Microplastics in Stormwater: Sampling and Methodology Challenges
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Stormwater Samples Collection
2.3. Sample Treatment
2.4. MP Characterization
2.4.1. Visual Analysis
2.4.2. Fluorescence Analysis
2.4.3. Raman Analysis
2.5. Quality Assurance
3. Results
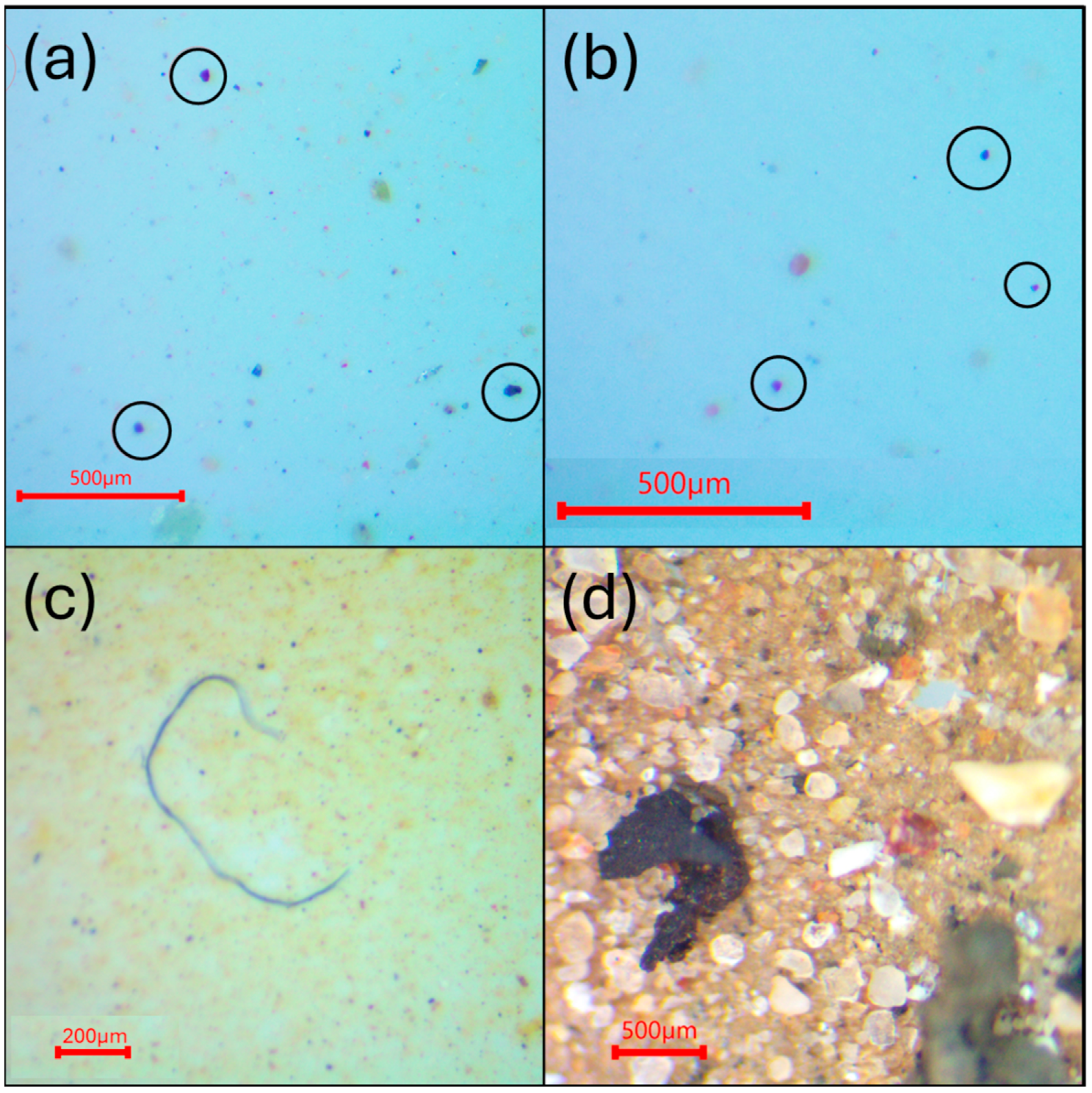
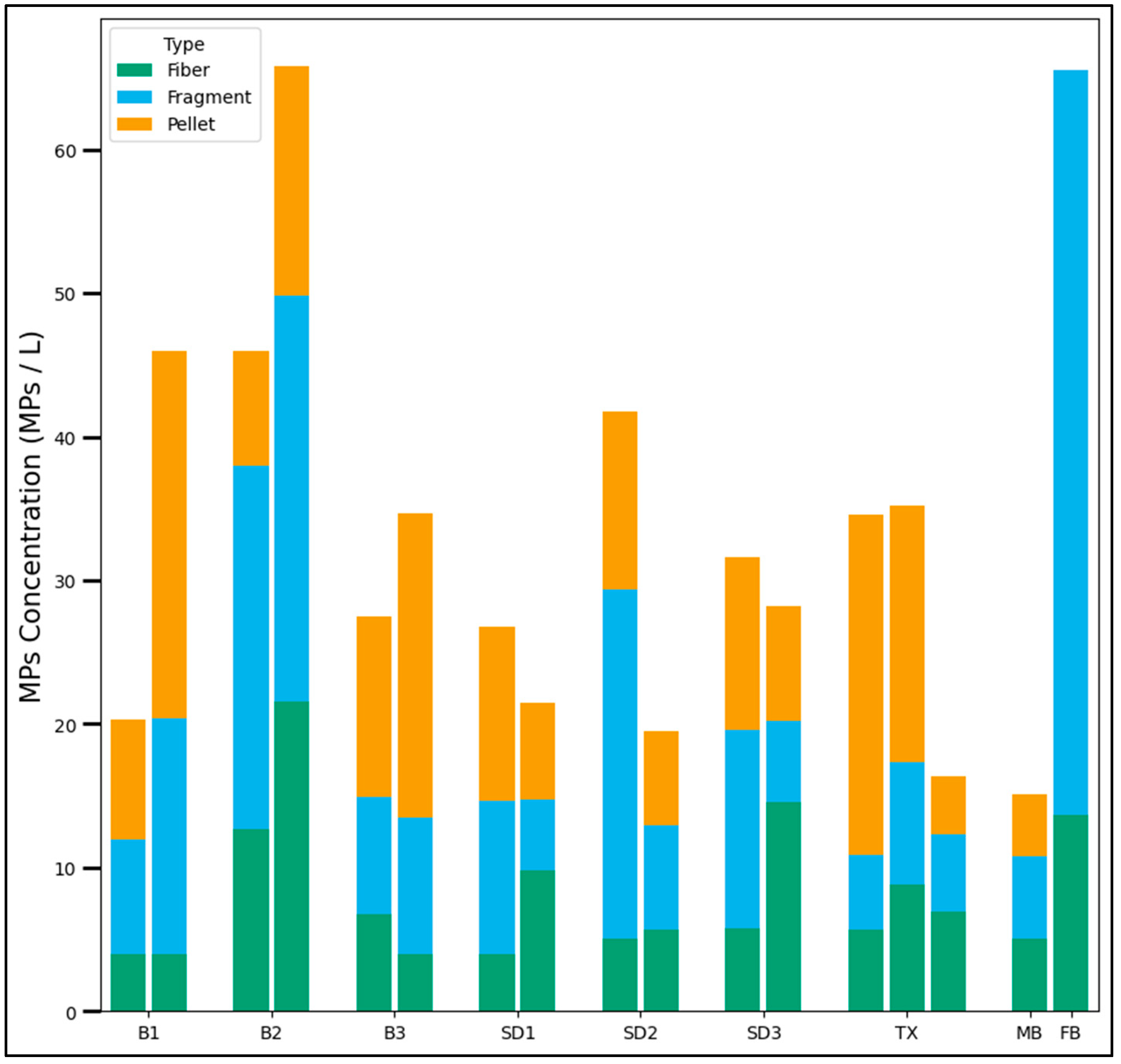
3.1. Visual Analysis
3.1.1. Stormwater Samples
