1. Introduction
In recent years, rapid industrialization and urbanization have significantly exacerbated the problem of heavy metal contamination in water systems [
1]. Among these pollutants, Cr
6+ and Pb
2+ are of particular concern due to their high toxicity, carcinogenicity, and persistent environmental impact [
1,
2]. Excessive discharge of Cr
6+ and Pb
2+ poses severe threats to human health and causes extensive damage to ecosystems [
3]. Traditional methods for removing heavy metals from water, such as chemical precipitation and ion exchange, are often costly and challenging to implement on a large scale [
4]. Therefore, there is an urgent need for cost-effective, efficient, and environmentally friendly solutions [
5]. Biochar derived from the thermochemical conversion of biomass waste has garnered significant research interest due to its cost-effectiveness, renewable nature, and exceptional adsorption capacity for heavy metal remediation. Recent advancements demonstrate the remarkable potential of modified biochar materials in wastewater treatment applications. Shahriyari Far et al. (2021) developed a composite adsorbent combining activated carbon with metal–organic frameworks, achieving a maximum lead ion adsorption capacity of 127 mg/L [
6]; similarly, iron-impregnated activated carbon synthesized from coconut shell biomass exhibited adsorption capacities of 11.9 mg/g for Pb
2+ and 22.1 mg/g for Cr
6+, respectively [
7]. Further enhancement was reported by Abushawish et al. (2022), where nitrogen-doped coconut shell activated carbon demonstrated a maximum Cr
6+ adsorption capacity of 15.15 mg/g [
8].
Biochar, with its high specific surface area, abundant functional groups, and excellent adsorption properties, has emerged as a promising material for heavy metal remediation [
9]. Among its variations, nitrogen-doped biochar (N-doped biochar) has shown enhanced adsorption capacity for Cr
6+ and Pb
2+ due to the introduction of nitrogen functional groups such as pyridine, pyrrole, and amines. These nitrogen groups not only adsorb heavy metals through coordination and electrostatic attraction but also promote the reduction in Cr
6+, further improving removal efficiency [
10].
For Pb
2+ removal, studies have demonstrated that amino, pyridinic nitrogen, and pyrrolic nitrogen functional groups on N-doped biochar significantly enhance adsorption capacity through chelation [
11]. Bakry et al. reported a maximum Pb
2+ adsorption capacity of 721 mg/g using nitrogen-doped porous carbon materials (ND-CPC), which surpasses the performance of conventional adsorbents. Moreover, the incorporation of nitrogen functional groups improves the selectivity and stability of the adsorption process, enabling efficient removal of Pb
2+ even in multi-metal ion systems [
11]. Further optimization of the nitrogen doping process has been shown to increase adsorption capacities. For instance, Li et al. found that nitrogen-doped biochar derived from lignocellulosic biomass achieved an adsorption capacity of 1466 mg/g for Pb
2+, attributed to its abundant microporous structure and high surface nitrogen content [
12].
China, as one of the world’s leading corn producers, generates approximately 220 million tons of corn stalks annually, accounting for around 30% of the country’s total crop straw production [
13]. In 2019, corn stalks represented 31.45% of the total straw yield from major grain crops in China, making them a vital biomass resource [
14]. Corn stalks are primarily concentrated in Northeast, North, and Southwest China, with Heilongjiang, Inner Mongolia, and Henan being major production regions, each exceeding 30 million tons [
15]. Despite their potential for energy and material utilization, challenges such as high collection and transportation costs have limited their practical usage [
16].
Similarly, apricot branches and shells are by-products of apricot cultivation and processing in regions such as Hebei, Shanxi, and Inner Mongolia, where mountain apricot plantations and almond processing industries are prominent [
17]. While detailed production data for apricot branches and shells are limited, Pingquan City in Hebei processes over 3 million kilograms of almonds annually, generating a substantial amount of these by-products [
18]. However, these resources are often underutilized and disposed of through burning or landfill, leading to resource waste and environmental pollution [
19].
Transforming corn stalks, apricot branches, and apricot shells into nitrogen-doped biochar not only enhances biomass resource utilization but also offers a cost-effective and efficient solution for heavy metal pollution in water systems. This approach aligns with sustainable development principles, delivering significant environmental and economic benefits [
20].
