1. Introduction
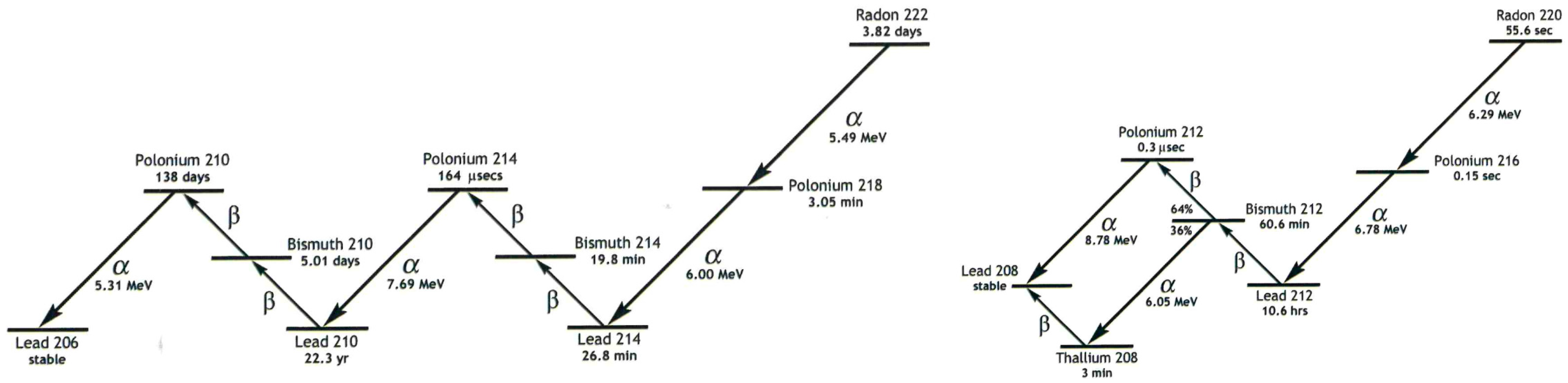
212Pb (half-life
t1/2 = 10.64 h; 0.57 MeV β
– emission; 100% intensity) is a promising radionuclide for targeted alpha-particle therapy [
1] that decays to
212Bi (
t1/2 = 60.6 min) and then emits one α-particle, either with a probability of 64% by decaying to
212Po (
t1/2 = 0.3 µs) and
208Pb or with a probability of 36% by decaying to
208Tl (
t1/2 = 3.1 min) and
208Pb [
2,
3]. Therefore, a
212Pb-labeled radiopharmaceutical, once accumulated in the tumor tissue, will deposit its highest dose in the form of the α-particle specifically in the tumor cells, with a lower probability of further α-decay occurring in healthy organs. Thus,
212Pb represents a more favorable choice for cancer α-therapy of patients who are naïve to (or who have progressed on) β–therapy, including patients with reduced renal function. Ongoing preclinical and clinical studies are investigating the potential of
212Pb-labeled peptides and antibodies (with administered radioactivity in the range of approximately 1–3 MBq/kg) [
4] (bridging the gap between administered activities of
177Lu and
225Ac). Recently, the true matched pair
203/212Pb has come into focus through several first in-human theranostic applications [
5,
6]. While
203Pb (
t1/2 = 51.9 h; 279 keV gamma ray; 81% intensity) represents an ideal elementally matched imaging surrogate,
212Pb itself can also be applied for SPECT imaging [
7,
8]. This true matched pair has the potential to overcome the differential pharmacokinetic/pharmacological properties observed between diagnostic and therapeutic radiotracers with unmatched radionuclide pairs like
68Ga/
177Lu [
9]. The radiopharmaceutical VMT-α-NET comprises a lead-specific chelator (PSC) conjugated to a [Tyr
3,Thr
8]octreotide backbone via a polyethylene glycol linker (PEG
2). Thus, this radiopharmaceutical is a somatostatin subtype 2 (SST2) receptor-targeting peptide that can be used for the imaging and treatment of neuroendocrine tumors (NETs) that exhibits rapid tumor accumulation, high tumor retention, and rapid renal excretion [
10]. It carries the chelator PSC [
10], which forms highly stable complexes with
203/212Pb and, in contrast to less stable 1,4,7,10-tetraazacyclododecane-
N,
N’,
N”,
N’”-tetraacetic acid (DOTA) complexes, remains intact even after β
– conversion to
212Bi [
11]. Another known chelator for
203/212Pb is DOTAM (also known as TCMC), which was already used in a clinical dose escalation trail with
212Pb-DOTAM-TATE [
12], for which instability of the
212Pb radionuclide progeny
212Bi has been reported [
13].
