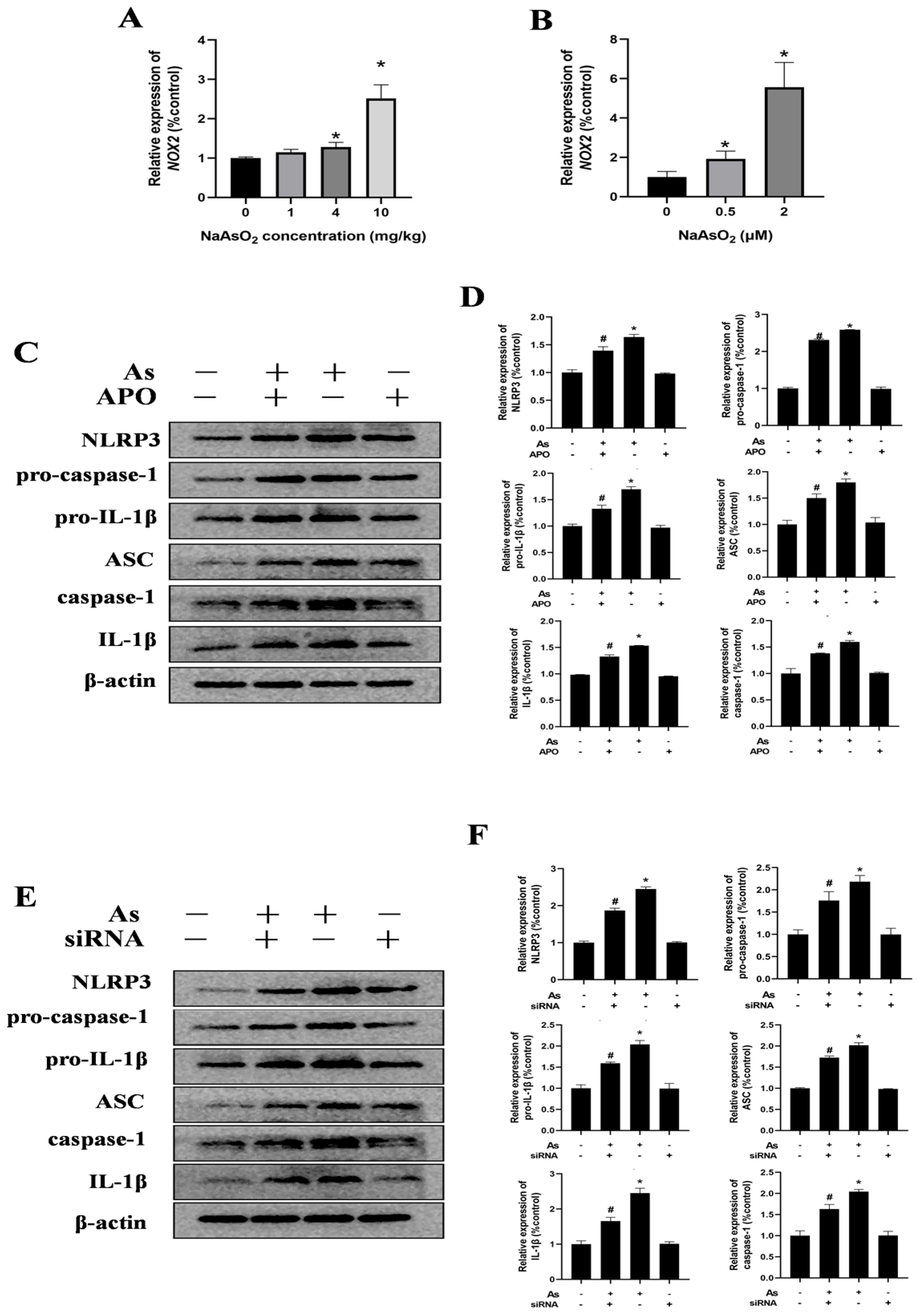
3.4. NOX2 Is Key to NLRP3 Inflammasome Activation in NaAsO2-Exposed Microglia
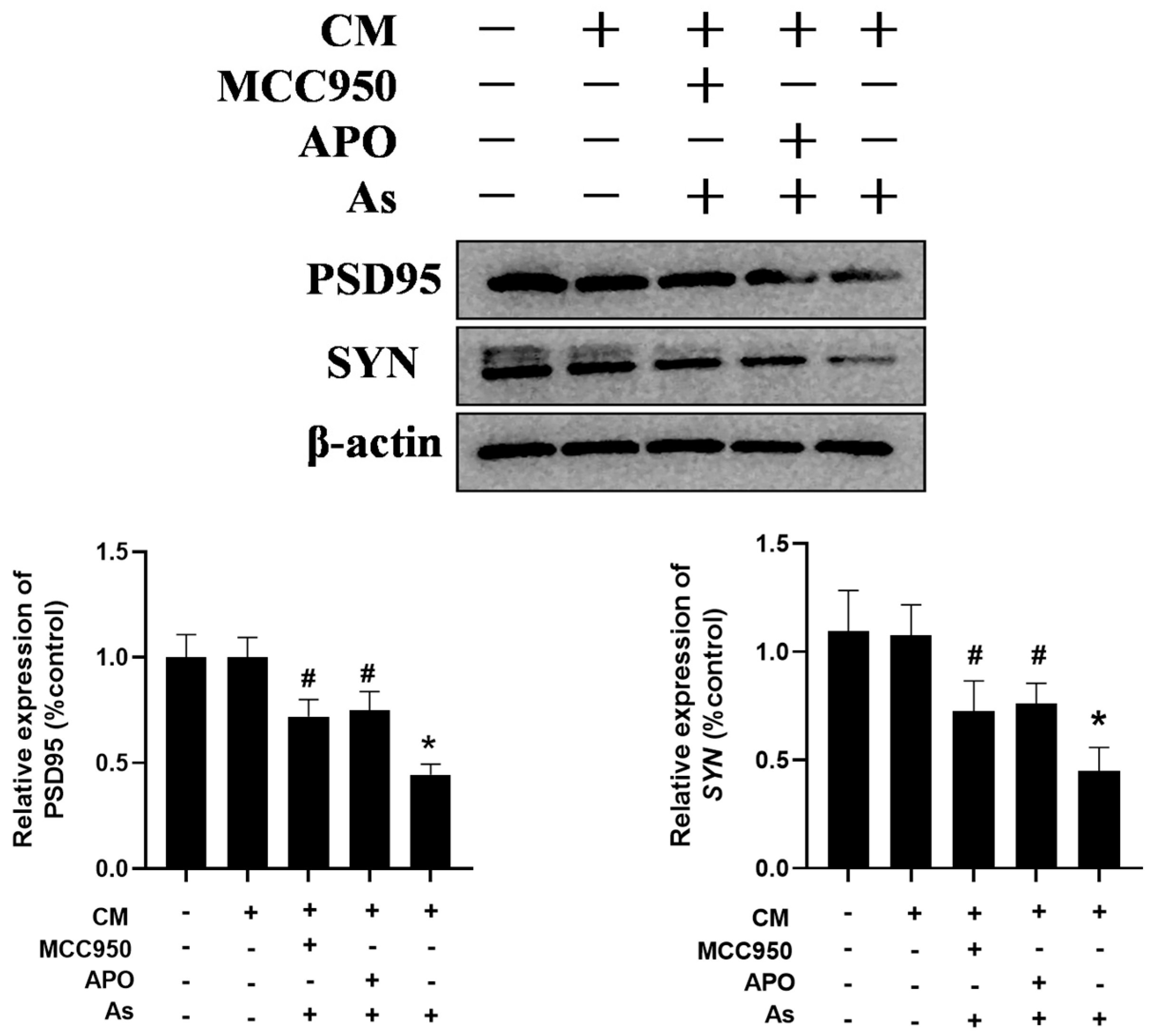
3.5. Inhibition of the Microglial NLRP3 Inflammasome or NOX2 Mitigates NaAsO2-Induced Impairment in Hippocampal HT22 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Rahman, M.M.; Mise, N.; Sikder, M.T.; Ichihara, G.; Uddin, M.K.; Kurasaki, M.; Ichihara, S. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ. Pollut. 2021, 289, 117940. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.D.; Lam, W.L. Health Effects associated with pre- and perinatal exposure to arsenic. Front. Genet. 2021, 12, 664717. [Google Scholar] [CrossRef]
- Sanders, A.P.; Desrosiers, T.A.; Warren, J.L.; Herring, A.H.; Enright, D.; Olshan, A.F.; Meyer, R.E.; Fry, R.C. Association between arsenic, cadmium, manganese, and lead levels in private wells and birth defects prevalence in North Carolina: A semi-ecologic study. BMC Public Health 2014, 14, 955. [Google Scholar] [CrossRef]
- Farzan, S.F.; Karagas, M.R.; Chen, Y. In utero and early life arsenic exposure in relation to long-term health and disease. Toxicol. Appl. Pharmacol. 2013, 272, 384–390. [Google Scholar] [CrossRef]
- Jin, Y.; Xi, S.; Li, X.; Lu, C.; Li, G.; Xu, Y.; Qu, C.; Niu, Y.; Sun, G. Arsenic speciation transported through the placenta from mother mice to their newborn pups. Environ. Res. 2006, 101, 349–355. [Google Scholar] [CrossRef]
- Cervantes, G.I.V.; Esquivel, D.F.G.; Ortega, D.R.; Ayala, T.B.; Chávez, L.A.R.; López-López, H.E.; Salazar, A.; Flores, I.; Pineda, B.; Gómez-Manzo, S.; et al. Mechanisms associated with cognitive and behavioral impairment induced by arsenic exposure. Cells 2023, 12, 2537. [Google Scholar] [CrossRef]
- Soler-Blasco, R.; Llop, S.; Riutort-Mayol, G.; Lozano, M.; Vallejo-Ortega, J.; Murcia, M.; Ballester, F.; Irizar, A.; Andiarena, A.; Fernandez-Jimenez, N.; et al. Genetic susceptibility to neurotoxicity related to prenatal inorganic arsenic exposure in young spanish children. Environ. Sci. Technol. 2023, 57, 15366–15378. [Google Scholar] [CrossRef]
