The Impact of Perfluoroalkyl Substances on the Clinical Manifestations of Primary Sjögren Syndrome
Abstract
1. Introduction
2. Methods
2.1. Patients and Serum Collection
2.2. Standards, Reagents and Nomenclatures
2.3. Sample Extraction
2.4. Instrumental Analysis
2.5. Quality Assurance and Quality Control
2.6. Statistical Analysis
3. Results
3.1. Demographic, Clinical and Immunologic Features of 136 Patients with Primary Sjogren’s Syndrome
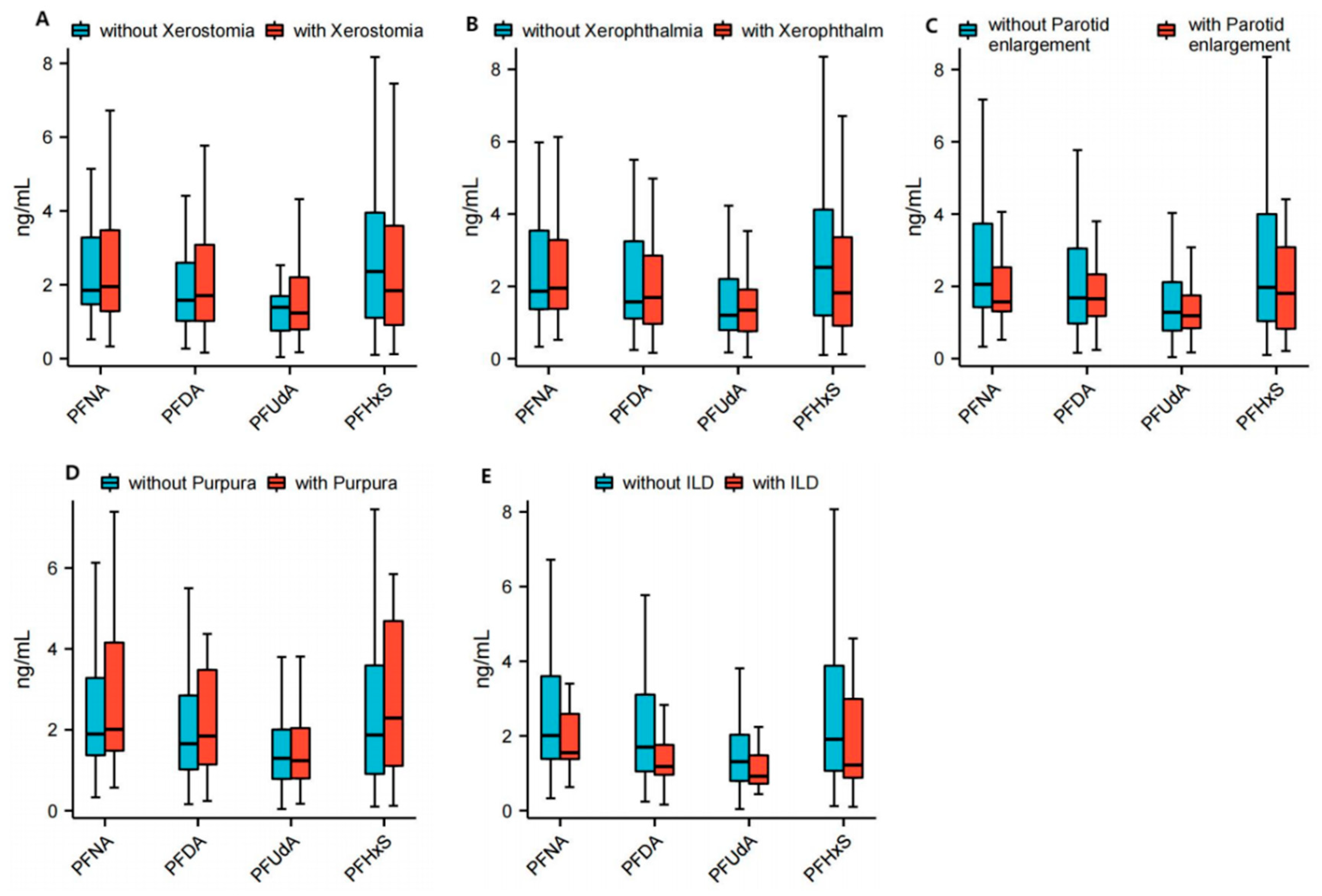
3.2. Correlation Between PFASs and Specific Clinical Manifestations in Patients with Primary Sjögren Syndrome
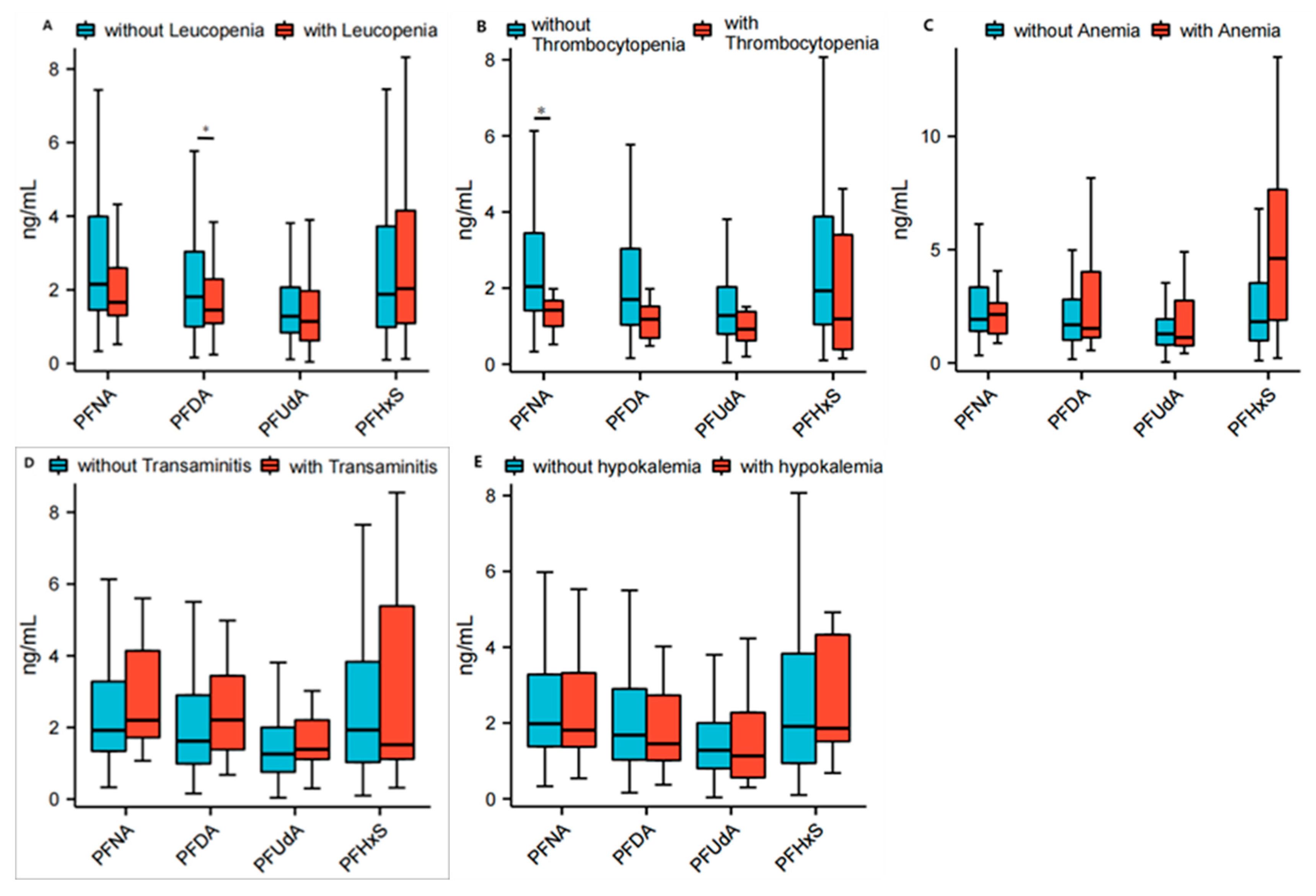
3.3. Correlation Between PFASs and Specific Blood Tests in Patients with Primary Sjögren Syndrome
3.4. Correlation Between PFASs and Some Specific Immunologic Indexes of Primary Sjögren Syndrome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Baldini, C.; Fulvio, G.; La Rocca, G.; Ferro, F. Update on the pathophysiology and treatment of primary Sjögren syndrome. Nat. Rev. Rheumatol. 2024, 20, 473–491. [Google Scholar] [CrossRef]
- Mariette, X.; Criswell, L.A. Primary Sjogren’s Syndrome. N. Engl. J. Med. 2018, 379, 97. [Google Scholar] [CrossRef] [PubMed]
- Odani, T.; Chiorini, J.A. Targeting primary Sjogren’s syndrome. Mod. Rheumatol. 2019, 29, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Bjork, A.; Mofors, J.; Wahren-Herlenius, M. Environmental factors in the pathogenesis of primary Sjogren’s syndrome. J. Intern. Med. 2020, 287, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, A.B.; Strynar, M.J.; Libelo, E.L. Polyfluorinated compounds: Past, present, and future. Environ. Sci. Technol. 2011, 45, 7954–7961. [Google Scholar] [CrossRef]