This study focuses on typical biomass wastes in China, including corn stalks, apricot branches, and apricot shells, to prepare biochar via nitrogen doping modification. The adsorption performance of the resulting biochars for Cr6+ and Pb2+ in aqueous solutions is systematically investigated. By comparing the adsorption rates of different biochar types, this research aims to identify highly efficient adsorbents and provide practical solutions for addressing Cr6+ and Pb2+ contamination in water systems.
3. Results and Discussion
3.1. Morphological Analysis by Scanning Electron Microscopy
Figure 1a–f present a comparative SEM analysis of biochar derived from apricot branches at 500 °C before and after nitrogen doping. The structural evolution induced by nitrogen doping was examined at various magnifications. As shown in
Figure 1a–c (500×, 2500×, and 10,000× magnification, respectively), the pristine biochar retains the intrinsic fibrous structure of the raw biomass. The surface morphology exhibits a bundled fiber arrangement with longitudinal grooves (
Figure 1a, 500×), a well-defined hollow tubular structure (
Figure 1b, 2500×), and a smooth, uniform surface texture (
Figure 1c, 10,000×).
Following nitrogen doping, significant morphological transformations are observed at corresponding magnifications (
Figure 1d–f). At 500× magnification (
Figure 1d), a substantial increase in fine particulate deposition is evident compared to the pristine biochar (
Figure 1a). At 2500× magnification (
Figure 1e), these particles appear densely packed and unevenly distributed, with an increased presence in the grooves and pores of the fibrous matrix, accompanied by observable agglomeration. Further magnification (
Figure 1f, 10,000×) reveals that these particles exhibit irregular shapes, classifying them as amorphous structures. The pronounced formation of amorphous particles significantly enhances the overall specific surface area of the nitrogen-doped biochar, which is expected to influence its physicochemical properties.
Figure 2a–f present the distribution and morphological characteristics of nitrogen-doped biochar particles, highlighting the potential correlation between adsorption properties and the type and quantity of surface functional groups. As observed in
Figure 2a, the carbonized biomass retains a heterogeneous fibrous matrix, exhibiting structural features such as sheet-like and fragmented fibrous morphologies. These features reflect the intrinsic fiber organization of the biomass as well as the extent of structural disruption induced by the carbonization process. Additionally, numerous fine particles are distributed across the biochar surface, appearing sparsely dispersed on larger fibrous structures while becoming increasingly dense in regions with smaller fiber fragments.
To further examine the morphology of these fine particles, a series of high-magnification SEM images were acquired at different scales: 1000× (
Figure 2a), 2000× (
Figure 2b), 2500× (
Figure 2c), 5000× (
Figure 2d), 10,000× (
Figure 2e), and 20,000× (
Figure 2f). The magnified images reveal that these particles predominantly accumulate within the grooves and porous regions of the fibrous matrix, forming localized agglomerates. Their irregular shape and non-uniform size distribution classify them as amorphous particles.
Figure 3a–c present SEM images (5000× magnification) of nitrogen-doped biochar derived from apricot branches, apricot shells, and corn stalks, respectively. The results reveal distinct morphological differences among the biomass-derived biochar, attributed to the inherent structural variations of the precursor materials. Despite these differences, all samples exhibit irregularly shaped particles surrounding the fragmented biomass matrix. High-magnification observations of sparsely distributed regions further demonstrate that these fine particles share a smooth surface morphology but lack a defined geometric shape, thus categorizing them as amorphous structures. Note:
Figure 3d–f represent the same samples as
Figure 3a–c but observed at higher magnification.