Access to
212Pb remains limited, and the available generators are in different experimental [
14,
15] or preclinical stages [
10]. For radiation safety and risk assessment, two
212Pb generators were obtained. Alpha and gamma dosimetry were performed during generator elution and radiosynthesis (
Figure 1). Key parameters in this context included observed emanation of
220Rn (Thoron) (
t1/2 = 55.6 s) during generator elution and monitoring of the 2.6 MeV gamma radiation from
208Tl during radiosynthesis, which were rated as critical for radiation exposure of personnel. Importantly, radiation safety measures can be easily taken to minimize the exposure to personnel for both of these parameters.
For the detection and measurement of
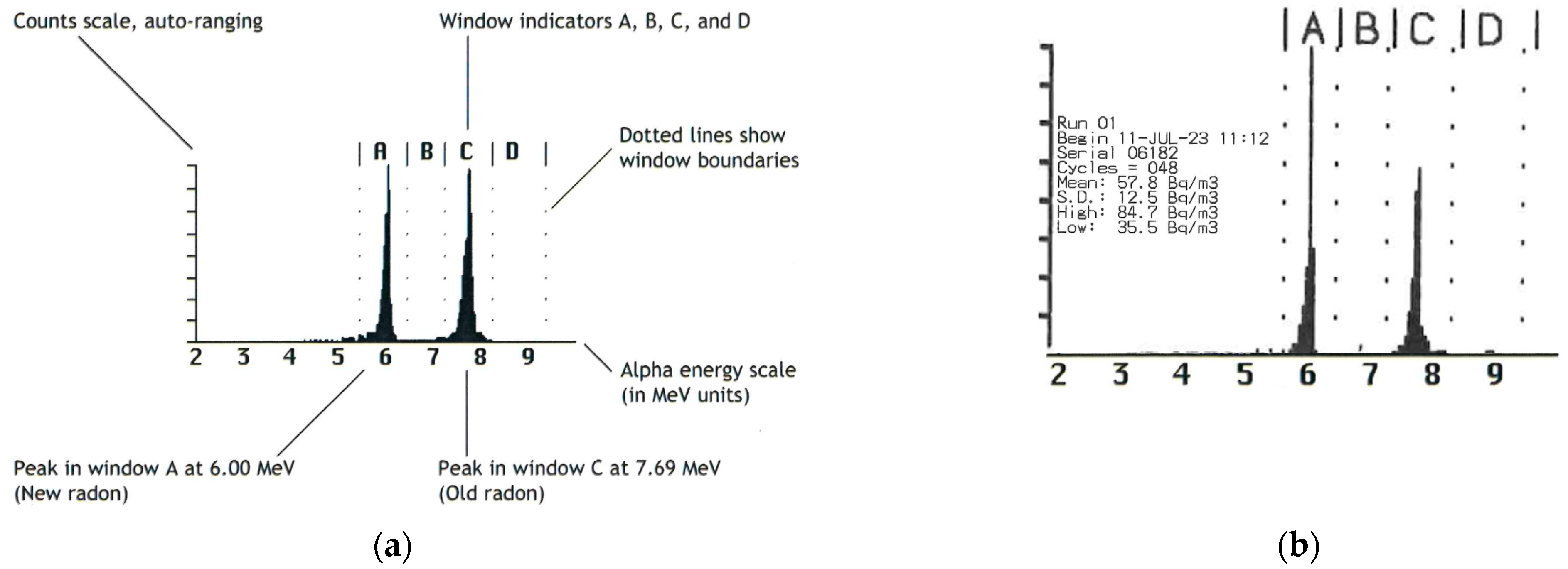
220Rn, an electronic radon detector (RAD7, DURRIDGE, Billerica, MA, USA) was used. The interworking of the RAD7 consisted of a solid-state, ion-implanted, planar, silicon-alpha detector housed within a 0.7 L hemisphere. The RAD7 specifically detects the alpha energies of
218Po (
t1/2 = 3.05 min, 6.00 MeV, channel A) and
216Po (
t1/2 = 0.15 s, 6.78 MeV, channel B) as decay products from
222Rn (
t1/2 = 3.82 d) and
220Rn, respectively, which can be used to quantify the concentration of each of these Rn isotopes (
Figure 2). For our measurements relevant here, due to the short half-life of
216Po, the response of the RAD7 to
220Rn is virtually instantaneous, while the response to
222Rn will take 15 min for the count rate to reach equilibrium. The upper limit of the RAD7 is 1 MBq/m