- Parajuli, R.P.; Fujiwara, T.; Umezaki, M.; Watanabe, C. Association of cord blood levels of lead, arsenic, and zinc with neurodevelopmental indicators in newborns: A birth cohort study in Chitwan Valley, Nepal. Environ. Res. 2013, 121, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Vahter, M.; Skröder, H.; Rahman, S.M.; Levi, M.; Derakhshani Hamadani, J.; Kippler, M. Prenatal and childhood arsenic exposure through drinking water and food and cognitive abilities at 10 years of age: A prospective cohort study. Environ. Int. 2020, 139, 105723. [Google Scholar] [CrossRef] [PubMed]
- Hovens, I.B.; van Leeuwen, B.L.; Nyakas, C.; Heineman, E.; van der Zee, E.A.; Schoemaker, R.G. Postoperative cognitive dysfunction and microglial activation in associated brain regions in old rats. Neurobiol. Learn. Mem. 2015, 118, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Y.; Tu, J.B.; Ran, R.T.; Zhang, W.X.; Tan, Q.; Tang, P.; Kuang, T.; Cheng, S.Q.; Chen, C.Z.; Jiang, X.J.; et al. Using the metabolome to understand the mechanisms linking chronic arsenic exposure to microglia activation, and learning and memory impairment. Neurotox. Res. 2021, 39, 720–739. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Bandyopadhyay, S. A comprehensive review of arsenic-induced neurotoxicity: Exploring the role of glial cell pathways and mechanisms. Chemosphere 2025, 372, 144046. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Liu, X.; Zhang, R.; Wang, X.; Zhang, Q.; Wei, Y.; Fang, F.; Yuan, Y.; Zhou, Q.; et al. TNF-α derived from arsenite-induced microglia activation mediated neuronal necroptosis. Ecotoxicol. Environ. Saf. 2022, 236, 113468. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Y.; Chen, X.; Jiang, D.; Zhang, F.; Guo, Y.; Hu, B.; Xu, G.; Peng, S.; Wu, L.; et al. NLRP3 inflammasome in cognitive impairment and pharmacological properties of its inhibitors. Transl. Neurodegener. 2023, 12, 49. [Google Scholar] [CrossRef]
- Heneka, M.T.; McManus, R.M.; Latz, E. Author Correction: Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 2019, 20, 187. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.Y.; Kim, J.B.; Kim, Y.; Park, S.M.; Chang, K.A. GPR40 agonist ameliorates neurodegeneration and motor impairment by regulating NLRP3 inflammasome in Parkinson’s disease animal models. Pharmacol. Res. 2024, 209, 107432. [Google Scholar] [CrossRef]
- Xu, W.; Huang, Y.; Zhou, R. NLRP3 inflammasome in neuroinflammation and central nervous system diseases. Cell Mol. Immunol. 2025, 22, 341–355. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Z.; Jin, J.; Zhao, C.; Zhao, B.; Liu, Y. NLRP3-GSDMD-dependent IL-1β secretion from microglia mediates learning and memory impairment in a chronic intermittent hypoxia-induced mouse model. Neuroscience 2024, 539, 51–65. [Google Scholar] [CrossRef]
