Hippocampal Proteomics Reveals the Novel Molecular Profiling of Postnatal Lead (Pb) Exposure on Autism-like Behaviors
Abstract
1. Introduction
2. Materials and Methods
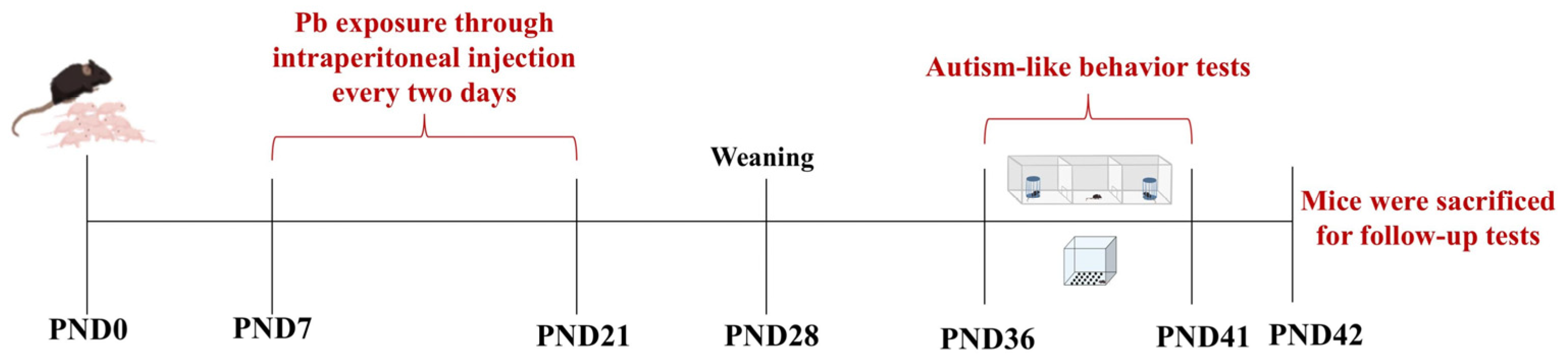
2.1. Establishment of Animal Models and Grouping
2.2. Three-Chamber Social Test
2.3. Marble-Burying Test
2.4. Pb Level Determination in Blood and Hippocampus
2.5. Histopathological Examination
2.6. TMT-Based Quantitative Proteomics
2.6.1. Protein Sample Preparation
2.6.2. TMT Labeling
2.6.3. LC-MS/MS Analysis
2.6.4. Processing and Analysis of the Spectral Data
2.7. Western Blotting Analysis
2.8. Statistical Analysis
3. Results
3.1. Postnatal Pb Exposure Induced Autism-like Behaviors in Mice
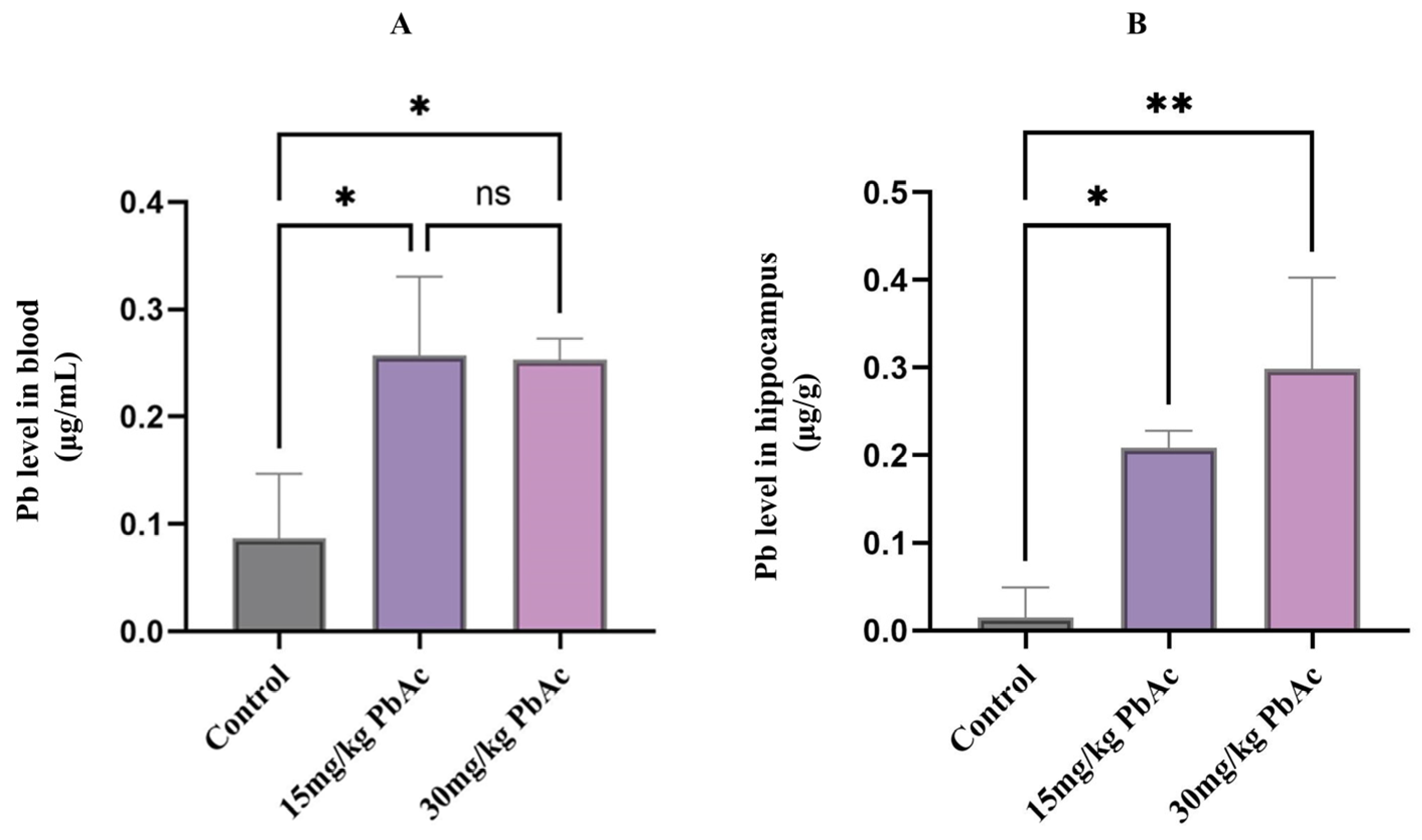
3.2. Pb Levels in Blood and the Hippocampus
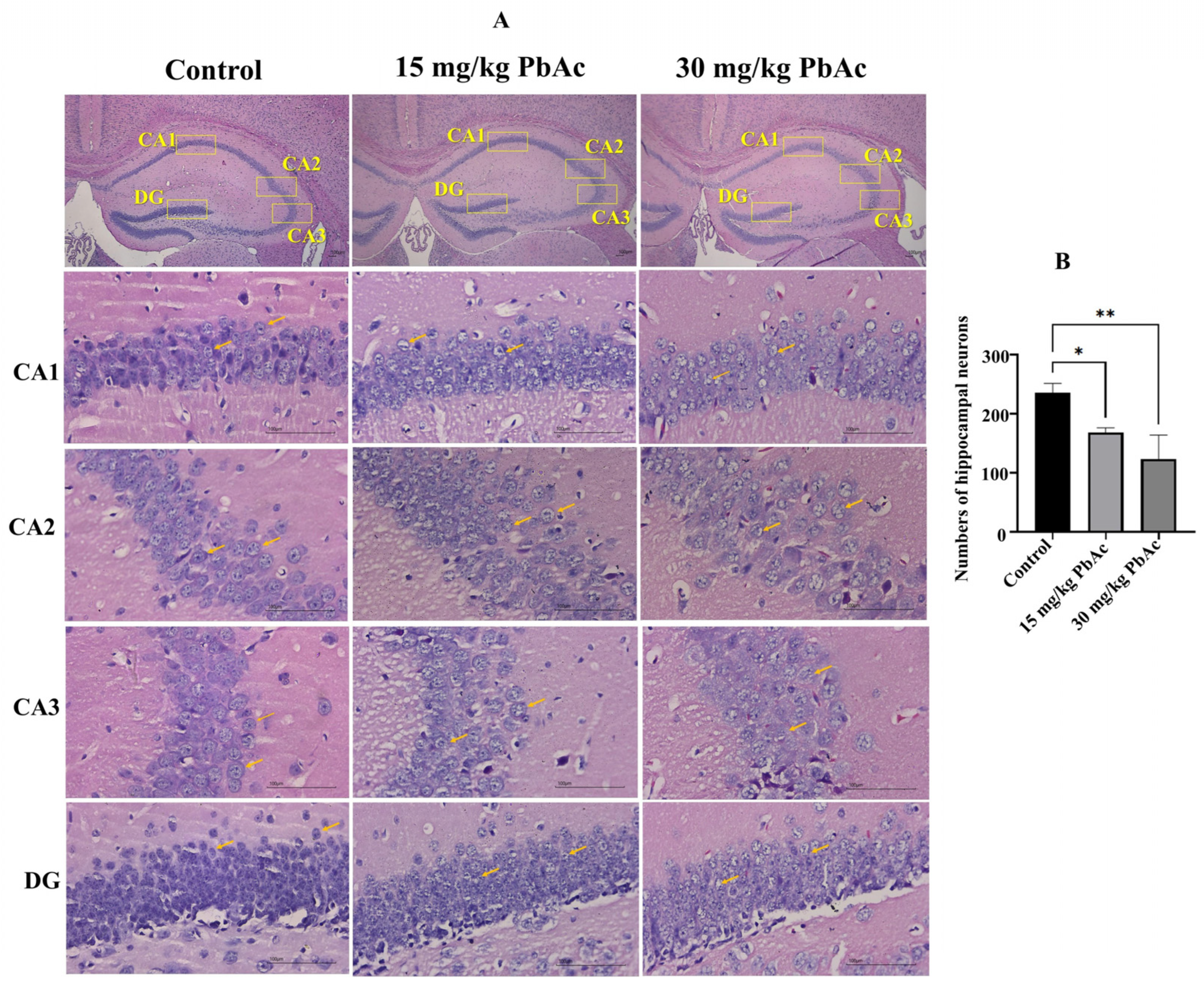
3.3. Postnatal Pb Exposure Induced Morphological Alterations in the Hippocampus
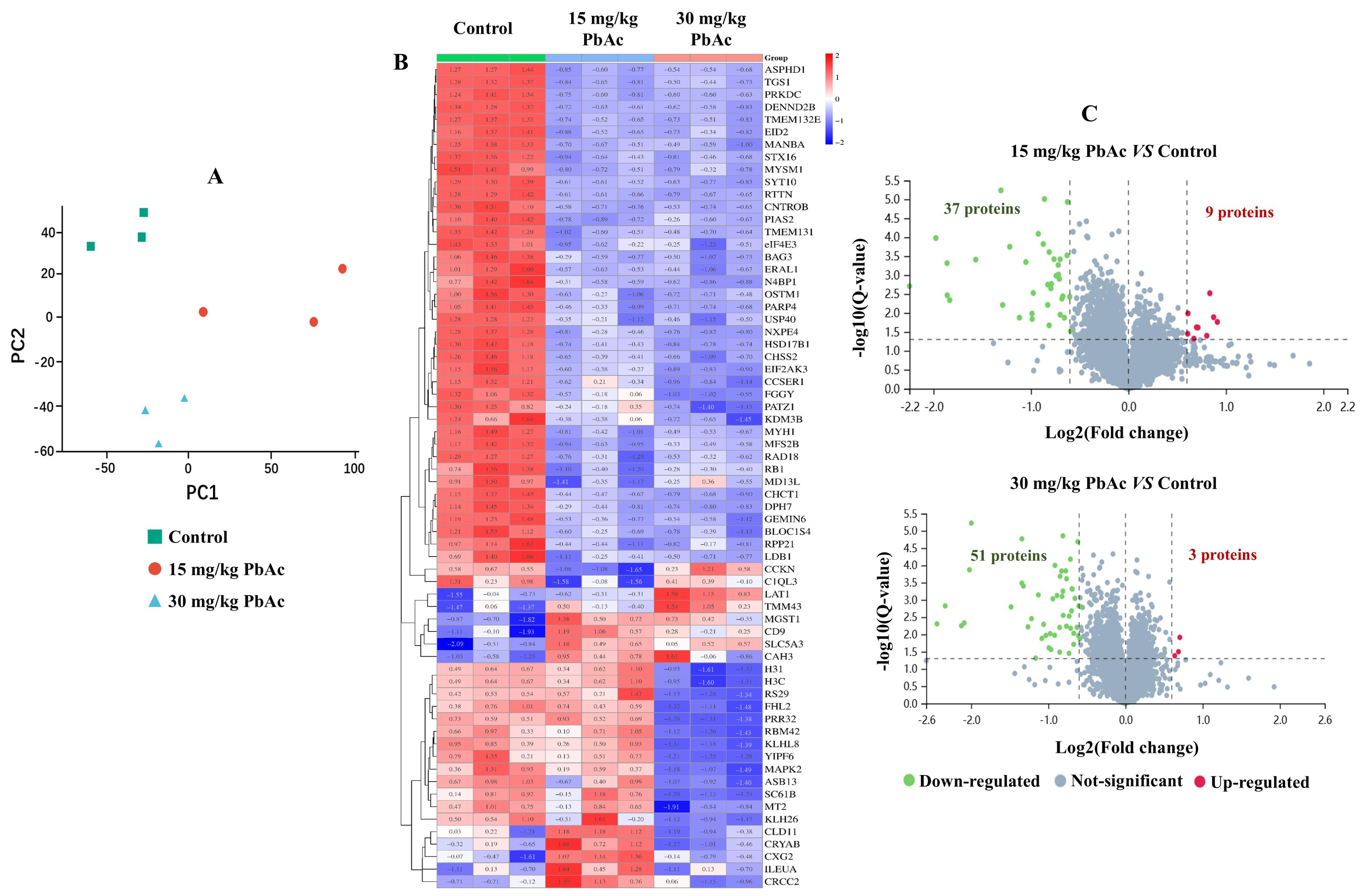
3.4. Postnatal Pb Exposure Affected the Proteome Profiling
3.5. Postnatal Pb Exposure Triggered Multi-Pathway Alterations
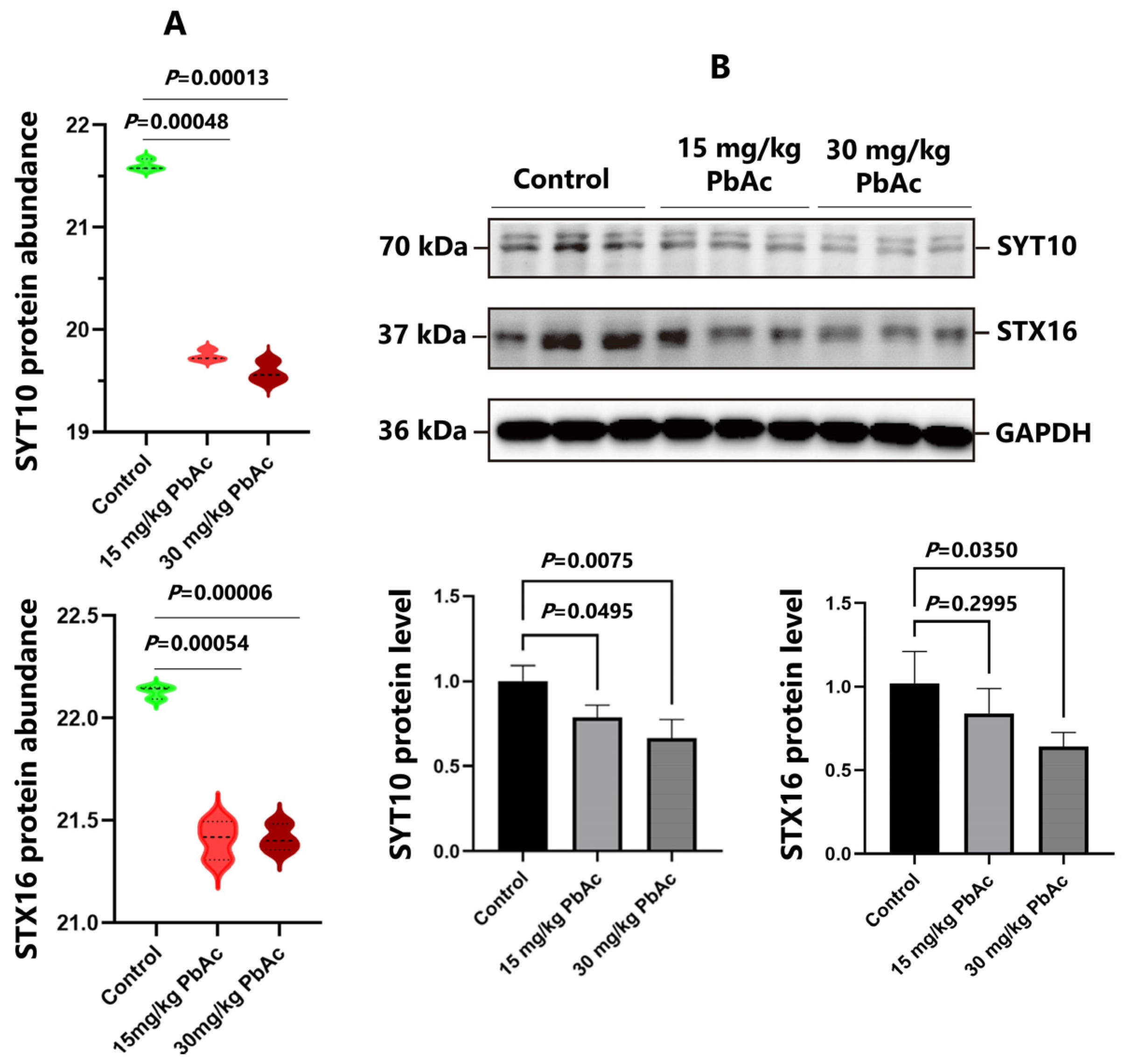
3.6. Postnatal Pb Exposure Decreased the Protein Levels of SYT10, STX16, and IGF-1
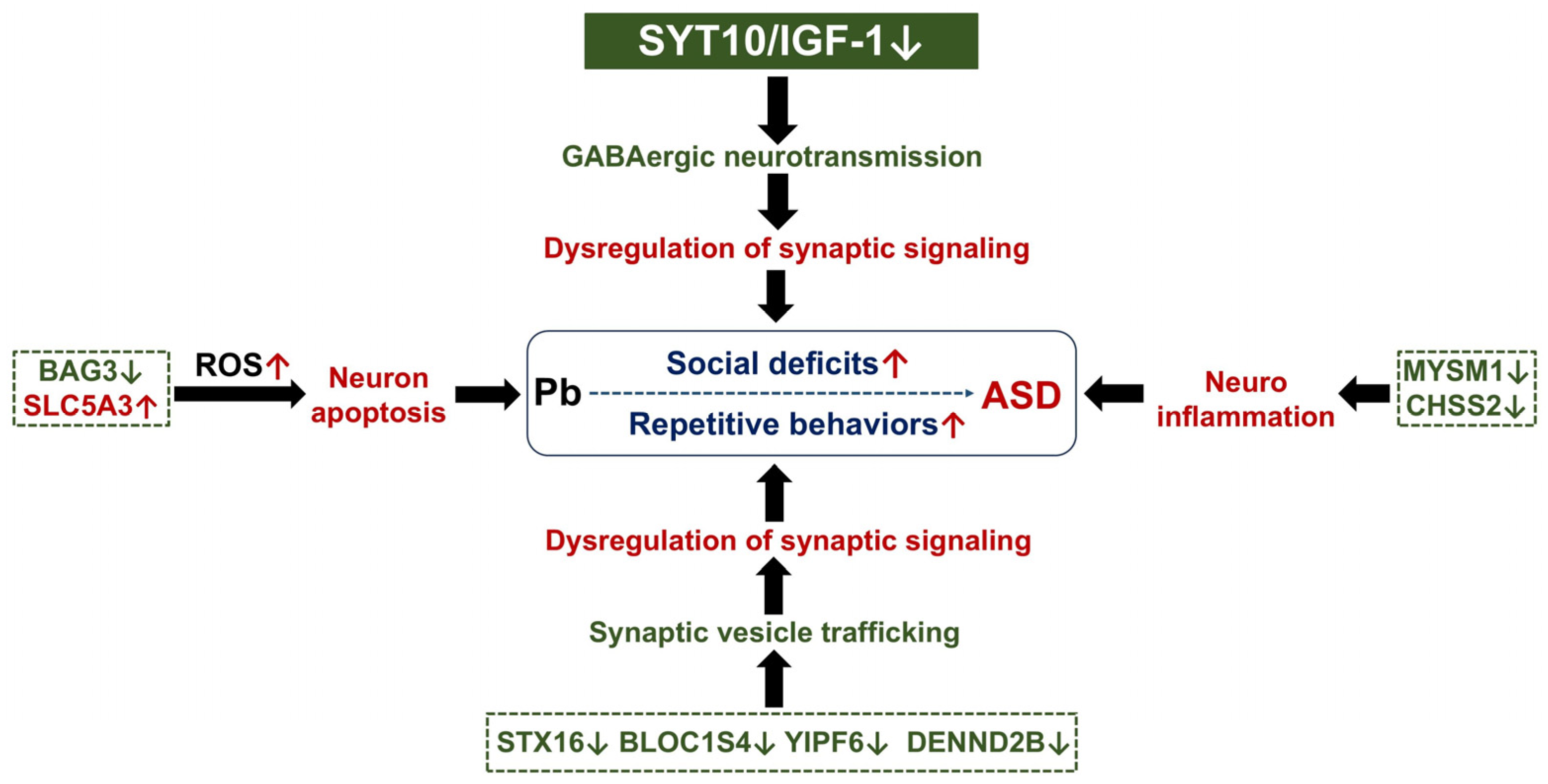
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASD | Autism spectrum disorder |
| SYT10 | Synaptotagmin-10 |
| STX16 | Syntaxin-16 |
| IGF-1 | Insulin-like growth factor 1 |
References
- Maenner, M.J.; Warren, Z.; Robinson Williams, A.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years-Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR Surveill. Summ. 2023, 72, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xu, X.; Yan, W.; Zou, X.; Wu, L.; Luo, X.; Li, T.; Huang, Y.; Guan, H.; Chen, X.; et al. Prevalence of Autism Spectrum Disorder in China: A Nationwide Multi-center Population-based Study Among Children Aged 6 to 12 Years. Neurosci. Bull. 2020, 36, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Study 2021 Autism Spectrum Collaborators. The global epidemiology and health burden of the autism spectrum: Findings from the Global Bur den of Disease Study 2021. Lancet Psychiatry 2025, 12, 111–121. [Google Scholar] [CrossRef]
- Stojsavljevic, A.; Lakicevic, N.; Pavlovic, S. Does Lead Have a Connection to Autism? A Systematic Review and Meta-Analysis. Toxics 2023, 11, 753. [Google Scholar] [CrossRef]
- Smith, M.R.; Yevoo, P.; Sadahiro, M.; Austin, C.; Amarasiriwardena, C.; Awawda, M.; Arora, M.; Dudley, J.T.; Morishita, H. Inte grative bioinformatics identifies postnatal lead (Pb) exposure disrupts developmental cortical plasticity. Sci. Rep. 2018, 8, 12. [Google Scholar] [CrossRef]