3. The original tubing from the manufacturer was used, as the detector was calibrated to the length of the tubing.
In this work, the results of 220Rn measurements and dosimetry of personnel over two years of generator elution, radiosynthesis, and quality control are presented. Thus, we intend to provide an informed overview of the safe operations of the two generator systems and information from our experience that can be useful for planning the handling 212Pb sources in laboratories with similar fume hoods. Our radiation protection required handling under these conditions. It is expected that this work will help other departments to consider radiation safety measures to be implemented that have a scientific basis for discussion internally and with their local authorities.
2. Materials and Methods
All reagents and solvents were purchased from commercial suppliers at the highest purity and used without further purification. VMT-α-NET (PSC-PEG2-TOC) and 224Ra/212Pb generator (VMT-α-GEN) were obtained from Perspective Therapeutics Inc. (Coralville, IA, USA). Another 224Ra/212Pb generator (CERN generator) was obtained from MEDICIS (CERN, CH-1211 Geneve 23, Switzerland). Custom-made Pb resin cartridges were filled with 50 mg of powder (PB-B10-F, Triskem, Bruz, France). The RAD7 electronic radon detector was obtained from DURRIDGE (Billerica, MA 01821, USA). Radiochemical purity (RCP) was monitored by TLC on iTLC-SG plates (Agilent, Santa Clara, CA, USA). Measurement of the radionuclide purity (RNP) and evaluation of the radio-TLC was performed with a thin-layer scanner (MiniScanPRO+, Eckert&Ziegler Eurotope GmbH, Berlin, Germany) equipped with a Model 43-2 alpha detector ZnS(Ag) scintillator (Ludlum Measurements, Sweetwater, TX, USA) and a built-in multi-channel analyzer (MCA) for gamma spectroscopy. Radio-HPLC was performed on a Shimadzu HPLC system (Thermo Scientific, Dreieich, Germany) equipped with a reverse-phase column (Analytical: Merck Chromolith HighResolution RP-18e; 150 × 4.6 mm plus a 5 × 4.6 mm guard column, Darmstadt, Germany) and a UV diode array detector (220 nm). The solvent system used was a gradient of acetonitrile–water (containing 0.05% TFA) (0–13 min: 0–60% MeCN) at a flow rate of 1.6 mL/min, unless otherwise stated. The pH was measured using a reflectance photometer (QUANTOFIX Relax, Macherey-Nagel GmbH & Co. KG, Düren, Germany). For personal dosimetry, whole-body dosimeters (type: LPS-OSL-GD 01, LPS, Berlin, Germany) and ring dosimeters (type: LPS-TLD-TD 08, Thermo Fisher Scientific, Berlin, Germany) were used.
Radiochemistry
Radiolabeling of the tumor ligands conjugated either with the chelators TCMC (DOTAM) or PSC was performed according to reported standard protocols for these chelators [
11]. Briefly, 50 µg of precursor VMT-α-NET (PSC-TOC derivative, M = 1578.7 g/mol)) or 62 µg of PSV-402 (TCMC-PSMA derivative, M = 1675.7 g/mol) (1 µg/µL in H
2O
suprapure) was added to a 10 mL reaction vial together with 100 µL of EtOH
absolute, 290 µL of 1 M NaAc/AcOH buffer (pH 4, 99.99% trace metal), and 2 mg of sodium ascorbate (Ph.Eur.).
