Does Electromagnetic Pollution in the ART Laboratory Affect Sperm Quality? A Cross-Sectional Observational Study
Abstract
1. Introduction
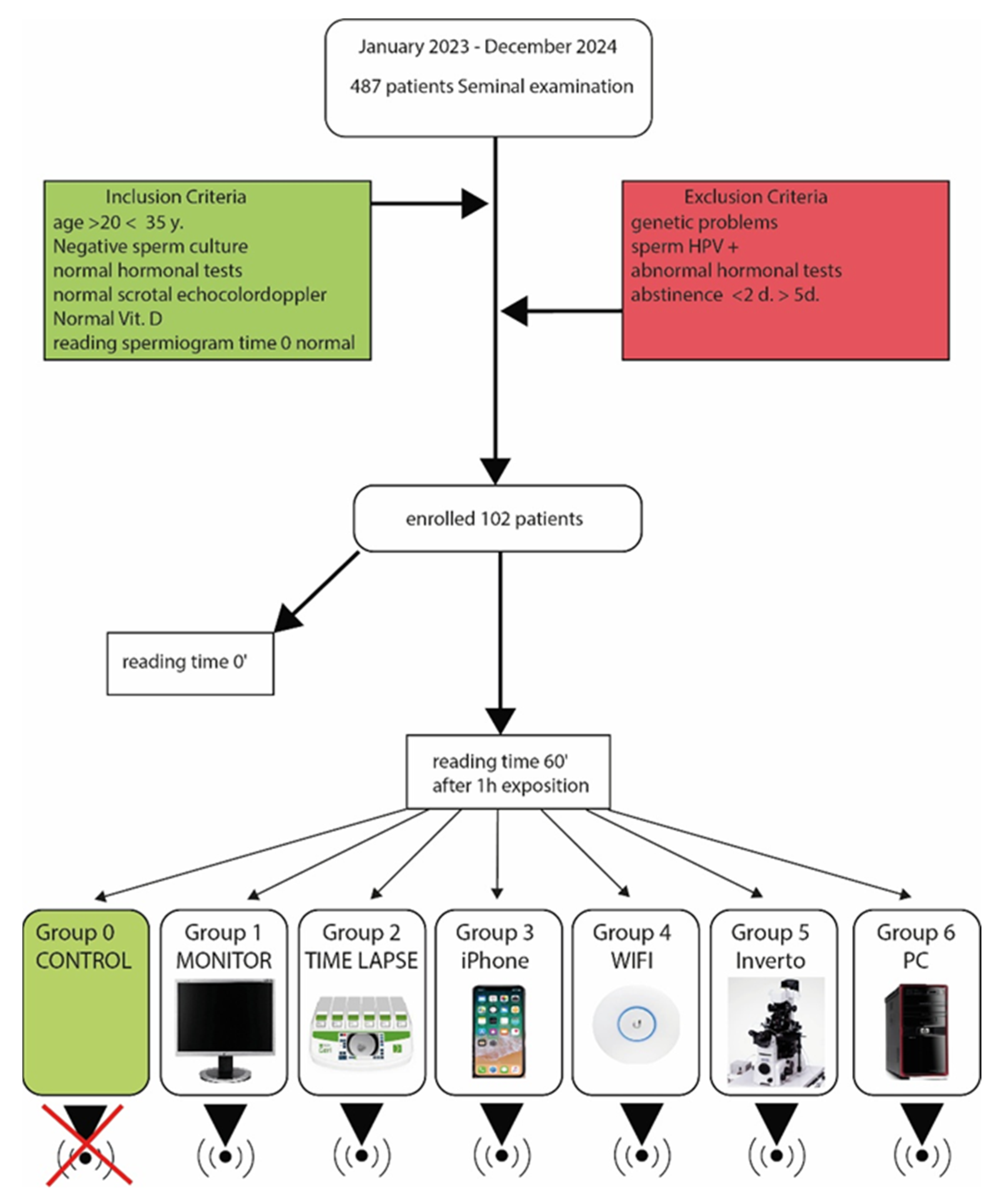
2. Materials and Methods
2.1. Exposure to Electromagnetic Fields (EMF)
2.2. Measuring Instruments
- Group 0—Control. Samples were stored under normal conditions, without exposure to EMF, at time 0 and time 60’ to ensure that any variations were solely due to exposure to electromagnetic fields.
