Abstract
Nitrous oxide (N2O) was originally used for medical and industrial purposes, but its recreational use has dramatically increased, raising a major global public health concern. Chronic inhalation is associated with neurological, metabolic, and psychiatric complications, as well as addiction. To address these challenges, the PROTOSIDE network was developed to provide a multidisciplinary approach to management and prevention. This initiative relies on competence centers integrating specialists in emergency medicine, neurology, clinical biochemistry, and addiction medicine. PROTOSIDE aims to standardize diagnostic protocols, optimize patient care pathways, and strengthen addictovigilance. A strong emphasis is placed on prevention, including awareness campaigns and collaboration with healthcare professionals and educators. By facilitating access to advanced biochemical analyses (homocysteine, methylmalonic acid) and promoting international guidelines, PROTOSIDE represents an innovative model for a global response to N2O misuse. This integrated approach enhances clinical management, reduces complications, and harmonizes public health strategies.
1. Context, State of the Art, and Challenges
The increasing recreational use of nitrous oxide (N2O), originally developed for medical and industrial purposes, has recently become a major global public health concern [1,2]. Scientific studies highlight the severe harms resulting from recreational N2O use, including neurological, metabolic, and psychiatric complications, as well as addiction [3].
1.1. A Historical Shift: From Medical Use to Recreational Misuse
- Origins of medical use and early recreational consumption
Nitrous oxide (N2O) was first identified in the late 18th century by Joseph Priestley and later popularized by Sir Humphry Davy, who experimented with its psychoactive properties in 1799. Davy’s observations of euphoria and dissociation led to its adoption as an anesthetic agent in dentistry and surgery by the mid-19th century. The substance quickly became a cornerstone of modern anesthetic practice, favored for its rapid onset, short duration of action, and minimal residual effects [4].
However, recreational misuse of N2O is not a new phenomenon. Historical accounts suggest that early anesthetic demonstrations in the 19th century often turned into public spectacles, where attendees inhaled the gas for entertainment, leading to fits of laughter—hence its nickname, “laughing gas”. By the early 20th century, sporadic cases of misuse among medical professionals and industrial workers were documented, particularly in settings where the gas was readily available [1].
Despite its long history in medicine, N2O was never classified as a controlled substance in most jurisdictions due to its legitimate medical applications and relatively low potential for acute toxicity. This regulatory leniency, combined with technological advances in gas storage and distribution, has played a pivotal role in facilitating its transition from clinical to recreational settings [2].
- The shift to mass recreational use
Although isolated cases of N2O abuse had been reported throughout the 20th century, widespread recreational consumption surged in the 21st century, fueled by several key factors (Figure 1).
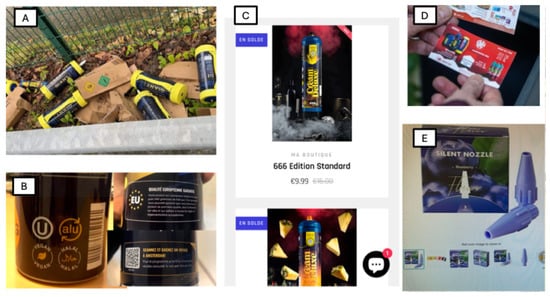
Figure 1.
Extensive Distribution and Aggressive Marketing Strategies. (A) Current forms of nitrous oxide consumption: large cylinders containing the equivalent of several hundred small cartridges. (B) Marketing labels on nitrous oxide canisters: “Vegan,” “Halal,” “European Quality,” and a promotional game offering a trip to Amsterdam. (C) Sale and aggressive marketing of nitrous oxide via social media platforms. (D) Leaflet advertising for nitrous oxide sales, distributed in mailboxes. (E) Canister adapter designed to add flavor to nitrous oxide.
One of the primary drivers is the mass commercial availability. N2O is legally sold for culinary and industrial purposes, particularly in the form of pressurized cartridges used in whipped cream dispensers [5]. The expansion of online marketplaces and non-traditional retail distributors has dramatically increased access [6].
In parallel, social media platforms such as TikTok, Instagram, and YouTube have glamorized N2O consumption, showcasing its effects while downplaying the potential dangers [7]. These platforms often showcase content featuring “balloon challenges” or binge-like consumption patterns at parties and music festivals [8].
Another key factor lies in the misconception of safety surrounding N2O. Compared to other inhalants (e.g., volatile solvents, nitrites, gasoline), N2O is perceived as “harmless” due to its medical applications and absence of strong withdrawal symptoms [9]. In addition, many users incorrectly assume that its rapid elimination from the body prevents long-term harm, unaware of the biochemical impact on vitamin B12 metabolism [10].
Compounding the issue is a notable shift toward high-dose and chronic use. Early recreational use primarily involved small “whippet” cartridges, limiting the amount of gas that could be consumed at one time [1]. However, the rise of large industrial-grade N2O canisters (containing several liters of gas) has led to prolonged sessions and higher cumulative exposure [1]. This transition is particularly concerning as chronic, high-dose use significantly increases the risk of neurological complications, including myelopathy and peripheral neuropathy [11].