- Shiani, A.; Sharafi, K.; Omer, A.K.; Kiani, A.; Karamimatin, B.; Massahi, T.; Ebrahimzadeh, G. A systematic literature review on the association between exposures to toxic elements and an autism spectrum disorder. Sci. Total Environ. 2023, 857, 159246. [Google Scholar] [CrossRef]
- Pillai, R.L.I.; Covington, M.; Visintainer, P.F.; Branch, H.J. Association of autism with lead poisoning in an environmental health clinic. Pediatr. Res. 2023, 94, 7–9. [Google Scholar] [CrossRef]
- Nakhaee, S.; Amirabadizadeh, A.; Farnia, V.; Azadi, N.A.; Mansouri, B.; Radmehr, F. Association Between Biological Lead Con centrations and autism spectrum disorder (ASD) in Children: A Systematic Review and Meta-Analysis. Biol. Trace Elem. Res. 2023, 201, 1567–1581. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Rezaei, A.; Esmaeili, A.; Nakhaee, S.; Azadi, N.A.; Mansouri, B. A case-control study on the relationship between urine trace element levels and autism spectrum disorder among Iranian children. Environ. Sci. Pollut. Res. 2022, 29, 57287–57295. [Google Scholar] [CrossRef]
- Zhou, Q.F.; Huang, D.N.; Xu, C.L.; Wang, J.L.; Jin, Y.T. Hair levels of heavy metals and essential elements in Chinese children with autism spectrum disorder. J. Trace Elem. Med. Biol. 2021, 66, 126748. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Shen, L.M.; Khan, N.U.; Zhang, X.; Chen, L.L.; Zhao, H.; Luo, P. Trace elements in children with autism spec trum disorder: A meta-analysis based on case-control studies. J. Trace Elem. Med. Biol. 2021, 67, 126782. [Google Scholar] [CrossRef] [PubMed]
- Rashaid, A.H.B.; Nusair, S.D.; Alqhazo, M.T.; Adams, J.B.; Abu-Dalo, M.A.; Bashtawi, M.A. Heavy metals and trace elements in scalp hair samples of children with severe autism spectrum disorder: A case-control study on Jordanian children. J. Trace Elem. Med. Biol. 2021, 67, 126790. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, A.S.; Rahbar, M.H.; Bakian, A.V.; Bilder, D.A.; Harrington, R.A.; Pettygrove, S.; Kirby, R.S.; Durkin, M.S.; Han, I.; Moye, L.A.; et al. Autism spectrum disorder prevalence and associations with air concentrations of lead, mercury, and arsenic. Environ. Monit. Assess. 2016, 188, 407. [Google Scholar] [CrossRef]
