In Vitro Study of the Effects of Pesticide Mixtures Used in Maize Cultivation in Ecuador
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment
2.2. Cell Culture
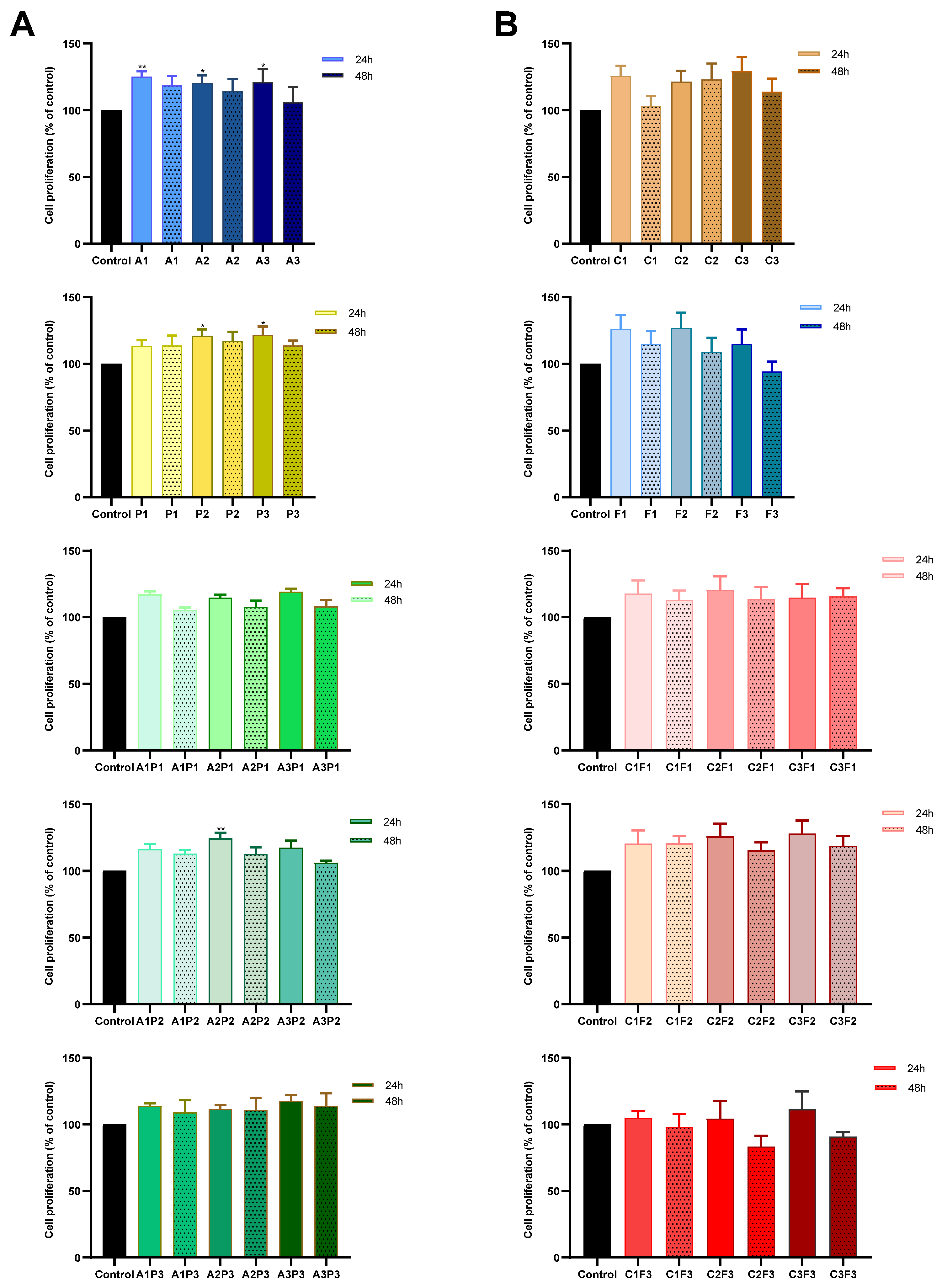
2.3. Cell Proliferation Assay
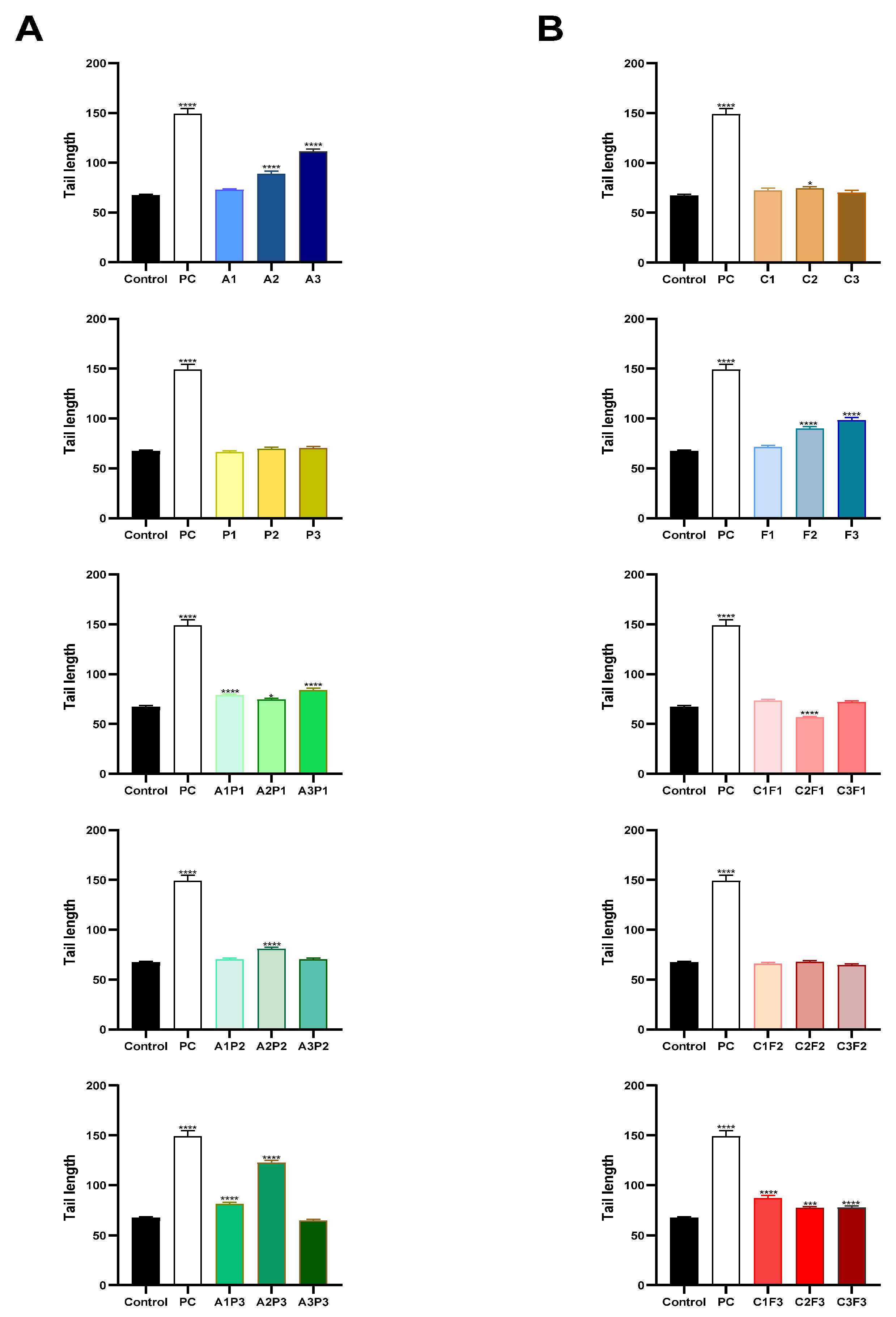
2.4. Comet Assay
2.5. Micronucleus Assay
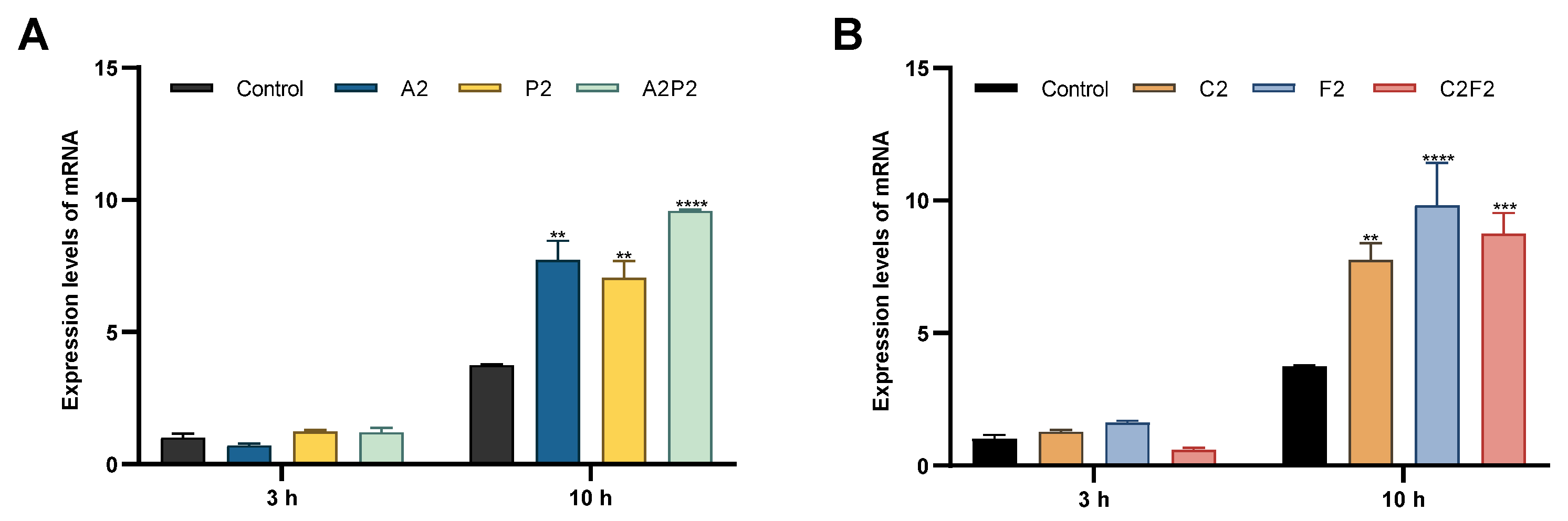
2.6. H2AX Expression
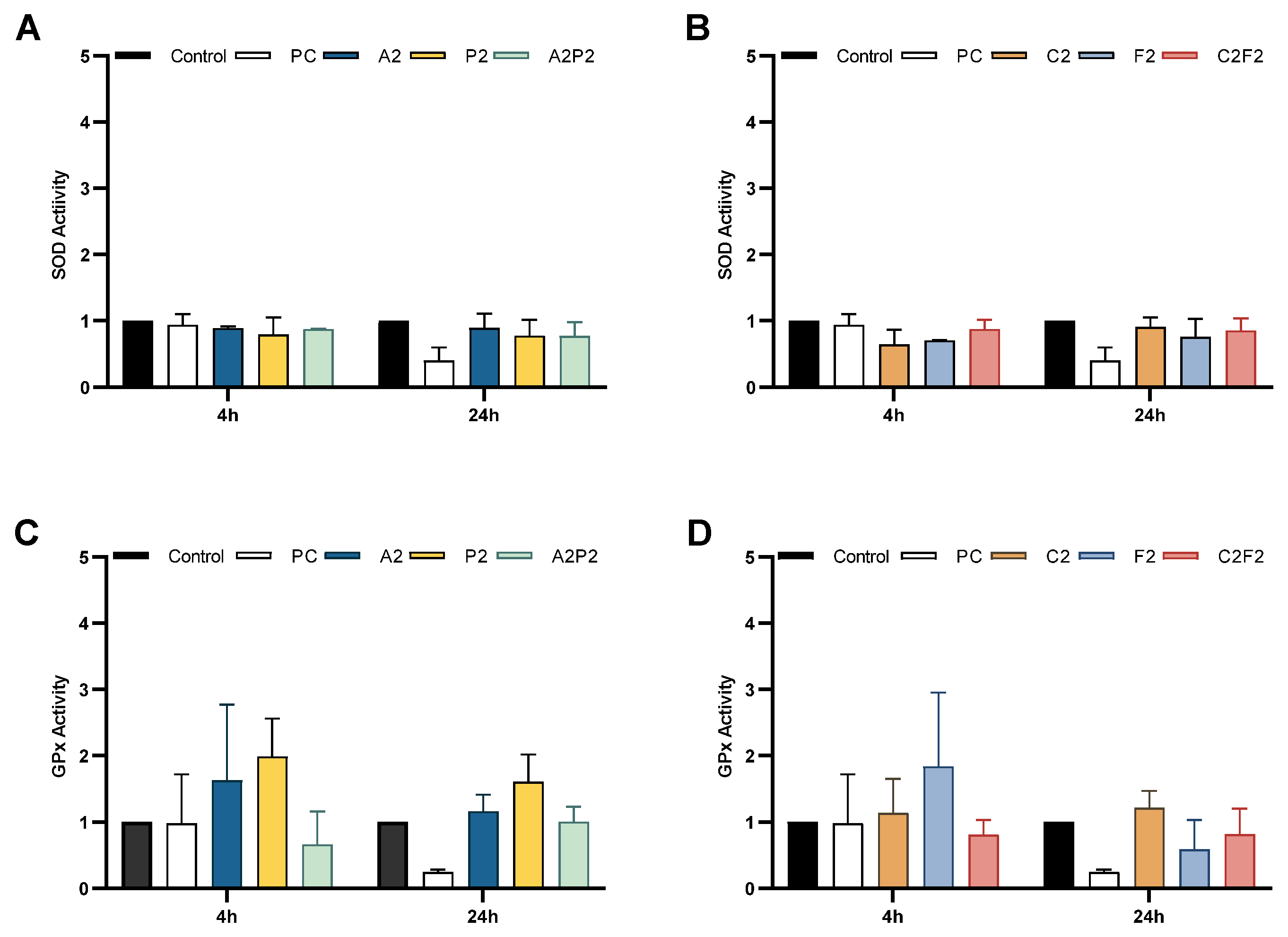
2.7. Antioxidant Enzyme Activities
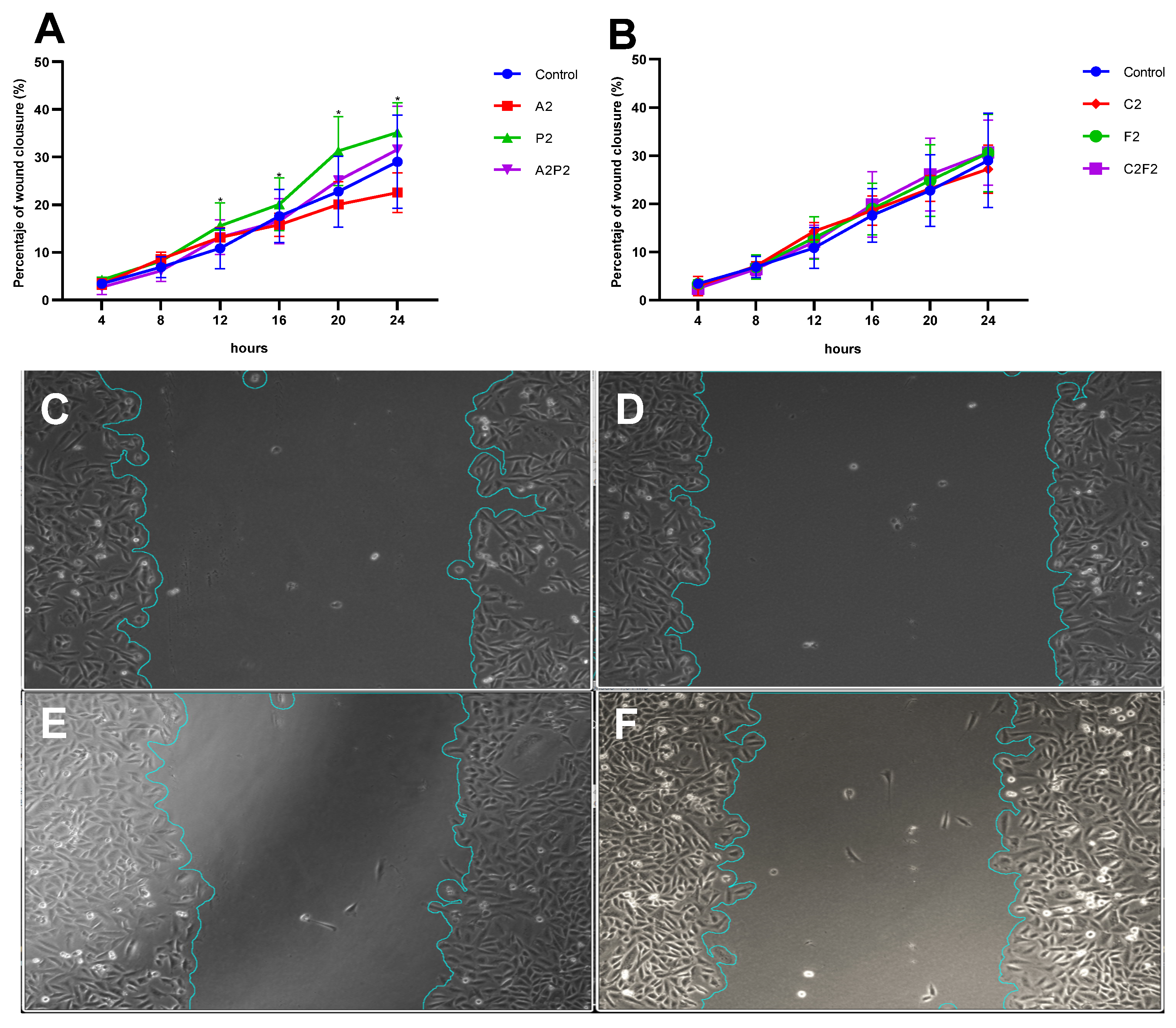
2.8. Scratch Wound Healing Assay
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Atrazine |
| P | Pendimethalin |
| C | Chlorpyrifos/cypermethrin |
| F | Fertilizer |
| MTS | [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS] |
| SOD | Superoxide dismutase |
| GPx | Glutathione peroxidase |
| H2AX | H2A.X variant histone |
| MN | Micronuclei |
| NPBs | Nucleoplasmic bridges |
References
- FAO. Pesticides Use and Trade, 1990–2021; FAOSTAT Analytical Briefs Series No. 70; FAO: Rome, Italy, 2023. [Google Scholar]
- INEC. Encuesta de Superficie y Producción Agropecuaria Continua (ESPAC); Instituto Nacional de Estadística y Censos (INEC): Quito, Ecuador, 2023. [Google Scholar]
- Ochoa-Cueva, P.A.; Arteaga, J.; Arévalo, A.P.; Kolok, A.S. A Potential Pesticides Exposure Index (PPEI) for Developing Countries: Applied in a Transboundary Basin. Integr. Environ. Assess. Manag. 2022, 18, 187–197. [Google Scholar] [CrossRef]
- Bhargavi, B.R.; Akurathi, R.; Vellanki, R.K.; Kanala, J.R.; Vellanki, R.K.; Kanala, J.R. Genotoxic Effect of Certain Pesticide Mixtures in CHO Cell Lines. Appl. Ecol. Environ. Sci. 2020, 8, 166–173. [Google Scholar] [CrossRef]
