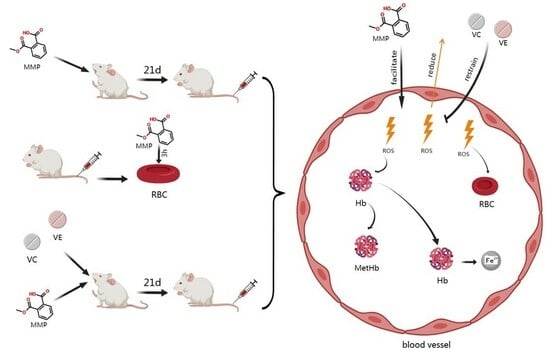
Metabolite Monomethyl Phthalate (MMP) Induces Oxidative Damage in Rat Erythrocytes: Role of Vitamins C and E
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. In Vivo Exposure Experiments
2.3. In Vitro Toxicity Experiments
2.4. Blood Indicator Tests
2.5. Detection of Oxidative Stress Indicators
2.6. Statistics and Data Analysis
3. Results and Discussion
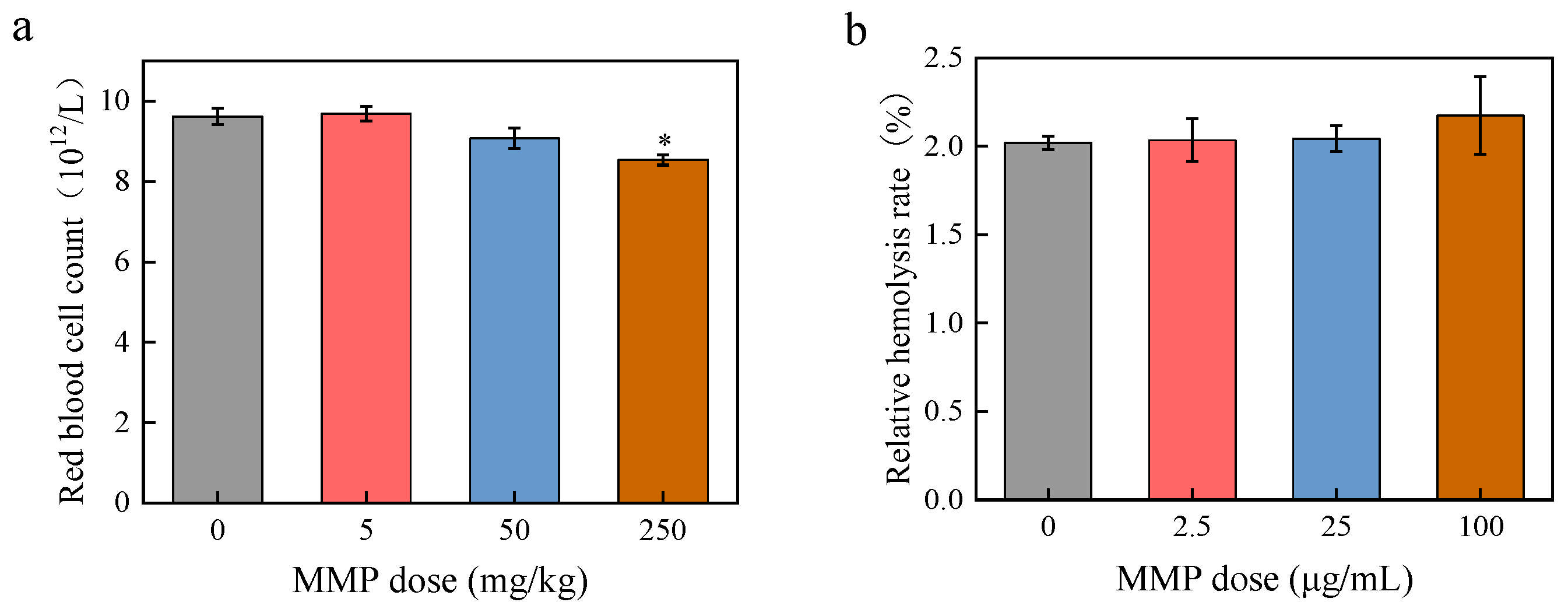
3.1. Toxic Effects of MMP on Erythrocytes Regarding Blood Indexes
3.2. Exploring Toxic Effects of MMP on Erythrocytes Regarding Oxidative Stress
3.3. Intervention with Antioxidants Against Toxic Effects of MMP on Erythrocytes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PAEs | Phthalate esters |
| DMP | Dimethyl phthalate |
| MMP | Monomethyl phthalate |
| BSA | Bovine serum albumin |
| RBCs | Red blood cells |
| SD rats | Sprague–Dawley rats |
| Fe2+ | Ferrous ions |
| Fe3+ | Ferric ions |
| VC | Vitamin C |
| VE | Vitamin E |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| MDA | Malondialdehyde |
| SD | Standard deviation |
| ROS | Reactive oxygen species |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| GPX | Glutathione peroxidase |
| NO | Nitric oxide |
References
- Gao, D.W.; Wen, Z.D. Phthalate esters in the environment: A critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Sci. Total Environ. 2016, 541, 986–1001. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.X.; Mo, C.H.; Zhao, H.M.; Xiang, L.; Katsoyiannis, A.; Li, Y.W.; Cai, Q.Y.; Wong, M.H. Soil contamination and sources of phthalates and its health risk in China: A review. Environ. Res. 2018, 164, 417–429. [Google Scholar] [CrossRef]
- Mariani, M.B.; Giannetti, V.; Mannino, P.; Ceccarelli, V. Enhanced Quality Control of Recycled Paperboard for Food Packaging. Anal. Lett. 2015, 48, 1720–1737. [Google Scholar] [CrossRef]
- Pivnenko, K.; Eriksen, M.K.; Martin-Fernandez, J.A.; Eriksson, E.; Astrup, T.F. Recycling of plastic waste: Presence of phthalates in plastics from households and industry. Waste Manag. 2016, 54, 44–52. [Google Scholar] [CrossRef]
- Net, S.; Sempéré, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, Fate, Behavior and Ecotoxicological State of Phthalates in Different Environmental Matrices. Environ. Sci. Technol. 2015, 49, 4019–4035. [Google Scholar] [CrossRef]
- Net, S.; Rabodonirina, S.; Ben Sghaier, R.; Dumoulin, D.; Chbib, C.; Tlili, I.; Ouddane, B. Distribution of phthalates, pesticides and drug residues in the dissolved, particulate and sedimentary phases from transboundary rivers (France-Belgium). Sci. Total Environ. 2015, 521, 152–159. [Google Scholar] [CrossRef]
- Selvaraj, K.K.; Sundaramoorthy, G.; Ravichandran, P.K.; Girijan, G.K.; Sampath, S.; Ramaswamy, B.R. Phthalate esters in water and sediments of the Kaveri River, India: Environmental levels and ecotoxicological evaluations. Environ. Geochem. Health 2015, 37, 83–96. [Google Scholar] [CrossRef]