- Zhou, X.L.; Xia, X.C.; Li, L.M.; Ye, Y.H.; Chen, Q.H.; Ke, M.Y.; Cui, Q.; He, Y.L.; Chen, Y.T.; Lin, S.Q.; et al. Evaluation of Heavy Metals and Essential Minerals in the Hair of Children with Autism Spectrum Disorder and Their Association with Symptom Severity. Biol. Trace Elem. Res. 2025, 203, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Barone, R.; Copat, C.; Grasso, A.; Cristaldi, A.; Rizzo, R.; Ferrante, M. Metal and essential element levels in hair and association with autism severity. J. Trace Elem. Med. Biol. 2020, 57, 99–103. [Google Scholar] [CrossRef]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Toxicological Status of Children with Autism vs. Neurotypical Children and the Association with Autism Severity. Biol. Trace Elem. Res. 2013, 151, 171–180. [Google Scholar] [CrossRef]
- Lin, H.H.; Jung, C.R.; Lin, C.Y.; Chang, Y.C.; Hsieh, C.Y.; Hsu, P.C.; Chuang, B.R.; Hwang, B.F. Prenatal and postnatal exposure to heavy metals in PM2.5 and autism spectrum disorder. Environ. Res. 2023, 237, 116874. [Google Scholar] [CrossRef]
- Arora, M.; Reichenberg, A.; Willfors, C.; Austin, C.; Gennings, C.; Berggren, S.; Lichtenstein, P.; Anckarsäter, H.; Tammimies, K.; Bölte, S. Fetal and postnatal metal dysregulation in autism. Nat. Commun. 2017, 8, 15493. [Google Scholar] [CrossRef]
- Zhou, Q.F.; Tian, Y.; Xu, C.L.; Wang, J.L.; Jin, Y.T. Prenatal and postnatal traffic pollution exposure, DNA methylation in Shank3 and MeCP2 promoter regions, H3K4me3 and H3K27me3 and sociability in rats’ offspring. Clin. Epigenetics 2021, 13, 12. [Google Scholar] [CrossRef]
- Assiri, M.A.; Albekairi, T.H.; Ansari, M.A.; Nadeem, A.; Attia, S.M.; Bakheet, S.A.; Shahid, M.; Aldossari, A.A.; Almutairi, M.M.; Almanaa, T.N.; et al. The Exposure to Lead (Pb) Exacerbates Immunological Abnormalities in BTBR T+ Itpr3tf/J Mice through the Regulation of Signaling Pathways Relevant to T Cells. Int. J. Mol. Sci. 2023, 24, 16218. [Google Scholar] [CrossRef]
- Almutairi, M.M.; Nadeem, A.; Ansari, M.A.; Bakheet, S.A.; Attia, S.M.; Albekairi, T.H.; Alhosaini, K.; Algahtani, M.; Alsaad, A.M.S.; Al-Mazroua, H.A.; et al. Lead (Pb) exposure exacerbates behavioral and immune abnormalities by upregulating Th17 and NF-kB-related signaling in BTBR T+Itpr 3tf/J autistic mouse model. Neurotoxicology 2022, 91, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Lim, G.; Hong, Y.S.; Kim, S.; Hwang, S.; Lee, J.; Sin, S.; Cho, A.; Kim, Y.; Gautam, R.; et al. Exposure to lead on expression levels of brain immunoglobulins, inflammatory cytokines, and brain-derived neurotropic factor in fetal and postnatal mice with autism-like characteristics. J. Toxicol. Environ. Health A 2021, 84, 891–900. [Google Scholar] [CrossRef]
- Shilpa, O.; Anupama, K.P.; Antony, A.; Gurushankara, H.P. Lead (Pb)-induced oxidative stress mediates sex-specific autistic-like behaviour in Drosophila melanogaster. Mol. Neurobiol. 2021, 58, 6378–6393. [Google Scholar] [CrossRef]
