Yucasin Alleviates Aluminum Toxicity Associated with Regulating Reactive Oxygen Species Homeostasis in Tomato Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions
2.2. Treatment with AlCl3, IAA and Yucasin
2.3. Al Content Determination
2.4. ROS Assays
2.5. Evaluation of Malondialdehyde (MDA) and Proline Content
2.6. TTC and Evans Blue Staining
2.7. Enzyme Activity Assays
2.8. Statistical Analysis
3. Results
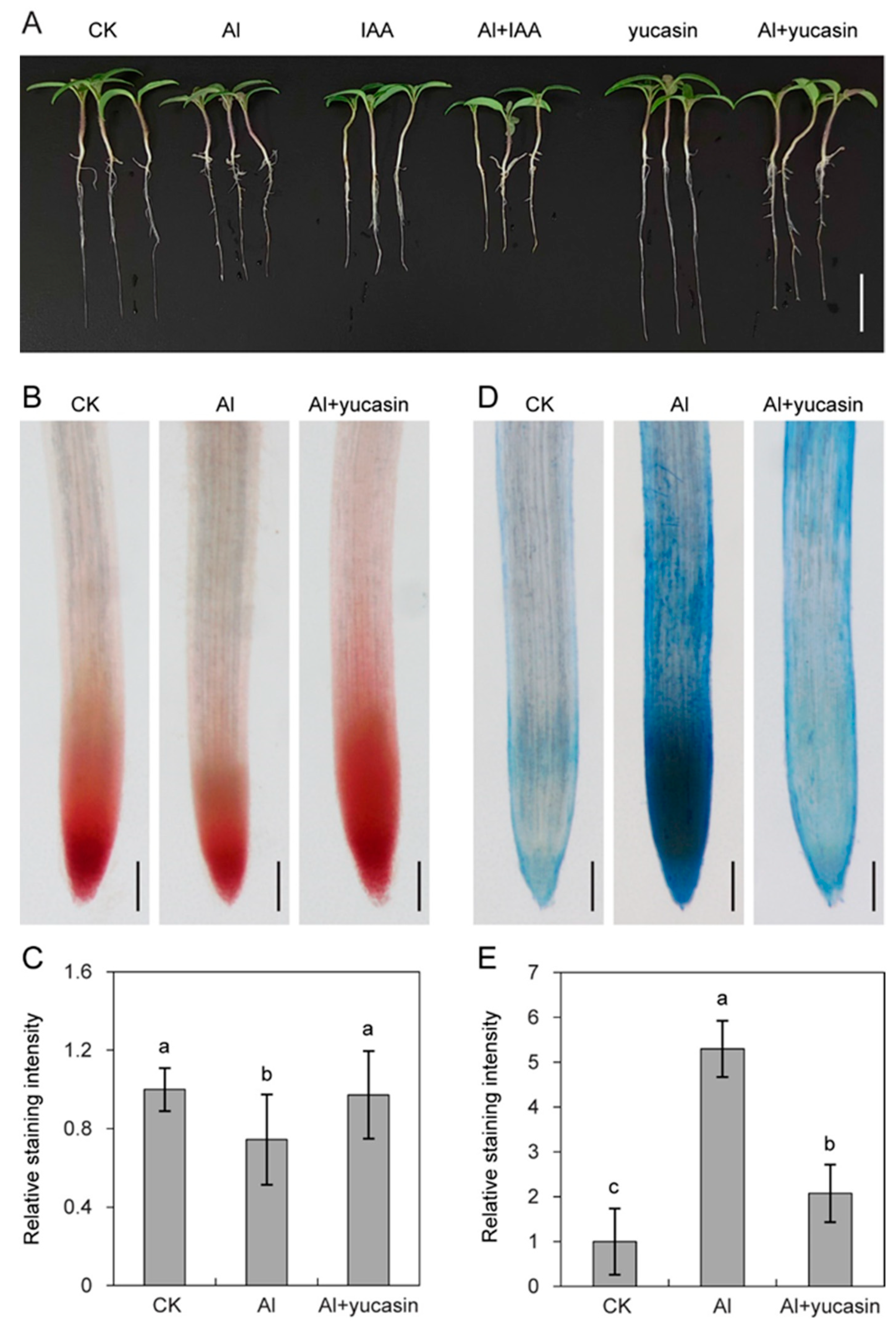
3.1. Yucasin Enhances Tomato Seedling Tolerance to Al Stress by Maintaining Root Viability
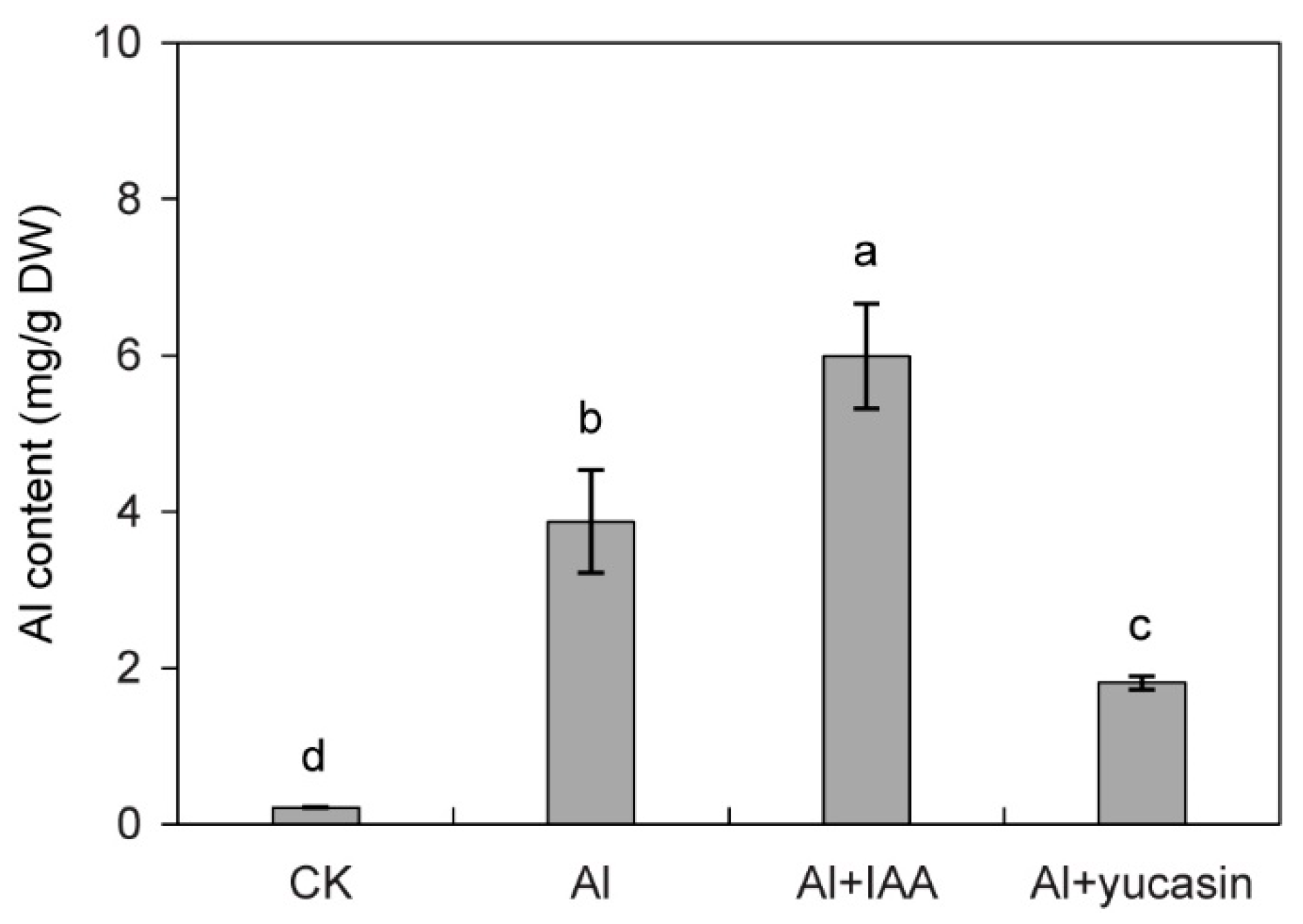
3.2. Yucasin Reduces Al Accumulation in Tomato Seedling Roots
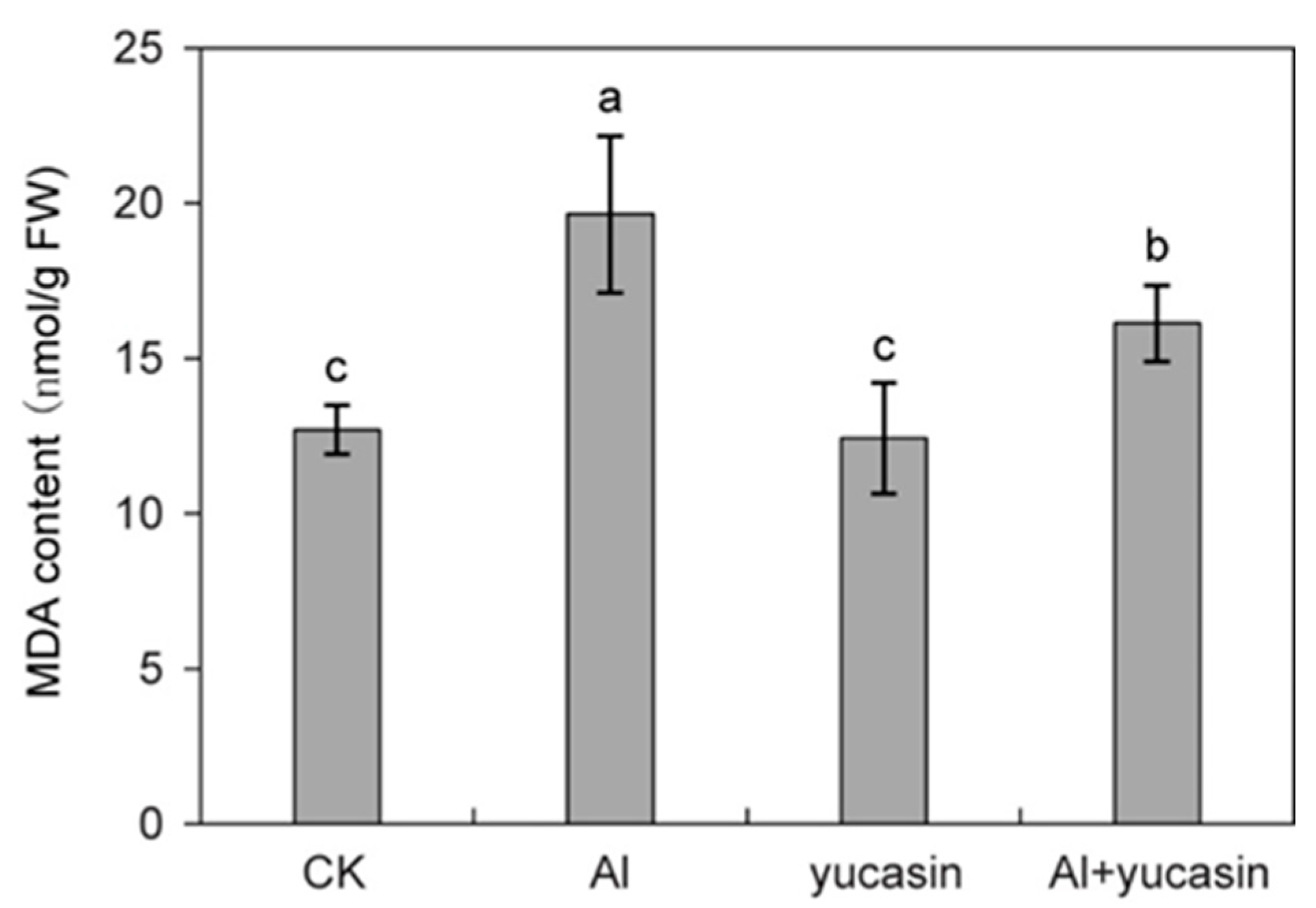
3.3. Yucasin Reduces ROS Accumulation and Oxidative Damage Under Al Toxicity
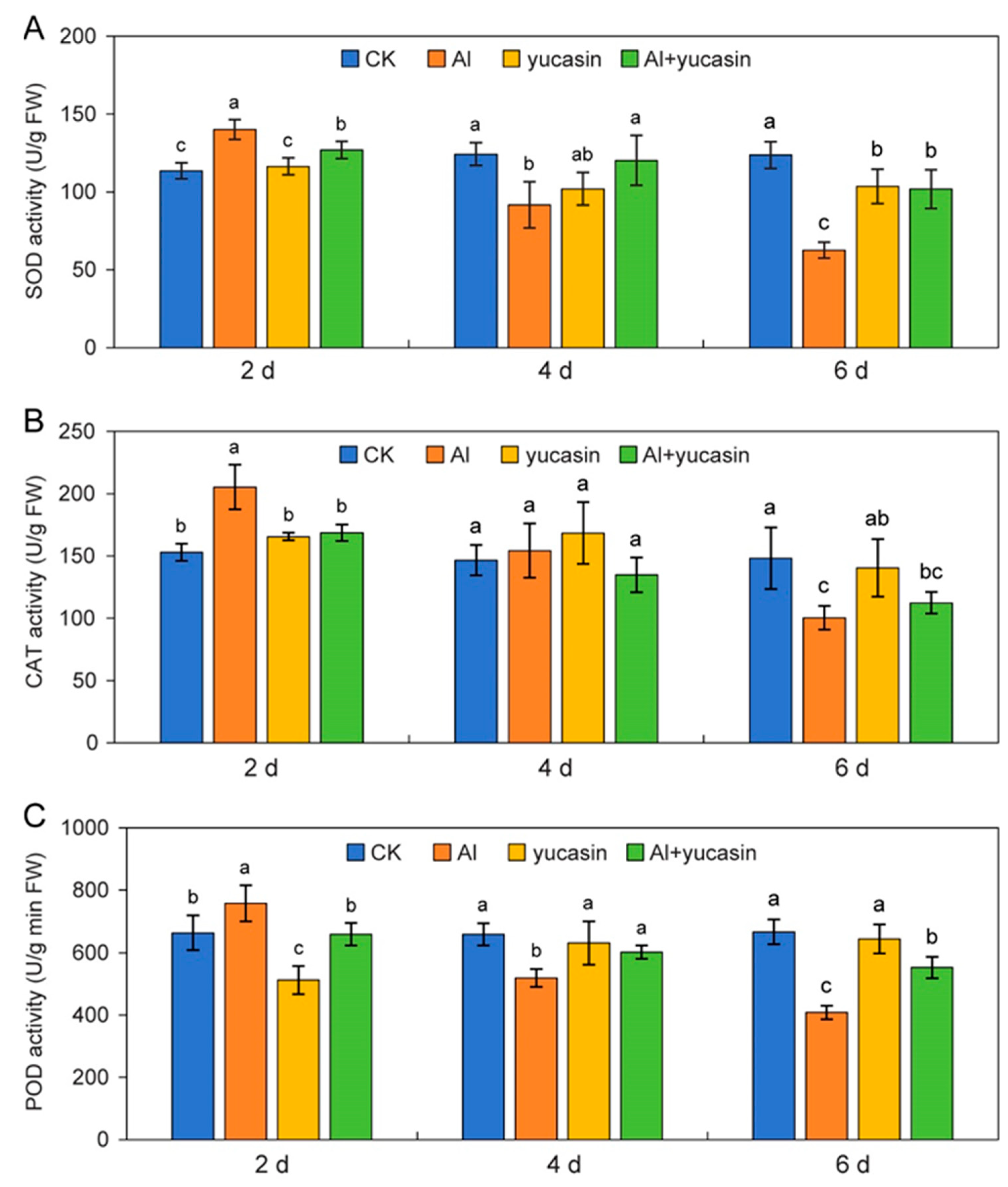
3.4. Yucasin Maintains Antioxidant Enzyme Activities in Tomato Seedlings
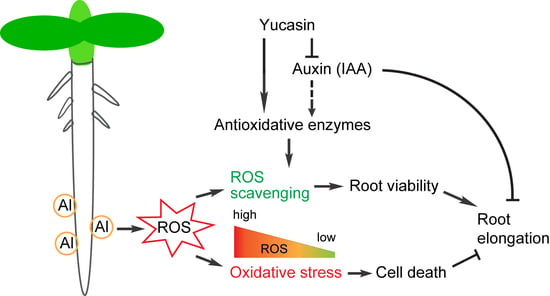
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kinraide, T.B. Identity of the rhizotoxic aluminium species. Plant Soil 1991, 134, 167–178. [Google Scholar] [CrossRef]
- Ofoe, R.; Thomas, R.H.; Asiedu, S.K.; Wang-Pruski, G.; Fofana, B.; Abbey, L. Aluminum in plant: Benefits, toxicity and tolerance mechanisms. Front. Plant Sci. 2023, 13, 1085998. [Google Scholar] [CrossRef]
- Silva, T.F.; Ferreira, B.G.; Dos Santos Isaias, R.M.; Alexandre, S.S.; França, M.G.C. Immunocytochemistry and density functional theory evidence the competition of aluminum and calcium for pectin binding in urochloa decumbens roots. Plant Physiol. Biochem. 2020, 153, 64–71. [Google Scholar] [CrossRef]
- Jaskowiak, J.; Tkaczyk, O.; Slota, M.; Kwasniewska, J.; Szarejko, I. Analysis of aluminum toxicity in Hordeum vulgare roots with an emphasis on DNA integrity and cell cycle. PLoS ONE 2018, 13, e0193156. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, Z.; Qin, R.; Zhang, H.; Zou, J.; Jiang, W.; Liu, D. Accumulation and cellular toxicity of aluminum in seedling of Pinus massoniana. BMC Plant Biol. 2014, 14, 264. [Google Scholar] [CrossRef]