In general, the elution of the generators was performed within a safety fume hood in order to avoid the release of 220Rn into the laboratory. During elution, the RAD7 was in sniffing mode with 220Rn detection activated. The sniff tube was attached in the vicinity of the respective generator outlet. During elution, the personnel wore additional FFP2 masks and arm cuffs in order to prevent contamination by skin adsorption of 212Pb from 220Rn.
Elution of VMT-α-GEN was performed with 4 mL of 2 M HClsuprapure, followed by 4 mL of air and an additional 1 mL of H2Osuprapure in order to wet the generator and avoid further 220Rn release from the generator.
Elution of CERN generator was performed by rapidly changing the glass vial with another one and letting the glass vial containing the 220Rn stay on top for about 10 min in order to let the 220Rn decay. Subsequently, the glass vial was rinsed 1–4 times with 1 mL 0.1 M HClsuprapure.
The collected generator eluates of 212Pb in 3–5 mL 0.1–1.6 M HClsuprapure were trapped on a custom-made Pb resin cartridge (50 mg PB-B10-F, Triskem, Bruz, France) preconditioned with 1 mL of 2 M HClsuprapure. The captured activity was rinsed with 1 mL of 2 M HClsuprapure. The activity was eluted with 2 mL of NaAc/AcOH buffer (pH 6, 99.99% trace metal) directly into the reaction vial. The solution was heated at 95 °C for 30–60 min. The reaction solution was then diluted with 4 mL of 0.9% NaCl solution and cooled.
Finally, the product was purified by using a C18 Plus light cartridge (WAT023501, Waters, Eschborn, Germany) preconditioned with 1 mL of EtOH and 3 mL of H
2O (wet condition). The cooled and diluted product solution (4 mL of 0.9% NaCl) was slowly passed through the C18 cartridge. The C18 cartridge containing the product was rinsed with 2 mL of 0.9% NaCl solution and was directly eluted with 1 mL of 50% EtOH for injection directly through a vented sterile filter (0.22 µm, SLGVV255 F, Millex-GV, Merck-Millipore) into a product vial. Finally, the product was diluted with 7 mL of 0.9% NaCl solution. The radiochemical yield for
212Pb-VMT-α-NET and
212Pb-PSV-402 were >95%, and the radiochemical purities were >99% (
Figure 3), respectively.
3. Results
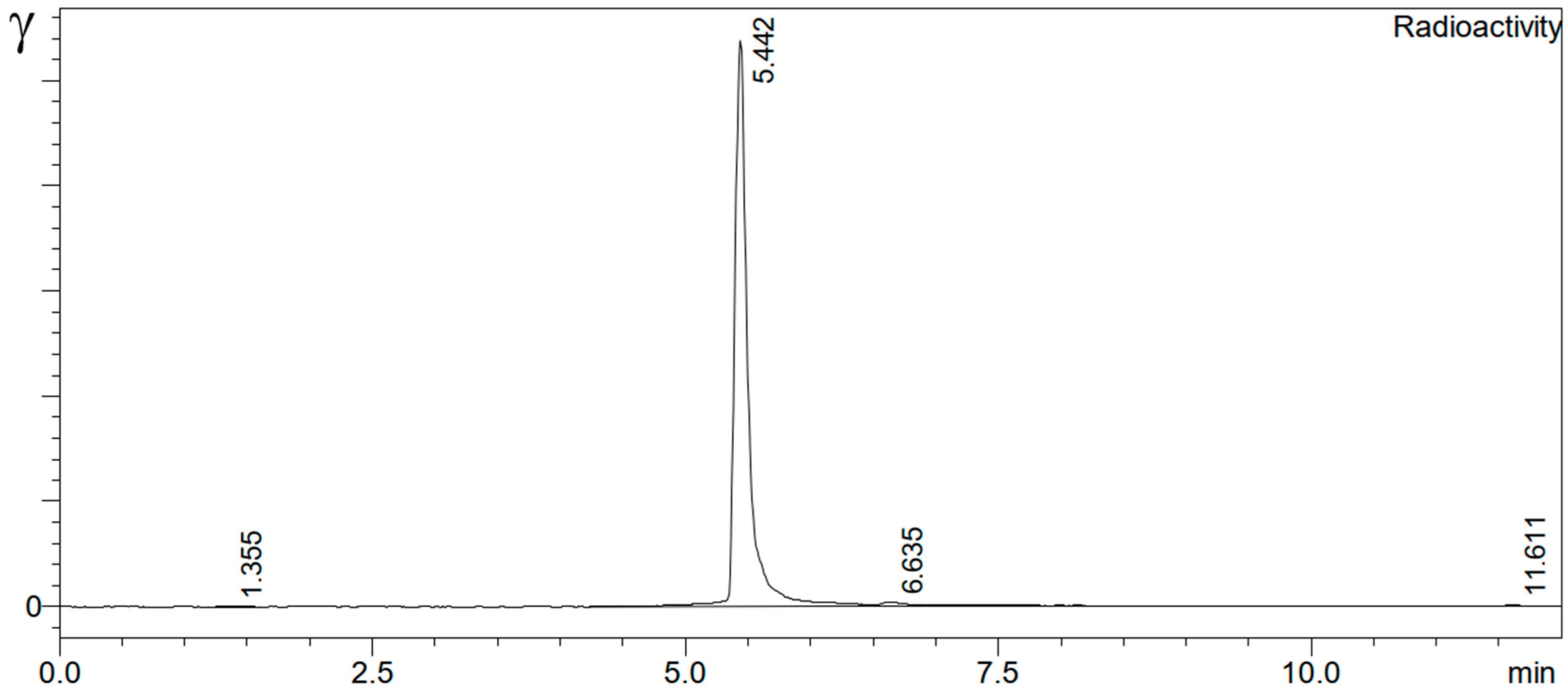