- The role of unregulated distribution channels
One of the most pressing challenges in mitigating N2O misuse is the unregulated nature of its distribution, which occupies a legal gray zone. Unlike alcohol or tobacco, it is widely available for legitimate industrial, medical, and culinary purposes, yet its recreational use is rarely addressed in legislation. Online retailers and international suppliers have exploited legal loopholes, making bulk purchases easier than ever, and there is limited enforcement of existing regulations, particularly in countries where recreational use is not explicitly prohibited. This lack of oversight has fueled an underground market where users can purchase large quantities without restriction, contributing to the rapid normalization of recreational N2O consumption.
1.2. The Epidemiological Surge: A Growing Global Concern
1.2.1. Europe: A Hub for Nitrous Oxide Consumption
Over the past decade, Europe has emerged as a central hotspot for N2O misuse, with alarming trends reported across multiple countries. The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) has identified N2O as the most widely used psychoactive substance among young adults [2,5].
According to the Global Drug Survey 2021, nitrous oxide ranked as the 14th most commonly used recreational substance globally. Its use is especially prevalent among adolescents and young adults under the age of 25, with studies cited in the 2023 WHO report estimating past-year prevalence between 4% and 15% in countries such as Denmark, the Netherlands, and the UK. In France, over 134 cases of nitrous-oxide-related neurological complications were reported to poison control centers in 2020 alone, and hospital admissions for N2O toxicity continue to rise across Europe [2,12].
This widespread use is likely driven by several factors: the legal status of N2O in many countries, its accessibility through whipped cream chargers and larger canisters, low cost, and its misleading reputation as a “safe” or “legal” high. Notably, the shift from recreational use of small whipped cream cartridges to high-volume canisters and tanks has dramatically increased the risk of severe toxic effects, including irreversible neurological damage and even death. Despite its trivialized image, N2O poses real and escalating dangers that warrant urgent and coordinated public health responses.
- Key epidemiological findings according to the EMCDDA report
According to the latest report from the EMCDDA, several key epidemiological trends have emerged in recent years regarding nitrous oxide misuse.
One of the most alarming findings is a five-fold increase in emergency room admissions linked to N2O-related complications. This sharp rise underscores a growing public health concern that spans across multiple European countries.
Among the nations most affected, the Netherlands, France, and the United Kingdom report the highest prevalence rates of recreational N2O use [12]. This pattern is particularly pronounced in urban areas where nightlife culture thrives. Indeed, the clustering of N2O misuse is frequently observed in environments such as music festivals, nightclubs, and university parties, where peer influence and easy access converge to drive consumption.
Compounding this issue is a notable shift in the method of use. Whereas users once relied on small whipped cream cartridges, the market has seen a transition toward large industrial-sized canisters. These high-volume containers enable prolonged inhalation sessions, significantly increasing both the intensity and duration of intoxication. This change in usage patterns has been directly associated with a rise in severe neurological outcomes, marking a dangerous evolution in the recreational use of nitrous oxide.
- Regulatory responses and effectiveness
In response to the growing misuse of nitrous oxide, some European countries have implemented regulatory measures aimed at curbing its recreational use. The Netherlands was among the first to act decisively, banning sales of N2O for recreational purposes in January 2023. Despite this legislative move, illegal distribution networks have quickly adapted, and unregulated markets continue to thrive [13].
France has taken a more moderated approach, introducing age restrictions and setting limits on the quantity of N2O that can be purchased. However, these measures suffer from inconsistent enforcement, diminishing their overall impact.
Similarly, the United Kingdom reclassified nitrous oxide as a Class C drug in 2023, effectively criminalizing its recreational use. Yet, early evidence indicates that illicit sales remain readily accessible through online platforms, underscoring the limitations of prohibition-based strategies.
Preliminary data suggest that such restrictions have not led to a substantial decline in use. This reinforces the urgent need for complementary harm reduction policies, including education, awareness campaigns, and community-based interventions, to address the multifaceted nature of N2O misuse.
1.2.2. Emerging Concerns: From Acute to Chronic Health Consequences
Historically, most N2O-related medical cases involved acute intoxication, presenting as transient dizziness, nausea, or mild euphoria. However, recent clinical reports suggest a shift toward severe, long-term complications [14]. Increasingly, clinicians are reporting chronic neurological syndromes associated with cumulative exposure [11]. Notably, a growing number of cases involve irreversible nerve damage in daily users [11]. These outcomes are often exacerbated by delayed diagnosis and mismanagement, as symptoms may be nonspecific and unfamiliar to many healthcare providers, leading to progressive disability [15,16].
These trends underscore the urgent need for enhanced surveillance, clinical awareness, and standardized treatment guidelines.
1.3. Clinical and Biological Consequences of Nitrous Oxide Misuse [1]
1.3.1. Neurological Manifestations: Myeloneuropathy and Central Nervous System Impairment
One of the most alarming consequences of chronic N2O exposure is its profound impact on the nervous system, leading to peripheral neuropathy and myelopathy [17]. The mechanism of action involves an irreversible oxidation of vitamin B12 (cobalamin), leading to a functional deficiency that disrupts key metabolic pathways [1]. This process also causes demyelination of the spinal cord (subacute combined degeneration, SCD), leading to motor dysfunction, paresthesia, and proprioceptive ataxia [18].
- Clinical presentations
The impact of N2O misuse on the nervous system can lead to myelopathies, characterized by spinal cord degeneration, affecting vibration sense, coordination, and motor function [19], as well as peripheral neuropathies, responsible for paresthesia, numbness, muscle weakness, and loss of deep tendon reflexes [20].