Figure 4a–c present high-magnification (20,000×) SEM images of nitrogen-doped biochar derived from corn stalks, apricot branches, and apricot shells, respectively. Particle size analysis was conducted on larger individual particles and agglomeration sites within the images. The results indicate that the maximum observed particle size for nitrogen-doped biochar derived from corn stalks reaches approximately 600 nm, whereas biochar from apricot branches exhibits a maximum particle size of 500 nm. In contrast, the largest particles in nitrogen-doped apricot shell biochar measure around 200 nm, with some agglomerated clusters also reaching 600 nm. These findings suggest that nitrogen-doped biochar derived from different biomass sources predominantly forms amorphous, irregularly shaped particles. However, the size distribution, degree of uniformity, and agglomeration tendency of these amorphous particles are significantly influenced by the intrinsic characteristics of the biomass precursor.
3.2. Characterization of Fourier Transform Infrared Spectroscopic
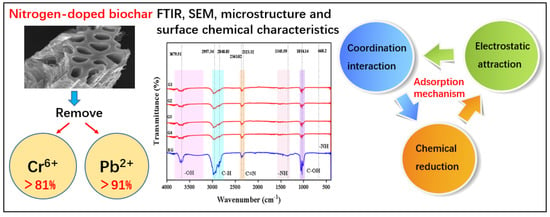
FT-IR spectroscopy is an effective tool for identifying surface functional groups on biochar. The infrared spectra of the 12 samples analyzed were generally similar (
Figure 5). The spectrum reveals characteristic peaks at 3697.51 cm
−1, corresponding to the stretching vibration of –OH bonds, at approximately 2957.34 cm
−1 for C-H bonds, around 2363.82 and 2323.32 cm
−1 for C
N bonds, and at 1345.59 cm
−1, indicating the presence of –NH bonds, and at 1034.14 cm
−1, indicating the presence of C–OH bonds, in the biochar [
30].
As nitrogen-doped biochar was prepared, the presence of C
N, N–H bonds confirms the existence of active functional groups on the biochar surface [
31]. During the adsorption process, the lone pair of electrons in amino groups can interact with certain heavy metal ions [
32]. For instance, Pb
2+ can form coordination complexes with amino groups, and amino groups with reducing properties can serve as electron donors, directly reducing Cr
6+ to Cr
3+ [
32]. Thus, the presence of amino groups enables biochar to undergo not only physical adsorption but also chemical adsorption during the adsorption process [
33].
3.3. Elemental Analysis
Elemental analysis was conducted on three nitrogen-doped biochars—G-600 (corn straw-derived, pyrolyzed at 600 °C, characterized by high adsorption performance), G-300 (corn straw-derived, 300 °C, low adsorption performance), and Z-500 (apricot branch-derived, 500 °C, the best among its type but inferior to G-600)—to elucidate their adsorption behavior toward Cr6+ and Pb2+.
To explore the influence of pyrolysis temperature and biomass type, comparisons were made between G-600 and G-300 (same biomass, different temperatures), and between G-600 and Z-500 (different biomass, both with relatively high adsorption). The elemental composition (C, H, N, S) was determined for each sample, and relevant parameters including C/H, C/N ratios, and the total elemental proportion (Sum%, the combined wt.% of C, H, N, and S) were calculated. These data were then correlated with the adsorption removal efficiencies (AR%) of Cr
6+ and Pb
2+, as illustrated in
Figure 6.
The results demonstrated that C%, N%, C/H ratio, and Sum% showed trends consistent with the AR% values of both Cr6+ and Pb2+. A higher C% and C/H ratio reflect a greater degree of carbonization, indicating that increased carbonization enhances adsorption performance. Similarly, elevated N% corresponds to higher nitrogen doping levels, which also contribute positively to adsorption capacity. In all three biochars, the combined content of C, H, N, and S was significantly below 100%, suggesting the presence of ash or inorganic constituents. The lower the Sum% value, the more anionic residues (e.g., negatively charged species) may be inferred. These anions are presumed to enhance electrostatic attraction toward positively charged Cr6+ and Pb2+ ions.
Interestingly, despite theoretical expectations that electrostatic interactions should favor Cr6+ adsorption over Pb2+, the experimental results showed stronger Pb2+ removal. This indicates that other factors, such as the degree of carbonization or nitrogen functionalization, may play a more dominant role than electrostatic interactions in this system.
