The Metal Oxidation State in Cu, CuO, and Cu2O Nanoparticles Plays a Key Role in Toxicity to Sea Urchin Arbacia lixula, Paracentrotus lividus, and Sphaerechinus granularis Embryos
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoparticles and Chemicals
2.2. Determination of Nanoparticle Colloidal Stability
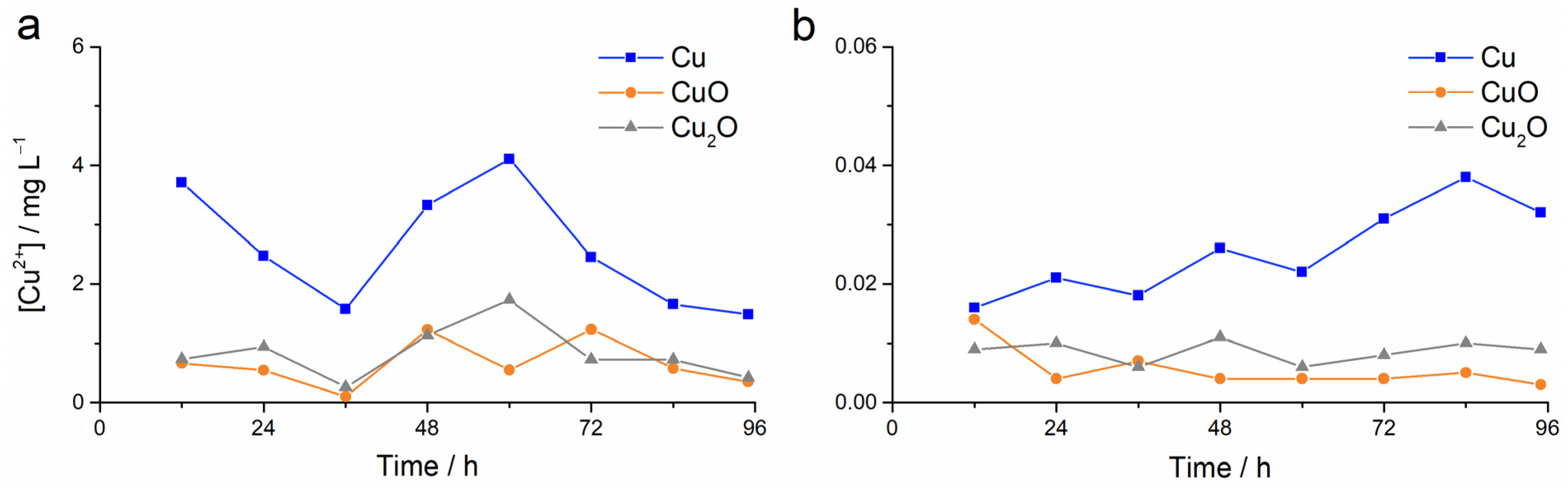
2.3. Nanoparticle Dissolution
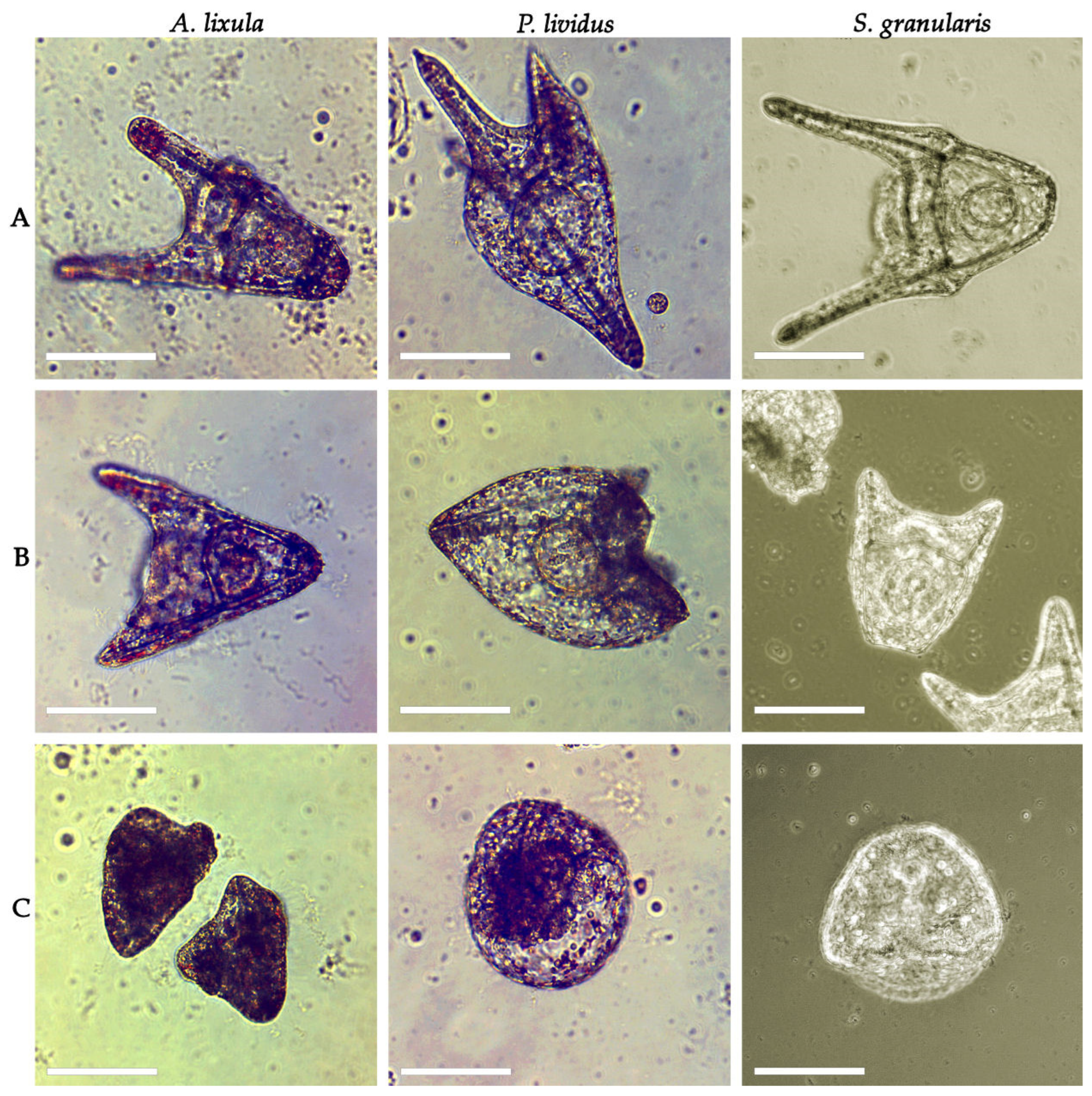
2.4. Embryotoxicity Assay
2.5. Statistical Analysis
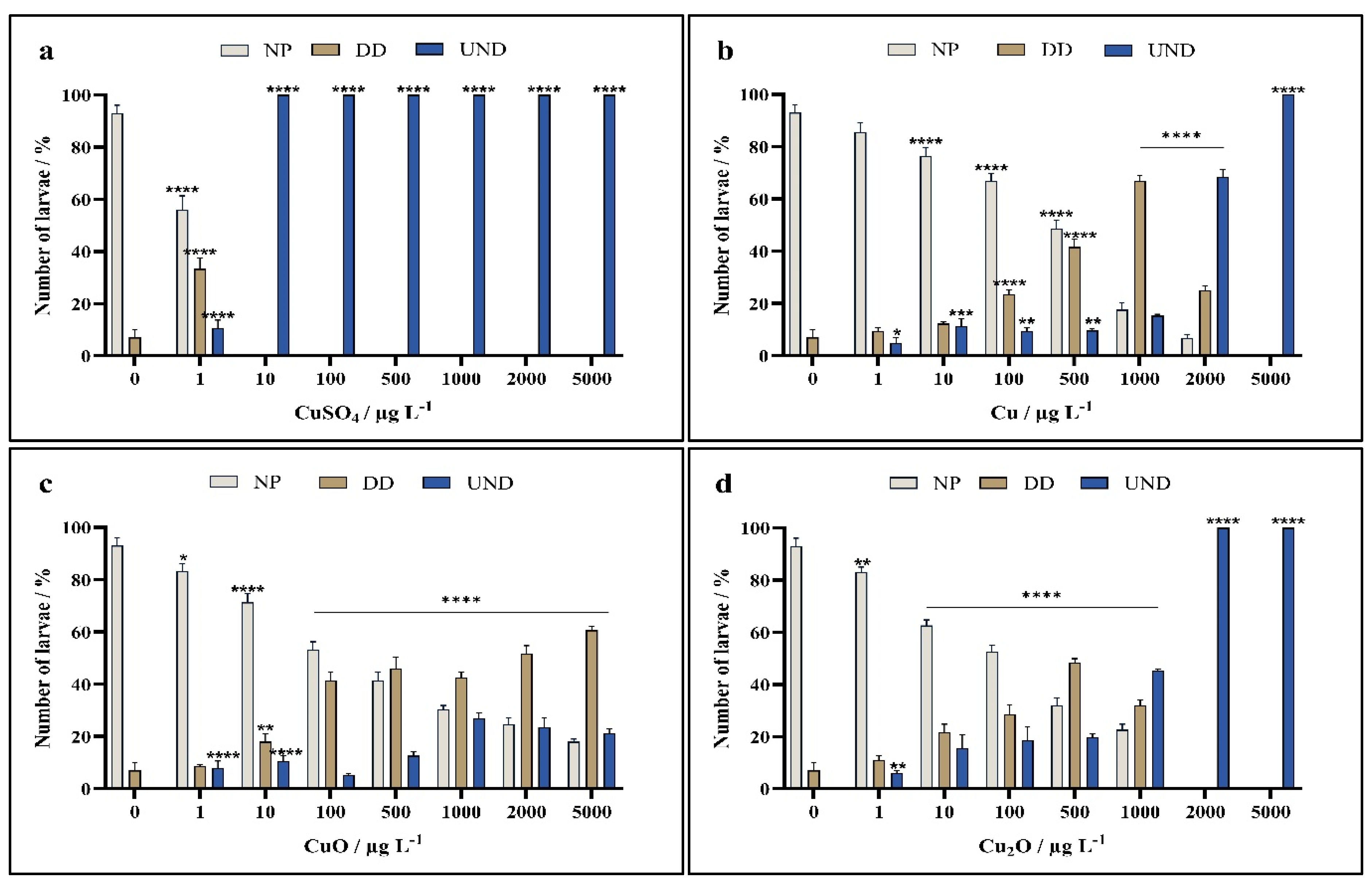
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raffi, M.; Mehrwan, S.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Yawar, W.; Ul Hasan, M.M. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Pariona, N.; Mtz-Enriquez, A.I.; Sánchez-Rangel, D.; Carrión, G.; Paraguay-Delgado, F.; Rosas-Saito, G. Green-synthesized copper nanoparticles as a potential antifungal against plant pathogens. RSC Adv. 2019, 9, 18835–18843. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Oranu, E.A.; Tao, M.; Keller, A.A. Release and detection of nanosized copper from a commercial antifouling paint. Water Res. 2016, 102, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Avelelas, F.; Martins, R.; Oliveira, T.; Maia, F.; Malheiro, E.; Soares, A.M.V.M.; Loureiro, S.; Tedim, J. Efficacy and ecotoxicity of novel anti-fouling nanomaterials in target and non-target marine species. Mar. Biotechnol. 2017, 19, 164–174. [Google Scholar] [CrossRef]
- Amara, I.; Miled, W.; Slama, R.B.; Ladhari, N. Antifouling processes and toxicity effects of antifouling paints on marine environment. A review. Environ. Toxicol. Pharmacol. 2018, 57, 115–130. [Google Scholar] [CrossRef]
- Lagerström, M.; Ytreberg, E.; Wiklund, A.E.; Granhag, L. Antifouling paints leach copper in excess—Study of metal release rates and efficacy along a salinity gradient. Water Res. 2020, 186, 116383. [Google Scholar] [CrossRef]
- Miller, R.J.; Adeleye, A.S.; Page, H.M.; Kui, L.; Lenihan, H.S.; Keller, A.A. Nano and traditional copper and zinc antifouling coatings: Metal release and impact on marine sessile invertebrate communities. J. Nanopart. Res. 2020, 22, 129. [Google Scholar] [CrossRef]