Cognitive and psychiatric impairment may also occur, including memory loss, mood disturbances, anxiety, and, in severe cases, psychotic symptoms [21,22].
Electrophysiological findings often reveal prolonged motor and sensory conduction velocities, reflecting axonal damage [20].
1.3.2. Biochemical and Metabolic Disruptions: Methylmalonic Acid and Homocysteine Dysregulation
The major biochemical disruption caused by N2O misuse is the functional inactivation of vitamin B12, also called cobalamin [23,24,25]. Vitamin B12 is a crucial cofactor for two essential enzymes: methionine synthase (MS) and methylmalonyl-CoA mutase (MM-CoAM). Thus, this oxidation disrupts both the methylation and mitochondrial metabolic cycles:
- -
- Methionine synthase, which requires methylcobalamin as a cofactor, catalyzes the remethylation of homocysteine into methionine. When MS activity is blocked, homocysteine accumulates in plasma—a biochemical hallmark of impaired one-carbon metabolism. This elevation is clinically relevant due to its association with vascular and neurological complications.
- -
- Methylmalonyl-CoA mutase, on the other hand, depends on adenosylcobalamin (another active form of vitamin B12) and facilitates the conversion of methylmalonyl-CoA to succinyl-CoA in mitochondria. Inactivation of this enzyme leads to accumulation of methylmalonic acid (MMA), a marker of intracellular B12 dysfunction.
- As a consequence, nitrous oxide intoxication can lead to (Table 1):
- Increased plasma homocysteine (marker of impaired methylation cycles) [26,27].
- Accumulation of methylmalonic acid (MMA) [10,28,29].
- Reduction in methionine synthesis, affecting myelin integrity [30].

Table 1.
Key biochemical markers in N2O toxicity.
Table 1.
Key biochemical markers in N2O toxicity.
| Biological Markers | Pathophysiological Significance |
|---|---|
| Plasma homocysteine | Sensitive marker of N2O exposure; reflects impaired methionine metabolism [10]. |
| Methylmalonic acid (MMA) | More specific to B12-related neurological dysfunction; correlated with clinical severity [10]. |
| Methionine levels | Low methionine is associated with myelin damage, linked to neurological outcomes [30]. |
- Key Takeaways:
- Homocysteine rises rapidly post-exposure, returning to normal within days [1,31].
- MMA remains elevated longer and correlates better with clinical severity [10,32,33].
- Vitamin B12 levels alone are unreliable, as they may appear normal despite severe toxicity [10].
1.3.3. Hematological and Bone Marrow Effects
Chronic N2O exposure could in theory also mimic megaloblastic anemia [34] due to its impact on vitamin B12 related to DNA synthesis and erythropoiesis; however, studies have not reported this effect yet [35].
1.3.4. Cardiovascular and Thromboembolic Complications
Recent research has demonstrated that N2O misuse increases thrombotic risk, primarily via hyperhomocysteinemia-induced endothelial dysfunction [36,37]. The literature reports cerebral venous thrombosis (CVT) [38]; myocardial infarction and deep vein thrombosis (DVT), likely due to homocysteine-related vascular inflammation and endothelial dysfunction [39]; and ischemic strokes associated with elevated homocysteine levels and reduced nitric oxide bioavailability, leading to prothrombotic states [40].
The hypothetical mechanisms of vascular injury caused by N2O misuse are multiple (Figure 2). First, the hyperhomocystenemia caused by consumption can lead to endothelial dysfunction as homocysteine promotes oxidative stress, thus damaging blood vessels [41,42,43]. Moreover, high levels of homocysteine are responsible for pro-coagulant effects via the activation of platelets and coagulation cascades [44]. Finally, impaired NO signaling leads to reduced vasodilation and increased blood pressure [45].
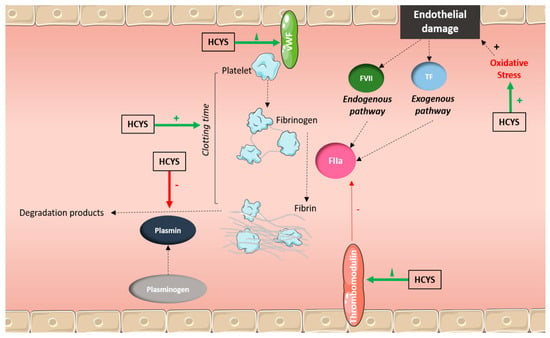
Figure 2.
Possible mechanisms of vascular injury and cardiovascular events related to high homocysteine (HCYS) caused by nitrous oxide (N2O) abuse. Endothelial damages: Homocysteine promotes oxidative stress, damaging blood vessels. Pro-coagulant effects: Elevated homocysteine activates platelets and coagulation cascades.
1.3.5. Psychiatric and Cognitive Consequences
Beyond its neurometabolic toxicity, N2O misuse is associated with psychiatric disorders, including anxiety and depressive symptoms (reported in chronic users) [46]; psychotic episodes and hallucinations, especially in those with underlying psychiatric conditions [22]; and memory impairments and executive dysfunction, possibly due to N2O-induced oxidative stress and impaired methylation pathways [47].