3.1.2. Contamination Source Trials
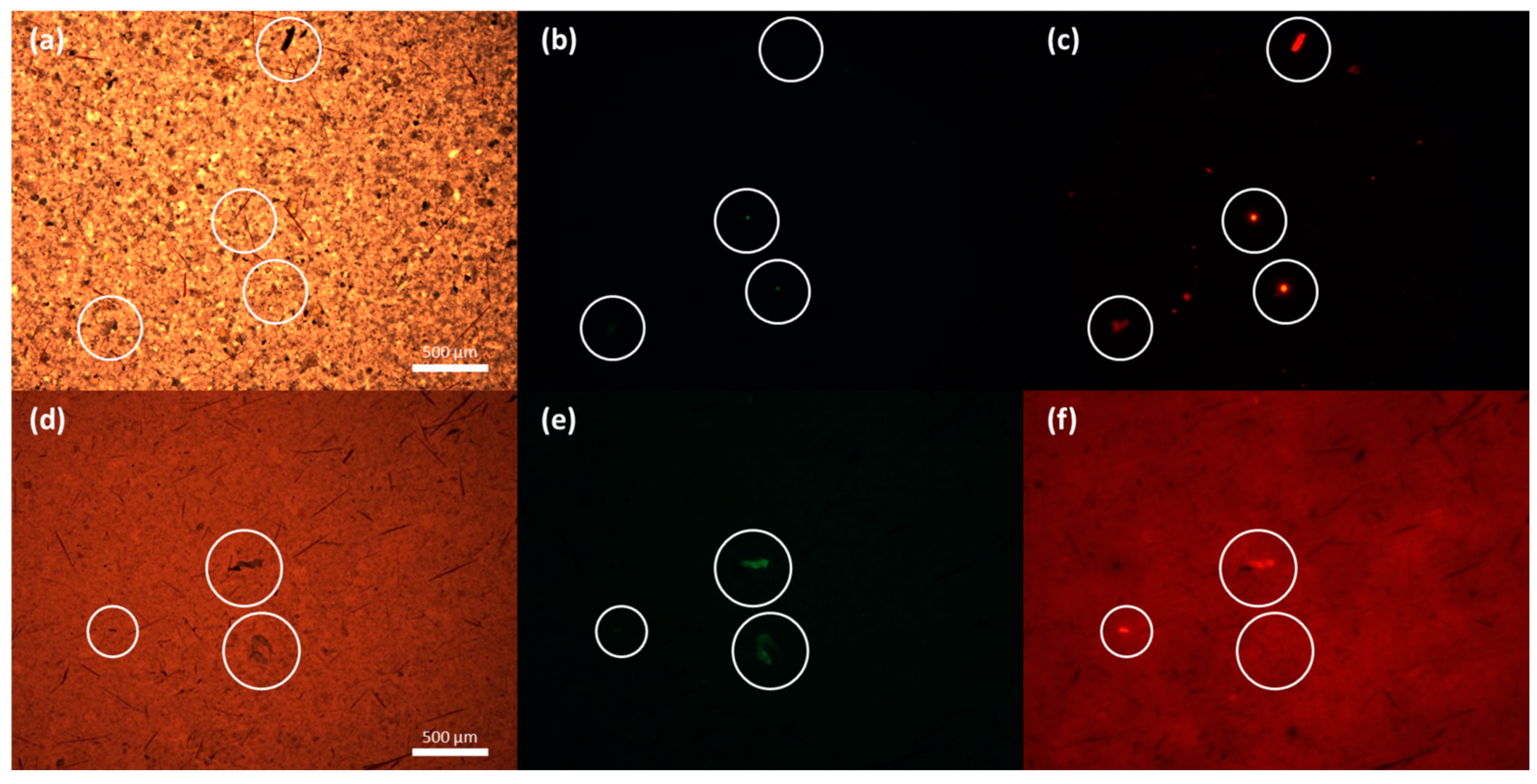
3.2. Fluorescence Microscopy
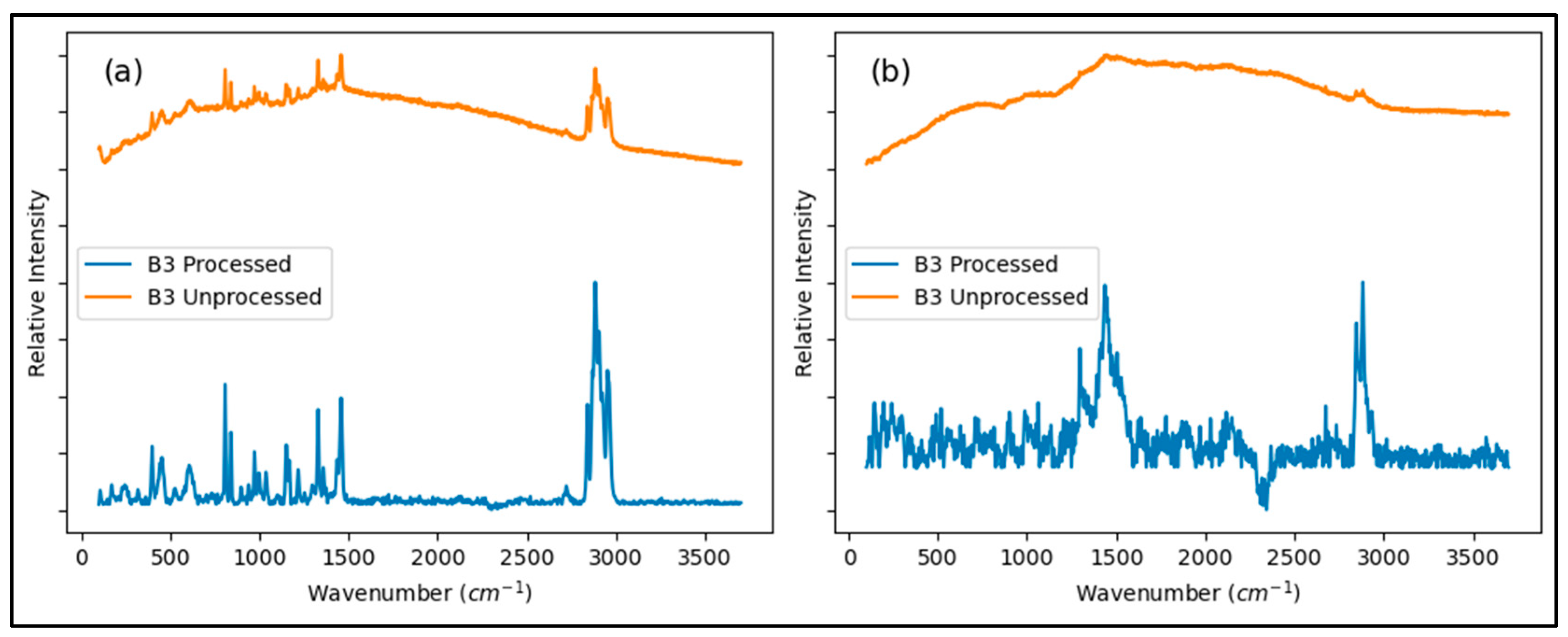
3.3. Raman Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MPs | Microplastics |
| TRWPs | Tire and road wear particles |
| PE | Polyethylene |
| PP | Polypropylene |
| PS | Polystyrene |
| PVC | Polyvinyl chloride |
| PUR | Polyurethane |
| PET | Polyethylene terephthalate |
| PAH | Polycyclic aromatic hydrocarbons |
| PCB | Polychlorinated biphenyls |
| OCP | Organochlorine pesticides |
| DDT | Dichloro-diphenyl-trichloroethane |
| HCH | Hexachlorocyclohexane |
| FTIR | Fourier transform infrared |
| PTFE | Polytetrafluoroethylene |
| AlOx | Aluminum oxide |
| DI | Deionized |
| NR | Nile red |
| SCM | Stormwater control measures |
| HDPE | High-density polyethylene |
| GFP | Green fluorescent protein |
| TxRed | Texas red |
| CB | Carbon black |
| ANOVA | Analysis of variance |
| HSD | Honestly Significant Difference |
| BF | Bright field |
| FB | Field blank |
| MB | Method (laboratory) blank |
References
- Arthur, C.; Baker, J.E.; Bamford, H.A. International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris, Tacoma, WA, USA, 9–11 September 2008; National Oceanic and Atmospheric Administration: Silver Spring, MA, USA, 2009.