- Olsen, G.W.; Burris, J.M.; Ehresman, D.J.; Froehlich, J.W.; Seacat, A.M.; Butenhoff, J.L.; Zobel, L.R. Half-Life of Serum Elimination of Perfluorooctanesulfonate, Perfluorohexanesulfonate, and Perfluorooctanoate in Retired Fluorochemical Production Workers. Environ. Health Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2018, 29, 131–147. [Google Scholar] [CrossRef]
- Verreault, J.; Houde, M.; Gabrielsen, G.W.; Berger, U.; Haukås, M.; Letcher, R.J.; Muir, D.C. Perfluorinated Alkyl Substances in Plasma, Liver, Brain, and Eggs of Glaucous Gulls (Larus hyperboreus) from the Norwegian Arctic. Environ. Sci. Technol. 2005, 39, 7439–7445. [Google Scholar] [CrossRef]
- Braun, J.M. Early-life exposure to EDCs: Role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2016, 13, 161–173. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, R.; Hua, L.; Guo, Y.; Huang, L.; Zhao, Y.; Wang, X.; Zhang, J. Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and childhood atopic dermatitis: A prospective birth cohort study. Environ. Health 2018, 17, 8. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, H.; Wen, Z.; Wang, Y.; Li, X.; Huang, T.; Mo, J.; Wu, Y.; Zhong, Y.; Ge, R.-S. Perfluoroalkyl substances cause Leydig cell dysfunction as endocrine disruptors. Chemosphere 2020, 253, 126764. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, S.; Huang, Z.; Fu, Y.; Fei, J.; Liu, X.; He, Z. Associations between the serum levels of PFOS/PFOA and IgG N-glycosylation in adult or children. Environ. Pollut. 2020, 265, 114285. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2020, 40, 606–630. [Google Scholar] [CrossRef]
- Steenland, K.; Zhao, L.; Winquist, A.; Parks, C. Ulcerative Colitis and Perfluorooctanoic Acid (PFOA) in a Highly Exposed Population of Community Residents and Workers in the Mid-Ohio Valley. Environ. Health Perspect. 2013, 121, 900–905. [Google Scholar] [CrossRef]