Some case studies suggest that N2O dependence and withdrawal symptoms may develop, particularly in high-frequency users, although clear diagnostic criteria for N2O addiction remain debated.
To address these growing challenges, the PROTOSIDE network (https://protoside.com/en/) was developed to formulate proposals for a coordinated, multidisciplinary, and global response.
1.4. Road Safety and Neurocognitive Risks of Nitrous Oxide Use
Recreational use of nitrous oxide (N2O) not only presents neurological and metabolic risks but also poses a significant emerging threat to road safety. Although its euphoric effects are short-lived, its impact on cognitive and motor functions may last well beyond the acute phase, impairing driving performance and increasing accident risk [48].
Recent studies and accident reports across Europe—particularly in France, the Netherlands, and the UK—highlight the growing involvement of N2O in traffic incidents. For instance, the French National Road Safety Observatory (ONISR) estimates that up to 11% of severe accidents in 2023 involved psychoactive substances, including N2O. In the Netherlands, N2O-related road accidents rose by 80% between 2019 and 2021, underscoring the scale of the problem [48].
The mechanisms of impairment [48] are multiple. First, the short-term effects of N2O include dizziness and mental confusion, which can impair spatial orientation and attention. Then, mid- to long-term effects include loss of motor coordination, which affects precise control of vehicle handling. Slowed reaction times can also occur and compromise the driver’s ability to respond to sudden obstacles. Euphoria and overconfidence can lead to risk-taking behaviors, including speeding and reckless maneuvers. Polydrug use, especially with alcohol or cannabis, further exacerbates driving impairment.
Despite these risks, N2O remains difficult to detect in roadside testing due to its rapid elimination and the absence of standardized field detection tools. This creates a critical gap in enforcement and makes it difficult to sanction intoxicated drivers.
- Current challenges in detection:
N2O is not detectable by standard alcohol or drug screening tools used in roadside tests. Indeed, the rapid absorption and elimination of this anesthetic gas complicates its detection in biological matrices, as N2O has a very short half-life of only a few minutes. Moreover, blood and urine analyses require advanced laboratory methods not available in field settings. Gas chromatography–mass spectrometry (GC-MS) is one of the available methods, but it faces several limitations, including difficulty in selecting an optimal internal standard, limited sensitivity, and potential risk of leaking during sampling, extraction, and analysis. Headspace-GC-MS appears promising but requires further validation for routine laboratory use. Infrared spectroscopy techniques offer high sensitivity for measuring N2O in air but are not applicable to biological matrices. Neither of these sophisticated methods are conducive to use roadside and by non-laboratorians.
Given these challenges, indirect markers reflecting the physiological impact of N2O exposure may need to be explored for reliable detection in forensic and clinical settings. However, indirect markers (homocysteine, methylmalonic acid) are not specific enough for acute detection.
Finally, there is no established legal limit to define N2O intoxication relevant for driving regulation.
- Public health implications:
Given the prevalence of use among adolescents and young adults and its rising association with road traffic fatalities, road safety must be a key target of public prevention campaigns. The PROTOSIDE network integrates this dimension by including road safety awareness in public educational efforts; collaborating with law enforcement and forensic toxicology experts to develop detection protocols; and advocating for regulatory reforms to classify N2O driving under drug-impaired driving laws.
Further research is urgently needed to define psychomotor thresholds of impairment under N2O; validate point-of-care detection tools; and propose legal frameworks and sanctions.
2. Why a Global Network Was Needed
Medical literature has established a sharp rise in N2O recreational use worldwide, with areas all over the world reporting exponential increases in related complications (1). The complications associated with recreational N2O use include subacute combined degeneration of the spinal cord, vitamin B12 deficiency, and thromboembolic events. All these complications often require specialized care, which is not widely accessible or consensual (3).
Gaps in diagnosis protocols, management, and prevention strategies represent additional difficulties. Founding PROTOSIDE was driven by the need to offer guidance on clinical practices and protocols for diagnosing and managing N2O misuse (4); offer an integrated network for comprehensive patient care [49]; and raise awareness among healthcare professionals and the public on the dangers of recreational N2O use [50].
3. Multidisciplinary Competence Centers: The Foundation of PROTOSIDE
The strength of PROTOSIDE (Platforms and Resources for Orientation, Treatment, and Organization of Solutions for Intoxications with N2O, Diagnostics, and Education) lies in its network of competence centers, which integrate various expertise to ensure comprehensive care (5). Centers are required to include specialists such as (Figure 3):
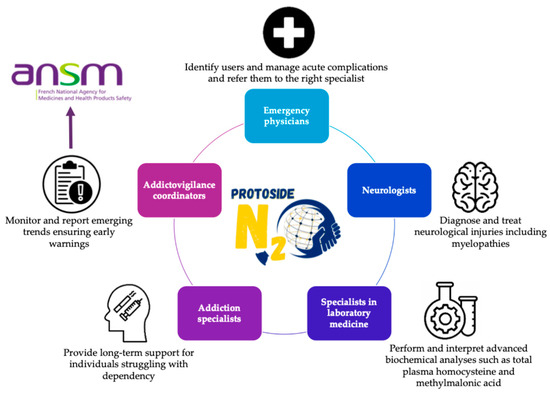
Figure 3.