- Ledda, C.; Cannizzaro, E.; Cinà, D.; Filetti, V.; Vitale, E.; Paravizzini, G.; Naso, C.D.; Iavicoli, I.; Rapisarda, V. Oxidative Stress and DNA Damage in Agricultural Workers after Exposure to Pesticides. J. Occup. Med. Toxicol. 2021, 16. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Quintana, R.; López-Durán, R.M.; Milić, M.; Bonassi, S.; Ochoa-Ocaña, M.A.; Uriostegui-Acosta, M.O.; Pérez-Flores, G.A.; Gómez-Olivares, J.L.; Sánchez-Alarcón, J. Assessment of Cytogenetic Damage and Cholinesterases’ Activity in Workers Occupationally Exposed to Pesticides in Zamora-Jacona, Michoacan, Mexico. Int. J. Environ. Res. Public Health 2021, 18, 6269. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide Dismutases: Dual Roles in Controlling ROS Damage and Regulating ROS Signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Katsuta, E.; Dessai, A.S.; Ebos, J.M.; Yan, L.; Ouchi, T.; Takabe, K. Original Article H2AX mRNA Expression Reflects DNA Repair, Cell Proliferation, Metastasis, and Worse Survival in Breast Cancer. Am J Cancer Res 2022, 12, 793–804. [Google Scholar]
- Mah, L.-J.; El-Osta, A.; Karagiannis, T.C. γH2AX: A Sensitive Molecular Marker of DNA Damage and Repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef]
- Cavalier, H.; Trasande, L.; Porta, M. Exposures to Pesticides and Risk of Cancer: Evaluation of Recent Epidemiological Evidence in Humans and Paths Forward. Int. J. Cancer 2023, 152, 879–912. [Google Scholar] [CrossRef]
- Dhankhar, N.; Kumar, J. Impact of Increasing Pesticides and Fertilizers on Human Health: A Review. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Kalyabina, V.P.; Esimbekova, E.N.; Kopylova, K.V.; Kratasyuk, V.A. Pesticides: Formulants, Distribution Pathways and Effects on Human Health – a Review. Toxicol. Rep. 2021, 8, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Arévalo-Jaramillo, P.; Idrobo, A.; Salcedo, L.; Cabrera, A.; Vintimilla, A.; Carrión, M.; Bailon-Moscoso, N. Biochemical and Genotoxic Effects in Women Exposed to Pesticides in Southern Ecuador. Environ. Sci. Pollut. Res. 2019, 26, 24911–24921. [Google Scholar] [CrossRef] [PubMed]