- Al-Shami, A.S.; Abd Elkader, H.A.E.; Moussa, N.; Essawy, A.E.; Haroun, M. Early-life bisphenol A exposure causes neuronal pyroptosis in juvenile and adult male rats through the NF-κB/IL-1β/NLRP3/caspase-1 signaling pathway: Exploration of age and dose as effective covariates using an in vivo and in silico modeling approach. Mol. Cell Biochem. 2025, 480, 2301–2330. [Google Scholar] [CrossRef]
- Pajarillo, E.; Kim, S.; Digman, A.; Dutton, M.; Son, D.S.; Aschner, M.; Lee, E. The role of microglial LRRK2 kinase in manganese-induced inflammatory neurotoxicity via NLRP3 inflammasome and RAB10-mediated autophagy dysfunction. J. Biol. Chem. 2023, 299, 104879. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, X.; Xu, C.; Gao, M.; Wang, S.; Zhang, J.; Tong, H.; Wang, L.; Han, Y.; Cheng, N.; et al. Inhibiting NLRP3 inflammasome activation prevents copper-induced neuropathology in a murine model of Wilson’s disease. Cell Death Dis. 2021, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- de Melo, F.M.; Kawasaki, K.; Sellani, T.A.; Bonifácio, B.S.; Mortara, R.A.; Toma, H.E.; de Melo, F.M.; Rodrigues, E.G. Quantum-dot-based iron oxide nanoparticles activate the NLRP3 inflammasome in murine Bone marrow-derived dendritic Cells. Nanomaterials 2022, 12, 3145. [Google Scholar] [CrossRef]
- Aschner, M.; Skalny, A.V.; Martins, A.C.; Tizabi, Y.; Zaitseva, I.P.; Santamaria, A.; Lu, R.; Gluhcheva, Y.Y.; Tinkov, A.A. The role of NLRP3 inflammasome activation in proinflammatory and cytotoxic effects of metal nanoparticles. Arch. Toxicol. 2025, 99, 1287–1314. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, F.; Zhou, Q.; Xu, Y.; Li, Y.; Huang, D.; Chen, L.; Liu, A.; Zou, F.; Meng, X. NLRP3 activation in microglia contributes to learning and memory impairment induced by chronic lead exposure in mice. Toxicol. Sci. 2023, 191, 179–191. [Google Scholar] [CrossRef]