- Li, C.; Liu, G.; Geng, X.; He, C.; Quan, T.; Hayashi, K.I.; De Smet, I.; Robert, H.S.; Ding, Z.; Yang, Z.B. Local regulation of auxin transport in root-apex transition zone mediates aluminium-induced Arabidopsis root-growth inhibition. Plant J. 2021, 108, 55–66. [Google Scholar] [CrossRef]
- Yang, Z.B.; Geng, X.; He, C.; Zhang, F.; Wang, R.; Horst, W.J.; Ding, Z. TAA1-regulated local auxin biosynthesis in the root-apex transition zone mediates the aluminum-induced inhibition of root growth in Arabidopsis. Plant Cell 2014, 26, 2889–2904. [Google Scholar] [CrossRef]
- Ranjan, A.; Sinha, R.; Sharma, T.R.; Pattanayak, A.; Singh, A.K. Alleviating aluminum toxicity in plants: Implications of reactive oxygen species signaling and crosstalk with other signaling pathways. Physiol. Plant 2021, 173, 1765–1784. [Google Scholar] [CrossRef]
- Yang, Z.B.; Liu, G.; Liu, J.; Zhang, B.; Meng, W.; Müller, B.; Hayashi, K.I.; Zhang, X.; Zhao, Z.; De Smet, I.; et al. Synergistic action of auxin and cytokinin mediates aluminum-induced root growth inhibition in Arabidopsis. EMBO Rep. 2017, 18, 1213–1230. [Google Scholar] [CrossRef]
- Liu, G.; Gao, S.; Tian, H.; Wu, W.; Robert, H.S.; Ding, Z. Local transcriptional control of YUCCA regulates auxin promoted root-growth inhibition in response to aluminium stress in Arabidopsis. PLoS Genet. 2016, 12, e1006360. [Google Scholar] [CrossRef]