- Group 1—Display (HP Monitor, HP Enterprise, Palo Alto, CA, USA). The samples were exposed to EMFs emitted by a 24-inch LCD monitor, placed at a distance of 10 cm from the semen samples for 1 h. The electric field power of the monitor was around 0.1 W.
- Group 2—Time-Lapse Incubator. The samples were exposed to EMFs from a time-lapse incubator (Incubator Geri®, Genea Biomedx, Kent Street, Sidney, Australia) (output power: 0.5 W) at a distance of 10 cm from the sperm samples for 1 h.
- Group 3—iPhone Cell Phone. The samples were exposed to radiation emitted by an Apple iPhone 12 mobile phone (output power: 0.1 W) (Apple Computer, Cupertino, CA, USA). The device was kept at a distance of 10 cm from the semen sample for 1 h.
- Group 4—Ubiquiti Wi-Fi Repeater. The samples were exposed to EMFs emitted by a Ubiquiti UniFi 6 long-range Wi-Fi repeater (Ubiquiti, 685 Third Avenue, New York, NY, USA), utilising 2.4 GHz and 5 GHz Wi-Fi technology with an emission power of approximately 20 dBm (decibel milliwatts), which corresponds to approximately 100 mW (milliwatts). As in all other cases, the sample was placed at a distance of 10 cm for 1 h
- Group 5—Nikon Invertoscope Model Ti (Nikon Instruments, Tokyo, Japan). The samples were exposed to EMFs generated by an invertoscope (semen analysis microscope) with an output power of 0.15 W, placed 10 cm away from the semen samples for 1 h.
- Group 6—HP Pavillon TP 01 PC (HP Enterprise, Palo Alto, CA, USA). The samples were exposed to EMFs emitted by a laptop, as Wi-Fi or Bluetooth devices typically emit at very low powers (around 0.2 W), and placed at a distance of 10 cm from the semen samples for 1 h.
2.3. Evaluation of Sperm Motility
- Progressive motility: percentage of spermatozoa that move in a straight line (progressive);
- Non-progressive motility;
- Immotile.
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Limitations of the Study
4.2. Implications and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agarwal, A.; Deepinder, F.; Sharma, R.K.; Ranga, G.; Li, J. Effect of cell phone usage on semen analysis in men attending infertility clinic: An observational study. Fertil. Steril. 2008, 89, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Sivani, S.; Sudarsanam, D. Impacts of radio-frequency electromagnetic field (RF-EMF) from cell phone towers and wireless devices on biosystem and ecosystem—A review. Biol. Med. 2012, 4, 202–216. [Google Scholar]
- Fejes, I.; Zavaczki, Z.; Szollosi, J.; Koloszar, S.; Daru, J.; Kovacs, L.; Pal, A. Is there a relationship between cell phone use and semen quality? Arch. Androl. 2005, 51, 385–393. [Google Scholar] [CrossRef]
- Agarwal, A.; Desai, N.R.; Makker, K.; Varghese, A.; Mouradi, R.; Sabanegh, E.; Sharma, R. Effects of radiofrequency electromagnetic waves (RF-EMW) from cellular phones on human ejaculated semen: An in vitro pilot study. Fertil. Steril. 2009, 92, 1318–1325. [Google Scholar] [CrossRef]
