Exposure to a Multitude of Environmental Chemicals During Pregnancy and Its Association with the Risk of Gestational Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Populations and Sample Collection
2.2. Sample Analysis
2.3. Quality Assurance and Control
2.4. Data Analysis
3. Results
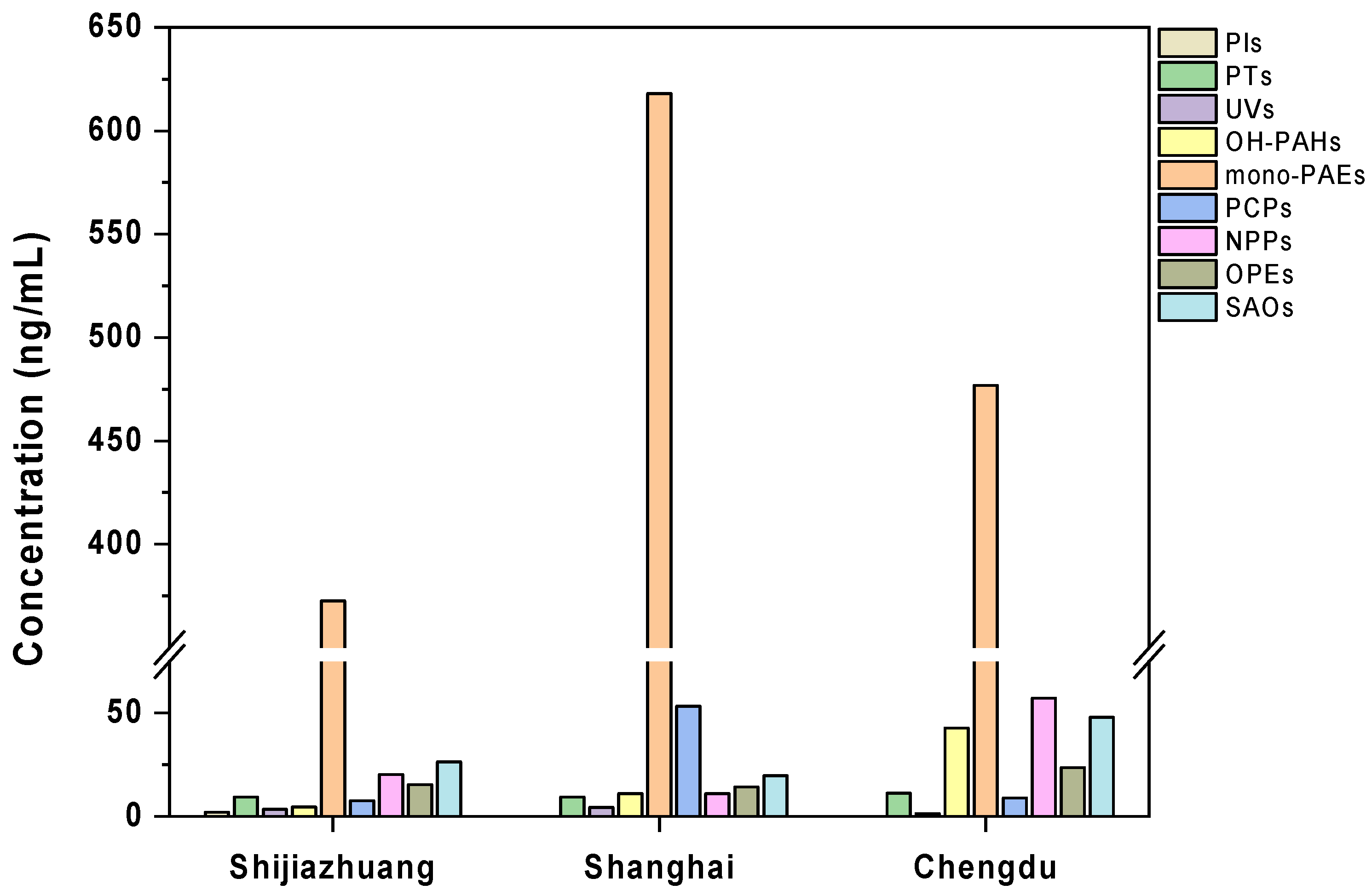
3.1. Characterization of Exposure Profiles
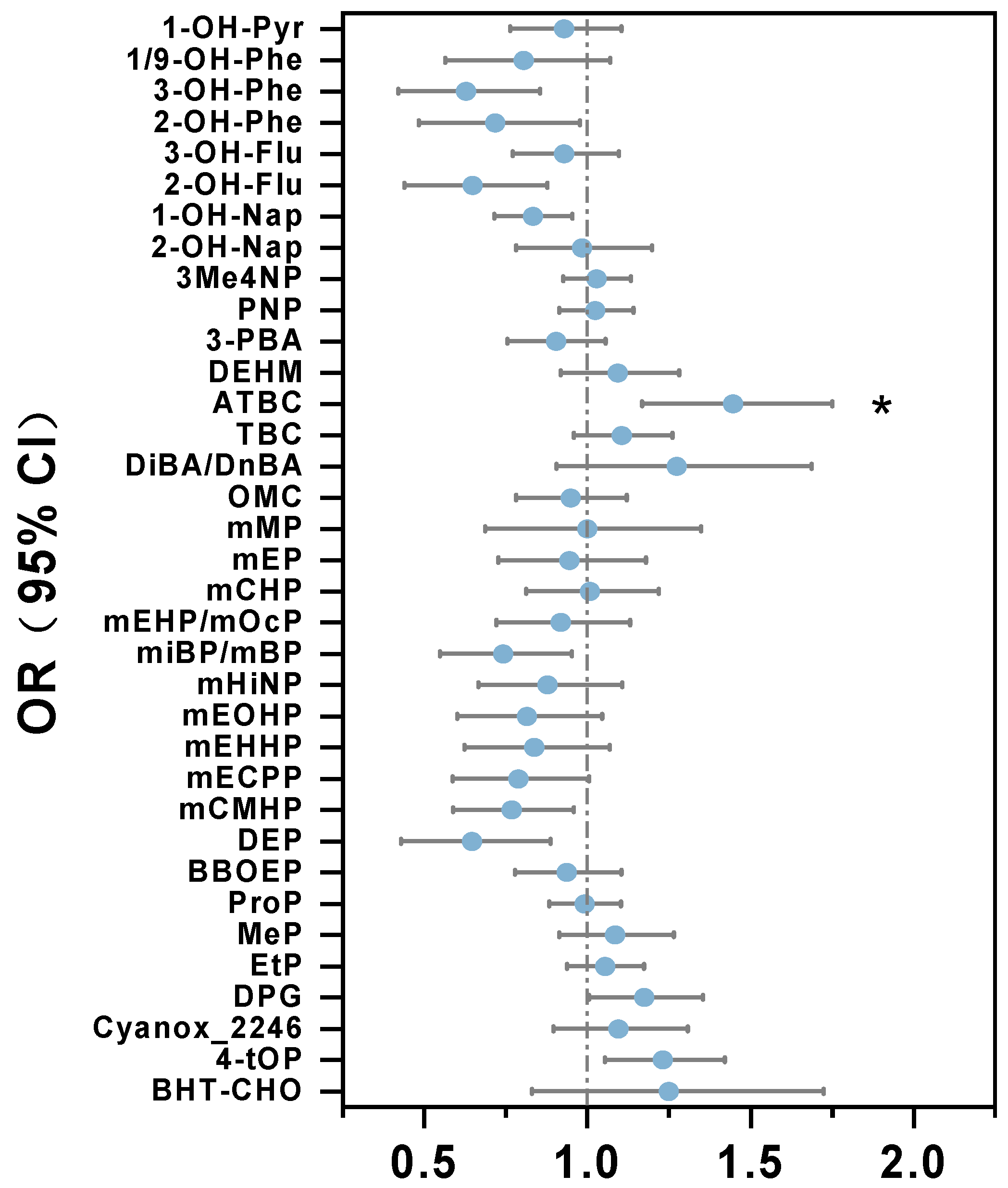
3.2. Association Between Chemicals Exposure and GDM
3.3. Implications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bline, A.P.; Ellis, L.B.; Pelch, K.E.; Lam, J.; Sen, S.; Zlatnik, M.; Varshavsky, J. The effect of per and polyfluoroalkyl substance (PFAS) exposure on gestational diabetes mellitus and its subclinical risk factors: A systematic review and meta-analysis protocol. Environ. Int. 2024, 188, 108711. [Google Scholar] [CrossRef]
- Huang, W.; Pan, X.; Tang, S.; Sun, F.; Wu, P.; Yuan, J.; Sun, W.; Pan, A.; Chen, D. Target Exposome for Characterizing Early Gestational Exposure to Contaminants of Emerging Concern and Association with Gestational Diabetes Mellitus. Environ. Sci. Technol. 2023, 57, 13408–13418. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Song, J.; Wu, Y.; Zhang, R.; Zhu, S.; Han, M.; Wang, B.; Liang, Z.; Liu, J. First Evidence of the Associations of Exposure to Pyrethroid Insecticides with the Risk of Gestational Diabetes Mellitus. Environ. Sci. Technol. Lett. 2023, 11, 418–425. [Google Scholar] [CrossRef]
- Wesley, S.; Gallo, M.; Apata, T.; Dis, J.; Hollenbach, S. Impact of Endocrine-Disrupting Chemicals, Climate, and Air Pollution on Pregnancy Outcomes: A Scoping Review. Semin. Reprod. Med. 2024, 42, 312–329. [Google Scholar] [CrossRef] [PubMed]
- Cathey, A.; Watkins, D.; Rosario, Z.; Vélez, C.; Mukherjee, B.; Alshawabkeh, A.; Cordero, J.; Meeker, J. Biomarkers of Exposure to Phthalate Mixtures and Adverse Birth Outcomes in a Puerto Rico Birth Cohort. Environ. Health Perspect. 2022, 130, 037009. [Google Scholar] [CrossRef]
