Modification of Biochar Catalyst Using Copper for Enhanced Catalytic Oxidation of VOCs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Modified Biochar Catalyst Using Copper
2.3. Catalytic Experiment
2.4. Theoretical Calculations
2.5. Analysis Method
3. Results and Discussion
3.1. Study on Preparation Method of Catalyst
3.1.1. Influence of Cu Loading on Catalyst Performance
3.1.2. Influence of Enzymatic Treatment on Catalyst Performance
3.1.3. Thermal Stability of Catalytic Materials at Different Reaction Temperatures
3.2. Effect of Operation Parameters on Catalyst Performance
3.3. Characterization
3.3.1. Surface Structure Analysis
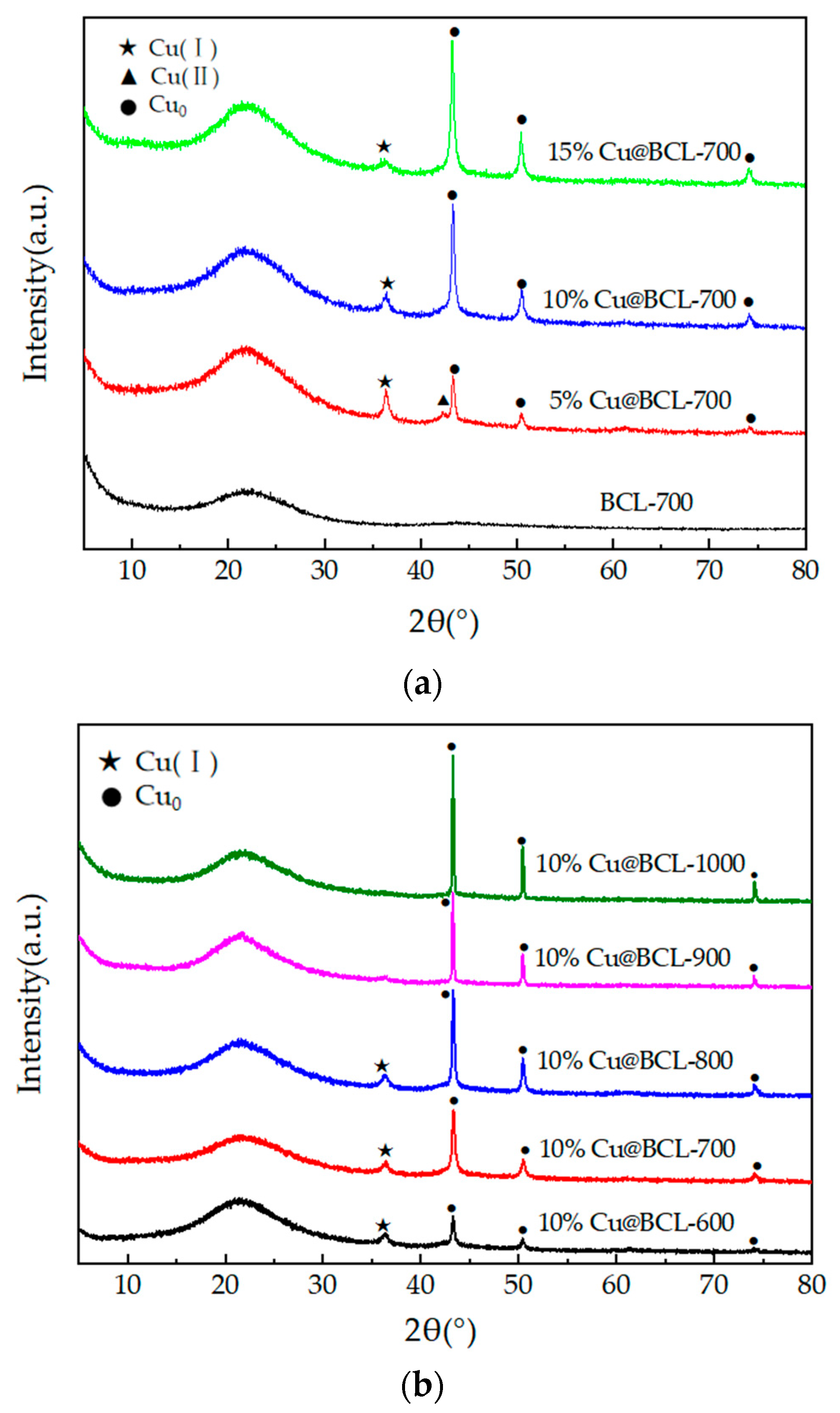
3.3.2. XRD Analysis
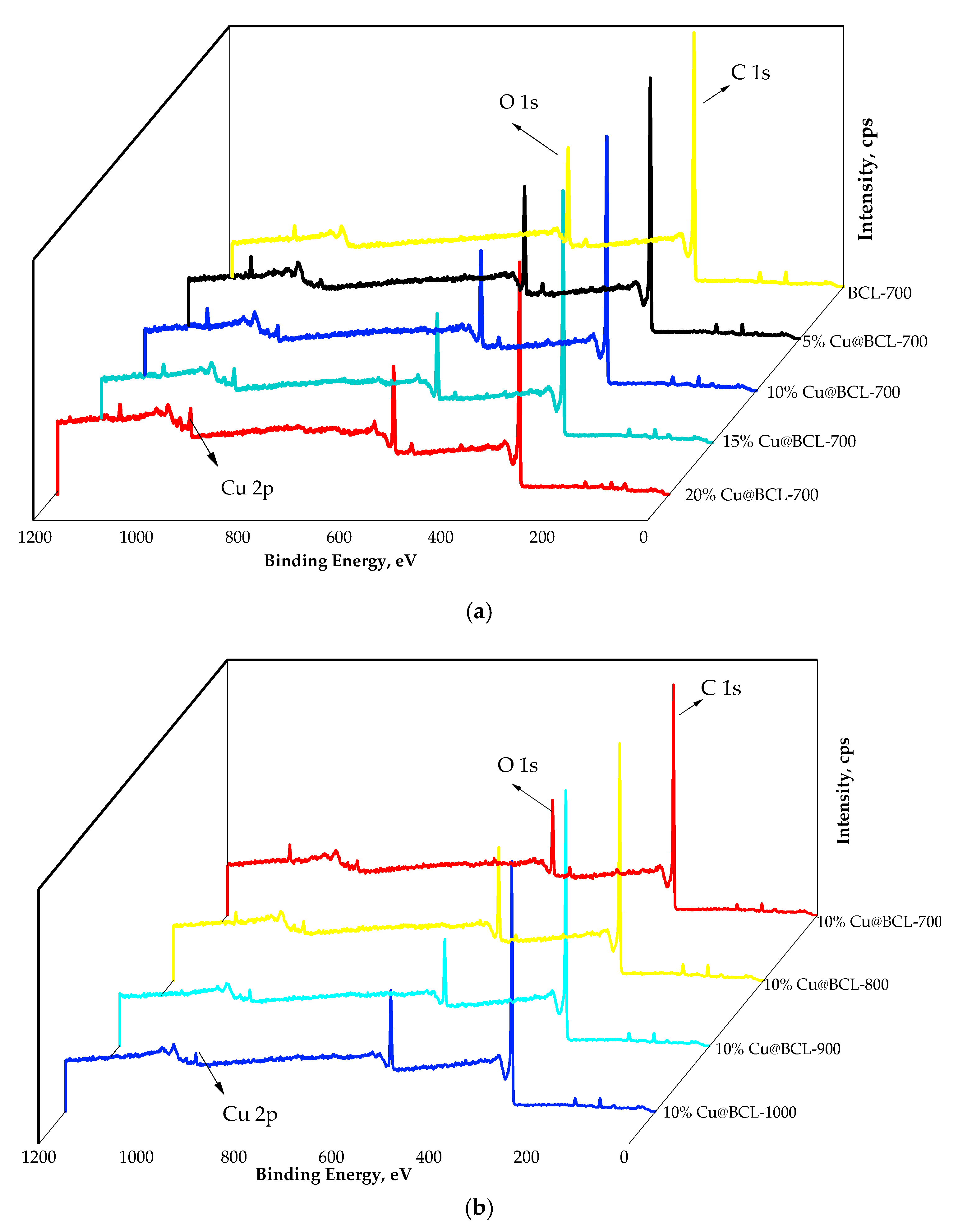
3.3.3. XPS Analysis
3.3.4. Functional Group Analysis
3.4. Macroscopic Catalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, K.; Zhou, X.Y.; Wang, Y.; Li, J.Y.; Yu, S.G.; Liu, H.J.; Jiang, J.S.; Yi, P. Analysis of emission characteristics and driving forces of air pollutants and GHG from coal-fired industrial boilers in China. J. Clean. Prod. 2023, 430, 139768. [Google Scholar] [CrossRef]
- Gong, P.J.; He, F.; Xie, J.L.; Fang, D. Catalytic removal of toluene using MnO2-based catalysts: A review. Chemosphere 2023, 318, 137938. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Scott, J.; Bagheri, A.; Dai, H.X.; Amal, R. Recent advances in ordered meso/macroporous metal oxides for heterogeneous catalysis: A review. J. Mater. Chem. A 2017, 5, 8825–8846. [Google Scholar] [CrossRef]
- Wissem, B.S.; Sun, J.; Wang, W.L.; Song, Z.L.; Zhao, X.Q.; Mao, Y.P.; Zhang, Z.C. Discovering the key role of MnO2 and CeO2 particles in the Fe2O3 catalysts for enhancing the catalytic oxidation of VOC: Synergistic effect of the lattice oxygen species and surface-adsorbed oxygen. Sci. Total Environ. 2022, 819, 152844. [Google Scholar] [CrossRef]