- Tao, L.; Xiao, X.; Huang, Q.; Zhu, H.; Feng, Y.; Li, Y.; Li, X.; Guo, Z.; Liu, J.; Wu, F.; et al. Boron supply restores aluminum-blocked auxin transport by the modulation of PIN2 trafficking in the root apical transition zone. Plant J. 2023, 114, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Kollmeier, M.; Felle, H.H.; Horst, W.J. Genotypical differences in aluminum resistance of maize are expressed in the distal part of the transition zone. Is reduced basipetal auxin flow involved in inhibition of root elongation by aluminum? Plant Physiol. 2000, 122, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Lei, G.J.; Wang, Z.W.; Shi, Y.Z.; Braam, J.; Li, G.X.; Zheng, S.J. Coordination between apoplastic and symplastic detoxification confers plant aluminum resistance. Plant Physiol. 2013, 162, 1947–1955. [Google Scholar] [CrossRef]
- Mashiguchi, K.; Tanaka, K.; Sakai, T.; Sugawara, S.; Kawaide, H.; Natsume, M.; Hanada, A.; Yaeno, T.; Shirasu, K.; Yao, H.; et al. The main auxin biosynthesis pathway in Arabidopsis. Proc. Nat. Acad. Sci. USA 2011, 108, 18512–18517. [Google Scholar] [CrossRef]
- Nishimura, T.; Hayashi, K.; Suzuki, H.; Gyohda, A.; Takaoka, C.; Sakaguchi, Y.; Matsumoto, S.; Kasahara, H.; Sakai, T.; Kato, J.; et al. Yucasin is a potent inhibitor of YUCCA, a key enzyme in auxin biosynthesis. Plant J. 2014, 77, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.Z.; Wang, X.; Liu, J.J.; Chen, Y.X.; Wang, A.Q.; Zhan, J.; Han, Z.Q.; He, L.F.; Xiao, D. AhASRK1, a peanut dual-specificity kinase that activates the Ca2+-ROS-MAPK signalling cascade to mediate programmed cell death induced by aluminium toxicity via ABA. Plant Physiol. Biochem. 2025, 220, 109538. [Google Scholar] [CrossRef]
- Ding, Z.J.; Xu, C.; Yan, J.Y.; Wang, Y.X.; Cui, M.Q.; Yuan, J.J.; Wang, Y.N.; Li, G.X.; Wu, J.X.; Wu, Y.R.; et al. The LRR receptor-like kinase ALR1 is a plant aluminum ion sensor. Cell Res. 2024, 34, 281–294. [Google Scholar] [CrossRef]
- Matsumoto, H.; Motoda, H. Oxidative stress is associated with aluminum toxicity recovery in apex of pea root. Plant Soil 2013, 363, 399–410. [Google Scholar] [CrossRef]