- Steenland, K.; Zhao, L.; Winquist, A. A cohort incidence study of workers exposed to perfluorooctanoic acid (PFOA). Occup. Environ. Med. 2015, 72, 373–380. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, W.; Qu, J.; Hu, S.; Zhang, L.; Zhao, M.; Wu, P.; Xue, J.; Hangbiao, J. Per-/polyfluoroalkyl substance concentrations in human serum and their associations with immune markers of rheumatoid arthritis. Chemosphere 2022, 298, 134338. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, Y.; Zhang, L.; Hu, S.; Liao, K.; Zhao, M.; Wu, P.; Jin, H. Evaluated serum perfluoroalkyl acids and their relationships with the incidence of rheumatoid arthritis in the general population in Hangzhou, China. Environ. Pollut. 2022, 307, 119505. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jin, H.; Qu, J.; Zhang, S.; Hu, S.; Xue, J.; Zhao, M. The influences of perfluoroalkyl substances on the rheumatoid arthritis clinic. BMC Immunol. 2022, 23, 10. [Google Scholar] [CrossRef] [PubMed]
- Ateia, M.; Maroli, A.; Tharayil, N.; Karanfil, T. The overlooked short- and ultrashort-chain poly- and perfluorinated substances: A review. Chemosphere 2019, 220, 866–882. [Google Scholar] [CrossRef]
- Pulster, E.L.; Wichterman, A.E.; Snyder, S.M.; Fogelson, S.; Da Silva, B.F.; Costa, K.A.; Aufmuth, J.; Deak, K.L.; Murawski, S.A.; Bowden, J.A. Detection of long chain per- and polyfluoroalkyl substances (PFAS) in the benthic Golden tilefish (Lopholatilus chamaeleonticeps) and their association with microscopic hepatic changes. Sci. Total Environ. 2022, 809, 151143. [Google Scholar] [CrossRef]