- Xia, Y.M.; Huang, X.L.; Mo, L.D.; Wang, C.; Fan, W.J.; Huang, H.L. TMT-based proteomics analysis of the cerebral cortex of TauT knockout rats. Proteome Sci. 2022, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Karri, V.; Ramos, D.; Bauza Martinez, J.; Odena, A.; Oliveira, E.; Coort, S.L.; Evelo, C.T.; Mariman, E.C.M.; Schuhmacher, M.; Kumar, V. Differential protein expression of hippocampal cells associated with heavy metals (Pb, As, and MeHg) neurotoxicity: Deepening into the molecular mechanism of neurodegenerative diseases. J. Proteom. 2018, 187, 106–125. [Google Scholar] [CrossRef] [PubMed]
- Mota, F.S.B.; Nascimento, K.S.; Oliveira, M.; Osterne, V.J.S.; Clemente, J.C.M.; Correia-Neto, C.; Lima-Neto, A.B.; van Tilburg, M.F.; Leal-Cardoso, J.H.; Guedes, M.I.F.; et al. Potential protein markers in children with Autistic Spectrum Disorder (ASD) revealed by salivary proteomics. Int. J. Biol. Macromol. 2022, 199, 243–251. [Google Scholar] [CrossRef]
- Carbonell, A.U.; Freire-Cobo, C.; Deyneko, I.V.; Dobariya, S.; Erdjument-Bromage, H.; Clipperton-Allen, A.E.; Page, D.T.; Neubert, T.A.; Jordan, B.A. Comparing synaptic proteomes across five mouse models for autism reveals converging molecular similarities including deficits in oxidative phosphorylation and Rho GTPase signaling. Front. Aging Neurosci. 2023, 15, 14. [Google Scholar] [CrossRef]
- Ben-Azu, B.; Adebayo, O.G.; Wopara, I.; Aduema, W.; Onyeleonu, I.; Umoren, E.B.; Kolawole, T.A.; Ebo, O.T.; Akpotu, A.E.; Ajibo, D.N.; et al. Lead acetate induces hippocampal pyramidal neuron degeneration in mice via up-regulation of executioner caspase-3, oxido-inflammatory stress expression and decreased BDNF and cholinergic activity: Reversal effects of Gingko biloba supplement. J. Trace Elem. Med. Biol. 2022, 71, 10. [Google Scholar] [CrossRef]
- Hosseini-Sharifabad, A.; Naghibzadeh, S.; Hajhashemi, V. The effect of lead, restraint stress or their co-exposure on the move ment disorders incidence in male mice. Res. Pharm. Sci. 2019, 14, 343–350. [Google Scholar] [CrossRef]
- Mitsuhashi, T.; Hattori, S.; Fujimura, K.; Shibata, S.; Miyakawa, T.; Takahashi, T. In utero exposure to valproic acid throughout pregnancy causes phenotypes of autism in offspring mice. Dev. Neurosci. 2023, 45, 223–233. [Google Scholar] [CrossRef]
- Liu, C.; Hu, Q.; Chen, Y.; Wu, L.; Liu, X.; Liang, D. Behavioral and Gene Expression Analysis of Stxbp6-Knockout Mice. Brain Sci. 2021, 11, 436. [Google Scholar] [CrossRef]