Multidisciplinary approach and team for patients with nitrous oxide care related to PROTOSIDE (www.protoside.com/en/) network competence centers; integration of expertise from various sources to ensure comprehensive care.
- Emergency physicians, to identify users, manage acute complications, and refer them to the right specialists;
- Neurologists, to diagnose and treat neurological injuries, including myelopathies [51];
- Specialists in laboratory medicine, equipped to perform advanced biochemical analyses such as total plasma homocysteine and MMA concentration measurements (4,6);
- Addiction specialists, to provide long-term support for individuals struggling with dependency [52];
- Addictovigilance coordinators, to monitor and report emerging trends, ensuring early warnings.
This multidisciplinary structure ensures that all aspects of patient care are addressed, from acute treatment to rehabilitation.
4. Comprehensive Patient Care and Prevention
PROTOSIDE adopts a holistic approach to patient care, aiming to offer an integrated pathway that includes:
- Early detection and diagnosis, leveraging multidisciplinary expertise and advanced analytical tools.
- Management, addressing acute complications, metabolic impairments, and neurological damage.
- Addiction support, including tailored programs to help patients overcome dependency on nitrous oxide.
- Long-term follow-up and coordination with primary care to ensure continuity of care through collaboration with general practitioners and specialists.
In addition to clinical care, PROTOSIDE places significant emphasis on prevention and public awareness. Key initiatives include first educational campaigns targeting the public, particularly young people, to highlight the risks of recreational N2O use. There is also an important need for media outreach to inform communities based on articles, interviews, and social media about the health dangers associated with N2O misuse. Finally, collaboration with schools and community organizations is essential, as is engaging with educators and youth leaders to promote safe behaviors and discourage N2O misuse.
5. Knowledge Sharing and Global Collaboration
PROTOSIDE is also committed to advancing knowledge and harmonizing global practices using:
- Scientific conferences, bringing together experts to share research, discuss best practices, and align strategies to manage N2O misuse.
- Training programs for healthcare professionals, providing comprehensive modules on diagnosing, treating, and preventing N2O-related complications.
- Publications and research, contributing to the medical literature on mechanisms, health impacts, and management of N2O misuse.
6. A Global Model for Public Health Action
By combining multidisciplinary expertise, international collaboration, and a strong emphasis on public education, PROTOSIDE sets a benchmark for addressing complex public health challenges. The network provides a robust framework for tackling the growing problem of N2O misuse while fostering innovation in diagnostics, treatment, and prevention.
Healthcare professionals and organizations interested in joining this effort or accessing resources can visit the official PROTOSIDE website at https://protoside.com/en/.
Author Contributions
Conceptualization, G.G., R.D., T.D. and J.P.N.; methodology, G.G., E.G. and T.D.; software, E.G.; validation, G.G., E.G. and B.M.; formal analysis, G.G. and D.D.; investigation, G.G., E.G., J.P.N., C.B., D.S., A.B. (Anas Bennis), and A.B. (Alexandra Boucher); resources, G.G., T.D., I.R.-V., E.C., S.D. and C.R.; writing—original draft preparation, G.G. and E.G.; writing—review and editing, G.G., R.D., T.D., J.P.N., D.D., S.D., J.C.G., D.S., B.M., I.R.-V., A.B. (Alexandra Boucher), A.B. (Anas Bennis), L.K., E.C., B.R., C.R., C.B. and A.C.; visualization, G.G. and E.G.; supervision, G.G., B.M. and L.K.; project administration, G.G. and E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable (only appropriate if no new data is generated or the article describes entirely theoretical research).
Acknowledgments
PROTOSIDE expresses its sincere gratitude to Laura Danvers for her invaluable contributions and dedication to the organization and development of the network. We would also like to thank Gwenaelle Vautrin, who recently joined the network as a legal and prevention expert, to reinforce our actions in the field and in the fight against the disease. The authors also thank the many clinicians, biologists, and public health stakeholders who support the mission of PROTOSIDE across France and Europe.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lucas, A.; Noyce, A.J.; Gernez, E.; Khoury, J.M.E.; Garcon, G.; Cavalier, E.; Antherieu, S.; Grzych, G. Nitrous Oxide Abuse Direct Measurement for Diagnosis and Follow-up: Update on Kinetics and Impact on Metabolic Pathways. Clin. Chem. Lab. Med. (CCLM) 2024, 62, 2356–2372. [Google Scholar] [CrossRef] [PubMed]
- Recreational Use of Nitrous Oxide—A Growing Concern for Europe | www.emcdda.europa.eu. Available online: https://www.emcdda.europa.eu/publications/rapid-communication/recreational-use-nitrous-oxide-growing-concern-europe_en (accessed on 2 June 2024).