- Brase, R.A.; Mullin, E.J.; Spink, D.C. Legacy and Emerging Per- and Polyfluoroalkyl Substances: Analytical Techniques, Environmental Fate, and Health Effects. Int. J. Mol. Sci. 2021, 22, 995. [Google Scholar] [CrossRef]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjögren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, Y.; Jiang, W.; Zhu, L.; Martin, J.W. Isomer-Specific Distribution of Perfluoroalkyl Substances in Blood. Environ. Sci. Technol. 2016, 50, 7808–7815. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Heilmann, C.; Weihe, P.; Nielsen, F.; Mogensen, U.B.; Budtz-Jorgensen, E. Serum Vaccine Antibody Concentrations in Adolescents Exposed to Perfluorinated Compounds. Environ. Health Perspect. 2017, 125, 077018. [Google Scholar] [CrossRef] [PubMed]
- Thorlacius, G.E.; Bjork, A.; Wahren-Herlenius, M. Genetics and epigenetics of primary Sjogren syndrome: Implications for future therapies. Nat. Rev. Rheumatol. 2023, 19, 288–306. [Google Scholar] [CrossRef] [PubMed]
- Perricone, C.; Bruno, L.; Cafaro, G.; Latini, A.; Ceccarelli, F.; Borgiani, P.; Ciccacci, C.; Bogdanos, D.; Novelli, G.; Gerli, R.; et al. Sjogren’s syndrome: Everything you always wanted to know about genetic and epigenetic factors. Autoimmun. Rev. 2024, 23, 103673. [Google Scholar] [CrossRef]
- Ulff-Møller, C.J.; Svendsen, A.J.; Viemose, L.N.; Jacobsen, S. Concordance of autoimmune disease in a nationwide Danish systemic lupus erythematosus twin cohort. Semin. Arthritis Rheum. 2018, 47, 538–544. [Google Scholar] [CrossRef]
- Brodin, P.; Jojic, V.; Gao, T.; Bhattacharya, S.; Angel, C.J.L.; Furman, D.; Shen-Orr, S.; Dekker, C.L.; Swan, G.E.; Butte, A.J.; et al. Variation in the Human Immune System Is Largely Driven by Non-Heritable Influences. Cell 2015, 160, 37–47. [Google Scholar] [CrossRef]
- Gandolfo, S.; Bombardieri, M.; Pers, J.-O.; Mariette, X.; Ciccia, F. Precision medicine in Sjögren’s disease. Lancet Rheumatol. 2024, 6, e636–e647. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Perfluoroalkyls; Agency for toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2021.