- De Iuliis, G.N.; Newey, R.J.; King, B.V.; Aitken, R.J. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS ONE 2009, 4, e6446. [Google Scholar] [CrossRef]
- Houston, B.J.; Nixon, B.; King, B.V.; De Iuliis, G.N.; Aitken, R.J. The effects of radiofrequency electromagnetic radiation on sperm function. Reproduction 2016, 152, R263–R276. [Google Scholar] [CrossRef]
- Falzone, N.; Huyser, C.; Becker, P.; Leszczynski, D.; Franken, D.R. The effect of pulsed 900-MHz GSM mobile phone radiation on the acrosome reaction, head morphometry and zona binding of human spermatozoa. Int. J. Androl. 2011, 34, 20–26. [Google Scholar] [CrossRef]
- Mailankot, M.; Kunnath, A.P.; Jayalekshmi, H.; Koduru, B.; Valsalan, R. Radio frequency electromagnetic radiation (RF-EMR) from GSM (0.9/1.8 GHz) mobile phones induces oxidative stress and reduces sperm motility in rats. Clinics 2009, 64, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Dasdag, S.; Akdag, M.Z.; Aksen, F.; Yilmaz, F.; Bashan, M.; Mutlu Dasdag, M.; Salih Celik, M. Whole body exposure of rats to microwaves emitted from a cell phone does not affect the testes. Bioelectromagnetics 2009, 24, 182–188. [Google Scholar] [CrossRef]
- Kesari, K.K.; Kumar, S.; Nirala, J.; Siddiqui, M.H.; Behari, J. Biophysical evaluation of radiofrequency electromagnetic field effects on male reproductive pattern. Cell Biochem. Biophys. 2013, 65, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Dastgheib, S.A. Electromagnetic fields and male fertility: A systematic review of the literature. Int. J. Reprod. Biomed. 2020, 18, 325–330. [Google Scholar]
- Yildirim, M.E.; Kaynar, M.; Badem, H.; Cavis, M.; Karatas, O.F. What is harmful for male fertility: Cell phone or the wireless internet? Kaohsiung J. Med. Sci. 2015, 31, 480–484. [Google Scholar] [CrossRef]
- Avendano, C.; Mata, A.; Sanchez Sarmiento, C.A.; Doncel, G.F. Use of laptop computers connected to internet through Wi-Fi decreases human sperm motility and increases sperm DNA fragmentation. Fertil. Steril. 2012, 97, 39–45.e2. [Google Scholar] [CrossRef]
- Kesari, K.K.; Kumar, S.; Behari, J. Mobile phone usage and male infertility in Wistar rats. Indian J. Exp. Biol. 2010, 48, 987–992. [Google Scholar]
- Agarwal, A.; Singh, A.; Hamada, A.; Kesari, K. Cell phones and male infertility: A review of recent innovations in technology and consequences. Int. Braz. J. Urol. 2011, 37, 432–454. [Google Scholar] [CrossRef]
- Falzone, N.; Huyser, C.; Becker, P.; Leszczynski, D. The effect of pulsed 900 MHz GSM mobile phone radiation on the acrosome reaction of human spermatozoa. Int. J. Androl. 2008, 33, 88–94. [Google Scholar]