- Song, H.; Hu, F.Y.; Peng, Y.; Li, K.Z.; Bai, S.P.; Li, J.H. Non-thermal plasma catalysis for chlorobenzene removal over CoMn/TiO2 and CeMn/TiO2: Synergistic effect of chemical catalysis and dielectric constant. Chem. Eng. J. 2018, 347, 447–454. [Google Scholar] [CrossRef]
- Zhu, X.B.; Zhang, S.; Yang, Y.; Zheng, C.H.; Zhou, J.S.; Gao, X.; Tu, X. Enhanced performance for plasma-catalytic oxidation of ethyl acetate over La1−x CexCoO3+δ catalysts. Appl. Catal. B Environ. 2017, 213, 97–105. [Google Scholar] [CrossRef]
- Georgiou, Y.; Smyrnioti, M.; Ioannides, T. Co3O4 catalysts for complete toluene oxidation: Review including meta-analysis of catalyst activity data. Catalysts 2023, 13, 1454. [Google Scholar] [CrossRef]
- Li, J.T.; Xu, Z.L.; Wang, T.; Xie, X.W.; Li, D.D.; Wang, J.G.; Huang, H.B.; Ao, Z.M. A versatile route to fabricate Metal/UiO-66 (Metal = Pt, Pd, Ru) with high activity and stability for the catalytic oxidation of various volatile organic compounds. Chem. Eng. J. 2022, 448, 136900. [Google Scholar] [CrossRef]
- Gupta, N.; Santhiya, D.; Murugavel, S.; Kumar, A.; Aditya, A.; Ganguli, M.; Gupta, S. Effects of transition metal ion dopants (Ag, Cu and Fe) on the structural, mechanical and antibacterial properties of bioactive glass. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 393–403. [Google Scholar] [CrossRef]
- Ma, J.Z.; Wang, C.X.; He, H. Transition metal doped cryptomelane-type manganese oxide catalysts for ozone decomposition. Appl. Catal. B Environ. 2017, 201, 503–510. [Google Scholar] [CrossRef]
- Xiao, G.F.; Guo, Z.Y.; Lin, B.L.; Fu, M.L.; Ye, D.Q.; Hu, Y. Cu-WVT Catalysts for Synergistic Elimination of NOx and Volatile Organic Compounds from Coal-Fired Flue Gas. Environ. Sci. Technol. 2022, 56, 10095–10104. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Zhang, H.X.; Hong, Q.L.; Yi, L.C.; Li, Q.H.; Zhang, J. Enhancing CO2 electroreduction to ethylene via copper-silver tandem catalyst in boron-imidazolate framework nanosheet. Adv. Energy Mater. 2023, 13, 2300088. [Google Scholar] [CrossRef]