- Yan, N.; Wang, Z.; Li, Z.; Zheng, Y.; Chang, N.; Xu, K.; Wang, Q.; Duan, X. Arsenic Exposure Induces Neuro-immune Toxicity in the Cerebral Cortex and the Hippocampus via Neuroglia and NLRP3 Inflammasome Activation in C57BL/6 Mice. Biol. Trace Elem. Res. 2024, 202, 4554–4566. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; Fan, J.; Chen, Y.; Wang, H.; Ge, Y.; Liang, H.; Li, W.; Liu, H.; Lv, Z.; et al. The role of ROS/p38 MAPK/NLRP3 inflammasome cascade in arsenic-induced depression-/anxiety-like behaviors of mice. Ecotoxicol. Environ. Saf. 2023, 261, 115111. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Liu, P.; Zhou, Y.; Ding, X.; Liu, H.; Yang, J. Prenatal Sevoflurane Exposure Impairs the Learning and Memory of Rat Offspring via HMGB1-Induced NLRP3/ASC Inflammasome Activation. ACS Chem. Neurosci. 2023, 14, 699–708. [Google Scholar] [CrossRef]
- Wang, X.; Tian, S.; Qu, Z.; Meng, R.; Ni, G.; Liu, M.; Cao, H. Investigating the protective effect of hydroxylated fullerenes on cognitive function in rats with temporal lobe epilepsy. Sci. Rep. 2025, 15, 14142. [Google Scholar] [CrossRef]
- Ji, S.; Dong, Y.; Wang, Z.; Zhu, R.; Jiang, Y.; Li, S.; Ma, X. Edaravone attenuates sleep deprivation-induced memory deficits by inhibiting oxidative stress and neuroinflammation in murine models. Biomedicines 2025, 13, 1047. [Google Scholar] [CrossRef]
- Nicoll, R.A.; Schulman, H. Synaptic memory and CaMKII. Physiol. Rev. 2023, 103, 2877–2925. [Google Scholar] [CrossRef]
- Wang, H.; Ma, L.; Su, W.; Liu, Y.; Xie, N.; Liu, J. NLRP3 inflammasome in health and disease (Review). Int. J. Mol. Med. 2025, 55, 48. [Google Scholar] [CrossRef]
- Henn, A.; Lund, S.; Hedtjärn, M.; Schrattenholz, A.; Pörzgen, P.; Leist, M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. Altex 2009, 26, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Peng, Y.; Yan, H. Commentary: Neuroinflammatory in vitro cell culture models and the potential applications for neurological disorders. Front. Pharmacol. 2021, 12, 792614. [Google Scholar] [CrossRef]
- Murumulla, L.; Bandaru, L.J.M.; Challa, S. Heavy metal mediated progressive degeneration and its noxious effects on brain microenvironment. Biol. Trace Elem. Res. 2024, 202, 1411–1427. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Liu, J.; Zhang, X.; Huang, A.; Yu, X.; Wu, K.; Huang, Y. Association between heavy metals exposure and risk of attention deficit hyperactivity disorder (ADHD) in children: A systematic review and meta-analysis. Eur. Child. Adolesc. Psychiatry 2025, 34, 921–941. [Google Scholar] [CrossRef]