- Rudzanova, B.; Vlaanderen, J.; Kalina, J.; Piler, P.; Zvonar, M.; Klanova, J.; Blaha, L.; Adamovsky, O. Impact of PFAS exposure on prevalence of immune-mediated diseases in adults in the Czech Republic. Environ. Res. 2023, 229, 115969. [Google Scholar] [CrossRef]
- Stein, C.R.; McGovern, K.J.; Pajak, A.M.; Maglione, P.J.; Wolff, M.S. Perfluoroalkyl and polyfluoroalkyl substances and indicators of immune function in children aged 12–19 y: National Health and Nutrition Examination Survey. Pediatr. Res. 2015, 79, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Andersen, E.W.; Budtz-Jørgensen, E.; Nielsen, F.; Mølbak, K.; Weihe, P.; Heilmann, C. Serum Vaccine Antibody Concentrations in Children Exposed to Perfluorinated Compounds. JAMA 2012, 307, 391–397. [Google Scholar] [CrossRef]
- Smit, L.A.; Lenters, V.; Hoyer, B.B.; Lindh, C.H.; Pedersen, H.S.; Liermontova, I.; Jonsson, B.A.; Piersma, A.H.; Bonde, J.P.; Toft, G.; et al. Prenatal exposure to environmental chemical contaminants and asthma and eczema in school-age children. Allergy 2015, 70, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, H.; Miyashita, C.; Okada, E.; Kashino, I.; Chen, C.J.; Ito, S.; Araki, A.; Kobayashi, S.; Matsuura, H.; Kishi, R. Prenatal exposure to perfluoroalkyl acids and prevalence of infectious diseases up to 4years of age. Environ. Int. 2017, 104, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Qin, X.D.; Zeng, X.W.; Paul, G.; Morawska, L.; Su, M.W.; Tsai, C.H.; Wang, S.Q.; Lee, Y.L.; Dong, G.H. Associations of serum perfluoroalkyl acid levels with T-helper cell-specific cytokines in children: By gender and asthma status. Sci. Total Environ. 2016, 559, 166–173. [Google Scholar] [CrossRef]
- Barton, K.E.; Zell-Baran, L.M.; DeWitt, J.C.; Brindley, S.; McDonough, C.A.; Higgins, C.P.; Adgate, J.L.; Starling, A.P. Cross-sectional associations between serum PFASs and inflammatory biomarkers in a population exposed to AFFF-contaminated drinking water. Int. J. Hyg. Environ. Health 2022, 240, 113905. [Google Scholar] [CrossRef]
- Pennings, J.L.; Jennen, D.G.; Nygaard, U.C.; Namork, E.; Haug, L.S.; van Loveren, H.; Granum, B. Cord blood gene expression supports that prenatal exposure to perfluoroalkyl substances causes depressed immune functionality in early childhood. J. Immunotoxicol. 2016, 13, 173–180. [Google Scholar] [CrossRef]
- NTP (National Toxicology Program). TOX-97: Perfuorohexanoic acid (307-24-4), perfuorooctanoic acid (335-67-1), perfuorononanoic acid (375-95-1), perfuorodecanoic acid (335-76-2), WY-14643 (50892-23-4). In Chemical Efects in Biological Systems (CEBS); National Toxicology Program: Research Triangle Park, NC, USA, 2019. [Google Scholar]
- Fang, X.; Zhang, L.; Feng, Y.; Zhao, Y.; Dai, J. Immunotoxic Effects of Perfluorononanoic Acid on BALB/c Mice. Toxicol. Sci. 2008, 105, 312–321. [Google Scholar] [CrossRef]