- Huang, W.J.; Yang, X.D.; Yao, S.C.; LwinOo, T.; He, H.Y.; Wang, A.Q.; Li, C.Z.; He, L.F. Reactive oxygen species burst induced by aluminum stress triggers mitochondria-dependent programmed cell death in peanut root tip cells. Plant Physiol. Bioch. 2014, 82, 76–84. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kobayashi, Y.; Devi, S.R.; Rikiishi, S.; Matsumoto, H. Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol. 2002, 128, 63–72. [Google Scholar] [CrossRef]
- Jiang, D.; Ou, Y.; Jiang, G.; Dai, G.; Liu, S.; Chen, G. Melatonin-priming ameliorates aluminum accumulation and toxicity in rice through enhancing aluminum exclusion and maintaining redox homeostasis. Plant Physiol. Biochem. 2025, 219, 109433. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cheng, H.; Wei, Y. Supplemental silicon and boron alleviates aluminum-induced oxidative damage in soybean roots. Plants 2024, 13, 821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Guo, J.; Zhou, F.; Singh, S.; Xu, X.; Xie, Q.; Yang, Z.; Huang, C.F. F-box protein RAE1 regulates the stability of the aluminum-resistance transcription factor STOP1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2019, 116, 319–327. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, Y.; Xie, W.; Ren, W.; Zhang, Y.; Zhang, H.; Dai, S.; Huang, C.F. H2O2 negatively regulates aluminum resistance via oxidation and degradation of the transcription factor STOP1. Plant Cell 2024, 36, 688–708. [Google Scholar] [CrossRef]
- Huang, C.F.; Ma, Y. Aluminum resistance in plants: A critical review focusing on STOP1. Plant Commun. 2025, 6, 101200. [Google Scholar] [CrossRef]