- Meng, J.C.; Zhou, F.; Ma, H.X.; Yuan, X.Z.; Wang, Y.J.; Zhang, J. A review of catalysts for methylcyclohexane dehydrogenation. Top. Catal. 2021, 64, 509–520. [Google Scholar] [CrossRef]
- Liu, Y.D.; Li, B.; Tong, W.K.; Tang, H.; Ping, Z.L.; Wang, W.J.; Gao, M.T.; Dai, C.M.; Liu, N.; Hu, J.J.; et al. Eco-friendly, stable, and high-performance biochar prepared by a twice-modification scheme: Saccharification of raw materials & thermal air oxidation of biochar. J. Environ. Manag. 2024, 371, 123226. [Google Scholar] [CrossRef]
- Huang, H.L.; Huang, Z.H.; Zhou, W.J. Comparative study of Ti-alkaline biochar and Ti-acidic biochar photo catalysts for degradation of methyl orange. Clean Technol. Environ. Policy 2023, 25, 1069–1077. [Google Scholar] [CrossRef]
- Du, J.L.; Shen, T.H.; Hu, J.H.; Zhang, F.X.; Yang, S.L.; Liu, H.L.; Wang, H. Study on thermochemical conversion of triglyceride biomass catalyzed by biochar catalyst. Energy 2023, 277, 127733. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, Y.T.; Kwon, E.E.; Lee, J. Biochar as a catalytic material for the production of 1, 4-butanediol and tetrahydrofuran from furan. Environ. Res. 2020, 184, 109325. [Google Scholar] [CrossRef]
- Yang, R.J.; Fan, Y.Y.; Ye, R.Q.; Tang, Y.X.; Cao, X.H.; Yin, Z.Y.; Zeng, Z.Y. MnO2-based materials for environmental applications. Adv. Mater. 2021, 33, 2004862. [Google Scholar] [CrossRef]
- Wang, J.; Cai, J.Y.; Zhou, X.Q.; Wang, S.Q.; Luo, F.; Yang, L.; Yu, J.X.; Chi, R.; Chen, Z.Q. Accelerating of Fe2+ regeneration in Fenton reaction by biochar: Pivotal roles of carbon defects as electron donor and shuttle. Sep. Purif. Technol. 2025, 354, 128945. [Google Scholar] [CrossRef]
- Qi, G.D.; Pan, Z.F.; Zhang, X.Y.; Miao, X.D.; Xiang, W.; Gao, B. Effect of ball milling with hydrogen peroxide or ammonia hydroxide on sorption performance of volatile organic compounds by biochar from different pyrolysis temperatures. Chem. Eng. J. 2022, 450, 138027. [Google Scholar] [CrossRef]