Comparative analysis of G-600 vs. G-300 and G-600 vs. Z-500 revealed an inverse correlation between H% and AR% for both Cr6+ and Pb2+. Since H% indirectly reflects the abundance of H-containing functional groups such as –OH and –NH2, this finding suggests that these groups did not significantly contribute to enhanced adsorption in this study. Although the C/N ratio followed the same trend as the AR% values, the difference in C/N between G-600 and G-300 (same feedstock) was minimal, indicating that pyrolysis temperature had limited impact on nitrogen incorporation. In contrast, the C/N ratio differed substantially between G-600 (corn straw) and Z-500 (apricot branch), highlighting that nitrogen doping efficiency is more sensitive to feedstock type than to pyrolysis temperature.
3.4. Specific Surface Area
The relevant data are summarized in
Table 1 according to the analysis and test report
The results of the analysis are as follows:
(1). BET Surface Area G (Corn straw) > Z (apricot branch): the specific surface area of G-300 (raw material: corn stalk, biochar preparation temperature 300 °C)and G-600 (raw material: corn stalk, biochar preparation temperature 600 °C) is much higher than that of Z-500 (raw material: apricot branch, biochar preparation temperature 500 °C). It is shown that corn straw is more likely to form a developed pore structure in the roasting process, especially when it is not excessively sintered at 300 °C.
(2) Pore volume
The G-300 (300 °C) has a significantly higher total pore volume (0.045 cm3/g), possibly due to the retention of more large mesopores or incomplete release of some volatiles, resulting in more pores.
(3) Percentage of micropores
The G-600 has a higher proportion of micropores (0.00305 cm3/g), indicating that the increase in roasting temperature is conducive to the generation of more micropores. Micropores are the key to adsorption capacity and are suitable for small molecules. In contrast, although G-300 has the highest specific surface area, it contributes little to micropores, indicating that it is mainly composed of medium and large pores.
(4) Average Pore Size
The average pore size of G-300 is the largest (nearly 14 nm), indicating that it is dominated by large mesopores; the average pore size of G-600 and Z-500 is 4–7 nm, which belongs to small mesopores.
(5) Comparative analysis of G-600 and G-300
Characteristics and adsorption advantages of G-600: the proportion of micropores is large (about 62%), which is conducive to enhancing the adsorption capacity; the pore size is smaller (4.66 nm), which is suitable for adsorption of small molecules;
(6) Characteristics and adsorption advantages of G-300:The specific surface area is slightly higher, but most of it is contributed by medium and large pores; the pore volume is large (0.045 cm3/g), which is beneficial to the adsorption of large molecules; the pore size is large (13.99 nm), which is conducive to rapid diffusion, but the adsorption force may be insufficient for small molecules.
(7) Summary
Corn stalk biochar calcined at 300 °C exhibited the highest BET surface area (12.76 m2/g) and a significantly larger total pore volume (0.045 cm3/g), with an average pore diameter of ~14 nm. This meso-to-macroporous structure is better suited for the adsorption of large molecules but limited micropore content may reduce the affinity for small molecule adsorption. In contrast, the 600 °C sample developed more micropores (micropore area accounting for ~62% of BET area) with a smaller average pore diameter of 4.66 nm. It leads to a higher micropore contribution and better small molecule absorption. The apricot branch biochar calcined at 500 °C showed a much lower surface area (3.70 m2/g) and pore volume (0.006 cm3/g). It suggests a relatively underdeveloped pore structure after thermal treatment.
3.5. Removal Efficiency of Cr6+ and Pb2+
Biochar was prepared from apricot branches, apricot shells, and corn stalks at pyrolysis temperatures of 300 °C, 400 °C, 500 °C, and 600 °C, followed by nitrogen doping. The resulting nitrogen-doped carbon materials were denoted as N-XZ-ZnBC-T (300 °C/400 °C/500 °C/600 °C). The removal efficiency of Cr6+ and Pb2+ from aqueous solutions was evaluated based on the adsorption rate, where a higher adsorption rate indicates superior removal performance.
3.5.1. Removal Efficiency of Cr6+
The concentration and absorbance of Cr
6+ before and after adsorption experiments were measured, and the adsorption rate was calculated using Equation (
),
.