- Torres, F.G.; De-la-Torre, G.E. Environmental pollution with antifouling paint particles: Distribution, ecotoxicology, and sustainable alternatives. Mar. Pollut. Bull. 2021, 169, 112529. [Google Scholar] [CrossRef]
- Loredo-Becerra, G.M.; Durán-Almendárez, A.; Calvillo-Anguiano, A.K.; DeAlba-Montero, I.; Hernández-Arteaga, L.O.; Ruiz, F. Waterborne antifouling paints containing nanometric copper and silver against marine Bacillus species. Bioinorg. Chem. Appl. 2022, 2022, 2435756. [Google Scholar] [CrossRef]
- Keller, A.A.; Ehrens, A.; Zheng, Y.; Nowack, B. Developing trends in nanomaterials and their environmental implications. Nat. Nanotechnol. 2023, 18, 834–837. [Google Scholar] [CrossRef]
- Camakaris, J.; Voskoboinik, I.; Mercer, J.F. Molecular mechanisms of copper homeostasis. Biochem. Biophys. Res. Commun. 1999, 261, 225–232. [Google Scholar] [CrossRef]
- Speisky, H.; Gómez, M.; Burgos-Bravo, F.; López-Alarcón, C.; Jullian, C.; Olea-Azar, C.; Aliaga, M.E. Generation of superoxide radicals by copper-glutathione complexes: Redox-consequences associated with their interaction with reduced glutathione. Bioorg. Med. Chem. 2009, 17, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, H.; Osborne, M.R.; Phillips, D.H. Effects of mixing metal ions on oxidative DNA damage mediated by a Fenton-type reduction. Toxicol. Vitr. 2008, 22, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Chebotaryova, S.P.; Zakharova, O.V.; Gusev, A.A.; Baranchikov, P.A.; Kolesnikov, E.A.; Yakusheva, A.S.; Skripnikova, E.V.; Lobakova, E.S.; Xu, J.; Alam, M.A.; et al. Assessment of the tolerance of a chlorophyte Desmodesmus to CuO-NP for evaluation of the nanopollution bioremediation potential of this microalga. Nanomaterials 2023, 13, 737. [Google Scholar] [CrossRef]
- Rotini, A.; Gallo, A.; Parlapiano, I.; Berducci, M.T.; Boni, R.; Tosti, E.; Prato, E.; Maggi, C.; Cicero, A.M.; Migliore, L.; et al. Insights into the CuO nanoparticle ecotoxicity with suitable marine model species. Ecotoxicol. Environ. Saf. 2018, 147, 852–860. [Google Scholar] [CrossRef]