- Calzada, J.; Gisbert, M.; Moscoso, B. The hidden cost of bananas: Pesticide effects on newborns’ health. J. Assoc. Environ. Resour. Econ. 2023, 10. [Google Scholar] [CrossRef]
- Hutter, H.P.; Poteser, M.; Lemmerer, K.; Wallner, P.; Sanavi, S.S.; Kundi, M.; Moshammer, H.; Weitensfelder, L. Indicators of Genotoxicity in Farmers and Laborers of Ecological and Conventional Banana Plantations in Ecuador. Int. J. Environ. Res. Public Health 2020, 17, 1435. [Google Scholar] [CrossRef]
- Menadi, S.; Boubidi, F.S.; Bouchelaghem, R.; Messarah, M.; Boumendjel, A. Occupational Exposure to the Dust of Chemical Fertilizers (NPK 15.15.15): Effect on Biochemical Parameters and Oxidative Stress Status among Workers in Annaba. Comp. Clin. Pathol. 2024, 33, 437–444. [Google Scholar] [CrossRef]
- Tasleem, S.; Masud, S.; Habib, S.S.; Naz, S.; Fazio, F.; Aslam, M.; Ullah, M.; Attaullah, S. Investigation of the Incidence of Heavy Metals Contamination in Commonly Used Fertilizers Applied to Vegetables, Fish Ponds, and Human Health Risk Assessments. Environ. Sci. Pollut. Res. 2023, 30, 100646–100659. [Google Scholar] [CrossRef]
- INEC. Módulo de Información Agroambiental y Tecnificación 2021; Instituto Nacional de Estadística y Censos (INEC): Quito, Ecuador, 2022. [Google Scholar]
- Nagy, K.; Duca, R.C.; Lovas, S.; Creta, M.; Scheepers, P.T.J.; Godderis, L.; Ádám, B. Systematic Review of Comparative Studies Assessing the Toxicity of Pesticide Active Ingredients and Their Product Formulations. Environ. Res. 2020, 181, 108926. [Google Scholar] [CrossRef]