- Deng, Y.; Yi, S.; Liu, W.; Yang, L.; Zhu, L.; Zhang, Q.; Jin, H.; Yang, R.; Wang, R.; Tang, N. Identification of Primary Organophosphate Esters Contributing to Enhanced Risk of Gestational Diabetes Mellitus Based on a Case-Control Study. Environ. Sci. Technol. 2024, 58, 17352–173542. [Google Scholar] [CrossRef]
- Oh, J.; Bennett, D.; Tancredi, D.; Calafat, A.; Schmidt, R.; Hertz-Picciotto, I.; Shin, H. Longitudinal Changes in Maternal Serum Concentrations of Per- and Polyfluoroalkyl Substances from Pregnancy to Two Years Postpartum. Environ. Sci. Technol. 2022, 56, 11449–11459. [Google Scholar] [CrossRef]
- Lin, S.; Li, J.; Yan, X.; Pei, L.; Shang, X. Maternal pesticide exposure and risk of preterm birth: A systematic review and meta-analysis. Environ. Int. 2023, 178, 108043. [Google Scholar] [CrossRef]
- Tao, L.; Tang, W.; Xia, Z.; Wu, B.; Liu, H.; Fu, J.; Lu, Q.; Guo, L.; Gao, C.; Zhou, Q.; et al. Machine learning predicts the serum PFOA and PFOS levels in pregnant women: Enhancement of fatty acid status on model performance. Environ. Int. 2024, 190, 108837. [Google Scholar] [CrossRef]
- Hall, A.; Ashley-Martin, J.; Liang, C.; Papandonatos, G.; Arbuckle, T.; Borghese, M.; Buckley, J.; Cecil, K.; Chen, A.; Dodds, L.; et al. Personal care product use and per- and polyfluoroalkyl substances in pregnant and lactating people in the Maternal-Infant Research on Environmental Chemicals study. Environ. Int. 2024, 193, 109094. [Google Scholar] [CrossRef]
- Jin, H.; Gao, Y.; Chen, R.; Zhang, Y.; Qu, J.; Bai, X.; Zhao, M. A preliminary report on the association between maternal serum organophosphate ester concentrations and gestational diabetes mellitus. Heliyon 2023, 9, e14302. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Xiong, S.; An, S.; Tao, L.; Dai, L.; Tian, Y.; Chen, W.; He, C.; Wu, N.; Liu, X.; et al. Association of urinary polycyclic aromatic hydrocarbon metabolites with gestational diabetes mellitus and gestational hypertension among pregnant women in Southwest China: A cross-sectional study. Environ. Pollut. 2024, 343, 123206. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothinen, N.; Kovelamudi, S.; Mehta, J. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Gu, W.; Liu, S.; Wang, Y.; Jiao, B.; Wang, M.; Long, Y.; Miao, K.; Duan, H.; et al. The key metabolic signatures and biomarkers of polycyclic aromatic hydrocarbon-induced blood glucose elevation in chinese individuals exposed to diesel engine exhaust. Ecol. Environ. Saf. 2024, 284, 116997. [Google Scholar] [CrossRef] [PubMed]
- Patel, C. Analytic Complexity and Challenges in Identifying Mixtures of Exposures Associated with Phenotypes in the Exposome Era. Curr. Epidemiol. Rep. 2017, 4, 22–30. [Google Scholar] [CrossRef]
- Mehaidli, A.; Mandal, R.; Simha, P. Selective degradation of endogenous organic metabolites in acidified fresh human urine using sulphate radical-based advanced oxidation. Water Res. 2024, 257, 121751. [Google Scholar] [CrossRef] [PubMed]
- O’ Lenick, C.; Pleil, J.; Stiegel, M.; Sobus, J.; Wallace, M. Detection and analysis of endogenous polar volatile organic compounds (PVOCs) in urine for human exposome research. Biomarkers 2019, 24, 240–248. [Google Scholar] [CrossRef]
- Řimnáčová, L.; Moos, M.; Opekar, S.; Vodrázka, P.; Pejchal, V.; Mráz, J.; Simek, P. Ethyl chloroformate mediated gas chromatographic-mass spectrometric biomonitoring of acidic biomarkers of occupational exposure and endogenous metabolites in human urine. J. Chromatogr. A 2021, 1656, 462547. [Google Scholar] [CrossRef]
- Dubin, R.; Rhee, E. Proteomics and Metabolomics in Kidney Disease, including Insights into Etiology, Treatment, and Prevention. Clin. J. Am. Soc. Nephrol. 2020, 15, 404–411. [Google Scholar] [CrossRef]
- Ryan, D.; Robards, K.; Prenzler, P.; Kendall, M. Recent and potential developments in the analysis of urine: A review. Anal. Chim. Acta 2011, 684, 17–29. [Google Scholar] [CrossRef]
- Song, G.; Liu, X.; Lei, K.; Li, T.; Li, W.; Chen, D. ExpoNano: A Strategy Based on Hyper-Cross-Linked Polymers Achieves Urinary Exposome Assessment for Biomonitoring. Environ. Sci. Technol. 2024, 58, 14088–14097. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Eichler, C.; Cohen, H.; Little, J. Assessing Human Exposure to Chemicals in Materials, Products and Articles: The International Risk Management Landscape for Phthalates. Environ. Sci. Technol. 2019, 53, 13583–13597. [Google Scholar] [CrossRef]