Figure 7 presents the statistical analysis of Cr
6+ removal efficiency for the twelve biochar samples. As shown in
Figure 7a, only N-JG-ZnBC-T (500 °C/600 °C) achieved an adsorption rate exceeding 80%.
Figure 7b illustrates the relationship between pyrolysis temperature and adsorption efficiency, revealing that nitrogen-doped biochar derived from corn stalks exhibited the highest Cr
6+ adsorption rate of 81.09% at 600 °C, followed closely by an adsorption rate of 80.77% at 500 °C. Given that both rates are approximately 81% (AC40.5mg/g), the 500 °C condition is considered the optimal choice in terms of energy efficiency. The adsorption rate of both N-XZ-ZnBC and N-XK-ZnBC increased with pyrolysis temperature up to 500 °C, after which a decline was observed. Therefore, 500 °C was identified as the optimal pyrolysis temperature for the preparation of nitrogen-doped biochars N-XZ-ZnBC and N-XK-ZnBC. Among the three biomass feedstocks, nitrogen-doped biochar from corn stalks demonstrated the highest Cr
6+ removal efficiency, followed by apricot shells, while apricot branches exhibited the lowest performance.
3.5.2. Removal Efficiency of Pb2+
The concentration and absorbance of Pb
2+ before and after the adsorption experiments were measured, and the adsorption rate was calculated using equation
.
.
Figure 8 presents the statistical analysis of Pb
2+ removal efficiency for the twelve biochar samples.
As shown in
Figure 8a, eight nitrogen-doped biochar samples, including N-XK-ZnBC-T (300 °C/400 °C/500 °C/600 °C) and N-JG-ZnBC-T (300 °C/400 °C/500 °C/600 °C), exhibited consistently high Pb
2+ removal efficiency, with adsorption rates ranging from a minimum of 84.47% to a maximum of 91.61%(AC-45.81 mg/g). This result suggests that nitrogen-doped biochar derived from apricot shells and corn stalks serves as an effective adsorbent for Pb
2+ removal from aqueous solutions. Notably, these materials maintained high adsorption efficiency even at lower pyrolysis temperatures.
Figure 8b illustrates the effect of temperature on the adsorption performance of nitrogen-doped biochar derived from the three biomass feedstocks. The results indicate that, for all three feedstocks, biochar prepared at 500 °C achieved the highest adsorption rate. Among them, corn stalk-derived biochar exhibited the best Pb
2+ adsorption performance, followed by apricot shells, while apricot branches performed the worst. Additionally, nitrogen-doped biochar from apricot branches did not achieve an adsorption rate exceeding 80% under any pyrolysis temperature. In summary,
Figure 8 highlights that corn stalks and apricot shells are the most suitable biomass feedstocks for producing nitrogen-doped biochar with high Pb
2+ removal efficiency. Even at a low pyrolysis temperature of 300 °C, biochar derived from these materials demonstrated effective Pb
2+ adsorption performance.
Apricot shell, apricot branch, and corn stalk were selected as raw materials to prepare biochar. The adsorption performance of biochar varies depending on the raw material [
34]. A comparison of these results indicates that biochar derived from apricot shell (XK-N-ZnBC) and corn stalk (JG-N-ZnBC) consistently demonstrated high Pb
2+ adsorption rates (>84.47%) across all temperatures (300–600 °C), with similar overall performance for both raw materials. In contrast, biochar derived from apricot branch (XZ-N-ZnBC) showed significantly lower Pb
2+ adsorption rates, with a maximum value of 77.76%.
For Cr6+ adsorption, only 600 °C-JG-N-ZnBC and 500 °C-JG-N-ZnBC exhibited relatively high adsorption rates of 81.09% and 80.77%, respectively. Biochars derived from apricot shell and apricot branch under all temperature conditions exhibited comparatively low Cr6+ adsorption rates. In summary, the selection of raw materials for biochar preparation should consider both the type of target ion and the availability of agricultural residues in different regions. For Cr6+ adsorption, corn stalk is recommended as the preferred raw material. For Pb2+ adsorption, regions like Chengde that produce abundant apricots can utilize apricot shells, while regions like Northeast China, rich in corn production, can opt for corn stalks as a biomass source.