- Huang, L.; Li, H.; Luo, Y.; Shi, J.; Kong, L.; Teng, W. Exogenous silicon alleviates aluminum stress in Eucalyptus species by enhancing the antioxidant capacity and improving plant growth and tolerance quality. BMC Plant Biol. 2024, 24, 997. [Google Scholar] [CrossRef]
- Wu, D.; Shen, H.; Yokawa, K.; Baluška, F. Alleviation of aluminium-induced cell rigidity by overexpression of OsPIN2 in rice roots. J. Exp. Bot. 2014, 65, 5305–5315. [Google Scholar] [CrossRef]
- Yuan, H.M.; Liu, W.C.; Jin, Y.; Lu, Y.T. Role of ROS and auxin in plant response to metal-mediated stress. Plant Signal Behav. 2013, 8, e24671. [Google Scholar] [CrossRef]
- Bai, B.; Bian, H.; Zeng, Z.; Hou, N.; Shi, B.; Wang, J.; Zhu, M.; Han, N. miR393-mediated auxin signaling regulation is involved in root elongation inhibition in response to toxic aluminum stress in Barley. Plant Cell Physiol. 2017, 58, 426–439. [Google Scholar] [CrossRef]
- Wang, M.; Qiao, J.; Yu, C.; Chen, H.; Sun, C.; Huang, L.; Li, C.; Geisler, M.; Qian, Q.; Jiang, A.; et al. The auxin influx carrier, OsAUX3, regulates rice root development and responses to aluminium stress. Plant Cell Environ. 2019, 42, 1125–1138. [Google Scholar] [CrossRef]
- Ivanchenko, M.G.; den Os, D.; Monshausen, G.B.; Dubrovsky, J.G.; Bednárová, A.; Krishnan, N. Auxin increases the hydrogen peroxide (H2O2) concentration in tomato (Solanum lycopersicum) root tips while inhibiting root growth. Ann. Bot. 2013, 112, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Jia, N.; Wei, S.; Xu, W.; Lv, T.; Bai, J.; Li, B. Mitochondrial HSC70-1 regulates polar auxin transport through ROS homeostasis in Arabidopsis roots. Antioxidants 2022, 11, 2035. [Google Scholar] [CrossRef]
- Wang, H.Q.; Zhao, X.Y.; Xuan, W.; Wang, P.; Zhao, F.J. Rice roots avoid asymmetric heavy metal and salinity stress via an RBOH-ROS-auxin signaling cascade. Mol. Plant 2023, 16, 1678–1694. [Google Scholar] [CrossRef]