- Back, S.; Kroon, E.; Colyer-Patel, K.; Cousijn, J. Does Nitrous Oxide Addiction Exist? An Evaluation of the Evidence for the Presence and Prevalence of Substance Use Disorder Symptoms in Recreational Nitrous Oxide Users. Addiction 2024, 119, 609–618. [Google Scholar] [CrossRef]
- Scherer, C.; Lanz, L.A.; Liebs, T.R.; Kaiser, N.; Zindel, M.; Berger, S.M. A Randomized Controlled Trial Comparing Immersive Virtual Reality Games versus Nitrous Oxide for Pain Reduction in Common Outpatient Procedures in Pediatric Surgery. Trials 2025, 26, 21. [Google Scholar] [CrossRef]
- Kronenberg, G.; Schoretsanitis, G.; Seifritz, E.; Olbrich, S. The Boon and Bane of Nitrous Oxide. Eur. Arch. Psychiatry Clin. Neurosci. 2024. [Google Scholar] [CrossRef]
- Hout, M.C.V.; Bingham, T. ‘Surfing the Silk Road’: A Study of Users’ Experiences. Int. J. Drug Policy 2013, 24, 524–529. [Google Scholar] [CrossRef]
- MacLean, S.J.; Riddell, O.; Herold, M.D.; Frank, V.A. Becoming a Nitrous Oxide User on Social Media: Learning to Maximise Pleasures and Minimise Harms. Int. J. Drug Policy 2022, 109, 103861. [Google Scholar] [CrossRef] [PubMed]
- Benschop, A.; van Bakkum, F.; Noijen, J. Changing Patterns of Substance Use During the Coronavirus Pandemic: Self-Reported Use of Tobacco, Alcohol, Cannabis, and Other Drugs. Front. Psychiatry 2021, 12, 633551. [Google Scholar] [CrossRef]
- Whelan, J.; John, T.; Crossin, R.; Ward, R.D. Consuming and Thinking About Nangs: A Survey of Nitrous Oxide Use, Knowledge, Attitudes and Perceptions in Aotearoa New Zealand. J. Psychoact. Drugs 2025, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Grzych, G.; Deheul, S.; Gernez, E.; Davion, J.-B.; Dobbelaere, D.; Carton, L.; Kim, I.; Guichard, J.C.; Girot, M.; Humbert, L.; et al. Comparison of Biomarker for Diagnosis of Nitrous Oxide Abuse: Challenge of Cobalamin Metabolic Parameters, a Retrospective Study. J. Neurol. 2023, 270, 2237–2245. [Google Scholar] [CrossRef]
- Largeau, B.; Karam, A.; Potey, C.; Caous, A.-S.; Tard, C.; Carton, L.; Kuchcinski, G.; Gautier, S.; Deheul, S.; Bordet, R. Myeloneuropathy Induced by Recreational Nitrous Oxide Use with Variable Exposure Levels. Eur. J. Neurol. 2022, 29, 2173–2180. [Google Scholar] [CrossRef]
- Guerlais, M.; Deheul, S.; Le Boisselier, R.; Victorri-Vigneau, C. Non-Medical Nitrous Oxide Misuse: From Identifying a Signal to Unprecedented Addictovigilance Network Communication. Therapies 2024, 80, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Netherlands to Ban Laughing Gas from January 2022. Available online: https://www.rijksoverheid.nl/onderwerpen/drugs/documenten/brochures/2022/12/07/lachgasverbod-per-1-januari-2023 (accessed on 2 June 2024).
- Gernez, E.; Lee, G.R.; Niguet, J.-P.; Zerimech, F.; Bennis, A.; Grzych, G. Nitrous Oxide Abuse: Clinical Outcomes, Pharmacology, Pharmacokinetics, Toxicity and Impact on Metabolism. Toxics 2023, 11, 962. [Google Scholar] [CrossRef] [PubMed]
- Fortanier, E.; Delmont, E.; Corazza, G.; Kouton, L.; Micallef, J.; Attarian, S. Longitudinal Follow-up and Prognostic Factors in Nitrous Oxide-Induced Neuropathy. J. Peripher. Nerv. Syst. 2024, 29, 252–261. [Google Scholar] [CrossRef]
- Berling, E.; Fargeot, G.; Aure, K.; Tran, T.H.; Kubis, N.; Lozeron, P.; Zanin, A. Nitrous Oxide-Induced Predominantly Motor Neuropathies: A Follow-up Study. J. Neurol. 2022, 269, 2720–2726. [Google Scholar] [CrossRef]
- Zaloum, S.A.; Paris, A.; Mair, D.; Gutteridge, C.; Ayling, R.M.; Onen, B.L.; Walton, J.; Workman, A.; Villanueva, N.; Noyce, A.J. Evaluation of an Ambulatory Care Pathway for Patients with Nitrous Oxide-Induced Myeloneuropathy. BMJ Neurol. Open 2024, 6, e000737. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Klein, J.P. Subacute Combined Degeneration from Nitrous Oxide Use. N. Engl. J. Med. 2022, 387, 832. [Google Scholar] [CrossRef]
- Meißner, J.N.; Neuneier, J.; Bartzokis, I.; Rehm, M.; Al-Hayali, A.; Müller, M.; Paus, S.; Limmroth, V.; Fink, G.R.; Petzold, G.C.; et al. Increase of Nitrous Oxide-Induced Neurological Disorders—A German Multicenter Experience. Neurol. Res. Pract. 2025, 7, 3. [Google Scholar] [CrossRef]