- Bjørklund, G.; Tippairote, T.; Rahaman, M.S.; Aaseth, J. Developmental toxicity of arsenic: A drift from the classical dose-response relationship. Arch. Toxicol. 2020, 94, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ge, X.; Yu, S.; Cheng, Q. Achyranthes bidentata polypeptide alleviates neurotoxicity of lipopolysaccharide-activated microglia via PI3K/Akt dependent NOX2/ROS pathway. Ann. Transl. Med. 2021, 9, 1522. [Google Scholar] [CrossRef]
- Cai, Q.; Shen, L.; Zhang, X.; Zhang, Z.; Wang, T. The IRE1-XBP1 axis regulates NLRP3 inflammasome-mediated microglia activation in hypoxic ischemic encephalopathy. Crit. Rev. Immunol. 2025, 45, 55–64. [Google Scholar] [CrossRef]
- Santos-García, I.; Bascuñana, P.; Brackhan, M.; Villa, M.; Eiriz, I.; Brüning, T.; Pahnke, J. The ABC transporter A7 modulates neuroinflammation via NLRP3 inflammasome in Alzheimer’s disease mice. Alzheimers Res. Ther. 2025, 17, 30. [Google Scholar] [CrossRef]
- Chen, T.; Han, T.; Miao, Y.; Yan, L.; Liu, Z.; Dong, H.; Cheng, T.; Liu, Y.; Yang, Y.; Fei, S.; et al. Cadmium exposure induced spleen inflammation by activating the MAPK/NF-κB/ NLRP3 signaling pathway and the intervention effect of astilbin. Vet. Immunol. Immunopathol. 2025, 281, 110889. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, Y.; Sun, S.; Zhao, P.; Zhou, X.; Liang, C.; Ma, Y.; Li, S.; Zhu, X.; Hao, X.; et al. NLRP3 inflammasome-mediated pyroptosis involvement in cadmium exposure-induced cognitive deficits via the Sirt3-mtROS axis. Sci. Total Environ. 2023, 903, 166478. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yao, Y.X.; Lu, X.; Peng, K.; He, Y.Z.; Liu, Z.B.; Zhao, H.; Wang, H.; Xu, D.X.; Tan, Z.X. Short-term respiratory cadmium exposure partially activates pulmonary NLRP3 inflammasome by inducing ferroptosis in mice. Ecotoxicol. Environ. Saf. 2024, 285, 117106. [Google Scholar] [CrossRef]
- Deng, A.; Yi, M.; Wang, Y.; Mo, P.; Huang, K.; Xie, P.; Fan, S.; Xue, M.; Ding, X.; Wang, Y.; et al. Artichoke water extract protects against Lead-induced hepatotoxicity by activating Nrf2 signaling and inhibiting NLRP3/caspase-1/GSDMD-mediated pyroptosis. J. Ethnopharmacol. 2025, 346, 119654. [Google Scholar] [CrossRef]
- Tuncer, S.; Akarsu, S.A.; Küçükler, S.; Gür, C.; Kandemir, F.M. Effects of sinapic acid on lead acetate-induced oxidative stress, apoptosis and inflammation in testicular tissue. Environ. Toxicol. 2023, 38, 2656–2667. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Zhong, S.; Zhang, X.; Yang, J.; Zhou, Q.; Wang, D.; Chang, X.; Wang, H. Fecal microbiome transplantation alleviates manganese-induced neurotoxicity by altering the composition and function of the gut microbiota via the cGAS-STING/NLRP3 pathway. Sci. Total Environ. 2024, 951, 175681. [Google Scholar] [CrossRef]