- Rockwell, C.E.; Turley, A.E.; Cheng, X.; Fields, P.E.; Klaassen, C.D. Persistent alterations in immune cell populations and function from a single dose of perfluorononanoic acid (PFNA) in C57Bl/6 mice. Food Chem. Toxicol. 2017, 100, 24–33. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J.J. The immunology of rheumatoid arthritis. Nat. Immunol. 2020, 22, 10–18. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Feng, Y.; Shi, Z.; Dai, J. Alterations of Cytokines and MAPK Signaling Pathways are Related to the Immunotoxic Effect of Perfluorononanoic Acid. Toxicol. Sci. 2009, 108, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Frawley, R.P.; Smith, M.; Cesta, M.F.; Hayes-Bouknight, S.; Blystone, C.; Kissling, G.E.; Harris, S.; Germolec, D. Immunotoxic and hepatotoxic effects of perfluoro-n-decanoic acid (PFDA) on female Harlan Sprague–Dawley rats and B6C3F1/N mice when administered by oral gavage for 28 days. J. Immunotoxicol. 2018, 15, 41–52. [Google Scholar] [CrossRef]
- Li, K.; Zhao, Q.; Fan, Z.; Jia, S.; Liu, Q.; Liu, F.; Liu, S. The toxicity of perfluorodecanoic acid is mainly manifested as a deflected immune function. Mol. Biol. Rep. 2022, 49, 4365–4376. [Google Scholar] [CrossRef]
- Song, X.; Ye, T.; Jing, D.; Wei, K.; Ge, Y.; Bei, X.; Qi, Y.; Wang, H.; Li, J.; Zhang, Y. Association between exposure to per- and polyfluoroalkyl substances and levels of lipid profile based on human studies. Rev. Environ. Health 2024, 40, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Ogunsuyi, O.M.; Fasakin, P.T.; Ajibiye, O.P.; Ogunsuyi, O.I.; Adekoya, K.O. Perfluoroundecanoic acid induces DNA damage, reproductive and pathophysiological dysfunctions via oxidative stress in male Swiss mice. Chemosphere 2023, 338, 139491. [Google Scholar] [CrossRef]
- Weatherly, L.M.; Shane, H.L.; Jackson, L.G.; Lukomska, E.; Baur, R.; Cooper, M.P.; Anderson, S.E. Systemic and immunotoxicity induced by topical application of perfluorohexane sulfonic acid (PFHxS) in a murine model. Food Chem. Toxicol. 2024, 186, 114578. [Google Scholar] [CrossRef]
- Narizzano, A.M.; Bohannon, M.E.; East, A.G.; Guigni, B.A.; Quinn, M.J. Reproductive and immune effects emerge at similar thresholds of PFHxS in deer mice. Reprod. Toxicol. 2023, 120, 108421. [Google Scholar] [CrossRef]
- Buser, M.C.; Scinicariello, F. Perfluoroalkyl substances and food allergies in adolescents. Environ. Int. 2016, 88, 74–79. [Google Scholar] [CrossRef]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; et al. Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J. 2020, 18, e06223. [Google Scholar] [CrossRef]



| Variables in Protocol * | n = 136 |
|---|---|
| Gender, male, n (%) | 4 (2.9) |
| Age at onset, mean (S.D.), years | 42 ± 11 |
| Disease duration, mean (S.D.), years | 6.3 ± 3.7 |
| Initial symptoms | |
| Sicca symptoms, n (%) | 97 (71.3) |
| Articular involvement, n (%) | 54 (39.7) |
| Parotid enlargement, n (%) | 17 (12.5) |
| Hypocytosis, n (%) | 15 (11.0) |
| Saprodontia, n (%) | 12 (8.8) |