- Ranjan, A.; Sinha, R.; Lal, S.K.; Bishi, S.K.; Singh, A.K. Phytohormone signalling and cross-talk to alleviate aluminium toxicity in plants. Plant Cell Rep. 2021, 40, 1331–1343. [Google Scholar] [CrossRef]
- Li, L.; Fu, Q.L.; Achal, V.; Liu, Y. A comparison of the potential health risk of aluminum and heavy metals in tea leaves and tea infusion of commercially available green tea in Jiangxi, China. Environ. Monit. Assess. 2015, 187, 228. [Google Scholar] [CrossRef]
- Lv, W.T.; Lin, B.; Zhang, M.; Hua, X.J. Proline accumulation is inhibitory to Arabidopsis seedlings during heat stress. Plant Physiol. 2011, 156, 1921–1933. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Teare. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Islam, E.; Yang, X.; Li, T.; Liu, D.; Jin, X.; Meng, F. Effect of Pb toxicity on root morphology, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J. Hazard. Mater. 2007, 147, 806–816. [Google Scholar] [CrossRef]
- Motoda, H.; Kano, Y.; Hiragami, F.; Kawamura, K.; Matsumoto, H. Morphological changes in the apex of pea roots during and after recovery from aluminium treatment. Plant Soil 2010, 333, 49–58. [Google Scholar] [CrossRef]
- Yang, W.; Wen, D.; Yang, Y.; Li, H.; Yang, C.; Yu, J.; Xiang, H. Metabolomics and transcriptomics combined with physiology reveal key metabolic pathway responses in tobacco roots exposed to NaHS. BMC Plant Biol. 2024, 24, 680. [Google Scholar] [CrossRef]
- Sun, P.; Tian, Q.Y.; Chen, J.; Zhang, W.H. Aluminium-induced inhibition of root elongation in Arabidopsis is mediated by ethylene and auxin. J. Exp. Bot. 2010, 61, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lu, X.; Li, C.; Zhang, B.; Zhang, C.; Zhang, X.S.; Ding, Z. Auxin efflux carrier ZmPGP1 mediates root growth inhibition under aluminum stress. Plant Physiol. 2018, 177, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E.A.; Quinn, C.F.; Tapken, W.; Malagoli, M.; Schiavon, M. Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 2009, 12, 267–274. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, M.; Liu, X.; Mao, Q.; Shi, C.; Kochian, L.V.; Liao, H. Aluminium is essential for root growth and development of tea plants (Camellia sinensis). J. Integr. Plant Biol. 2020, 62, 984–997. [Google Scholar] [CrossRef]
- Jiang, X.; Lai, S.; Kong, D.; Hou, X.; Shi, Y.; Fu, Z.; Liu, Y.; Gao, L.; Xia, T. Al-induced CsUGT84J2 enhances flavonol and auxin accumulation to promote root growth in tea plants. Hortic. Res. 2023, 10, uhad095. [Google Scholar] [CrossRef]
- Liu, J.P.; Magalhaes, J.V.; Shaff, J.; Kochian, L.V. Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J. 2009, 57, 389–399. [Google Scholar] [CrossRef]
- Kochian, L.V.; Pineros, M.A.; Liu, J.P.; Magalhaes, J.V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Zhang, F.; Yan, X.; Han, X.; Tang, R.; Chu, M.; Yang, Y.; Yang, Y.H.; Zhao, F.; Fu, A.; Luan, S.; et al. A defective vacuolar proton pump enhances aluminum tolerance by reducing vacuole sequestration of organic acids. Plant Physiol. 2019, 181, 743–761. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Qu, M.; Xiao, H.; Feng, Y.; Liu, J.; Wu, L.; Yu, M. Cell wall pectin and its methyl-esterification in transition zone determine Al resistance in cultivars of pea (Pisum sativum). Front. Plant Sci. 2016, 7, 177833. [Google Scholar] [CrossRef]
- Demecsová, L.; Zelinová, V.; Liptáková, L.; Tamás, L. Mild cadmium stress induces auxin synthesis and accumulation, while severe cadmium stress causes its rapid depletion in barley root tip. Environ. Exp. Bot. 2020, 175, 104038. [Google Scholar] [CrossRef]