- Zha, S.; Rong, J.; Guan, X.; Tang, Y.; Han, Y.; Liu, G. Immunotoxicity of four nanoparticles to a marine bivalve species, Tegillarca granosa. J. Hazard. Mater. 2019, 377, 237–248. [Google Scholar] [CrossRef]
- Pagano, G.; Thomas, P.; Guida, M.; Palumbo, A.; Romano, G.; Oral, R.; Trifuoggi, M. Sea urchin bioassays in toxicity testing: II. Sediment evaluation. Expert Opin. Environ. Biol. 2017, 6, 1. [Google Scholar] [CrossRef]
- Pagano, G.; Guida, M.; Trifuoggi, M.; Thomas, P.; Palumbo, A.; Romano, G.; Oral, R. Sea urchin bioassays in toxicity testing: I. Inorganics, organics, complex mixtures and natural products. Expert Opin. Environ. Biol. 2017, 6, 1. [Google Scholar] [CrossRef]
- Fernandez, N.; Beiras, R. Combined toxicity of dissolved mercury with copper, lead and cadmium on embryogenesis and early larval growth of the Paracentrotus lividus sea urchin. Ecotoxicology 2001, 10, 263–271. [Google Scholar] [CrossRef]
- Morroni, L.; Pinsino, A.; Pellegrini, D.; Regoli, F. Reversibility of trace metals effects on sea urchin embryonic development. Ecotoxicol. Environ. Saf. 2018, 148, 923–929. [Google Scholar] [CrossRef]
- Manzo, S.; Buono, S.; Cresimini, C. Predictability of copper, Irgarol, and diuron combined effects on sea urchin Paracentrotus lividus. Arch. Environ. Contam. Toxicol. 2008, 54, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Manfra, L.; Boni, R.; Rotini, A.; Migliore, L.; Tosti, E. Cytotoxicity and genotoxicity of CuO nanoparticles in sea urchin spermatozoa through oxidative stress. Environ. Int. 2018, 118, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Torres-Duarte, C.; Cole, B.J.; Cherr, G.N. Copper oxide and zinc oxide nanomaterials act as inhibitors of multidrug resistance transport in sea urchin embryos: Their role as chemosensitizers. Environ. Sci. Technol. 2015, 49, 5760–5770. [Google Scholar] [CrossRef]
- Torres-Duarte, C.; Adeleye, A.S.; Pokhrel, S.; Mädler, L.; Keller, A.A.; Cherr, G.N. Developmental effects of two different copper oxide nanomaterials in sea urchin (Lytechinus pictus) embryos. Nanotoxicology 2016, 10, 671–679. [Google Scholar] [CrossRef]