| Purpura, n (%) | 10 (7.4) |
| Flaccid paralysis due to hypokalemia | 7 (5.1) |
| Fever | 4 (2.9) |
| Xerostomia, n (%) | 104 (76.5) |
| Xerophthalmia, n (%) | 84 (61.8) |
| Saprodontia, n (%) | 26 (19.1) |
| Parotid enlargement, n (%) | 28 (20.6) |
| Altered ocular tests, n (%) | 132 (96.4) |
| Schirmer’s I test (≤5 mm in 5 min), n (%) | 123 (90.4) |
| Ocular dye-positive, n (%) # | 114 (83.8) |
| Altered oral tests, n (%) | 116 (85.3) |
| WUSF (≤1.5 mL in 15 min) | 99 (72.8) |
| Parotid sialography positive $ | 109 (94.0) |
| Positive salivary gland biopsy !, n (%) | 121/129 (93.8) |
| Arthritis | 24 (17.6) |
| Fever | 14 (10.3) |
| Fatigue | 18 (13.2) |
| Purpura, n (%) | 13 (9.6) |
| Flaccid paralysis due to hypokalemia | 11 (8.1) |
| Heart involvement | 44 (32.4) |
| Pulmonary involvement | 39 (28.7) |
| Liver involvement | 35 (25.7) |
| Renal involvement | 13 (9.6) |
| Autoimmune thyroiditis | 17 (12.5) |
| Family history of rheumatic disease | 13 (9.6) |
| Cytopenia | 69 (50.7) |
| Leucopenia (<4 × 109/L), n (%) | 41 (30.1) |
| Anemia (Hb <110 g/L), n (%) | 17(12.5) |
| Thrombocytopenia (<100 × 109/L), n (%) | 17 (12.5) |
| Lymphopenia (<0.8 × 109/L), n (%) | 11 (8.1) |
| ANA positive, n (%) | 125 (91.9) |
| Anti-Ro/SS-A positive, n (%) | 106 (77.9) |
| Anti-La/SS-B positive, n (%) | 65 (48.5) |
| RF positive, n (%) | 89 (65.4) |
| High IgG levels (>17 g/L), n (%) | 105 (77.2) |
| High IgA levels (>4 g/L), n (%) | 45 (33.1) |
| High IgM levels (>2.3 g/L), n (%) | 30 (22.1) |
| Low C3 levels (<0.9 g/L), n (%) | 28 (20.6) |
| Low C4 levels (<0.1 g/L), n (%) | 9 (6.6) |
| Group | PFNA (M (Q1, Q3)) | PFDA (M (Q1, Q3)) | PFUdA (M (Q1, Q3)) | PFHxS (M (Q1, Q3)) |
|---|---|---|---|---|
| Control (n = 148) | 1.19 (0.77, 1.57) | 1.9 (1.2, 3.1) | 1.5 (0.9, 2.3) | 2.5 (1.3, 4.3) |
| pSS (n = 136) | 1.9 (1.4, 3.3) | 1.7 (1.0, 3.0) | 3.3 (2.8, 4.9) | 1.9 (1.0, 3.9) |
| p & | 0.0796 | 0.2990 | 0.0117 * | 0.1278 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Jin, H.; Hu, S.; Zhang, S.; Zhao, M.; Xue, J. The Impact of Perfluoroalkyl Substances on the Clinical Manifestations of Primary Sjögren Syndrome. Toxics 2025, 13, 570. https://doi.org/10.3390/toxics13070570
Zhao Y, Jin H, Hu S, Zhang S, Zhao M, Xue J. The Impact of Perfluoroalkyl Substances on the Clinical Manifestations of Primary Sjögren Syndrome. Toxics. 2025; 13(7):570. https://doi.org/10.3390/toxics13070570
Chicago/Turabian StyleZhao, Yun, Hangbiao Jin, Shetuan Hu, Songzhao Zhang, Meirong Zhao, and Jing Xue. 2025. "The Impact of Perfluoroalkyl Substances on the Clinical Manifestations of Primary Sjögren Syndrome" Toxics 13, no. 7: 570. https://doi.org/10.3390/toxics13070570
APA StyleZhao, Y., Jin, H., Hu, S., Zhang, S., Zhao, M., & Xue, J. (2025). The Impact of Perfluoroalkyl Substances on the Clinical Manifestations of Primary Sjögren Syndrome. Toxics, 13(7), 570. https://doi.org/10.3390/toxics13070570