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-Q. Occurrence of microplastics and its pollution in the environment: A review. Sustain. Prod. Consum. 2018, 13, 16–23. [Google Scholar] [CrossRef]
- Laskar, N.; Kumar, U. Plastics and microplastics: A threat to environment. Environ. Technol. Innov. 2019, 14, 100352. [Google Scholar] [CrossRef]
- Kovochich, M.; Parker, J.A.; Oh, S.C.; Lee, J.P.; Wagner, S.; Reemtsma, T.; Unice, K.M. Characterization of individual tire and road wear particles in environmental road dust, tunnel dust, and sediment. Environ. Sci. Technol. Lett. 2021, 8, 1057–1064. [Google Scholar] [CrossRef]
- Rosso, B.; Gregoris, E.; Litti, L.; Zorzi, F.; Fiorini, M.; Bravo, B.; Barbante, C.; Gambaro, A.; Corami, F. Identification and quantification of tire wear particles by employing different cross-validation techniques: FTIR-ATR Micro-FTIR, Pyr-GC/MS, and SEM. Environ. Pollut. 2023, 326, 121511. [Google Scholar] [CrossRef]
- Eisentraut, P.; Dümichen, E.; Ruhl, A.S.; Jekel, M.; Albrecht, M.; Gehde, M.; Braun, U. Two birds with one stone—Fast and simultaneous analysis of microplastics: Microparticles derived from thermoplastics and tire wear. Environ. Sci. Technol. Lett. 2018, 5, 608–613. [Google Scholar] [CrossRef]
- Alfonso, M.B.; Arias, A.H.; Ronda, A.C.; Piccolo, M.C. Continental microplastics: Presence, features, and environmental transport pathways. Sci. Total Environ. 2021, 799, 149447. [Google Scholar] [CrossRef]
- Fahrenfeld, N.L.; Arbuckle-Keil, G.; Beni, N.N.; Bartelt-Hunt, S.L. Source tracking microplastics in the freshwater environment. TrAC Trends Anal. Chem. 2019, 112, 248–254. [Google Scholar] [CrossRef]
- Leusch, F.D.; Lu, H.C.; Perera, K.; Neale, P.A.; Ziajahromi, S. Analysis of the literature shows a remarkably consistent relationship between size and abundance of microplastics across different environmental matrices. Environ. Pollut. 2022, 319, 120984. [Google Scholar] [CrossRef]
- Lutz, N.; Fogarty, J.; Rate, A. Accumulation and potential for transport of microplastics in stormwater drains into marine environments, Perth region, Western Australia. Mar. Pollut. Bull. 2021, 168, 112362. [Google Scholar] [CrossRef] [PubMed]
- Sang, W.; Chen, Z.; Mei, L.; Hao, S.; Zhan, C.; Zhang, W.B.; Li, M.; Liu, J. The abundance and characteristics of microplastics in rainwater pipelines in Wuhan, China. Sci. Total Environ. 2021, 755, 142606. [Google Scholar] [CrossRef] [PubMed]
- Herath, S.; Hagare, D.; Siddiqui, Z.; Maheshwari, B. Microplastics in urban stormwater—Developing a methodology for its monitoring. Environ. Monit. Assess. 2022, 194, 173. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Olesen, K.B.; Borregaard, A.R.; Vollertsen, J. Microplastics in urban and highway stormwater retention ponds. Sci. Total Environ. 2019, 671, 992–1000. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Cverenkárová, K.; Valachovičová, M.; Mackuľak, T.; Žemlička, L.; Bírošová, L. Microplastics in the food chain. Life 2021, 11, 1349. [Google Scholar] [CrossRef]
- Yu, Q.; Hu, X.; Yang, B.; Zhang, G.; Wang, J.; Ling, W. Distribution, abundance and risks of microplastics in the environment. Chemosphere 2020, 249, 126059. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Blair, R.M.; Waldron, S.; Phoenix, V.R.; Gauchotte-Lindsay, C. Microscopy and elemental analysis characterisation of microplastics in sediment of a freshwater urban river in Scotland, UK. Environ. Sci. Pollut. Res. 2019, 26, 12491–12504. [Google Scholar] [CrossRef]