- Bui, T.; Giovanoulis, G.; Cousins, A.; Magner, J.; Cousins, I.; de Wit, C. Human exposure, hazard and risk of alternative plasticizers to phthalate esters. Sci. Total Environ. 2016, 541, 451–467. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, Q.; Wang, H.; Shang, H.; Liu, C. Research progress of the synthesis of green plasticizer tributyl citrate catalyzed by solid acid. Sci. Technol. Rev. 2019, 37, 79–85. [Google Scholar]
- Schossler, P.; Schripp, T.; Salthammer, T.; Bahadir, M. Beyond phthalates: Gas phase concentrations and modeled gas/particle distribution of modern plasticizers. Sci. Total Environ. 2011, 409, 4031–4038. [Google Scholar] [CrossRef] [PubMed]
- Fiume, M.; Eldreth, H.; Bergfeld, W.; Belsito, D.; Hill, R.; Klaassen, C.; Liebler, D.; Marks, J.; Shank, R., Jr.; Slaga, T.; et al. Final Report of the Cosmetic Ingredient Review Expert Panel on the Safety Assessment of Dicarboxylic Acids, Salts, and Esters. Int. J. Toxicol. 2012, 31, 5S–76S. [Google Scholar] [CrossRef] [PubMed]
- Glazko, I.; Gur’yanova, O.; Levanova, S.; Sokolov, A. Kinetic characteristics of the manufacture of esters from isoprene production waste. Pet. Chem. 2010, 50, 395–401. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, P.; Zhang, H.; Zhu, Q.; Liao, C.; Jiang, G. Analysis, occurrence, toxicity and environmental health risks of synthetic phenolic antioxidants: A review. Environ. Res. 2021, 201, 111531. [Google Scholar] [CrossRef]
- Liu, R.; Mabury, S. Synthetic Phenolic Antioxidants and Transformation Products in Human Sera from United States Donors. Environ. Sci. Technol. Lett. 2018, 5, 419–423. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, W.; Huang, Y.; Liu, Y.; Liang, X. Research Progress on the Toxicity of Synthetic Phenolic Antioxidants and Their Metabolites to Fish. Environ. Monit. Forewarn. 2020, 12, 49–57. [Google Scholar]