- Eshraghi, A.A.; Memis, I.; Wang, F.; White, I.; Furar, E.; Mittal, J.; Moosa, M.; Atkins, C.M.; Mittal, R. Genetic ablation of metabo tropic glutamate receptor 5 in rats results in an autism-like behavioral phenotype. PLoS ONE 2022, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Angoa-Perez, M.; Kane, M.J.; Briggs, D.I.; Francescutti, D.M.; Kuhn, D.M. Marble burying and nestlet shredding as tests of repet itive, compulsive-like behaviors in mice. J. Vis. Exp. JoVE 2013, 82, 50978. [Google Scholar] [CrossRef]
- Lane, J.M.; Merced-Nieves, F.M.; Midya, V.; Liu, S.H.; Martinez-Medina, S.; Wright, R.J.; Téllez-Rojo, M.M.; Wright, R.O. Prena tal exposure to metal mixtures and childhood temporal processing in the PROGRESS Birth Cohort Study: Modification by childhood obesity. Sci. Total Environ. 2024, 917, 10. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Wang, W.; Hao, H.; Zhang, L.; Liu, G.; Wang, J.; Zhang, Y. Role of NF-κB in lead exposure-induced activation of astrocytes based on bioinformatics analysis of hippocampal proteomics. Chem.-Biol. Interact. 2023, 370, 110310. [Google Scholar] [CrossRef]
- Xu, Z.H.; Song, T.Y.; Yang, X.F.; Cong, L.H.; Yin, L.H.; Xu, Y.W.; Han, X.; Gao, M.; Xu, L.N. TMT-based proteomics reveals methylprotodioscin alleviates oxidative stress and inflammation via COX6C in myocardial infraction. Biomed. Pharmacother. 2024, 180, 15. [Google Scholar] [CrossRef]
- Wei, W.D.; Zhang, Q.N.; Jin, T.T.; Zhu, L.; Zhao, J.L.; Li, F.; Zhao, S.; Kong, D.Z.; Hao, J. Quantitative Proteomics Characteriza tion of the Effect and Mechanism of Trichostatin A on the Hippocampus of Type II Diabetic Mice. Cell. Mol. Neurobiol. 2023, 43, 4309–4332. [Google Scholar] [CrossRef]
- Ru, M.Y.; He, J.; Bai, Y.A.; Zhang, K.; Shi, Q.Q.; Gao, F.; Wang, Y.Y.; Li, B.L.; Shen, L. Integration of Proteomic and Metabolomic Data Reveals the Lipid Metabolism Disorder in the Liver of Rats Exposed to Simulated Microgravity. Biomolecules 2024, 14, 682. [Google Scholar] [CrossRef]
- Tang, D.D.; Chen, M.J.; Huang, X.H.; Zhang, G.C.; Zeng, L.; Zhang, G.S.; Wu, S.J.; Wang, Y.W. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, 8. [Google Scholar] [CrossRef]
- Loisy, M.; Bouisset, G.; Lopez, S.; Muller, M.; Spitsyn, A.; Duval, J.; Piskorowski, R.A.; Verret, L.; Chevaleyre, V. Sequential inhib itory plasticities in hippocampal area CA2 and social memory formation. Neuron 2022, 110, 2854–2866. [Google Scholar] [CrossRef]
- Barnea-Goraly, N.; Frazier, T.W.; Piacenza, L.; Minshew, N.J.; Keshavan, M.S.; Reiss, A.L.; Hardan, A.Y. A preliminary longitude nal volumetric MRI study of amygdala and hippocampal volumes in autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Hitti, F.L.; Siegelbaum, S.A. The hippocampal CA2 region is essential for social memory. Nature 2014, 508, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Piskorowski, R.A.; Nasrallah, K.; Diamantopoulou, A.; Mukai, J.; Hassan, S.I.; Siegelbaum, S.A.; Gogos, J.A.; Chevaleyre, V. Age-Dependent Specific Changes in Area CA2 of the Hippocampus and Social Memory Deficit in a Mouse Model of the 22q11.2 Deletion Syndrome. Neuron 2016, 89, 163–176. [Google Scholar] [CrossRef]