- Hanvey, J.S.; Lewis, P.J.; Lavers, J.L.; Crosbie, N.D.; Pozo, K.; Clarke, B.O. A review of analytical techniques for quantifying microplastics in sediments. Anal. Methods 2017, 9, 1369–1383. [Google Scholar] [CrossRef]
- Kabir, M.S.; Wang, H.; Luster-Teasley, S.; Zhang, L.; Zhao, R. Microplastics in landfill leachate: Sources, detection, occurrence, and removal. Environ. Sci. Ecotechnol. 2023, 16, 100256. [Google Scholar] [CrossRef] [PubMed]
- Hatinoğlu, M.D.; Sanin, F.D. Sewage sludge as a source of microplastics in the environment: A review of occurrence and fate during sludge treatment. J. Environ. Manag. 2021, 295, 113028. [Google Scholar] [CrossRef] [PubMed]
- Parashar, N.; Hait, S. Recent advances on microplastics pollution and removal from wastewater systems: A critical review. J. Environ. Manag. 2023, 340, 118014. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.J.; Song, Y.K.; Hong, S.H.; Jang, M. Identification and quantification of microplastics using Nile Red staining. Mar. Pollut. Bull. 2016, 113, 469–476. [Google Scholar] [CrossRef]
- Shruti, V.C.; Pérez-Guevara, F.; Roy, P.D.; Kutralam-Muniasamy, G. Analyzing microplastics with Nile Red: Emerging trends, challenges, and prospects. J. Hazard. Mater. 2022, 423, 127171. [Google Scholar] [CrossRef]
- Anger, P.M.; von der Esch, E.; Baumann, T.; Elsner, M.; Niessner, R.; Ivleva, N.P. Raman microspectroscopy as a tool for microplastic particle analysis. TrAC Trends Anal. Chem. 2018, 109, 214–226. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef]
- Parmar, S.; Arbuckle-Keil, G.; Kumi, G.; Fahrenfeld, N.L. Urban stormwater microplastic size distribution and impact of subsampling on polymer diversity. Environ. Sci. Process. Impacts 2023, 25, 1374–1384. [Google Scholar] [CrossRef]
- Rosso, B.; Corami, F.; Vezzaro, L.; Biondi, S.; Bravo, B.; Barbante, C.; Gambaro, A. Quantification and characterization of additives, plasticizers, and small microplastics (5–100 μm) in highway stormwater runoff. J. Environ. Manag. 2022, 324, 116348. [Google Scholar] [CrossRef]
- Thaysen, C.; Munno, K.; Hermabessiere, L.; Rochman, C.M. Towards Raman automation for microplastics: Developing strategies for particle adhesion and filter subsampling. Appl. Spectrosc. 2020, 74, 976–988. [Google Scholar] [CrossRef]
- Grbić, J.; Helm, P.; Athey, S.; Rochman, C.M. Microplastics entering northwestern Lake Ontario are diverse and linked to urban sources. Water Res. 2020, 174, 115623. [Google Scholar] [CrossRef] [PubMed]
- Kovochich, M.; Liong, M.; Parker, J.A.; Oh, S.C.; Lee, J.P.; Xi, L.; Kreider, M.L.; Unice, K.M. Chemical mapping of tire and road wear particles for single particle analysis. Sci. Total Environ. 2021, 757, 144085. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Sedlak, M.; Lin, D.; Box, C.; Holleman, C.; Rochman, C.M.; Sutton, R. Recommended best practices for collecting, analyzing, and reporting microplastics in environmental media: Lessons learned from comprehensive monitoring of San Francisco Bay. J. Hazard. Mater. 2021, 409, 124770. [Google Scholar] [CrossRef] [PubMed]
- Munno, K.; Helm, P.A.; Jackson, D.A.; Rochman, C.; Sims, A. Impacts of temperature and selected chemical digestion methods on microplastic particles. Environ. Toxicol. Chem. 2018, 37, 91–98. [Google Scholar] [CrossRef]