- Torres-Duarte, C.; Ramos-Torres, K.M.; Rahimoff, R.; Cherr, G.N. Stage specific effects of soluble copper and copper oxide nanoparticles during sea urchin embryo development and their relation to intracellular copper uptake. Aquat. Toxicol. 2017, 189, 134–141. [Google Scholar] [CrossRef]
- Blossom, N.; Szafranski, F.; Lotz, A. Use of copper-based antifouling paint: A U.S. regulatory update. CoatingsTech 2018, 15, 63–68. [Google Scholar]
- Cima, F.; Varello, R. Potential disruptive effects of copper-based antifouling paints on the biodiversity of coastal macrofouling communities. Environ. Sci. Pollut. Res. Int. 2023, 30, 8633–8646. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Chang, Y.Q.; Lawrence, J.M. Disease in sea urchins. In Sea Urchins: Biology and Ecology. Developments in Aquaculture and Fisheries Science; Lawrence, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 38, pp. 179–186. [Google Scholar]
- Thomas, P.J.; Oral, R.; Pagano, G.; Tez, S.; Toscanesi, M.; Ranieri, P.; Trifuoggi, M.; Lyons, D.M. Mild toxicity in Paracentrotus lividus early life stages may indicate species-specific sensitivity to polystyrene and polymethylmethacrylate microplastics. Mar. Environ. Res. 2020, 161, 105132. [Google Scholar] [CrossRef]
- Prabhu, B.M.; Ali, S.F.; Murdock, R.C.; Hussain, S.M.; Srivatsan, M. Copper nanoparticles exert size and concentration dependent toxicity on somatosensory neurons of rat. Nanotoxicology 2009, 4, 150–160. [Google Scholar] [CrossRef]
- Maisano, M.; Cappello, T.; Catanese, E.; Vitale, V.; Natalotto, A.; Giannetto, A.; Barreca, D.; Brunelli, E.; Mauceri, A.; Fasulo, S. Developmental abnormalities and neurotoxicological effects of CuO NPs on the black sea urchin Arbacia lixula by embryotoxicity assay. Mar. Environ. Res. 2015, 111, 121–127. [Google Scholar] [CrossRef]
- Cappello, T.; Vitale, V.; Oliva, S.; Villari, V.; Mauceri, A.; Fasulo, S.; Maisano, M. Alteration of neurotransmission and skeletogenesis in sea urchin Arbacia lixula embryos exposed to copper oxide nanoparticles. Comp. Biochem. Physiol. C 2017, 199, 20–27. [Google Scholar] [CrossRef]