Prior to the generator handling, a two-day background measurement within the fume hood in the vicinity of the respective generator was performed and compared with an example spectrum without a generator (
Figure 4a), which resulted exclusively in the detection of natural
222Rn (57.8 ± 12.5 Bq/m
3) (
Figure 4b).
No
220Rn emanation was detected when the generators were stored within the fume hood (wet storage for VMT-α-GEN and dry for CERN generator) and without use. The concentrations of
220Rn and
222Rn in Bq/m
3 over the time of handling for both generators are displayed in
Figure 5. The measurements were stopped when an equilibrium was reached or after the end of a whole synthesis (120–135 min), including C18 purification and quality control by thin-layer chromatography (TLC).
220Rn emanation was measured during generator elution and radiolabeling. Measurable
220Rn release was observed only during generator elution (
Figure 6), and as soon as the eluate was trapped on the Pb resin, the
220Rn signal from the air decreased significantly. For example, the elution of 493 MBq
212Pb from VMT-α-GEN resulted in a release of
220Rn with >1 MBq/m
3 for 5 min, which then decreased to 0.4 MBq/m
3 after 10 min and <0.1 MBq/m
3 after 15 min.
Elution of
212Pb from CERN generator (~700 MBq EOB; ~600 MBq at shipping time; ~500 MBq at reception time) was carried out by rinsing the glass bottle with 1–4 mL of 0.1 M HCl. This resulted in a higher release of
220Rn with >1 MBq/m
3 for 55 min, reaching a lower plateau of <0.4 MBq/m
3 for the rest of the measurement. The recovery rate of the rinsing process is shown in
Table 1. Therefore, at least 3 mL of 0.1 M HCl had to be used for effective activity recovery from the glass bottle. Lower
220Rn release was observed when switching from rinsing the glass bottle with HCl to completely dissolving the
224Ra/
212Pb from the glass wool in 1 M HCl, passing the solution over the Pb resin (
212Pb trapping), and collecting the
224Ra-containing eluate back into the residual vial. Here,
220Rn with 0.6–0.66 MBq/m
3 in the first 10 min was measured, which dropped below 0.08 MBq/m
3 after 15 min and further. Therefore, a significant amount of
220Rn could be prevented from escaping into the air. Here, the recovery rate of
212Pb was at least 66 ± 2.5% (n = 3), and therefore, the procedure was superior to the glass bottle method.