- Yang, C.; Kang, B.; Cao, Z.; Zhang, J.; Zhao, F.; Wang, D.; Su, P.; Chen, J. Early-Life Pb Exposure Might Exert Synapse-Toxic Effects Via Inhibiting Synapse-Associated Membrane Protein 2 (VAMP2) Mediated by Upregulation of miR-34b. J. Alzheimer’s Dis. 2022, 87, 619–633. [Google Scholar] [CrossRef]
- Guang, S.Q.; Pang, N.; Deng, X.L.; Yang, L.F.; He, F.; Wu, L.W.; Chen, C.; Yin, F.; Peng, J. Synaptopathology Involved in Autism Spectrum Disorder. Front. Cell. Neurosci. 2018, 12, 16. [Google Scholar] [CrossRef]
- Lo, L.H.Y.; Lai, K.O. Dysregulation of protein synthesis and dendritic spine morphogenesis in ASD: Studies in human pluripo tent stem cells. Mol. Autism 2020, 11, 9. [Google Scholar] [CrossRef]
- Cao, P.; Maximov, A.; Südhof, T.C. Activity-Dependent IGF-1 Exocytosis Is Controlled by the Ca 2+-Sensor Synaptotagmin-10. Cell 2011, 145, 300–311. [Google Scholar] [CrossRef]
- Liu, Z.H.; Chen, Z.J.; Shang, C.P.; Yan, F.; Shi, Y.C.; Zhang, J.J.; Qu, B.L.; Han, H.L.; Wang, Y.Y.; Li, D.P.; et al. IGF1-Dependent Synaptic Plasticity of Mitral Cells in Olfactory Memory during Social Learning. Neuron 2017, 95, 106–122.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiong, B.R.; Zhang, L.Q.; Huang, X.; Yuan, X.M.; Tian, Y.K.; Tian, X.B. The Role of the GABAergic System in Diseases of the Central Nervous System. Neuroscience 2021, 470, 88–99. [Google Scholar] [CrossRef]
- Ghiani, C.A.; Starcevic, M.; Rodriguez-Fernandez, I.A.; Nazarian, R.; Cheli, V.T.; Chan, L.N.; Malvar, J.S.; de Vellis, J.; Sabatti, C.; Dell’Angelica, E.C. The dysbindin-containing complex (BLOC-1) in brain: Developmental regulation, interaction with SNARE proteins and role in neurite outgrowth. Mol. Psychiatr. 2010, 15, 204–215. [Google Scholar] [CrossRef]
- Zhuang, Z.; Jiang, L.; Yang, G.; Luo, X. Identifying BLOC1S4 as a prospective target and prognostic indicator in lung adenocarci noma. Asian J. Surg. 2024, 48, 2491–2493. [Google Scholar] [CrossRef]
- Chua, C.E.L.; Tang, B.L. Syntaxin 16 is enriched in neuronal dendrites and may have a role in neurite outgrowth. Mol. Membr. Biol. 2008, 25, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.A.; Gan, B.Q.; Tang, B.L. Syntaxin 16: Unraveling Cellular Physiology Through a Ubiquitous SNARE Molecule. J. Cell. Physiol. 2010, 225, 326–332. [Google Scholar] [CrossRef]