- Gutschi, T.; Mohamad Al-Ali, B.; Shamloul, R.; Pummer, K.; Trummer, H. Impact of cell phone use on men’s semen parameters. Andrology 2011, 43, 312–316. [Google Scholar] [CrossRef]
- Sepehrimanesh, M.; Kazemipour, N.; Saeb, M.; Nazifi, S.; Jelodar, G. Analysis of rat testicular proteome following 30 days exposure to 900 MHz electromagnetic field radiation. J. Proteome Res. 2014, 13, 4370–4379. [Google Scholar] [CrossRef]
- Jurewicz, J.; Radwan, M.; Sobala, W.; Hanke, W. Lifestyle and semen quality: Role of modifiable risk factors. Syst. Biol. Reprod. Med. 2013, 60, 43–51. [Google Scholar] [CrossRef]
- Cordelli, E.; Ardoino, L.; Benassi, B.; Consales, C.; Eleuteri, P.; Marino, C.; Sciortino, M.; Villani, P.; Brinkworth, M.; Chen, G.; et al. Effects of radiofrequency electromagnetic field (RF-EMF) exposure on male fertility: A systematic review of experimental studies on non-human mammals and human sperm in vitro. Environ. Int. 2024, 185, 108509. [Google Scholar] [CrossRef]
- Rahban, R.; Senn, A.; Nef, S.; Roosli, M. Association between self-reported mobile phone use and the semen quality of young men. Fertil. Steril. 2023, 120, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Rai, U.; Singh, R. Genotoxic Risks to Male Reproductive Health from Radiofrequency Radiation. Cells 2023, 12, 594. [Google Scholar] [CrossRef]
- Motchidlover, L.; Sari-Minodier, I.; Sunyach, C.; Metzler-Guillemain, C.; Perrin, J. Impact of non-ionising radiation of male fertility: A systematic review. Fr. J. Urol. 2025, 35, 102800. [Google Scholar] [CrossRef]
- Kenny, R.P.; Johnson, E.E.; Adesanya, A.M.; Richmond, C.; Beyer, F.; Calderon, C.; Rankin, J.; Pearce, M.S.; Toledano, M.; Craig, D.; et al. The effects of radiofrequency exposure on male fertility: A systematic review of human observational studies with dose-response meta-analysis. Environ. Int. 2024, 190, 108817. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.Y.; Khodamoradi, K.; Blachman-Braun, R.; Dullea, A.; Bidhan, J.; Campbell, K.; Zizzo, J.; Israeli, J.; Kim, M.; Petrella, F.; et al. Effect of Radiofrequency Electromagnetic Radiation Emitted by Modern Cellphones on Sperm Motility and Viability: An In Vitro Study. Eur. Urol. Focus 2023, 9, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Gharib, T.M.; Almekaty, K.; Abdel Aal, A.M.; Abdel-Al, I.; Deif, H.; Hassan, G.M.; Haty, A.; Alhefnawy, M.A. Effect of radiofrequency electromagnetic waves of mobile phone stations on male fertility. Arch. Ital. Urol. Androl. 2024, 96, 12595. [Google Scholar] [CrossRef]
- Keskin, I.; Karabulut, S.; Kaplan, A.A.; Alagoz, M.; Akdeniz, M.; Tufekci, K.K.; Davis, D.L.; Kaplan, S. Preliminary study on the impact of 900 MHz radiation on human sperm: An in vitro molecular approach. Reprod. Toxicol. 2024, 130, 108744. [Google Scholar] [CrossRef]