3.1. Direct Measurement of Radiation Exposure
Radiation exposure of a VMT-α-GEN generator (360 MBq) was measured with standard well-known dosimeters under different conditions. The dose rate at a distance of 1 m and on the surface was 18.2 µSv/hr and 3.5 mSv/hr, respectively. A dose rate of 10 µSv/h was measured at a distance of 1 m for an unshielded vial with 32 MBq. Attenuation factors of 1.5, 2.2, and 8.2 were determined for lead absorbers with thicknesses of 0.5 cm, 1 cm, and 5 cm. By means of a test irradiation on an Alderson phantom, various electronic personal dosimeters (EPDs) were examined with regard to their suitability and compared with the measured values of two official personal dosimeters. The mean value of the EPDs used was (0.72 ± 0.15) µSv, thus showing good agreement with the values of the official dosimeters (0.7 mSv and 0.8 mSv).
3.2. Radiation Exporsure to Medical Staff
Radiation exposure of personnel in our department is routinely monitored by measuring whole-body (film dosimeter type LPS-OSL-GD 01) and partial body (finger ring OSL dosimeter) doses. Looking at the values for nearly two years, it can be found that there were slightly higher effective and finger doses in the months when the
212Pb generators were handled (
Table 2, one person). It should be noted that our experience has assisted us in establishing procedures that minimize whole-body and finger ring exposures. On average, the starting activity was ~600 MBq, and the generators were eluted every 1–2 days for up to four weeks (first week for patient use and the following three weeks for experimental radiochemistry). The mean whole-body and finger doses accumulated during clinical routine production of radiotracers were (0.133 ± 0.047) mSv and (6.583 ± 5.649) mSv per month, respectively. The mean effective and finger doses accumulated in the months of generator handling were (0.250 ± 0.126) mSv and (8.083 ± 3.989) mSv per month, respectively. Therefore, an additional monthly effective dose of ~0.12 mSv can be expected when handling these types of generators. This would result in an additional dose of 1.44 mSv per year for one person if a fresh
224Ra/
212Pb generator were to be handled every month.
An attempt was made to assess potential 220Rn levels that might be observed in the breathing zone of operators for the case where a generator was located outside of an appropriate fume hood hot-cell working environment. In this case, generators were eluted, and operators wore FFP2-masks during the elution of approximately 600 MBq starting activity through the Pb resin trapping step. Subsequently, potential activity trapped by the masks was assayed by the alpha-detection mode of a CoMo-170 device (NUVIA Instruments, Dresden, Germany). Although this approach is a crude measurement of radioactivity, no signal above the 50 IPS (warning signal) was detected (background of ~10 IPS). These results demonstrate the importance of operation of generator technologies in an appropriate fume hood that is shielded according to ALARA principles.
3.3. 222Rn Exposure
As a higher
222Rn signal was detected during generator elution, an additional
222Rn dosimetry to the gamma dosimetry by three
222Rn exposimeters (type: B97, ALTRAC, Berlin, Germany) was performed during the experiments to rule out
222Ra emanation, since the generators are known to be absolutely free of any
226Ra due to the production process of
224Ra [
16]. One was positioned inside the fume hood within the vicinity of the
212Pb-generator, one was positioned outside of the fume hood, and one was attached to the lab coat of the personnel. Unfortunately, the exposimeters were restricted to detect
222Rn only. A slightly higher
222Rn level was measured within the fume hood in the vicinity of the
212Pb generator, which was expected due to a higher measured activity of
222Rn with the RAD7 during elution (see
Table 1). As the differences between the measured values were very small, this could have naturally occurred, since there was only one dosimeter used at the respective measuring points. However, the measured values of the dosimeters were 443 kBq h/m
3 in the vicinity of the
212Pb generator, 364 kBq h/m
3 outside the fume hood, and 111 kBq h/m
3 at the lab coat. Therefore, the detection of
222Rn during the detection of high concentration of
220Rn might have been due to measurement issues of the RAD7 itself and not because of some small impurities of
226Ra within the
224Ra.