- Brandl, K.; Tomisato, W.; Li, X.H.; Neppl, C.; Pirie, E.; Falk, W.; Xia, Y.; Moresco, E.M.Y.; Baccala, R.; Theofilopoulos, A.N.; et al. Yip1 domain family, member 6 (Yipf6) mutation induces spontaneous intestinal inflammation in mice. Proc. Natl. Acad. Sci. USA 2012, 109, 12650–12655. [Google Scholar] [CrossRef] [PubMed]
- Sheik, S.; Pandey, H.; Thirumalasetti, S.K.; Nakamura, N. Characteristics and Functions of the Yip1 Domain Family (YIPF), Multi-Span Transmembrane Proteins Mainly Localized to the Golgi Apparatus. Front. Cell. Dev. Biol. 2019, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Göhring, I.; Tagariello, A.; Endele, S.; Stolt, C.C.; Ghassibé, M.; Fisher, M.; Thiel, C.T.; Trautmann, U.; Vikkula, M.; Winterpacht, A.; et al. Disruption of ST5 is associated with mental retardation and multiple congenital anomalies. J. Med. Genet. 2010, 47, 91–98. [Google Scholar] [CrossRef]
- Kumar, R.; Francis, V.; Ioannou, M.; Kulasekaran, G.; Banks, E.; Cordova, A.; Khan, M.; McPherson, P. DENND2B functions as an activator of Rab35 to regulate cytokinesis. Mol. Biol. Cell 2023, 34, 639. [Google Scholar]
- Dong, D.Y.; Zielke, H.R.; Yeh, D.; Yang, P.X. Cellular stress and apoptosis contribute to the pathogenesis of autism spectrum disorder. Autism Res. 2018, 11, 1076–1090. [Google Scholar] [CrossRef]
- Wei, H.G.; Alberts, I.; Li, X.H. The apoptotic perspective of autism. Int. J. Dev. Neurosci. 2014, 36, 13–18. [Google Scholar] [CrossRef]
- Guo, J.C.; Li, R.K.; Ouyang, Z.Q.; Tang, J.W.; Zhang, W.; Chen, H.; Zhu, Q.; Zhang, J.; Zhu, G.C. Insights into the mechanism of transcription factors in Pb2+-induced apoptosis. Toxicology 2024, 503, 14. [Google Scholar] [CrossRef]
- Bussche, J.V.; Soares, E.V. Lead induces oxidative stress and phenotypic markers of apoptosis in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2011, 90, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.P.; Zhang, F.; Yang, F.; Huang, S.J.; Su, S.; Tan, X.M.; Zhong, L.L.; Deng, L.J.; Pang, L.H. METTL16 participates in haemo globin H disease through m6A modification. PLoS ONE 2024, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.L.; Li, L.M.; Xia, X.C.; Zhou, X.L.; Du, Y.K.; Yin, Z.Q.; Wang, J. Integration of Urine Proteomic and Metabolomic Profiling Reveals Novel Insights Into Neuroinflammation in Autism Spectrum Disorder. Front. Psychiatry 2022, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Gekara, N.O. The deubiquitinase MYSM1 dampens NOD2-mediated inflammation and tissue damage by inactivating the RIP2 complex. Nat. Commun. 2018, 9, 13. [Google Scholar] [CrossRef]
- Panda, S.; Nilsson, J.A.; Gekara, N.O. Deubiquitinase MYSM1 Regulates Innate Immunity through Inactivation of TRAF3 and TRAF6 Complexes. Immunity 2015, 43, 647–659. [Google Scholar] [CrossRef]