- Wang, X.K.; Tao, W.; Xu, Y.; Feng, J.T.; Wang, F.H. Indoor phthalate concentration and exposure in residential and office buildings in Xi’an, China. Atmos. Environ. 2014, 87, 146–152. [Google Scholar] [CrossRef]
- Ma, T.T.; Christie, P.; Luo, Y.M.; Teng, Y. Phthalate esters contamination in soil and plants on agricultural land near an electronic waste recycling site. Environ. Geochem. Health 2013, 35, 465–476. [Google Scholar] [CrossRef]
- Li, B.; Liu, R.; Gao, H.; Tan, R.; Zeng, P.; Song, Y. Spatial distribution and ecological risk assessment of phthalic acid esters and phenols in surface sediment from urban rivers in Northeast China. Environ. Pollut. 2016, 219, 409–415. [Google Scholar] [CrossRef]
- Fierens, T.; Van Holderbeke, M.; Willems, H.; De Henauw, S.; Sioen, I. Transfer of eight phthalates through the milk chain—A case study. Environ. Int. 2013, 51, 1–7. [Google Scholar] [CrossRef]
- Silva, M.J.; Barr, D.B.; Reidy, J.A.; Kato, K.; Malek, N.A.; Hodge, C.C.; Hurtz, D.; Calafat, A.M.; Needham, L.L.; Brock, J.W. Glucuronidation patterns of common urinary and serum monoester phthalate metabolites. Arch. Toxicol. 2003, 77, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Bloom, M.S.; Valachovic, E.L.; Begum, T.F.; Kucklick, J.R.; Brock, J.W.; Wenzel, A.G.; Wineland, R.J.; Cruze, L.; Unal, E.R.; Newman, R.B. Association between gestational phthalate exposure and newborn head circumference; impacts by race and sex. Environ. Res. 2021, 195, 110763. [Google Scholar] [CrossRef] [PubMed]
- Wineland, R.J.; Bloom, M.S.; Cruze, L.; Butts, C.D.; Wenzel, A.G.; Unal, E.R.; Kohno, S.; Willan, K.B.; Brock, J.W.; Newman, R.B. In utero effects of maternal phthalate exposure on male genital development. Prenat. Diagn. 2019, 39, 209–218. [Google Scholar] [CrossRef]
- Suzuki, T.; Yaguchi, K.; Suzuki, S.; Suga, T. Monitoring of phthalic acid monoesters in river water by solid-phase extraction and GC-MS determination. Environ. Sci. Technol. 2001, 35, 3757–3763. [Google Scholar] [CrossRef]
- Ye, D.M.; Yang, H.; Xu, T.T.; Lin, Z.Z.; Zhang, Y.J.; Liu, L.Y.; Guo, Y. Underlying Degradation of Phthalates via Microbials in Dust from Different Microenvironments. Environ. Sci. Technol. 2023, 57, 9744–9753. [Google Scholar] [CrossRef]
- Blair, J.D.; Ikonomou, M.G.; Kelly, B.C.; Surridge, B.; Gobas, F. Ultra-Trace Determination of Phthalate Ester Metabolites in Seawater, Sediments, and Biota from an Urbanized Marine Inlet by LC/ESI-MS/MS. Environ. Sci. Technol. 2009, 43, 6262–6268. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, H.; Gu, J.; Jiao, Y.; Han, S.; Akindolie, M.S.; Wang, Y.; Zhang, L.; Tao, Y. Anthraquinone-2,6-disulfonate enhanced biodegradation of dibutyl phthalate: Reducing membrane damage and oxidative stress in bacterial degradation. Bioresour. Technol. 2020, 302, 122845. [Google Scholar] [CrossRef]