4. Discussion
As discussed in the literature,
224Ra itself could be used for alpha therapy (DaRT) [
17], and therefore, the emission of
220Rn in vivo is of another level of importance. Recently, the first preclinical results of
220Rn diffusion in tissue were obtained [
18]. Based on this, calculations were performed in order to determine the dosimetry in vivo [
19]. Parallel to the brachytherapeutic concept of using alpha-particles for therapy, the approach presented here is based on the radiolabeling of tumor-specific ligands with
212Pb. The detection system employed (i.e., RAD7) is the only transportable system commercially available to our knowledge for the measurement of the radionuclide relevant to our application (i.e.,
220Rn), as other personal alpha detectors were only able to detect naturally occurring
222Rn and were therefore unsuitable for this purpose. Other departments like PTB are able to measure probes of
220/222Rn [
20].
In our study, the elution efficiency of
212Pb from the glass bottle was ~30%, in contrast to the 80–85% reported in the literature [
14]. The radiolabeling with the eluate from CERN generator for, e.g., DOTA peptide resulted in a ~50% radiochemical yield (RCY) when using the same precursor amounts. For a higher radiolabeling yield, the amount of precursor had to be increased to 50–62 µg vs. 20 µg. Therefore, and because of a high release of
220Rn during rinsing of the bottle, it cannot be recommended to use
212Pb in this form with our present infrastructure. In order to prevent
220Rn release, the glass wool containing the
224Ra was placed in 1–2 mL of 1 M HCl in order to dissolve the
224Ra and
220Rn. This resulted in an order of magnitude lower release of
220Rn by the wet method. However, from that experience onwards, further
224Ra derived from CERN generator was already provided in solution (1 M HCl), preventing higher
220Rn release by dissolving the glass wool containing
224Ra in the laboratory.
The elution yield of the
224Ra/
212Pb generator varied between 59–80% of the total loaded
224Ra activity, depending on days between consecutive generator elution [
21]. The recovery rate with 66% for
212Pb from the
224Ra solution (CERN generator) was in-between the recovery rate of VMT-α-GEN, whereas the recovery rate of
212Pb from CERN generator was the lowest observed at ~30%. Notably, the
220Rn release of VMT-α-GEN was the lowest of the three different types of
212Pb separation examined. From this work, VMT-α-GEN is suggested as the best and safest choice for handling
212Pb.
All isotopes of Pb were trapped by the Pb resin exclusively. The