- Renner, K.O.; Foster, H.A.; Routledge, E.J.; Scrimshaw, M.D. A comparison of different approaches for characterizing microplastics in selected personal care products. Environ. Toxicol. Chem. 2022, 41, 880–887. [Google Scholar] [CrossRef]
- Anguiano, G.; Foreman, M.; Pilkington, J. Hybrid Low Impact Development/Best Management Practices for DoD Industrial Site Stormwater Runoff; Naval Facilities Engineering and Expeditionary Warfare Center (EXWC): Port Hueneme, CA, USA, 2020. [Google Scholar]
- Drygiannaki, I.; Rao, B.; Dawson, J.A.; Rakowska, M.; Reible, D.D.; Hayman, N.T.; Rosen, G.H.; Colvin, M.A.; Chadwick, D.B.; Pitt, R.; et al. Assessing sediment recontamination from metals in stormwater. Sci. Total Environ. 2020, 737, 139726. [Google Scholar]
- Gómez-Ávila, C.; Rao, B.; Hussain, T.; Zhou, H.; Pitt, R.; Colvin, M.; Hayman, N.; DeMyers, M.; Reible, D. Particle size-based evaluation of stormwater control measures in reducing solids, polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs). Water Res. 2025, 277, 123299. [Google Scholar] [CrossRef]
- Houle, J.J.; Macadam, D.R.; Ballestero, T.P.; Puls, T.A. Utilizing in situ ultraviolet-visual spectroscopy to measure nutrients and sediment concentrations in stormwater runoff. J. Sustain. Water Built Environ. 2022, 8, 04022012. [Google Scholar] [CrossRef]
- Hussain, T.; Athanasiou, D.; Rao, B.; Bejar, M.; Rakowska, M.; Drygiannaki, I.; Chadwick, D.B.; Colvin, M.A.; Hayman, N.T.; Rosen, G.H.; et al. Sediment recontamination potential and biological impacts of hydrophobic organics from stormwater in a mixed-use watershed. Sci. Total Environ. 2024, 906, 167444. [Google Scholar] [CrossRef]
- Jani, J.; Lusk, M.G.; Yang, Y.Y.; Toor, G.S. Wet season nitrogen export from a residential stormwater pond. PLoS ONE 2020, 15, e0230908. [Google Scholar] [CrossRef]
- Thompson, J.; Schwartz, J.; Hathaway, J. Performance evaluation of a regenerative stormwater conveyance system: Case study in Knoxville, Tennessee. J. Environ. Eng. 2020, 146, 05020004. [Google Scholar] [CrossRef]
- Topaz, T.; Ben-Ari, J.; Banchik, E.K.; Bassa, O.; Egozi, R.; Suari, Y.; Sade, T.; Zedaka, H.; Gilboa, M.; Yahel, G.; et al. Pesticides and pharmaceuticals data collected during two consecutive years in a Mediterranean micro-estuary. Data Brief 2023, 50, 109456. [Google Scholar] [CrossRef] [PubMed]
- El Hayany, B.; El Fels, L.; Quénéa, K.; Dignac, M.F.; Rumpel, C.; Gupta, V.K.; Hafidi, M. Microplastics from lagooning sludge to composts as revealed by fluorescent staining-image analysis, Raman spectroscopy and pyrolysis-GC/MS. J. Environ. Manag. 2020, 275, 111249. [Google Scholar] [CrossRef] [PubMed]
- Stanton, T.; Johnson, M.; Nathanail, P.; Gomes, R.L.; Needham, T.; Burson, A. Exploring the efficacy of Nile red in microplastic quantification: A costaining approach. Environ. Sci. Technol. Lett. 2019, 6, 606–611. [Google Scholar] [CrossRef]
- Lenz, R.; Enders, K.; Stedmon, C.A.; Mackenzie, D.M.; Nielsen, T.G. A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Mar. Pollut. Bull. 2015, 100, 82–91. [Google Scholar] [CrossRef]
- Dutta, A.K.; Kamada, K.; Ohta, K. Spectroscopic studies of nile red in organic solvents and polymers. J. Photochem. Photobiol. A Chem. 1996, 93, 57–64. [Google Scholar] [CrossRef]
- Sturm, M.T.; Horn, H.; Schuhen, K. The potential of fluorescent dyes—Comparative study of Nile red and three derivatives for the detection of microplastics. Anal. Bioanal. Chem. 2021, 413, 1059–1071. [Google Scholar] [CrossRef]