- Zeljezic, D.; Garaj-Vrhovac, V.; Perkovic, P. Evaluation of DNA Damage Induced by Atrazine and Atrazine-Based Herbicide in Human Lymphocytes in Vitro Using a Comet and DNA Diffusion Assay. Toxicol. Vitr. 2006, 20, 923–935. [Google Scholar] [CrossRef]
- Ferguson, S.; Mesnage, R.; Antoniou, M.N. Cytotoxicity Mechanisms of Eight Major Herbicide Active Ingredients in Comparison to Their Commercial Formulations. Toxics 2022, 10, 711. [Google Scholar] [CrossRef]
- OMS. Guías Para La Calidad Del Agua de Consumo Humano: Cuarta Edición Que Incorpora La Primera Adenda; OMS: Ginebra, Switzerland, 2018; ISBN 978-92-4-354995-8. [Google Scholar]
- INEN. Norma Técnica Ecuatoriana NTE INEN 1108: Agua Potable. Requisitos; Quinta versión; Instituto Ecuatoriano de Normali-zación (INEN): Quito, Ecuador, 2014. [Google Scholar]
- Guamán-Ortiz, L.M.; Romero-Benavides, J.C.; Suarez, A.I.; Torres-Aguilar, S.; Castillo-Veintimilla, P.; Samaniego-Romero, J.; Ortiz-Diaz, K.; Bailon-Moscoso, N. Cytotoxic Property of Grias Neuberthii Extract on Human Colon Cancer Cells: A Crucial Role of Autophagy. Evid.-Based Complement. Altern. Med. 2020, 2020, 1565306. [Google Scholar] [CrossRef]
- Bailon-Moscoso, N.; Tinitana, F.; Martínez-Espinosa, R.; Jaramillo-Velez, A.; Palacio-Arpi, A.; Aguilar-Hernandez, J.; Romero-Benavides, J.C. Cytotoxic, Antioxidative, Genotoxic and Antigenotoxic Effects of Horchata, Beverage of South Ecuador. BMC Complement. Altern. Med. 2017, 17, 539. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K.; Abdel-Hamid, A.M.E.; El-Esawi, M.A.; Ali, H.; Elshikh, M.S. Seaweed Extracts Enhance Salam Turfgrass Performance during Prolonged Irrigation Intervals and Saline Shock. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, D.; Zhang, X.; Chen, M.; Zhi, Y.; Cui, W.; Li, S.; Xu, F.; Tan, Y.; Zhou, H.; et al. Screening of an FDA-Approved Compound Library Identifies Apigenin for the Treatment of Myocardial Injury. Int. J. Biol. Sci. 2023, 19, 5233–5244. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Figueroa, F.T.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An Image J Plugin for the High Throughput Image Analysis of in Vitro Scratch Wound Healing Assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
- Andrade-Rivas, F.; Paul, N.; Spiegel, J.; Henderson, S.B.; Parrott, L.; Delgado-Ron, J.A.; Echeverri, A.; Bosch, M. van den Mapping Potential Population-Level Pesticide Exposures in Ecuador Using a Modular and Scalable Geospatial Strategy. GeoHealth 2023, 7, e2022GH000775. [Google Scholar] [CrossRef] [PubMed]
- Kmetič, I.; Srček, V.G.; Slivac, I.; Šimić, B.; Kniewald, Z.; Kniewald, J. Atrazine Exposure Decreases Cell Proliferation in Chinese Hamster Ovary (CHO-K1) Cell Line. Bull. Environ. Contam. Toxicol. 2008, 81, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Manske, M.K.; Beltz, L.A.; Dhanwada, K.R. Low-Level Atrazine Exposure Decreases Cell Proliferation in Human Fibroblasts. Arch. Environ. Contam. Toxicol. 2004, 46, 438–444. [Google Scholar] [CrossRef]
- Tian, Y.; He, J.; Liu, N.; Huang, D.; Liu, Z.; Yang, Y.; Chen, J.; Zhao, B.; Zhao, S.; Liang, B. Atrazine Exposure Improves the Proliferation of H22 Cells: In Vitro and in Vivo. RSC Advances 2018, 8, 21759–21767. [Google Scholar] [CrossRef]
- González-Palomo, A.K.; Ruíz-Rodríguez, V.M.; Hernández-Blanco, D.V.; Vázquez, F.J.P.; Alcántara-Quintana, L.E.; Cortés-Garcia, J.D. Atrazine Modifies Markers of Melanocyte Maturation and Apoptosis in Primary Skin Cultures. Toxicol. Mech. Methods 2023, 33, 233–238. [Google Scholar] [CrossRef]
- Lee, H.S.; Amarakoon, D.; Tamia, G.; Park, Y.; Smolensky, D.; Lee, S.H. Pendimethalin Induces Apoptotic Cell Death through Activating ER Stress-Mediated Mitochondrial Dysfunction in Human Umbilical Vein Endothelial Cells. Food Chem. Toxicol. 2022, 168, 113370. [Google Scholar] [CrossRef]