- Guo, L.; Yang, S.; Tu, Z.; Yu, F.; Qiu, C.; Huang, G.; Fang, S. An indole-3-acetic acid inhibitor mitigated mild cadmium stress by suppressing peroxide formation in rice seedling roots. Plant Physiol. Biochem. 2024, 213, 108823. [Google Scholar] [CrossRef] [PubMed]
- Tamás, L.; Mistrík, I.; Zelinová, V. Heavy metal-induced reactive oxygen species and cell death in barley root tip. Environ. Exp. Bot. 2017, 140, 34–40. [Google Scholar] [CrossRef]
- Li, B.; Fu, Y.; Li, X.; Yin, H.; Xi, Z. Brassinosteroids alleviate cadmium phytotoxicity by minimizing oxidative stress in grape seedlings: Toward regulating the ascorbate-glutathione cycle. Sci. Hortic. 2022, 299, 111002. [Google Scholar] [CrossRef]
- Niu, K.; Zhu, R.; Wang, Y.; Zhao, C.; Ma, H. 2,4-epibrassinolide improves cadmium tolerance and lateral root growth associated with regulating endogenous auxin and ethylene in Kentucky bluegrass. Ecotoxicol. Environ. Saf. 2023, 249, 114460. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Bai, C.; Cai, J.; Wu, Y.; Zhu, C. Yucasin Alleviates Aluminum Toxicity Associated with Regulating Reactive Oxygen Species Homeostasis in Tomato Seedlings. Toxics 2025, 13, 406. https://doi.org/10.3390/toxics13050406
Liu H, Bai C, Cai J, Wu Y, Zhu C. Yucasin Alleviates Aluminum Toxicity Associated with Regulating Reactive Oxygen Species Homeostasis in Tomato Seedlings. Toxics. 2025; 13(5):406. https://doi.org/10.3390/toxics13050406
Chicago/Turabian StyleLiu, Huabin, Chuangyang Bai, Jiahui Cai, Yue Wu, and Changwei Zhu. 2025. "Yucasin Alleviates Aluminum Toxicity Associated with Regulating Reactive Oxygen Species Homeostasis in Tomato Seedlings" Toxics 13, no. 5: 406. https://doi.org/10.3390/toxics13050406
APA StyleLiu, H., Bai, C., Cai, J., Wu, Y., & Zhu, C. (2025). Yucasin Alleviates Aluminum Toxicity Associated with Regulating Reactive Oxygen Species Homeostasis in Tomato Seedlings. Toxics, 13(5), 406. https://doi.org/10.3390/toxics13050406