- Giannetto, A.; Cappello, T.; Oliva, S.; Parrino, V.; De Marco, G.; Fasulo, S.; Mauceri, A.; Maisano, M. Copper oxide nanoparticles induce the transcriptional modulation of oxidative stress-related genes in Arbacia lixula embryos. Aquat. Toxicol. 2018, 201, 187–197. [Google Scholar] [CrossRef]
- Morroni, L.; Sartori, D.; Costantini, M.; Genovesi, L.; Magliocco, T.; Ruocco, N.; Buttino, I. First molecular evidence of the toxicogenetic effects of copper on sea urchin Paracentrotus lividus embryo development. Water Res. 2019, 160, 415–423. [Google Scholar] [CrossRef]
- Garcia-Velasco, N.; Carrero, J.A.; Urionabarrenetxea, E.; Doni, L.; Zaldibar, B.; Izagirre, U.; Soto, M. Innovative in vivo and in vitro bioassays for the establishment of toxicity thresholds of pollutants in sediment quality assessment using polychaetes and their immune cells. Chemosphere 2023, 311, 136935. [Google Scholar] [CrossRef] [PubMed]
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Vija, H.; Käkinen, A.; Titma, T.; Heinlaan, M.; et al. Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro. PLoS ONE 2014, 9, e102108. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.R.; Adeleye, A.S.; Gardea-Torresdey, J.; Keller, A.A. Aggregation, dissolution, and transformation of copper nanoparticles in natural waters. Environ. Sci. Technol. 2015, 49, 2749–2756. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.; Hansen, D. The importance of trace metal speciation to water quality criteria. Water Environ. Res. 1996, 68, 42–54. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Conway, J.R.; Perez, T.; Rutten, P.; Keller, A.A. Influence of extracellular polymeric substances on the long-term fate, dissolution, and speciation of copper-based nanoparticles. Environ. Sci. Technol. 2014, 48, 12561–12568. [Google Scholar] [CrossRef]
- Arnold, W.R.; Cotsifas, J.S.; Ogle, R.S.; DePalma, S.G.S.; Smith, D.S. A comparison of the copper sensitivity of six invertebrate species in ambient salt water of varying dissolved organic matter concentrations. Environ. Toxicol. Chem. 2010, 29, 311–319. [Google Scholar] [CrossRef]