- Zhou, Y.; Shen, C.H.; Wang, T.; Xue, Y.J. Inhibition effect of three types of biochar on volatile organic compounds from asphalt: Revealing chemical adsorption as the primary mechanism. Constr. Build. Mater. 2024, 411, 134322. [Google Scholar] [CrossRef]
- Koltowski, M.; Charmas, B.; Skubiszewska-Zieba, J.; Oleszczuk, P. Effect of biochar activation by different methods on toxicity of soil contaminated by industrial activity. Ecotoxicol. Environ. Saf. 2017, 136, 119–125. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, S.Q.; Su, X.T.; Zhang, X.L.; Cao, W.M.; Xu, Y.F. Role of the biochar modified with ZnCl2 and FeCl3 on the electrochemical degradation of nitrobenzene. Chemosphere 2021, 275, 129966. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liu, Y.H.; Tu, C.; Deng, S.P.; Hao, D.D.; Xiao, L.; Mao, M. Preparation of iron-modified biochar and its application in arsenic contaminated soil remediation. Huanjing Kexue 2023, 44, 969–974. [Google Scholar] [CrossRef]
- Hua, L.; Cheng, T.Z.; Liang, Z.Y.; Wei, T. Investigation of the mechanism of phytate-modified biochar-catalyzed persulfate degradation of Ponceau 2R. Biochar 2022, 4, 6. [Google Scholar] [CrossRef]
- Cao, D.; Niu, R.P.; Mo, G.L.; Dong, H.W.; Liu, R.; Liu, J.; Fan, J.L. Adsorption properties and competitive adsorption mechanism exhibited by carbon-nanotube-modified biochar for removal of crude oil and Ni(II) pollutants from water. Ecotoxicol. Environ. Saf. 2025, 290, 117357. [Google Scholar] [CrossRef]
- Yorgun, S.; Vural, N.; Demiral, H. Preparation of high-surface area activated carbons from Paulownia wood by ZnCl2 activation. Microporous Mesoporous Mater. 2009, 122, 189–194. [Google Scholar] [CrossRef]
- Angin, D.; Altintig, E.; Köse, T.E. Influence of process parameters on the surface and chemical properties of activated carbon obtained from biochar by chemical activation. Bioresour. Technol. 2013, 148, 542–549. [Google Scholar] [CrossRef]