3.6. Effect of Adsorption Time on Adsorption Performance
To explore the adsorption rates at various time intervals, nitrogen-doped biochar prepared from three biomass feedstocks was tested at four time points: 6, 12, 18, and 24 h. Taking the adsorption of lead ions by biochar prepared at 500 °C as an example, the adsorption rates for apricot shell biochar were 33.02%, 56.79%, 81.32%, and 89.51% at the respective time intervals. For apricot branch biochar, the adsorption rates were 20.55%, 65.68%, 75.30%, and 77.76%. Corn stalk biochar exhibited adsorption rates of 48.06%, 77.96%, 90.06%, and 91.61% over the same time periods. The line chart illustrating the adsorption rates over time is shown in
Figure 9.
Based on
Figure 9, it can be observed that the adsorption rates of biochar derived from the three biomass feedstocks increased rapidly during the 6–12 h interval and then gradually approached equilibrium. Specifically, the adsorption rate for apricot shell biochar exhibited minimal growth between 18–24 h, reaching equilibrium after 18 h. For apricot branch biochar, the growth rate began to decrease after 12 h, achieving equilibrium within 24 h. Corn stalk biochar demonstrated a growth of less than 2% between 18–24 h, indicating that equilibrium was already achieved during this period. These findings confirm that the selected 24-h adsorption equilibrium time for the biochars is scientifically valid and reliable.
4. Conclusions
Nitrogen-doped biochar was successfully synthesized and evaluated for its adsorption performance toward Pb2+ and Cr6+. The nitrogen-doped biochar exhibited significantly higher adsorption efficiency for Pb2+ compared to Cr6+. FT-IR analysis indicated the introduction of -NH2 groups in the nitrogen-doped biochar, which can interact with heavy metals. The adsorption process involves both physical and chemical adsorption, the latter enhancing the overall adsorption capacity.
SEM images revealed the presence of numerous pores of varying sizes and shapes on the biochar surface. Post-adsorption, these pores were found to contain a substantial number of particles, confirming their role in the physical adsorption of heavy metal ions, thus improving adsorption performance. Among the eight nitrogen-doped biochars derived from apricot shells and corn stalks (activated at 300–600 °C), the materials showed high adsorption efficiency for Pb
2+, with adsorption rates exceeding 84.47%. The highest adsorption rates were observed for 500 °C-XK-N-ZnBC (89.51%) and 500 °C-JG-N-ZnBC (91.61%). The obtained adsorption capacity (45.81 mg/g) is significantly higher than that of recently reported modified coconut shell-derived carbon for Pb
2+ removal (11.9 mg/g) [
7].
For Cr
6+ adsorption, only two biochars—600 °C-JG-N-ZnBC (81.09%) and 500 °C-JG-N-ZnBC (80.77%)—demonstrated relatively high efficiencies. The adsorption capacity achieved in this study (40.5 mg/g) is substantially higher than those reported for Cr
6+ removal using modified coconut shell biochar (22.1 mg/g) [
7] and nitrogen-doped coconut shell activated carbon (15.15 mg/g) [
8], respectively.
For Cr6+ adsorption, only two biochars—600 °C-JG-N-ZnBC (81.09%) and 500 °C-JG-N-ZnBC (80.77%)—demonstrated relatively high efficiencies. Future studies should systematically investigate the influence of pH on Cr6+ adsorption. Additionally, improvements in testing methods for equilibrium adsorption capacity and adsorption rate could further refine the findings. While this study primarily focused on the performance evaluation of nitrogen-doped biochars for the removal of Cr6+ and Pb2+ from aqueous solutions, future work should include a techno-economic assessment to determine the feasibility of a large-scale application. The utilization of biomass waste not only adds value to agricultural residues but also contributes to environmental sustainability. Moreover, the incorporation of nitrogen into the biochar structure offers potential agronomic benefits when applied to soil, such as acting as a slow-release nutrient and reducing the risk of secondary pollution. Therefore, evaluating both the environmental and economic implications of this approach is essential to support its practical deployment in water and soil remediation systems.