- Zitoun, R.; Clearwater, S.J.; Hassler, C.; Thompson, K.J.; Albert, A.; Sander, S.G. Copper toxicity to blue mussel embryos (Mytilus galloprovincialis): The effect of natural dissolved organic matter on copper toxicity in estuarine waters. Sci. Total Environ. 2019, 653, 300–314. [Google Scholar] [CrossRef]
- Lorenzo, J.I.; Nieto, O.; Beiras, R. Effect of humic acids on speciation and toxicity of copper to Paracentrotus lividus larvae in seawater. Aquat. Toxicol. 2002, 58, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.I.; Nieto, O.; Beiras, R. Anodic stripping voltammetry measures copper bioavailability for sea urchin larvae in the presence of fulvic acids. Environ. Toxicol. Chem. 2006, 25, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, H.; von dem Bussche, A.; Creighton, M.; Hurt, R.H.; Kane, A.B.; Gao, H. Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proc. Nat. Acad. Sci. USA 2013, 110, 12295–12300. [Google Scholar] [CrossRef]
- Ivask, A.; Juganson, K.; Bondarenko, O.; Mortimer, M.; Aruoja, V.; Kasemets, K.; Blinova, I.; Heinlaan, M.; Slaveykova, V.; Kahru, A. Mechanisms of toxic action of Ag, ZnO and CuO nanoparticles to selected ecotoxicological test organisms and mammalian cells in vitro: A comparative review. Nanotoxicology 2014, 8, 57–71. [Google Scholar] [CrossRef]
- Solioz, M. Copper oxidation state and mycobacterial infection. Mycobact. Dis. 2016, 6, 1000210. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Wang, Z.; von dem Bussche, A.; Kabadi, P.K.; Kane, A.B.; Hurt, R.H. Biological and environmental transformations of copper-based nanomaterials. ACS Nano 2013, 7, 8715–8727. [Google Scholar] [CrossRef]
- Kukla, S.P.; Slobodskova, V.V.; Zhuravel, E.V.; Mazur, A.A.; Chelomin, V.P. Exposure of adult sand dollars (Scaphechinus mirabilis) (Agassiz, 1864) to copper oxide nanoparticles induces gamete DNA damage. Environ. Sci. Pollut. Res. Int. 2022, 29, 39451–39460. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, M.A.; Nicosia, A.; Costa, S.; Cuttitta, A.; Gianguzza, F. Metallothionein gene family in the sea urchin Paracentrotus lividus: Gene structure, differential expression and phylogenetic analysis. Int. J. Mol. Sci. 2017, 18, 812. [Google Scholar] [CrossRef]