- Wang, Y.X.; Liu, C.; Chen, Y.J.; Chen, H.G.; Yang, P.; Wang, P.; Huang, L.L.; Ai, S.H.; Duan, P.; Pan, A.; et al. Predictors and correlations of phthalate metabolite concentrations in urine and seminal plasma among reproductive-aged men. Environ. Res. 2018, 161, 336–344. [Google Scholar] [CrossRef]
- Olsén, L.; Lampa, E.; Birkholz, D.A.; Lind, L.; Lind, P.M. Circulating levels of bisphenol A (BPA) and phthalates in an elderly population in Sweden, based on the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS). Ecotoxicol. Environ. Saf. 2012, 75, 242–248. [Google Scholar] [CrossRef]
- Chen, G.; Huang, W.; Li, H.; Huang, W. Phthalate exposure during pregnancy and its relationship with birth outcomes in Guangzhou. J. Environ. Occup. Med. 2021, 38, 573–579. [Google Scholar] [CrossRef]
- You, L.; Wang, Y.X.; Zeng, Q.; Li, M.; Huang, Y.H.; Hu, Y.; Cao, W.C.; Liu, A.L.; Lu, W.Q. Semen Phthalate Metabolites, Spermatozoa Apoptosis, and DNA Damage: A Cross-Sectional Study in China. Environ. Sci. Technol. 2015, 49, 3805–3812. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.A.; Asimakopoulos, A.G.; Barbosa, F.; Kannan, K. Urinary concentrations of 25 phthalate metabolites in Brazilian children and their association with oxidative DNA damage. Sci. Total Environ. 2017, 586, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.L.; Zhao, H.; Liu, L.; Yang, D.; Chen, H.; Sun, T. Investigation of the binding interactions between dimethyl phthalate and its metabolite with bovine serum albumin by multispectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117771. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, L.M.; Cheng, H.; Huang, S.Z.; Feng, X.M.; Huang, L.L.; Wei, L.Y.; Cao, D.H.; Wang, S.D.; Tian, L.; et al. Gender-specific effects of prenatal mixed exposure to serum phthalates on neurodevelopment of children aged 2-3 years:the Guangxi Birth Cohort Study. Environ. Sci. Pollut. Res. 2022, 29, 85547–85558. [Google Scholar] [CrossRef]
- Huang, P.C.; Chang, W.H.; Wu, M.T.; Chen, M.L.; Wang, I.J.; Shih, S.F.; Hsiung, C.A.; Liao, K.W. Characterization of phthalate exposure in relation to serum thyroid and growth hormones, and estimated daily intake levels in children exposed to phthalate-tainted products: A longitudinal cohort study. Environ. Pollut. 2020, 264, 114648. [Google Scholar] [CrossRef]
- Deng, T.R.; Du, Y.Y.; Wang, Y.X.; Teng, X.M.; Hua, X.; Yuan, X.Q.; Yao, Y.C.; Guo, N.; Li, Y.F. The associations of urinary phthalate metabolites with the intermediate and pregnancy outcomes of women receiving IVF/ICSI treatments: A prospective single-center study. Ecotoxicol. Environ. Saf. 2020, 188, 109884. [Google Scholar] [CrossRef]