- Sarigöl-Kiliç, Z.; Ündeğer-Bucurgat, Ü. The Apoptotic and Anti-Apoptotic Effects of Pendimethalin and Trifluralin on A549 Cells in Vitro. Turk. J. Pharm. Sci. 2018, 15, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Yang, J.; Ning, J.; Wang, M.; Song, Q. Atrazine Triggers DNA Damage Response and Induces DNA Double-Strand Breaks in MCF-10A Cells. Int. J. Mol. Sci. 2015, 16, 14353–14368. [Google Scholar] [CrossRef] [PubMed]
- Demir, N.; Aydin, S.; Bucurgat, Ü.Ü. Pendimetalinin Genotoksik Etkilerinin Çin Hamster over Hücrelerinde Tek Hücre Jel Elektroforez (Comet) Yöntemiyle Değerlendirilmesi. Turk. J. Pharm. Sci. 2017, 14, 185–190. [Google Scholar] [CrossRef]
- Ahmad, M.I.; Zafeer, M.F.; Javed, M.; Ahmad, M. Pendimethalin-Induced Oxidative Stress, DNA Damage and Activation of Anti-Inflammatory and Apoptotic Markers in Male Rats. Sci. Rep. 2018, 8, 17139. [Google Scholar] [CrossRef]
- Serpa, E.A.; Schmitt, E.G.; Zuravski, L.; Machado, M.M.; Oliveira, L.F.S. de Chlorpyrifos Induces Genotoxic Effects in Human Leukocytes in Vitro at Low Concentrations. Acta Sci. Health Sci. 2019, 41, e44291. [Google Scholar] [CrossRef]
- Li, D.; Huang, Q.; Lu, M.; Zhang, L.; Yang, Z.; Zong, M.; Tao, L. The Organophosphate Insecticide Chlorpyrifos Confers Its Genotoxic Effects by Inducing DNA Damage and Cell Apoptosis. Chemosphere 2015, 135, 387–393. [Google Scholar] [CrossRef]
- Cuenca, J.B.; de Olivera Galvão, M.F.; Endirlik, B.Ü.; Tirado, N.; Dreij, K. In Vitro Cytotoxicity and Genotoxicity of Single and Combined Pesticides Used by Bolivian Farmers. Environ. Mol. Mutagen. 2022, 63, 4–17. [Google Scholar] [CrossRef]