- Burić, P.; Jakšić, Ž.; Štajner, L.; Dutour Sikirić, M.; Jurašin, D.; Cascio, C.; Calzolai, L.; Lyons, D.M. Effect of silver nanoparticles on Mediterranean sea urchin embryonal development is species specific and depends on moment of first exposure. Mar. Environ. Res. 2015, 111, 50–59. [Google Scholar] [CrossRef]
- Burić, P.; Čarapar, I.; Pavičić-Hamer, D.; Kovačić, I.; Jurković, L.; Dutour Sikirić, M.; Domazet Jurašin, D.; Mikac, N.; Bačić, N.; Lyons, D.M. Particle size modulates silver nanoparticle toxicity during embryogenesis of urchins Arbacia lixula and Paracentrotus lividus. Int. J. Mol. Sci. 2023, 24, 745. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Lin, S.; Ji, Z.; Thomas, C.; Li, L.; Mecklenburg, M.; Meng, H.; Wang, X.; Zhang, H.; Xia, T.; et al. Surface defects on plate-shaped silver nanoparticles contribute to its hazard potential in a fish cell line and zebrafish embryos. ACS Nano 2012, 6, 3745–3759. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, L.M.; Albalghiti, E.M.; Fishman, Z.S.; Perreault, F.; Corredor, C.; Posner, J.D.; Elimelech, M.; Pfefferle, L.D.; Zimmerman, J.B. Shape-dependent surface reactivity and antimicrobial activity of nano-cupric oxide. Environ. Sci. Technol. 2016, 50, 3975–3984. [Google Scholar] [CrossRef]
- Sartori, D.; Pellegrini, D.; Gaion, A. Analysis of variability in embryological response of two sea urchin species to spatial and temporal features—Can these factors influence responses in standardized ecotoxicological assays? Expert Opin. Environ. Biol. 2016, 5, S1. [Google Scholar] [CrossRef]
- Masullo, T.; Biondo, G.; Natale, M.D.; Tagliavia, M.; Bennici, C.D.; Musco, M.; Ragusa, M.A.; Costa, S.; Cuttitta, A.; Nicosia, A. Gene expression changes after parental exposure to metals in the sea urchin affect timing of genetic programme of embryo development. Biology 2021, 10, 103. [Google Scholar] [CrossRef]
- Pelikan, J.; Majnarić, N.; Maurić Maljković, M.; Pikelj, K.; Hamer, B. Physico-Chemical and Ecotoxicological Evaluation of Marine Sediments Contamination: A Case Study of Rovinj Coastal Area, NE Adriatic Sea, Croatia. Toxics 2022, 10, 478. [Google Scholar] [CrossRef]