- Yu, G.; Bai, Z.; Song, C.; Cheng, Q.; Wang, G.; Tang, Z.; Yang, S. Current progress on the effect of mobile phone radiation on sperm quality: An updated systematic review and meta-analysis of human and animal studies. Environ. Pollut. 2021, 282, 116952. [Google Scholar] [CrossRef]
- Sciorio, R.; Tramontano, L.; Esteves, S.C. Effects of mobile phone radiofrequency radiation on sperm quality. Zygote 2022, 30, 159–168. [Google Scholar] [CrossRef]
- Pacchierotti, F.; Ardoino, L.; Benassi, B.; Consales, C.; Cordelli, E.; Eleuteri, P.; Marino, C.; Sciortino, M.; Brinkworth, M.; Chen, G.; et al. Effects of Radiofrequency Electromagnetic Field (RF-EMF) exposure on male fertility and pregnancy and birth outcomes: Protocols for a systematic review of experimental studies in non-human mammals and in human sperm exposed in vitro. Environ. Int. 2021, 157, 106806. [Google Scholar] [CrossRef]
- Kim, S.; Han, D.; Ryu, J.; Kim, K.; Kim, Y.H. Effects of mobile phone usage on sperm quality—No time-dependent relationship on usage: A systematic review and updated meta-analysis. Environ. Res. 2021, 202, 111784. [Google Scholar] [CrossRef] [PubMed]
- Jaffar, F.H.F.; Osman, K.; Ismail, N.H.; Chin, K.Y.; Ibrahim, S.F. Adverse Effects of Wi-Fi Radiation on Male Reproductive System: A Systematic Review. Tohoku J. Exp. Med. 2019, 248, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Rai, U.; Singh, R. Radiofrequency radiation: A possible threat to male fertility. Reprod. Toxicol. 2021, 100, 90–100. [Google Scholar] [CrossRef]
- Zha, X.D.; Wang, W.W.; Xu, S.; Shang, X.J. Impacts of electromagnetic radiation from cellphones and Wi-Fi on spermatogenesis. Zhonghua Nan Ke Xue 2019, 25, 451–455. [Google Scholar]
- Sterling, L.; Harris, L.R.; Carroll, K. The effects of wireless devices on male reproductive health: A literature overview. Rev. Int. Androl. 2022, 20, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh-Taheri, M.; Khalili, M.A.; Hosseininejad Mohebati, A.; Zardast, M.; Hosseini, M.; Palmerini, M.G.; Doostabadi, M.R. The detrimental effect of cell phone radiation on sperm biological characteristics in normozoospermic. Andrology 2022, 54, e14257. [Google Scholar] [CrossRef]
- Okechukwu, C.E. Does the Use of Mobile Phone Affect Male Fertility? A Mini-Review. J. Hum. Reprod. Ski. 2020, 13, 174–183. [Google Scholar] [CrossRef]
- Santini, S.J.; Cordone, V.; Falone, S.; Mijit, M.; Tatone, C.; Amicarelli, F.; Di Emidio, G. Role of Mitochondria in the Oxidative Stress Induced by Electromagnetic Fields: Focus on Reproductive Systems. Oxidative Med. Cell. Longev. 2018, 2018, 5076271. [Google Scholar] [CrossRef]
- Gautam, R.; Priyadarshini, E.; Nirala, J.; Rajamani, P. Impact of nonionizing electromagnetic radiation on male infertility: An assessment of the mechanism and consequences. Int. J. Radiat. Biol. 2022, 98, 1063–1073. [Google Scholar] [CrossRef]