- Chen, X.; Wang, W.; Li, Y.F.; Huo, Y.; Zhang, H.; Feng, F.; Xi, W.J.; Zhang, T.Z.; Gao, J.J.; Yang, F.; et al. MYSM1 inhibits human colorectal cancer tumorigenesis by activating miR-200 family members/CDH1 and blocking PI3K/AKT signaling. J. Exp. Clin. Cancer Res. 2021, 40, 21. [Google Scholar] [CrossRef]
- Qin, Q.Z.; Ruan, H.Q.; Zhang, H.Y.; Xu, Z.H.; Pan, W.T.; Yan, X.L.; Jiang, X.X. Deubiquitinase MYSM1: An Important Tissue Development and Function Regulator. Int. J. Mol. Sci. 2024, 25, 13051. [Google Scholar] [CrossRef]
- Pomin, V.H. Sulfated glycans in inflammation. Eur. J. Med. Chem. 2015, 92, 353–369. [Google Scholar] [CrossRef]
- Rexrode, L.E.; Hartley, J.; Showmaker, K.C.; Challagundla, L.; Vandewege, M.W.; Martin, B.E.; Blair, E.; Bollavarapu, R.; Antonyraj, R.B.; Hilton, K.; et al. Molecular profiling of the hippocampus of children with autism spectrum disorder. Mol. Psychiatr. 2024, 29, 1968–1979. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Kavalioti, M.; Tsilioni, I. Mast Cells, Stress, Fear and Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 3611. [Google Scholar] [CrossRef]
- Macke, E.L.; Henningsen, E.; Jessen, E.; Zumwalde, N.A.; Landowski, M.; Western, D.E.; Lee, W.-H.; Liu, C.; Gruenke, N.P.; Doebley, A.-L.; et al. Loss of Chondroitin Sulfate Modification Causes Inflammation and Neurodegeneration in skt Mice. Genetics 2020, 214, 121–134. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Zhou, X.; Ma, Z.; Liu, R.; Zhang, Y.; Wang, Y.; Liu, Y.; Xia, X.; Wang, J. Hippocampal Proteomics Reveals the Novel Molecular Profiling of Postnatal Lead (Pb) Exposure on Autism-like Behaviors. Toxics 2025, 13, 465. https://doi.org/10.3390/toxics13060465
Liu L, Zhou X, Ma Z, Liu R, Zhang Y, Wang Y, Liu Y, Xia X, Wang J. Hippocampal Proteomics Reveals the Novel Molecular Profiling of Postnatal Lead (Pb) Exposure on Autism-like Behaviors. Toxics. 2025; 13(6):465. https://doi.org/10.3390/toxics13060465
Chicago/Turabian StyleLiu, Li, Xulan Zhou, Zihan Ma, Ruming Liu, Yuhan Zhang, Yaqi Wang, Yiwen Liu, Xiaochun Xia, and Juan Wang. 2025. "Hippocampal Proteomics Reveals the Novel Molecular Profiling of Postnatal Lead (Pb) Exposure on Autism-like Behaviors" Toxics 13, no. 6: 465. https://doi.org/10.3390/toxics13060465
APA StyleLiu, L., Zhou, X., Ma, Z., Liu, R., Zhang, Y., Wang, Y., Liu, Y., Xia, X., & Wang, J. (2025). Hippocampal Proteomics Reveals the Novel Molecular Profiling of Postnatal Lead (Pb) Exposure on Autism-like Behaviors. Toxics, 13(6), 465. https://doi.org/10.3390/toxics13060465