- Aniduzzaman, M.; Islam, A.K.M.S. Preparation of porous bio-char and activated carbon from rice husk by leaching ash and chemical activation. SpringerPlus 2016, 5, 1248. [Google Scholar] [CrossRef]
- Li, B.; Liu, Y.D.; Tong, W.K.; Zhang, J.B.; Tang, H.; Wang, W.J.; Gao, M.T.; Dai, C.M.; Liu, N.; Hu, J.J.; et al. Effects of cellulase treatment on properties of lignocellulose-based biochar. Bioresour. Technol. 2024, 413, 131452. [Google Scholar] [CrossRef]
- Chen, X.C.; Wang, Y.; Lv, J.Q.; Feng, Z.H.; Liu, Y.T.; Xia, H.T.; Li, Y.; Wang, C.F.; Zeng, K.; Liu, Y.; et al. Simple one-pot synthesis of manganese dioxide modified bamboo-derived biochar composites for uranium(vi) removal. New J. Chem. 2022, 46, 14427–14438. [Google Scholar] [CrossRef]
- Yang, B.W.; Dai, J.W.; Zhao, Y.; Wu, J.W.; Ji, C.Y.; Zhang, Y.H. Advances in preparation, application in contaminant removal, and environmental risks of biochar-based catalysts: A review. Biochar 2022, 4, 51. [Google Scholar] [CrossRef]
- Blöchl, P.E.; Först, C.J.; Schimpl, J. Projector augmented wave method: Ab initio molecular dynamics with full wave functions. Bull. Mater. Sci. 2003, 26, 33–41. [Google Scholar] [CrossRef]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Nardelli, M.B.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with QUANTUM ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef]
- Martínez-Cruz, M.A.; Ramos-Sanchez, G.; Oliver-Tolentino, M.; Pfeiffer, H.; Gonzalez, I. Improving the Structural Reversibility of LiNiO2 by Incorporation of Cu, an Electrochemical and In-Situ XRD Study. J. Alloys Compd. 2022, 923, 166328. [Google Scholar] [CrossRef]
- Wang, K.D.; Wu, C.; Wang, F.; Jing, N.; Jiang, G.Q. Co/Co3O4 nanoparticles coupled with hollow nanoporous carbon polyhedrons for the enhanced electrochemical sensing of acetaminophen. ACS Sustain. Chem. Eng. 2019, 7, 18582–18592. [Google Scholar] [CrossRef]