- Kamali, K.; Atarod, M.; Sarhadi, S.; Nikbakht, J.; Emami, M.; Maghsoudi, R.; Salimi, H.; Fallahpour, B.; Kamali, N.; Momtazan, A.; et al. Effects of electromagnetic waves emitted from 3G+wi-fi modems on human semen analysis. Urology 2017, 84, 209–214. [Google Scholar] [CrossRef]
- Chen, H.G.; Wu, P.; Sun, B.; Chen, J.X.; Xiong, C.L.; Meng, T.Q.; Huang, X.Y.; Su, Q.L.; Zhou, H.; Wang, Y.X.; et al. Association between electronic device usage and sperm quality parameters in healthy men screened as potential sperm donors. Environ. Pollut. 2022, 312, 120089. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.A.; Galloway, T.S.; Mondal, D.; Esteves, S.C.; Mathews, F. Effect of mobile telephones on sperm quality: A systematic review and meta-analysis. Environ. Int. 2014, 70, 106–112. [Google Scholar] [CrossRef]
- Houston, B.J.; Nixon, B.; King, B.V.; Aitken, R.J.; De Iuliis, G.N. Probing theProbing the Origins of 1,800 MHz Radio Frequency Origins of 1,800 MHz Radio Frequency Electromagnetic Radiation Induced Damage in Mouse Immortalized Germ Cells and Spermatozoa in vitro. Front Public Health 2018, 6, 270. [Google Scholar] [CrossRef]
- Nakatani-Enomoto, S.; Okutsu, M.; Suzuki, S.; Suganuma, R.; Groiss, S.J.; Kadowaki, S.; Enomoto, H.; Fujimori, K.; Ugawa, Y. Effects of 1950 MHz W-CDMA-like signal on human spermatozoa. Bioelectromagnetics 2016, 37, 373–381. [Google Scholar] [CrossRef]
- Liu, K.; Li, Y.; Zhang, G.; Liu, J.; Cao, J.; Ao, L.; Zhang, S. Association between mobile phone use and semen quality: A systemic review and meta-analysis. Andrology 2014, 2, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Erogul, O.; Oztas, E.; Yildirim, I.; Kir, T.; Aydur, E.; Komesli, G.; Irkilata, H.C.; Irmak, M.K.; Peker, A.F. Effects of electromagnetic radiation from a cellular phone on human sperm motility: An in vitro study. Arch. Med. Res. 2006, 37, 840–843. [Google Scholar] [CrossRef]
- Zalata, A.; El-Samanoudy, A.Z.; Shaalan, D.; El-Baiomy, Y.; Mostafa, T. In vitro effect of cell phone radiation on motility, DNA fragmentation and clusterin gene expression in human sperm. Int. J. Fertil. Steril. 2015, 9, 129–136. [Google Scholar]
- Deepinder, F.; Makker, K.; Agarwal, A. Cell phones and male infertility: Dissecting the relationship. Reprod. Biomed. Online 2007, 15, 266–270. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.A.; Vicari, E.; D’Agata, R.; Calogero, A.E. Effects of the exposure to mobile phones on male reproduction: A review of the literature. J. Androl. 2012, 33, 350–356. [Google Scholar] [CrossRef]
- Hagras, A.M.; Toraih, E.A.; Fawzy, M.S. Mobile phones electromagnetic radiation and NAD+-dependent isocitrate dehydrogenase as a mitochondrial marker in asthenozoospermia. Biochim. Open 2016, 3, 19–25. [Google Scholar] [CrossRef]