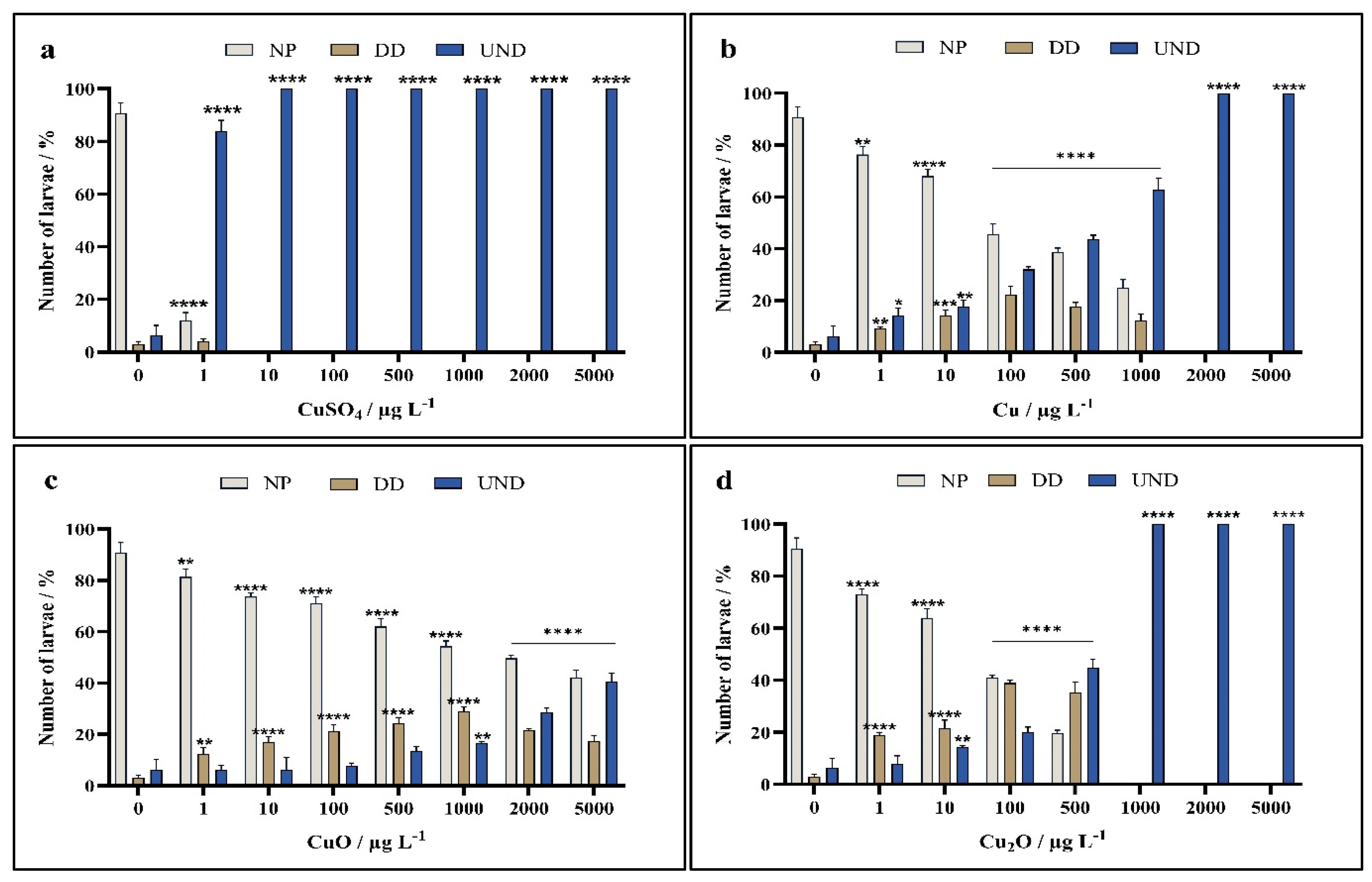
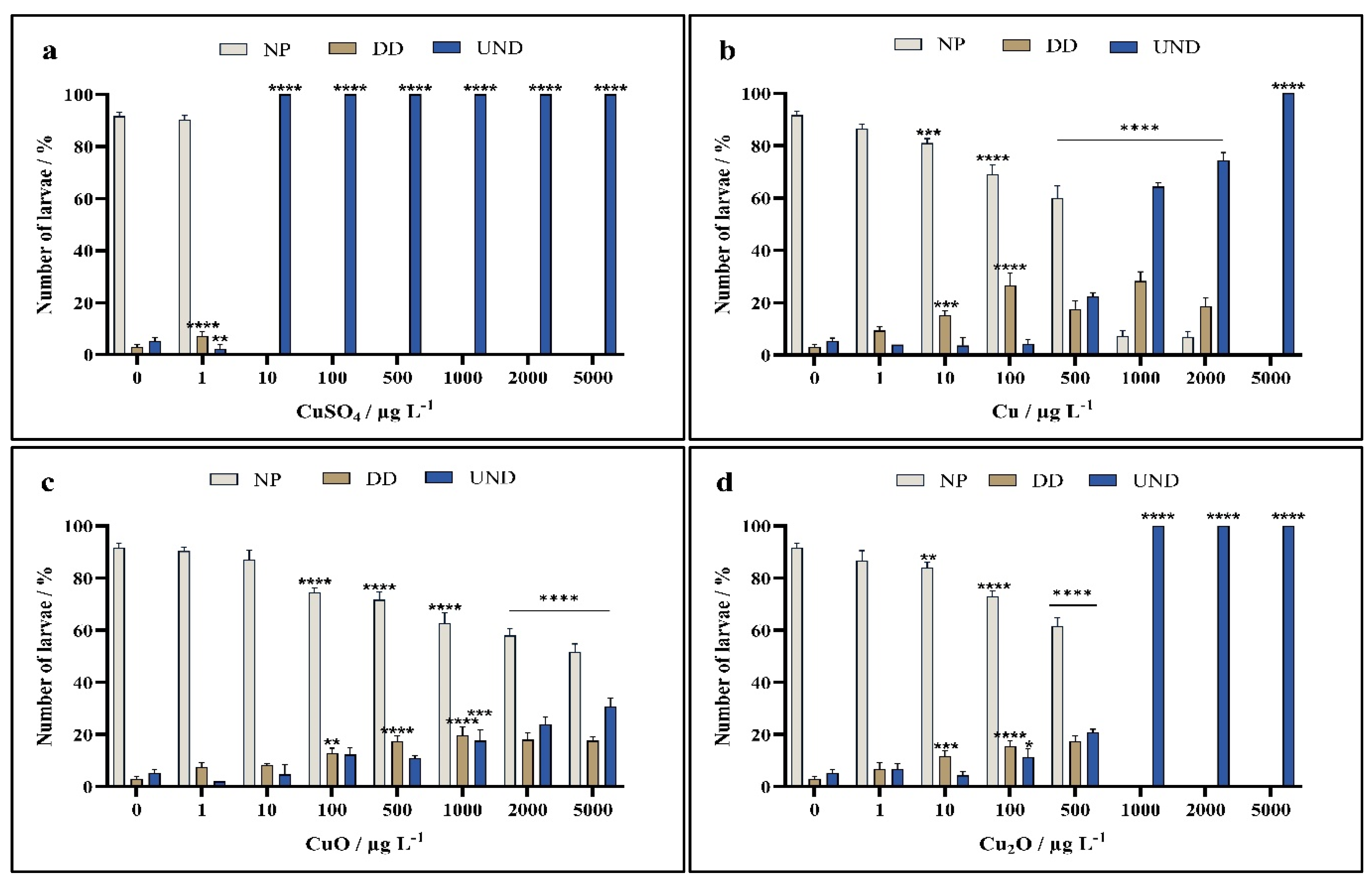
| EC50 | A. lixula | P. lividus | S. granularis |
|---|---|---|---|
| μg L−1 | μg L−1 | μg L−1 | |
| Cu2+ | 0.51 | 5.06 | 1.92 |
| Cu | 88 ± 16 | 617 ± 89 | 496 ± 116 |
| CuO | 1629 ± 548 | nd | 91 ± 47 |
| Cu2O | 99 ± 39 | 606 ± 90 | 371 ± 169 |
| LC50 | A. lixula | P. lividus | S. granularis |
|---|---|---|---|
| μg L−1 | μg L−1 | μg L−1 | |
| Cu2+ | 0.56 | 5.39 | 4.90 |
| Cu | 695 ± 86 | 837 ± 17 | 1764 ± 83 |
| CuO | nd | nd | nd |
| Cu2O | 559 ± 40 | 622 ± 124 | 1108 ± 115 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čarapar, I.; Jurković, L.; Pavičić-Hamer, D.; Jaklin, A.; Dutour Sikirić, M.; Hamer, B.; Lyons, D.M. The Metal Oxidation State in Cu, CuO, and Cu2O Nanoparticles Plays a Key Role in Toxicity to Sea Urchin Arbacia lixula, Paracentrotus lividus, and Sphaerechinus granularis Embryos. Toxics 2025, 13, 469. https://doi.org/10.3390/toxics13060469
Čarapar I, Jurković L, Pavičić-Hamer D, Jaklin A, Dutour Sikirić M, Hamer B, Lyons DM. The Metal Oxidation State in Cu, CuO, and Cu2O Nanoparticles Plays a Key Role in Toxicity to Sea Urchin Arbacia lixula, Paracentrotus lividus, and Sphaerechinus granularis Embryos. Toxics. 2025; 13(6):469. https://doi.org/10.3390/toxics13060469
Chicago/Turabian StyleČarapar, Ivana, Lara Jurković, Dijana Pavičić-Hamer, Andrej Jaklin, Maja Dutour Sikirić, Bojan Hamer, and Daniel Mark Lyons. 2025. "The Metal Oxidation State in Cu, CuO, and Cu2O Nanoparticles Plays a Key Role in Toxicity to Sea Urchin Arbacia lixula, Paracentrotus lividus, and Sphaerechinus granularis Embryos" Toxics 13, no. 6: 469. https://doi.org/10.3390/toxics13060469
APA StyleČarapar, I., Jurković, L., Pavičić-Hamer, D., Jaklin, A., Dutour Sikirić, M., Hamer, B., & Lyons, D. M. (2025). The Metal Oxidation State in Cu, CuO, and Cu2O Nanoparticles Plays a Key Role in Toxicity to Sea Urchin Arbacia lixula, Paracentrotus lividus, and Sphaerechinus granularis Embryos. Toxics, 13(6), 469. https://doi.org/10.3390/toxics13060469