- Tadee, A.; Mahakunakorn, P.; Porasuphatana, S. Oxidative Stress and Genotoxicity of Co-Exposure to Chlorpyrifos and Aflatoxin B1 in HepG2 Cells. Toxicol. Ind. Health 2020, 36, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, K.S.; Kuttikrishnan, S.; Ahmad, N.; Habeeba, U.; Mariyam, Z.; Suleman, M.; Bhat, A.A.; Uddin, S. H2AX: A Key Player in DNA Damage Response and a Promising Target for Cancer Therapy. Biomed. Pharmacother. 2024, 175, 116663. [Google Scholar] [CrossRef]
- Merola, C.; Fabrello, J.; Matozzo, V.; Faggio, C.; Iannetta, A.; Tinelli, A.; Crescenzo, G.; Amorena, M.; Perugini, M. Dinitroaniline Herbicide Pendimethalin Affects Development and Induces Biochemical and Histological Alterations in Zebrafish Early-Life Stages. Sci. Total Environ. 2022, 828, 154414. [Google Scholar] [CrossRef]
- Hernández-Toledano, D.S.; Vega, L. Methylated Dialkylphosphate Metabolites of the Organophosphate Pesticide Malathion Modify Actin Cytoskeleton Arrangement and Cell Migration via Activation of Rho GTPases Rac1 and Cdc42. Chem.-Biol. Interact. 2023, 382, 110593. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.; Malliri, A. GEFs: Dual Regulation of Rac1 Signaling. Small GTPases 2017, 8, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Cabello, G.; Galaz, S.; Botella, L.; Calaf, G.; Pacheco, M.; Stockert, J.C.; Villanueva, A.; Cañete, M.; Juarranz, A. The Pesticide Malathion Induces Alterations in Actin Cytoskeleton and in Cell Adhesion of Cultured Breast Carcinoma Cells. Int. J. Oncol. 2003, 23, 697–704. [Google Scholar] [CrossRef] [PubMed]
| Type | Pesticide | Dose (µg/mL) | ||
|---|---|---|---|---|
| Herbicide | Atrazine (A) | 0.01 A1 | 0.1 A2 | 1 A3 |
| Pendimethalin (P) | 0.002 P1 | 0.02 P2 | 0.2 P3 | |
| Insecticide | Chlorpyrifos/cypermethrin + (C) | 0.003 C1 | 0.03 C2 | 0.3 C3 |
| Fertilizer | Phosphorus * (F) | 1 F1 | 10 F2 | 100 F3 |