- Desai, N.R.; Kesari, K.K.; Agarwal, A. Pathophysiology of cell phone radiation: Oxidative stress and carcinogenesis with focus on male reproductive system. Reprod. Biol. Endocrinol. 2009, 7, 114. [Google Scholar] [CrossRef] [PubMed]


| Time 0’ | Time 60’ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abst. day | Vol. ml. | ph | Nor. Morp. In% | Conc. Mil/mL | Total Mil. | PR% | NP% | IM% | PR% | NP% | IM% | |
| average | 3.7 | 3.64 | 7.6 | 9.75% | 61.79 | 223.65 | 47.2% | 14.5% | 38.3% | 45.08% | 16.4% | 38.7% |
| SD | 0.7 | 1.22 | 0.2 | 3.41% | 30.55 | 142.77 | 10.3 | 6.12 | 11.7 | 9.242 | 5.6 | 9.73 |
| M_M | M_A | TL_M | TL_A | P_M | P_A | W_M | W_A | I_M | I_A | PC_M | PC_A | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I.M.F. mV | 1161 | 209.3 | 294.5 | 11.6 | 1610.6 | 295.0 | 4259.2 | 241.5 | 299.2 | 8.7 | 624.1 | 174.2 |
| SD | 88.2 | 8.3 | 15.2 | 2.4 | 122.2 | 12.9 | 229.4 | 17.2 | 22.6 | 2.1 | 15.7 | 11.2 |
| Group 1 Monitor | Group 2 Timelapse | Group 3 iPhone | Group 4 Wifi | Group 5 Invertoscope | Group 6 PC | Average of the Exposed Groups | Group 0 Control | Group 0 vs. Average Group 1 + 2 + 3 + 4 + 5 + 6 (Test t Student) | |
|---|---|---|---|---|---|---|---|---|---|
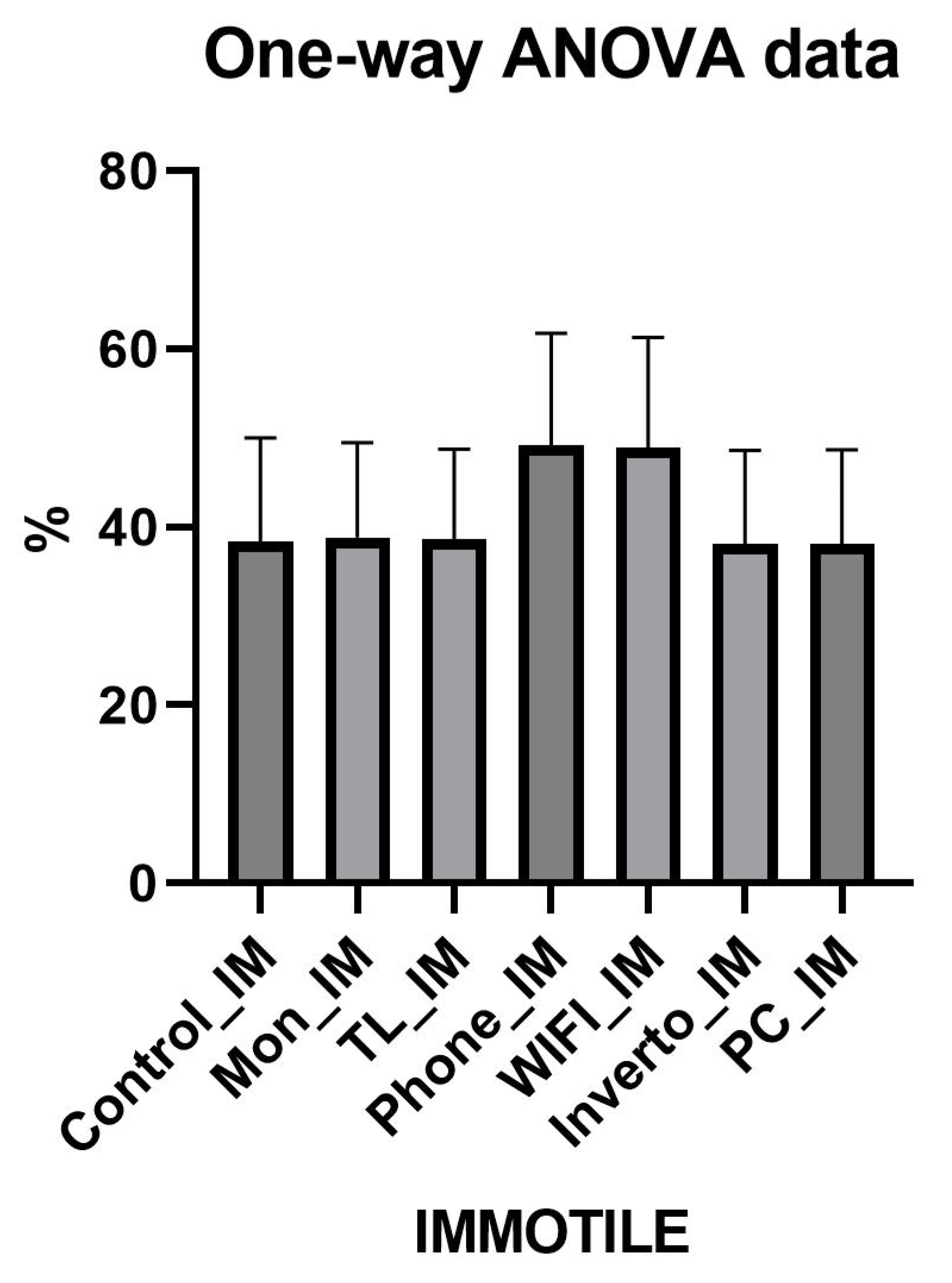
| PR | 44% | 44.7% | 25% | 25% | 45% | 45% | 38.11% | 45.1% | N.S. |
| NP | 16.9% | 17% | 25.9% | 26% | 17% | 17% | 19.9% | 16% | N.S. |
| IM | 38.9% | 39% | 49% | 49% | 38% | 38% | 41.9% | 39% | N.S. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldini, G.M.; Lot, D.; Ferri, D.; Montano, L.; Tartagni, M.V.; Malvasi, A.; Laganà, A.S.; Palumbo, M.; Baldini, D.; Trojano, G. Does Electromagnetic Pollution in the ART Laboratory Affect Sperm Quality? A Cross-Sectional Observational Study. Toxics 2025, 13, 510. https://doi.org/10.3390/toxics13060510
Baldini GM, Lot D, Ferri D, Montano L, Tartagni MV, Malvasi A, Laganà AS, Palumbo M, Baldini D, Trojano G. Does Electromagnetic Pollution in the ART Laboratory Affect Sperm Quality? A Cross-Sectional Observational Study. Toxics. 2025; 13(6):510. https://doi.org/10.3390/toxics13060510
Chicago/Turabian StyleBaldini, Giorgio Maria, Dario Lot, Daniele Ferri, Luigi Montano, Mario Valerio Tartagni, Antonio Malvasi, Antonio Simone Laganà, Mario Palumbo, Domenico Baldini, and Giuseppe Trojano. 2025. "Does Electromagnetic Pollution in the ART Laboratory Affect Sperm Quality? A Cross-Sectional Observational Study" Toxics 13, no. 6: 510. https://doi.org/10.3390/toxics13060510
APA StyleBaldini, G. M., Lot, D., Ferri, D., Montano, L., Tartagni, M. V., Malvasi, A., Laganà, A. S., Palumbo, M., Baldini, D., & Trojano, G. (2025). Does Electromagnetic Pollution in the ART Laboratory Affect Sperm Quality? A Cross-Sectional Observational Study. Toxics, 13(6), 510. https://doi.org/10.3390/toxics13060510