- Grzych, G.; Scuccimarra, M.; Plasse, L.; Gernez, E.; Cassim, F.; Touze, B.; Girot, M.; Bossaert, C.; Tard, C. Understanding Neuropathy Features in the Context of Nitrous Oxide Abuse: A Combined Electrophysiological and Metabolic Approach. Biomedicines 2024, 12, 429. [Google Scholar] [CrossRef] [PubMed]
- Paulus, M.C.; Wijnhoven, A.M.; Maessen, G.C.; Blankensteijn, S.R.; van der Heyden, M.A.G. Does Vitamin B12 Deficiency Explain Psychiatric Symptoms in Recreational Nitrous Oxide Users? A Narrative Review. Clin. Toxicol. 2021, 59, 947–955. [Google Scholar] [CrossRef]
- Blom, J.D. Hallucinations and Vitamin B12 Deficiency: A Systematic Review. Psychopathology 2024, 57, 492–503. [Google Scholar] [CrossRef]
- Ménétrier, T.; Denimal, D. Vitamin B12 Status in Recreational Users of Nitrous Oxide: A Systematic Review Focusing on the Prevalence of Laboratory Abnormalities. Antioxidants 2023, 12, 1191. [Google Scholar] [CrossRef]
- Kondo, H.; Osborne, M.L.; Kolhouse, J.F.; Binder, M.J.; Podell, E.R.; Utley, C.S.; Abrams, R.S.; Allen, R.H. Nitrous Oxide Has Multiple Deleterious Effects on Cobalamin Metabolism and Causes Decreases in Activities of Both Mammalian Cobalamin-Dependent Enzymes in Rats. J. Clin. Investig. 1981, 67, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Stabler, S.P. Vitamin B12 Deficiency. N. Engl. J. Med. 2013, 368, 149–160. [Google Scholar] [CrossRef]
- Grzych, G.; Zerimech, F.; Touze, B.; Descamps, C.; Bout, M.-A.; Joncquel, M.; Douillard, C.; Kim, I.; Tard, C.; Brousseau, T. Enhancing Differential Diagnosis Related to Oxidative Stress, Nitrous Oxide, and Nutrition by Rapid Plasma Homocysteine Measurement. J. Xenobiot. 2024, 14, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Grzych, G.; Douillard, C.; Lannoy, J.; Joncquel Chevalier Curt, M. Very High Plasma Homocysteine without Malnutrition or Inherited Disorder. Clin. Chem. 2020, 66, 1468–1469. [Google Scholar] [CrossRef]
- Waclawik, A.J.; Luzzio, C.C.; Juhasz-Pocsine, K.; Hamilton, V. Myeloneuropathy from Nitrous Oxide Abuse: Unusually High Methylmalonic Acid and Homocysteine Levels. WMJ 2003, 102, 43–45. [Google Scholar] [PubMed]
- Fu, X.; Francisco, C.; Pattengale, P.; O’Gorman, M.R.; Mitchell, W.G. An Adolescent with Increased Plasma Methylmalonic Acid and Total Homocysteine. Clin. Chem. 2017, 63, 1069–1072. [Google Scholar] [CrossRef]
- Gernez, E.; Deheul, S.; Tard, C.; Joncquel, M.; Douillard, C.; Grzych, G. Plasma Methionine and Clinical Severity in Nitrous Oxide Consumption. Toxics 2023, 11, 12. [Google Scholar] [CrossRef]
- Frontiera, M.S.; Stabler, S.P.; Kolhouse, J.F.; Allen, R.H. Regulation of Methionine Metabolism: Effects of Nitrous Oxide and Excess Dietary Methionine. J. Nutr. Biochem. 1994, 5, 28–38. [Google Scholar] [CrossRef]
- Adam, L.C.; Grobelny, A.; Hahn, K.; Audebert, H.J.; Krause, P.; Franke, C.; Ruprecht, K. Severe Subacute Combined Degeneration of the Spinal Cord Resulting from Nitrous Oxide (N2O) Abuse: A Case Series. Neurol. Res. Pract. 2025, 7, 7. [Google Scholar] [CrossRef]
- Kyaw, K.Y.; Lwin, M.T.; Lwin, Z.T. Correlation Between Nitrous Oxide and Functional Vitamin B12 Deficiency Resulting in Subacute Combined Degeneration of the Spinal Cord: A Case Report. Cureus 2024, 16, e74383. [Google Scholar] [CrossRef] [PubMed]
- Amess, J.A.L.; Rees, G.M.; Burman, J.F.; Nancekievill, D.G.; Mollin, D.L. Megaloblastic Hæmopoiesis in Patients Receiving Nitrous Oxide. Lancet 1978, 312, 339–342. [Google Scholar] [CrossRef]
- Wang, R.; Lan, L.; Xu, L.; Zhu, B.; Huang, Y. A Retrospective Cohort Study on Red Blood Cell Morphology Changes in Pre-School Age Children under Nitrous Oxide Anesthesia. BMC Anesthesiol. 2021, 21, 171. [Google Scholar] [CrossRef] [PubMed]
- Patyjewicz, M.; Mair, D.; Zaloum, S.A.; Onen, B.; Walton, J.; Dobson, R.; Joerres, C.; Shah, A.M.; MacCallum, P.; Massey, T.H.; et al. Recreational Nitrous Oxide and Thrombotic Events: A Case Series. BMJ Neurol. Open 2024, 6, e000619. [Google Scholar] [CrossRef] [PubMed]