- Marques dos Santos, M.; Cheriaux, C.; Jia, S.; Thomas, M.; Gallard, H.; Croue, J.; Carato, P.; Snyder, S. Genotoxic effects of chlorinated disinfection by-products of 1,3-diphenylguanidine (DPG): Cell-based in-vitro testing and formation potential during water disinfection. J. Hazard. Mater. 2022, 436, 129114. [Google Scholar] [CrossRef]
- Schulze, S.; Zahn, D.; Montes, R.; Rodil, R.; Quintana, J.; Knepper, T.; Reemtsma, T.; Berger, U. Occurrence of emerging persistent and mobile organic contaminants in European water samples. Water Res. 2019, 153, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Hübner, D.; Huppertsberg, S.; Knepper, T.; Zahn, D. Probing the chemical complexity of tires: Identification of potential tire-borne water contaminants with high-resolution mass spectrometry. Sci. Total Environ. 2022, 802, 149799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, W.; Liu, H.; Huang, S.; Huang, W.; Zhu, Y.; Ma, X.; Xia, Y.; Zhang, J.; Lu, W. Intergenerational metabolism-disrupting effects of maternal exposure to plasticizer acetyl tributyl citrate (ATBC). Environ. Int. 2024, 191, 108967. [Google Scholar] [CrossRef]
- Zhang, W.; Jie, J.; Xu, Q.; Wei, R.; Liao, X.; Zhang, D.; Zhang, Y.; Zhang, J.; Su, G.; Chen, Y.; et al. Characterizing the obesogenic and fatty liver-inducing effects of Acetyl tributyl citrate (ATBC) plasticizer using both in vivo and in vitro models. J. Hazard. Mater. 2023, 445, 130548. [Google Scholar] [CrossRef]

| Chemicals | non-GDM (n = 170) | GDM (n = 85) | FDR | ||
|---|---|---|---|---|---|
| Median (ng/mL) | DF (%) | Median (ng/mL) | DF (%) | ||
| BHT-CHO | 30.5 | 100% | 32.4 | 100% | 0.507 |
| 4-tOP | 6.04 | 75% | 9.75 | 88% | 0.090 |
| Cyanox_2246 | 0.350 | 98% | 0.420 | 94% | 0.261 |
| DPG | 1.98 | 82% | 3.04 | 89% | 0.343 |
| EtP | 0.972 | 90% | 1.11 | 93% | 0.968 |
| MePa | 6.50 | 99% | 6.74 | 100% | 0.513 |
| ProP | 0.662 | 89% | 0.722 | 93% | 0.625 |
| BBOEP | 1.44 | 91% | 1.60 | 91% | 0.872 |
| DEP | 24.6 | 100% | 16.2 | 100% | 0.000 |
| mCMHP | 5.70 | 97% | 4.26 | 96% | 0.064 |
| mECPP | 6.02 | 100% | 4.93 | 99% | 0.145 |
| mEHHP | 14.7 | 100% | 11.2 | 100% | 0.175 |
| mEOHP | 4.33 | 100% | 3.02 | 100% | 0.145 |
| mHiNP | 7.86 | 100% | 5.29 | 100% | 0.208 |