- Sarkar, S.; Rokad, D.; Malovic, E.; Luo, J.; Harischandra, D.S.; Jin, H.; Anantharam, V.; Huang, X.; Lewis, M.; Kanthasamy, A.; et al. Manganese activates NLRP3 inflammasome signaling and propagates exosomal release of ASC in microglial cells. Sci. Signal 2019, 12, eaat9900. [Google Scholar] [CrossRef] [PubMed]
- Bujko, K.; Adamiak, M.; Konopko, A.; Chumak, V.; Ratajczak, J.; Brzezniakiewicz-Janus, K.; Kucia, M.; Ratajczak, M.Z. Defect in migration of HSPCs in nox-2 deficient mice explained by impaired activation of nlrp3 inflammasome and impaired formation of membrane lipid rafts. Stem Cell Rev. Rep. 2025, 21, 45–58. [Google Scholar] [CrossRef]
- Sun, B.; Wang, X.; Ji, Z.; Wang, M.; Liao, Y.P.; Chang, C.H.; Li, R.; Zhang, H.; Nel, A.E.; Xia, T. NADPH oxidase-dependent NLRP3 inflammasome activation and its important role in lung fibrosis by multiwalled carbon nanotubes. Small 2015, 11, 2087–2097. [Google Scholar] [CrossRef]
- Han, Y.; Lu, Y.; Li, X.; Niu, X.; Chang, A.K.; Yang, Z.; Li, X.; He, X.; Bi, X. Novel organoselenides (NSAIDs-Se derivatives) protect against LPS-induced inflammation in microglia by targeting the NOX2/NLRP3 signaling pathway. Int. Immunopharmacol. 2021, 101, 108377. [Google Scholar] [CrossRef]
- Ahmed, G.; Rahaman, M.S.; Perez, E.; Khan, K.M. Associations of environmental exposure to arsenic, manganese, lead, and cadmium with Alzheimer’s Disease: A review of recent evidence from mechanistic studies. J. Xenobiot. 2025, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Frisbie, S.H.; Ortega, R.; Maynard, D.M.; Sarkar, B. The concentrations of arsenic and other toxic elements in Bangladesh’s drinking water. Environ. Health Perspect. 2002, 110, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.K.; Shin, E.J.; Kim, D.J.; Tran, H.Q.; Jeong, J.H.; Jang, C.G.; Ottersen, O.P.; Nah, S.Y.; Hong, J.S.; Nabeshima, T.; et al. PKCδ-dependent p47phox activation mediates methamphetamine-induced dopaminergic neurotoxicity. Free Radic. Biol. Med. 2018, 115, 318–337. [Google Scholar] [CrossRef]