- Lei, D.Y.; Liu, C.K.; Dong, C.L.; Wang, S.J.; Zhang, P.; Li, Y.; Liu, J.X.; Dong, Y.L.; Zhou, C.X. Correction to “Reduced Graphene Oxide/MXene/FeCoC Nanocomposite Aerogels Derived from Metal–Organic Frameworks toward Efficient Microwave Absorption”. ACS Appl. Nano Mater. 2024, 7, 21. [Google Scholar] [CrossRef]
- Chen, Q.; Meng, S.Y.; Liu, R.; Zhai, X.H.; Wang, X.K.; Wang, L.; Guo, H.C.; Yi, Y.H. Plasma-catalytic CO2 hydrogenation to methanol over CuO-MgO/Beta catalyst with high selectivity. Appl. Catal. B Environ. Energy 2024, 3, 342. [Google Scholar] [CrossRef]
- Wang, Y.C.; Zhu, L.; Liu, Y.; Vovk, E.I.; Lang, J.Y.; Zhou, Z.X.; Gao, P.; Li, S.G.; Yang, Y. Understanding surface structures of In2O3 catalysts during CO2 hydrogenation reaction using time-resolved IR, XPS with in situ treatment, and DFT calculations. Appl. Surf. Sci. 2023, 9, 631. [Google Scholar] [CrossRef]
- Behera, A.K.; Das, A.; Mallik, A.; Das, S. Electrochemically functionalized graphene as an Anti-corrosion reinforcement in Cu matrix composite thin Films. International J. Miner. Metall. Mater. 2021, 28, 1525–1533. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Li, Z.; Zheng, H.Y.; Hao, Z.Q.; Wang, X.; Wang, J.J. Influence of surface oxygenated groups on the formation of active Cu species and the catalytic activity of Cu/AC catalyst for the synthesis of dimethyl carbonate. Appl. Surf. Sci. 2016, 390, 68–77. [Google Scholar] [CrossRef]
- Peng, X.; Liu, S.; Li, X.J.; Wu, Z.X.; Liu, D.Q. High-efficiency transformation of O3 into ·OH via AgOx nanocatalysis in synergy with water falling film discharge for multicomponent VOCs degradation. Chem. Eng. J. 2023, 478, 147351. [Google Scholar] [CrossRef]
- Grams, J.; Ruppert, A.M. Catalyst stability-bottleneck of efficient catalytic pyrolysis. Catalysts 2021, 11, 265. [Google Scholar] [CrossRef]