| miBP_mBP | 62.5 | 100% | 44.8 | 100% | 0.145 |
| mEHP_mOcP | 0.865 | 79% | 0.712 | 82% | 0.508 |
| mCHP | 1.77 | 91% | 1.94 | 94% | 0.669 |
| mEP | 3.10 | 97% | 2.22 | 92% | 0.548 |
| mMP | 0390 | 100% | 0.369 | 100% | 0.815 |
| OMC | 1.19 | 86% | 1.69 | 88% | 0.160 |
| DiBA_DnBA | 35.5 | 100% | 46.7 | 99% | 0.330 |
| TBC | 0.712 | 74% | 1.12 | 78% | 0.064 |
| ATBC | 13.4 | 96% | 24.3 | 99% | 0.018 |
| DEHM | 0.444 | 88% | 0.779 | 88% | 0.145 |
| 3-PBA | 2.04 | 95% | 2.27 | 89% | 0.968 |
| PNP | 3.25 | 72% | 4.40 | 75% | 0.754 |
| 3Me4NP | 3.16 | 71% | 3.07 | 74% | 0.880 |
| 2-OH-Nap | 16.1 | 100% | 14.6 | 100% | 0.855 |
| 1-OH-Nap | 11.8 | 98% | 9.01 | 93% | 0.145 |
| 2-OH-Flu | 7.46 | 100% | 4.87 | 100% | 0.061 |
| 3-OH-Flu | 0.445 | 96% | 0.413 | 94% | 0.588 |
| 2-OH-Phe | 2.50 | 100% | 2.04 | 100% | 0.145 |
| 3-OH-Phe | 4.27 | 100% | 3.25 | 100% | 0.058 |
| 1/9-OH-Phe | 2.64 | 100% | 1.98 | 100% | 0.282 |
| 1-OH-Pyr | 0.193 | 97% | 0.157 | 92% | 0.855 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Pan, X.-F.; Miao, M.; Guo, H.; Meng, P.; Huang, W. Exposure to a Multitude of Environmental Chemicals During Pregnancy and Its Association with the Risk of Gestational Diabetes Mellitus. Toxics 2025, 13, 461. https://doi.org/10.3390/toxics13060461
Lin Y, Pan X-F, Miao M, Guo H, Meng P, Huang W. Exposure to a Multitude of Environmental Chemicals During Pregnancy and Its Association with the Risk of Gestational Diabetes Mellitus. Toxics. 2025; 13(6):461. https://doi.org/10.3390/toxics13060461
Chicago/Turabian StyleLin, Yuzhe, Xiong-Fei Pan, Maohua Miao, Huicai Guo, Peipei Meng, and Wei Huang. 2025. "Exposure to a Multitude of Environmental Chemicals During Pregnancy and Its Association with the Risk of Gestational Diabetes Mellitus" Toxics 13, no. 6: 461. https://doi.org/10.3390/toxics13060461
APA StyleLin, Y., Pan, X.-F., Miao, M., Guo, H., Meng, P., & Huang, W. (2025). Exposure to a Multitude of Environmental Chemicals During Pregnancy and Its Association with the Risk of Gestational Diabetes Mellitus. Toxics, 13(6), 461. https://doi.org/10.3390/toxics13060461