| Treatment | MN | NBUDs | NOTCH | PNB | NDI |
|---|---|---|---|---|---|
| A2 | 33.67 ± 3.48 | 222.00 ± 35.82 | 8.66 ± 1.97 | 6.83 ± 0.98 | 1.97 ± 0.02 |
| P2 | 25.17 ± 2.85 | 180.80 ± 40.01 | 8.33 ± 2.77 | 7.83 ± 0.54 | 1.98 ± 0.02 |
| A2P2 | 27.83 ± 1.88 | 183.80 ± 15.21 | 8.16 ± 2.12 | 9.00 ± 0.68 ** | 1.99 ± 0.01 |
| C2 | 25.33 ± 3.42 | 172.50 ± 16.36 | 5.16 ± 1.16 | 5.00 ± 0.93 | 1.93 ± 0.02 |
| F2 | 24.83 ± 3.43 | 132.00 ± 21.19 | 5.50 ± 1.31 | 4.66 ± 0.55 | 1.96 ± 0.03 |
| C2F2 | 25.83 ± 3.04 | 129.20 ± 21.11 | 4.50 ± 1.14 | 5.66 ± 0.84 | 1.95 ± 0.01 |
| PC | 35.00 ± 3.21 * | 198.00 ± 48.51 | 5.33 ± 2.33 | 4.33 ± 0.33 | 1.90 ± 0.04 |
| Control | 26.67 ± 1.38 | 210.80 ± 50.22 | 5.00 ± 0.85 | 5.16 ± 0.70 | 1.92 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arévalo-Jaramillo, A.P.; Guamán Hurtado, J.E.; Cevallos-Solorzano, G.; Bailon-Moscoso, N. In Vitro Study of the Effects of Pesticide Mixtures Used in Maize Cultivation in Ecuador. Toxics 2025, 13, 530. https://doi.org/10.3390/toxics13070530
Arévalo-Jaramillo AP, Guamán Hurtado JE, Cevallos-Solorzano G, Bailon-Moscoso N. In Vitro Study of the Effects of Pesticide Mixtures Used in Maize Cultivation in Ecuador. Toxics. 2025; 13(7):530. https://doi.org/10.3390/toxics13070530
Chicago/Turabian StyleArévalo-Jaramillo, Ana Paulina, Jackeline Elizabeth Guamán Hurtado, Gabriela Cevallos-Solorzano, and Natalia Bailon-Moscoso. 2025. "In Vitro Study of the Effects of Pesticide Mixtures Used in Maize Cultivation in Ecuador" Toxics 13, no. 7: 530. https://doi.org/10.3390/toxics13070530
APA StyleArévalo-Jaramillo, A. P., Guamán Hurtado, J. E., Cevallos-Solorzano, G., & Bailon-Moscoso, N. (2025). In Vitro Study of the Effects of Pesticide Mixtures Used in Maize Cultivation in Ecuador. Toxics, 13(7), 530. https://doi.org/10.3390/toxics13070530