- Yamane, I.; Sato, K.; Otomo, R.; Yanase, T.; Miura, A.; Nagahama, T.; Kamiya, Y.; Shimada, T. Ultrahigh-Pressure Preparation and Catalytic Activity of MOF-Derived Cu Nanoparticles. Nanomaterials 2021, 11, 1040. [Google Scholar] [CrossRef]
- Zhang, X.; Wan, D.Q.; Peng, K.; Zhang, W. Enhancement of Thermal Conductivity and Mechanical Properties of Cu-Reduced Graphene Oxide Composites by Interface Modification. J. Mater. Eng. Perform. 2019, 28, 5165–5171. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Li, S.J.; Zhang, Q.; Li, M.T.; Yang, L.; Yan, W.; Xu, H. Controlling the Phase Composition of Pre-Catalysts to Obtain Abundant Cu(111)/Cu(200) Grain Boundaries for Enhancing Electrocatalytic CO2 Reduction Selectivity to Ethylene. Small 2024, 21, 2409001. [Google Scholar] [CrossRef]
- Gurtner, D.; Kresta, M.; Hupfauf, B.; Götz, P.; Nussbaumer, R.; Hofmann, A.; Pfeifer, C. Mechanical Strength Char-acterisation of Pyrolysis Biochar from Woody Biomass. Energy 2023, 285, 129366. [Google Scholar] [CrossRef]
- Sun, F.W.; Chen, T.H.; Chu, Z.Y.; Zhai, P.X.; Liu, H.B.; Wang, Q.; Zou, X.H.; Chen, D. The synergistic effect of calcite and Cu2+ on the degradation of sulfadiazine via PDS activation: A role of Cu(III). Water Res. 2022, 219, 118529. [Google Scholar] [CrossRef]
- Liu, T.; Yin, K.; Li, N.; Liu, H.; Chen, J.; Zhou, X.; Zhang, Y. Efficient activation of peroxymonosulfate by copper supported on polyurethane foam for contaminant degradation: Synergistic effect and mechanism. Chem. Eng. J. 2022, 427, 131741. [Google Scholar] [CrossRef]
- Hu, Y.B.; Wang, Y.; Wang, Y.L.; Li, W.; Zhang, J.L.; Han, Y.H. High performance of supported Cu-based catalysts modulated via phosphamide coordination in acetylene hydrochlorination. Appl. Catal. A Gen. 2020, 591, 117468. [Google Scholar] [CrossRef]
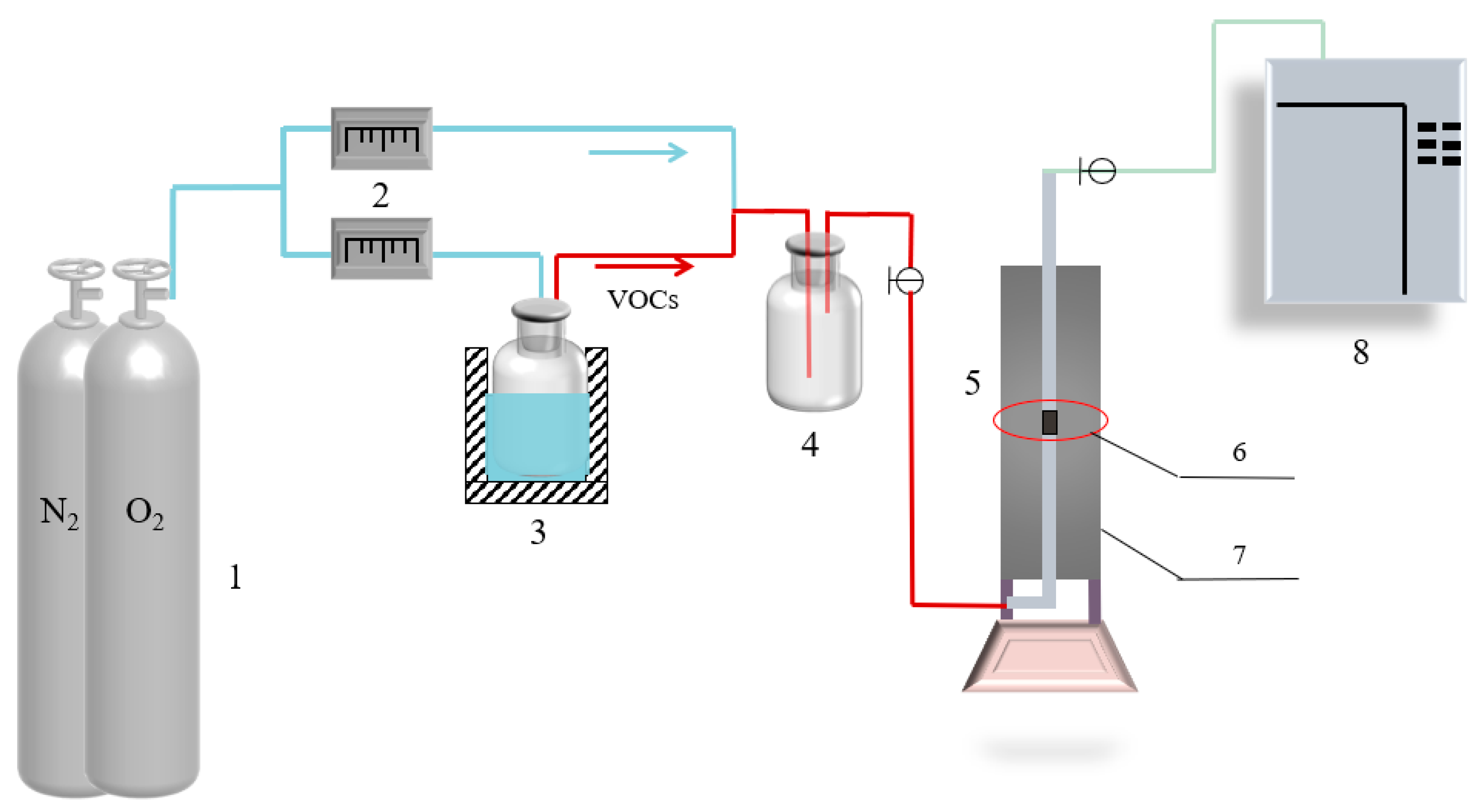


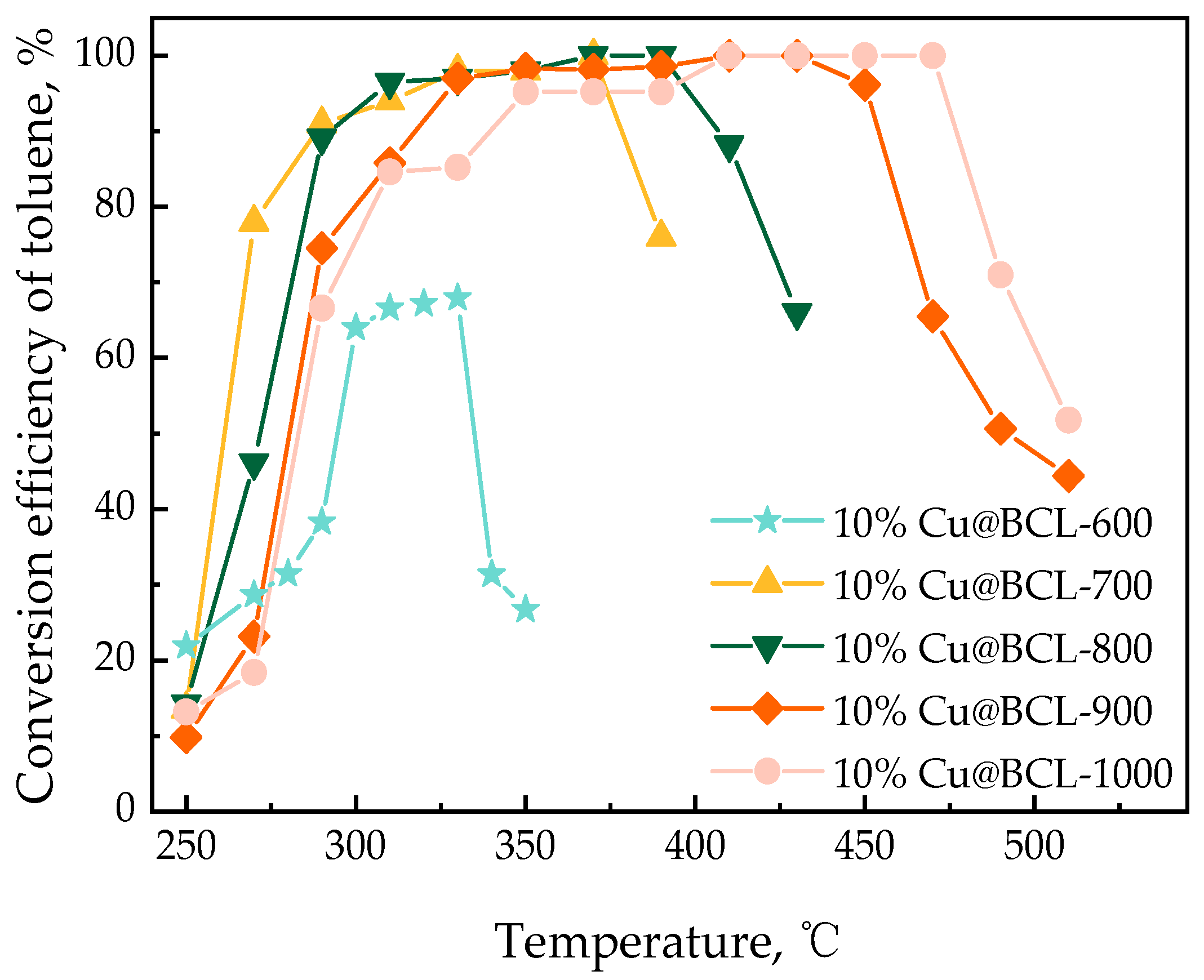
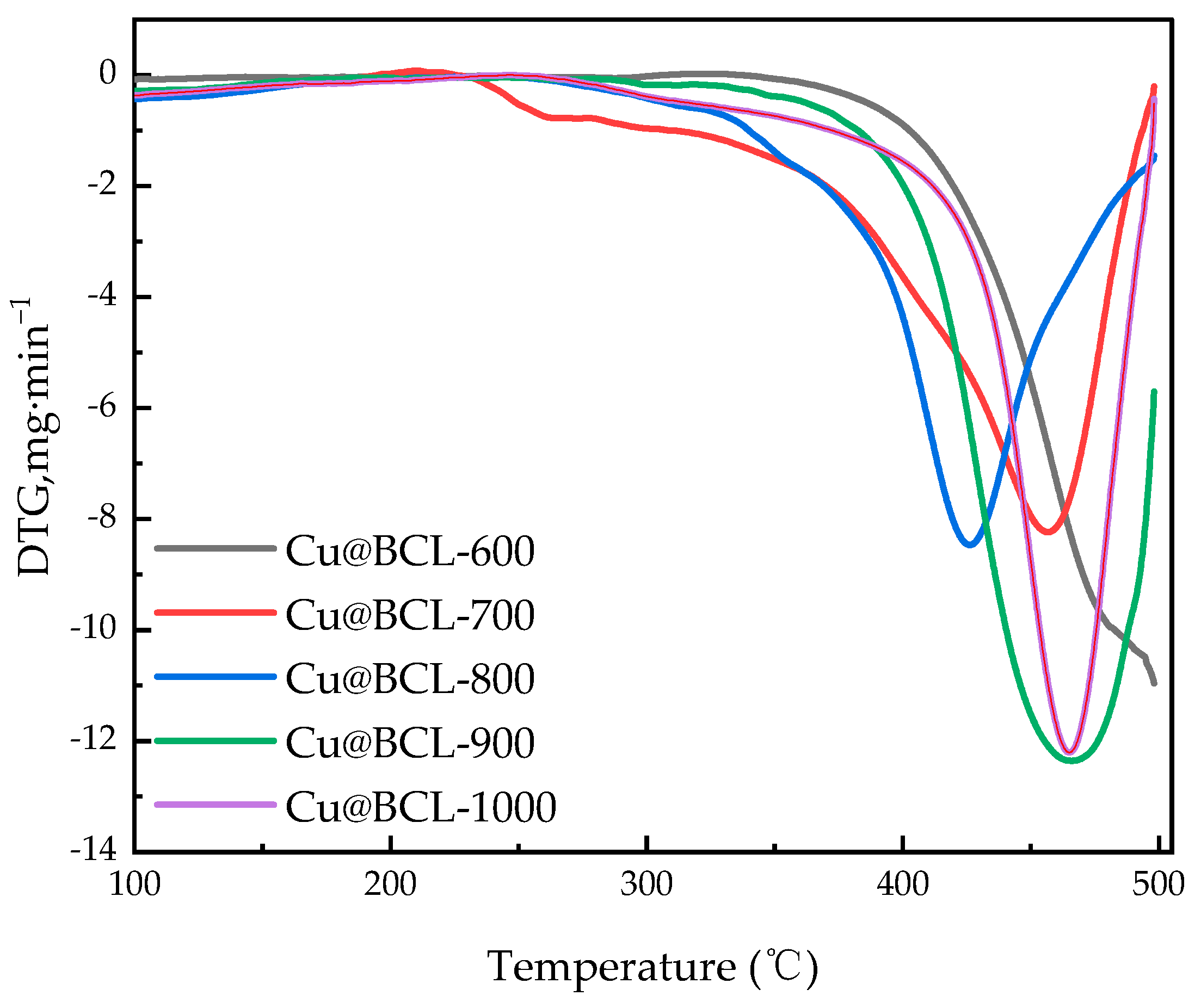
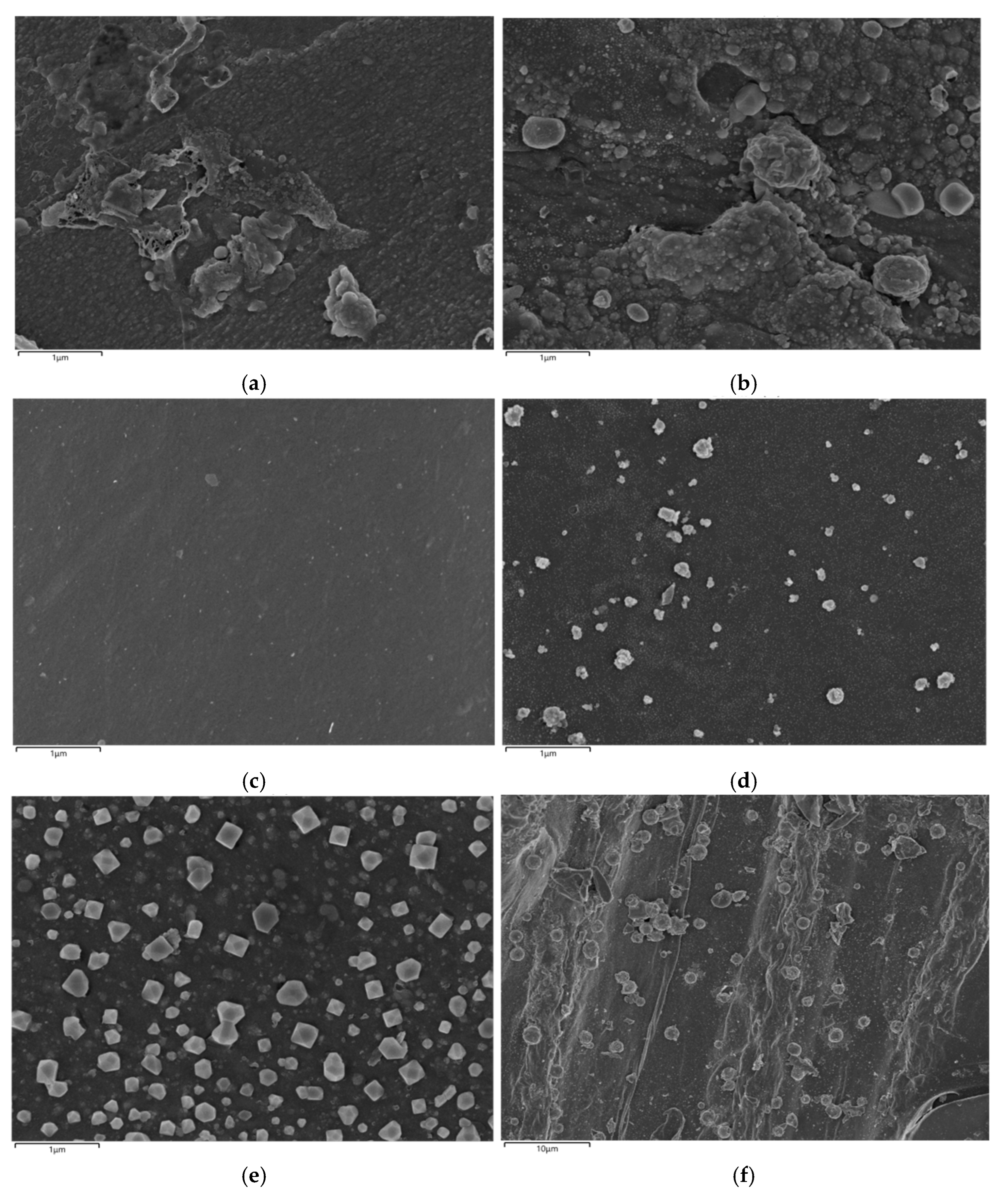