- Chillé, D.; Mollica-Nardo, V.; Giuffrè, O.; Ponterio, R.C.; Saija, F.; Sponer, J.; Trusso, S.; Cassone, G.; Foti, C. Binding of arsenic by common functional groups: An experimental and quantum-mechanical study. Appl. Sci. 2022, 12, 3210. [Google Scholar] [CrossRef]
- Gailer, J.; Ruprecht, L.; Reitmeir, P.; Benker, B.; Schramel, P. Mobilization of exogenous and endogenous selenium to bile after the intravenous administration of environmentally relevant doses of arsenite to rabbits. Appl. Organomet. Chem. 2004, 18, 670–675. [Google Scholar] [CrossRef]
- Gailer, J.; George, G.N.; Pickering, I.J.; Prince, R.C.; Ringwald, S.C.; Pemberton, J.E.; Glass, R.S.; Younis, H.S.; DeYoung, D.W.; Aposhian, H.V. A metabolic link between arsenite and selenite: The seleno-bis (S-glutathionyl) arsinium ion. J. Am. Chem. Soc. 2000, 122, 4637–4639. [Google Scholar] [CrossRef]

| Forward Primer | Reverse Primer | |
|---|---|---|
| IL-1β | CCCAAGCACCTTCTTTTCCTT | TCAGACAGCACGAGGCATTT |
| TNF-α | CAAGAGCCCTTGCCCTAAGG | GGACTCCGTGATGTCTAAGTACTT |
| iNOS | CCTCACCTACTTCCTGGACATCA | GGGTTGTTGCTGAACTTCCAA |
| NOX2 | GACCTCTGCCCCTGAGGAAA | CCAAAGGGCCCATCAACTG |
| GAPDH | ATGCCGCCTGGAGAAACC | GCATCAAAGGTGGAAGAATGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Xiao, Y.; Wang, D.; Han, X.; Zhou, R.; Zhang, H.; Zhu, K.; Wu, J.; Sun, X.; Li, S. NOX2/NLRP3-Inflammasome-Dependent Microglia Activation Promotes As(III)-Induced Learning and Memory Impairments in Developmental Rats. Toxics 2025, 13, 538. https://doi.org/10.3390/toxics13070538
Zhang L, Xiao Y, Wang D, Han X, Zhou R, Zhang H, Zhu K, Wu J, Sun X, Li S. NOX2/NLRP3-Inflammasome-Dependent Microglia Activation Promotes As(III)-Induced Learning and Memory Impairments in Developmental Rats. Toxics. 2025; 13(7):538. https://doi.org/10.3390/toxics13070538
Chicago/Turabian StyleZhang, Linlin, Yuyao Xiao, Dan Wang, Xuerong Han, Ruoqi Zhou, Huiying Zhang, Kexin Zhu, Junyao Wu, Xiance Sun, and Shuangyue Li. 2025. "NOX2/NLRP3-Inflammasome-Dependent Microglia Activation Promotes As(III)-Induced Learning and Memory Impairments in Developmental Rats" Toxics 13, no. 7: 538. https://doi.org/10.3390/toxics13070538
APA StyleZhang, L., Xiao, Y., Wang, D., Han, X., Zhou, R., Zhang, H., Zhu, K., Wu, J., Sun, X., & Li, S. (2025). NOX2/NLRP3-Inflammasome-Dependent Microglia Activation Promotes As(III)-Induced Learning and Memory Impairments in Developmental Rats. Toxics, 13(7), 538. https://doi.org/10.3390/toxics13070538