212Pb eluate measured ~60% of the starting activity, where all nuclides were in equilibrium. All other nuclides,
224Ra,
212Bi, and
208Tl, were found in the waste elution, which measured ~40% of the starting activity. Therefore, the waste elution could be recycled and reused for further experiments within the following days. In
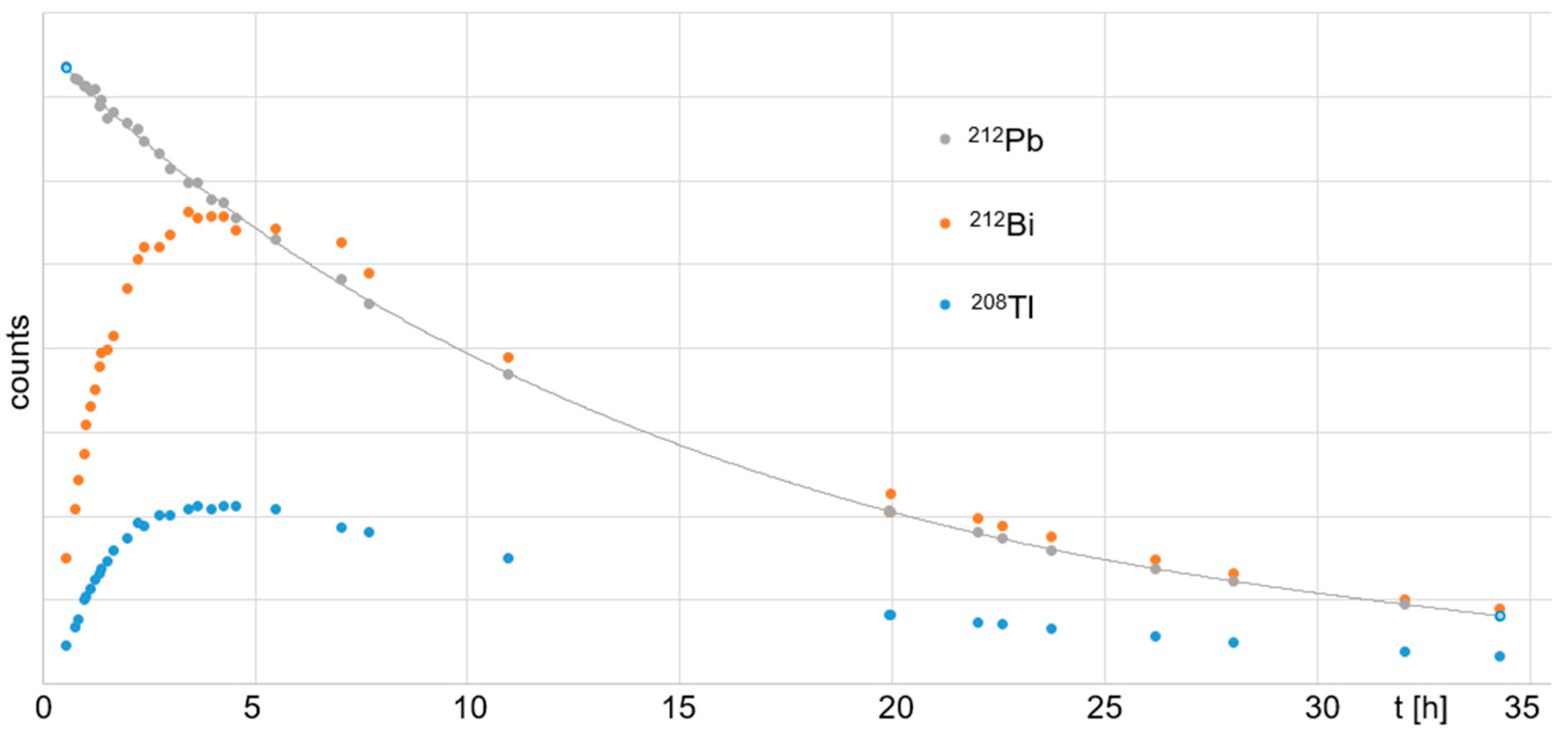
Figure 7, the ingrowth of daughter nuclides over time for the Pb-resin-eluted
212Pb solution is shown, as is the radioactive decay of the initial
212Pb with a half-life of (10.7 ± 0.2) h. The residual eluate that was separated from the
212Pb by the Pb resin contained
224Ra,
212Bi, and
208Tl (
Figure 8).
212Bi and
208Tl decayed initially, with their respective half-lives of
t1/2 = 60.6 min and 3.1 min. At ~0.2 d (~4.8 h), their decay reached equilibrium with the newly formed
212Pb, which resulted from the
224Ra decay.
212Pb showed a typical ingrowth to equilibrium with its mother nuclide
224Ra over time. However, the
212Pb eluate showed a typical ingrowth of the daughter nuclides
212Bi and
208Tl up to equilibrium with their mother nuclide
212Pb (~4 h after Pb resin purification). This provides a method for the straightforward handling of waste by fast decay in storage.
The measured additional monthly effective dose of ~0.12 mSv would result in a calculated additional dose of 1.44 mSv per year for the operator if a fresh 224Ra/212Pb generator were to be handled every month. In comparison, a monthly effective dose of ~0.54 mSv (mean value for three MTAs over two years exclusively working in our PET facility) can be found for handling 11C/18F/68Ga radiopharmaceuticals for patient application purposes. Personnel dose is restricted to 20 mSv per year; therefore, a dose of 1.44 mSv is 7% of the annual allowed dose, which indicates a very low additional risk. However, several methods such as varying the personnel or improvements to the time, shielding, and distance parameters are under consideration and will result in an even lower additional dose per year for one person.
The daughter nuclide
208Tl from
212Pb has a high gamma energy of 2.6 MeV [
22]. It is assumed that most of the gamma radiation is not detected by the whole-body dosimeter. However, the gamma radiation may trigger “Bremsstrahlung” at the walls of the laboratory, which might then be detected by the dosimeter and would thus have a higher impact on the whole-body dose. Nevertheless, the generator itself was shielded by an additional 10 cm lead wall to reduce the breakthrough of the gamma radiation of
208Tl. In addition, VMT-α-GEN itself has a more effective and broader shielding (weight 35 kg) as compared to common
188Re or
99mTc generators.