| Toluene Concentration, mg·m−3 | 500 | 1000 | 1500 | 2000 | 2500 | 3000 |
|---|---|---|---|---|---|---|
| T90, °C | 290 | 285 | 280 | 290 | 295 | 300 |
| GHSV, h−1 | 90,000 | 75,000 | 60,000 | 45,000 | 30,000 |
|---|---|---|---|---|---|
| Degradation rate of toluene, % | 84.32 | 89.09 | 95.28 | 88.63 | 83.97 |
| Sample | Specific Surface Area SBET (m2·g−1) | Total Pore Volume (cm2·g−1) | Micropore Volume (cm2·g−1) | Mean Aperture (nm) |
|---|---|---|---|---|
| BC | 254 | 0.15 | 0.11 | 2.4 |
| BCL | 216 | 0.13 | 0.09 | 2.4 |
| 5%Cu@BCL | 209 | 0.12 | 0.10 | 2.4 |
| 10%Cu@BCL | 200 | 0.11 | 0.09 | 2.3 |
| 20%Cu@BCL | 170 | 0.10 | 0.08 | 2.3 |
| 30%Cu@BCL | 153 | 0.09 | 0.07 | 2.3 |
| Cu@BCL-1000 | 302 | 0.19 | 0.12 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Zhang, J.; Cai, Y.-L.; Zhang, J.-G.; Ouyang, D.-J.; Wang, S.-B.; Xu, Q.-M.; Hu, J.-J.; Chen, D.-M.; Wang, G.-W.; et al. Modification of Biochar Catalyst Using Copper for Enhanced Catalytic Oxidation of VOCs. Toxics 2025, 13, 503. https://doi.org/10.3390/toxics13060503
Liu N, Zhang J, Cai Y-L, Zhang J-G, Ouyang D-J, Wang S-B, Xu Q-M, Hu J-J, Chen D-M, Wang G-W, et al. Modification of Biochar Catalyst Using Copper for Enhanced Catalytic Oxidation of VOCs. Toxics. 2025; 13(6):503. https://doi.org/10.3390/toxics13060503
Chicago/Turabian StyleLiu, Nan, Jin Zhang, Ya-Lan Cai, Ji-Guo Zhang, Du-Juan Ouyang, Shao-Bo Wang, Qi-Man Xu, Jia-Jun Hu, Di-Ming Chen, Guo-Wen Wang, and et al. 2025. "Modification of Biochar Catalyst Using Copper for Enhanced Catalytic Oxidation of VOCs" Toxics 13, no. 6: 503. https://doi.org/10.3390/toxics13060503
APA StyleLiu, N., Zhang, J., Cai, Y.-L., Zhang, J.-G., Ouyang, D.-J., Wang, S.-B., Xu, Q.-M., Hu, J.-J., Chen, D.-M., Wang, G.-W., & Li, J.-X. (2025). Modification of Biochar Catalyst Using Copper for Enhanced Catalytic Oxidation of VOCs. Toxics, 13(6), 503. https://doi.org/10.3390/toxics13060503