| Site ID | Site Location | Catchment Type | Type of SCM | No. of Rain Events Sampled |
|---|---|---|---|---|
| B1 | Bremerton area | Industrial | Hydrodynamic separator | 3 |
| B2 | Bremerton area | Industrial/loading dock | Hydrodynamic separator + media filter | 3 |
| B3 | Bremerton area | Industrial | Hydrodynamic separator + media filter | 2 |
| SD1 | San Diego area | Parking lot | Bio-infiltration | 3 |
| SD2 | San Diego area | Metal fabrication facility + parking lot | Bio-infiltration + media filter | 3 |
| SD3 | San Diego area | Parking lot | Bio-infiltration | 2 |
| TX | Texas | Mixed use | Retention pond | 3 |
| Treatment Level | n | Treatment Level Description | Group | Average MP Count | STD | ||
|---|---|---|---|---|---|---|---|
| 1 | 3 | Filter + Filtration + Digestion + Sampling | A | 71 | 13 | ||
| 2 | 9 | Filter + Filtration + Digestion | B | 24 | 9 | ||
| 3 | 6 | Filter + Filtration | B | 16 | 9 | ||
| 4 | 6 | Filter | C | 2 | 4 | ||
| Treatment Level | n | Treatment Level Description | Group | Average MP Count | STD | ||
|---|---|---|---|---|---|---|---|
| 1 | 3 | Filter + Filtration + Digestion + Sampling | A | 1219 | 97 | ||
| 2 | 9 | Filter + Filtration + Digestion | B | 214 | 83 | ||
| 3 | 3 | Filter + Filtration | B | C | 107 | 45 | |
| 4 | 3 | Filter | C | 75 | 24 | ||
| Stage | Site ID | Visual Microscopy (MPs/L) | Fluorescence Microscopy (MPs/L) | Concentration Ratio (Fluorescence/Visual) |
|---|---|---|---|---|
| Inlet | B1 | 21 | 411 | 19.6 |
| B2 | 51 | 362 | 7.10 | |
| B3 | 60 | 227 | 3.78 | |
| SD1 | 28 | 138 | 4.93 | |
| SD2 | 84 | 376 | 4.48 | |
| SD3 | 36 | 192 | 5.33 | |
| Outlet | B1 | 54 | 89 | 1.65 |
| B2 | 77 | 207 | 2.69 | |
| B3 | 112 | 316 | 2.82 | |
| SD1 | 21 | 183 | 8.71 | |
| SD2 | 22 | 120 | 5.45 | |
| SD3 | 34 | 217 | 6.38 | |
| Inlet 1 | TX | 64 | 41 | 0.64 |
| Inlet 2 | TX | 55 | 197 | 3.58 |
| Inlet 3 | TX | 34 | 33 | 0.97 |
| Blank | Laboratory | 24 | 214 | 8.92 |
| Field | 71 | 1219 | 17.2 |
| Location | Stage | No. of Particles Analyzed | No. of Particles Identified | Identification Method | MP Identified |
|---|---|---|---|---|---|
| SD1 | Inlet | 14 | 2 | Manually (1) Software (1) | TRWP TRWP |
| Outlet | 8 | 2 | Software (2) | PET and TWRP | |
| B3 | Inlet | 9 | 2 | Manually (2) | HDPE and PP |
| Outlet | 6 | 1 | Software (1) | TWRP | |
| TX | Field Blank | 6 | 1 | Manually (1) | PP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez Garcia, A.; Zhou, H.; Gomez-Avila, C.; Hussain, T.; Roghani, A.; Reible, D.; Rao, B.A. Microplastics in Stormwater: Sampling and Methodology Challenges. Toxics 2025, 13, 502. https://doi.org/10.3390/toxics13060502
Sanchez Garcia A, Zhou H, Gomez-Avila C, Hussain T, Roghani A, Reible D, Rao BA. Microplastics in Stormwater: Sampling and Methodology Challenges. Toxics. 2025; 13(6):502. https://doi.org/10.3390/toxics13060502
Chicago/Turabian StyleSanchez Garcia, Andres, Huayun Zhou, Cesar Gomez-Avila, Tariq Hussain, Aryan Roghani, Danny Reible, and Balaji Anandha Rao. 2025. "Microplastics in Stormwater: Sampling and Methodology Challenges" Toxics 13, no. 6: 502. https://doi.org/10.3390/toxics13060502
APA StyleSanchez Garcia, A., Zhou, H., Gomez-Avila, C., Hussain, T., Roghani, A., Reible, D., & Rao, B. A. (2025). Microplastics in Stormwater: Sampling and Methodology Challenges. Toxics, 13(6), 502. https://doi.org/10.3390/toxics13060502