- Oulkadi, S.; Peters, B.; Vliegen, A.-S. Thromboembolic Complications of Recreational Nitrous Oxide (Ab)Use: A Systematic Review. J. Thromb. Thrombolysis 2022, 54, 686–695. [Google Scholar] [CrossRef]
- Doukhi, D.; Siguret, V.; Vodovar, D.; Delrue, M.; Reiner, P.; Aghetti, A.; Guey, S.; Mazighi, M.; Crassard, I. Cerebral Venous Thrombosis and Nitrous Oxide Intoxication: Report of Two Cases and Review of the Literature. Brain Behav. 2025, 15, e70394. [Google Scholar] [CrossRef]
- Bizouard, T.; Caplette, C.; Duval, D.; Savary, D.; Douillet, D. Right Iliac Deep Vein Thrombosis and Pulmonary Embolism Associated with Recreational Nitrous Oxide: A Case Report. Int. J. Emerg. Med. 2024, 17, 169. [Google Scholar] [CrossRef]
- Bajaj, D.; Agrawal, A.; Gupta, S.; Bajaj, S.; Bajaj, D.; Agrawal, A.; Gupta, S.; Bajaj, S. Recreational Nitrous Oxide Abuse Causing Ischemic Stroke in a Young Patient: A Rare Case Report. Cureus 2018, 10, e3761. [Google Scholar] [CrossRef]
- Gueant, J.-L.; Gueant-Rodriguez, R.-M.; Oussalah, A.; Zuily, S.; Rosenberg, I. Hyperhomocysteinemia in Cardiovascular Diseases: Revisiting Observational Studies and Clinical Trials. Thromb. Haemost. 2022, 123, 270–282. [Google Scholar] [CrossRef]
- Lander, M.E.; Lander, S.J.; Park, J.; Chae, C. Recreational Nitrous Oxide Causing Deep Vein Thrombosis and Subacute Combined Degeneration: Whip It Real Good. Cureus 2024, 16, e65155. [Google Scholar] [CrossRef]
- McMahon, G.; Lönnberg, F.; Gautam, G.; Ågren, A.; Nordmark Grass, J.; Siddiqui, A.J. Life-Threatening Thrombosis After Large Amounts of Nitrous Oxide Use. JACC Case Rep. 2024, 29, 102312. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Suryadi, J.; Yang, Y.; Kucukkal, T.G.; Cao, W.; Alexov, E. Mutations in the KDM5C ARID Domain and Their Plausible Association with Syndromic Claes-Jensen-Type Disease. Int. J. Mol. Sci. 2015, 16, 27270–27287. [Google Scholar] [CrossRef] [PubMed]
- Posthuma, J.J.; Reesink, K.D.; Schütten, M.; Ghossein, C.; Spaanderman, M.E.; ten Cate, H.; Schep, G. A Rare Case of Intermittent Claudication Associated with Impaired Arterial Vasodilation. Case Rep. Vasc. Med. 2017, 2017, 4868123. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Hines, L.A.; Bonell, C.; Hickman, M.; Adara, L.; Townson, J.; Cannings-John, R.; Moore, L.; White, J. Association of Volatile Substance, Nitrous Oxide and Alkyl Nitrate Use with Mental Health in UK Adolescents. Br. J. Psychiatry 2025, 226, 10–15. [Google Scholar] [CrossRef]
- Sharpe, E.E.; Warner, L.L.; Brakke, B.D.; Davis, P.R.; Finkel, D.M.; Burkle, C.M.; Hanson, A.C.; Pompeian, R.J.; Arendt, K.W.; Butler Tobah, Y.S.; et al. Impact of Nitrous Oxide Use on Parturient Recall of Neuraxial Analgesia Risks. J. Clin. Anesth. 2024, 98, 111579. [Google Scholar] [CrossRef]
- Grzych, G.; Megarbane, B.; Diesnis, R.; Chauvin, A.; Denimal, D.; Diesnis, R.; Gernez, E.; Redonnet-Vernhet, I.; Blin, J.; Kim, I.; et al. Nitrous oxide and road safety: Challenges and opportunities for biological screening. SPECTRA Diagn. 2024; ahead of print. [Google Scholar]
- Gernez, E.; Bennis, A.; Diesnis, R.; Niguet, J.P.; Grzych, G. Awareness of Health Care Related to Nitrous Oxide Abuse for Diagnosis, Treatment and Follow-Up. Ir. J. Med. Sci. 2023, 192, 3087–3089. [Google Scholar] [CrossRef]
- Zaloum, S.A.; Mair, D.; Paris, A.; Smith, L.J.; Patyjewicz, M.; Onen, B.L.; Noyce, A.J. Tackling the Growing Burden of Nitrous Oxide-Induced Public Health Harms. Lancet Public Health 2025, 10, e257–e263. [Google Scholar] [CrossRef]
- Paris, A.; Lake, L.; Joseph, A.; Workman, A.; Walton, J.; Hayton, T.; Evangelou, N.; Lilleker, J.B.; Ayling, R.M.; Nicholl, D.; et al. Nitrous Oxide-Induced Subacute Combined Degeneration of the Cord: Diagnosis and Treatment. Pract. Neurol. 2023, 23, 222–228. [Google Scholar] [CrossRef]
- Diesing, D.; Neu, P. Trenddroge Lachgas (Distickstoffmonoxid, N2O) und die Abhängigkeitskriterien nach ICD-10. Nervenarzt 2024, 96, 284–289. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).