- Oliveira Pereira, E.A.; Labine, L.M.; Kleywegt, S.; Jobst, K.J.; Simpson, A.J.; Simpson, M.J. Daphnia magna sub-lethal exposure to phthalate pollutants elicits disruptions in amino acid and energy metabolism. Aquat. Toxicol. 2023, 257, 106432. [Google Scholar] [CrossRef]
- Mathieu-Denoncourt, J.; Martyniuk, C.J.; Loughery, J.R.; Yargeau, V.; de Solla, S.R.; Langlois, V.S. Lethal and sublethal effects of phthalate diesters in Silurana tropicalis larvae. Environ. Toxicol. Chem. 2016, 35, 2511–2522. [Google Scholar] [CrossRef]
- Kuhn, V.; Diederich, L.; Keller, T.C.S.; Kramer, C.M.; Lückstädt, W.; Panknin, C.; Suvorava, T.; Isakson, B.E.; Kelm, M.; Cortese-Krott, M.M. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxid. Redox Signal. 2017, 26, 718–742. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Gambardella, L.; Bozzuto, G.; Vona, R.; Caruso, D.; Villari, V.; Cappello, T.; Maisano, M.; Dossena, S.; et al. Internalization of nano- and micro-plastics in human erythrocytes leads to oxidative stress and estrogen receptor-mediated cellular responses. Free Radic. Biol. Med. 2024, 223, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rapa, M.; Darie-Nita, R.N.; Matei, E.; Predescu, A.M.; Berbecaru, A.C.; Predescu, C. Insights into Anthropogenic Micro- and Nanoplastic Accumulation in Drinking Water Sources and Their Potential Effects on Human Health. Polymers 2023, 15, 2425. [Google Scholar] [CrossRef] [PubMed]
- Niccolai, E.; Colzi, I.; Amedei, A. Adverse Effects of Micro- and Nanoplastics on Humans and the Environment. Int. J. Mol. Sci. 2023, 24, 15822. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.Q.; Li, M.R.; Xie, Y.J.; Guo, J.J. Investigating the Permeation Mechanism of Typical Phthalic Acid Esters (PAEs) and Membrane Response Using Molecular Dynamics Simulations. Membranes 2022, 12, 596. [Google Scholar] [CrossRef]
- Xu, G.; Xia, Y.; Xinwei, L.; Zhenxing, C. Effects of Monomethyl Phthalate on Blood Biochemical Indicators of Rats and Its Toxicology. Asian J. Ecotoxicol. 2022, 17, 389–399. [Google Scholar]
- Chi, Z.X.; Liu, R.T.; You, H.; Ma, S.S.; Cui, H.; Zhang, Q. Probing the In Vitro Cytotoxicity of the Veterinary Drug Oxytetracycline. PLoS ONE 2014, 9, e102334. [Google Scholar] [CrossRef]
- Kwack, S.J.; Kim, K.B.; Kim, H.S.; Lee, B.M. Comparative Toxicological Evaluation of Phthalate Diesters and Metabolites in Sprague-Dawley Male Rats for Risk Assessment. J. Toxicol. Environ. Health Part A Curr. Issues 2009, 72, 1446–1454. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem.-Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Jang, H.; Song, G.; Lim, W.; Park, S. Toxic effects of dibutyl phthalate on trophoblast through mitochondria mediated cellular dysfunction. Toxicol. Appl. Pharmacol. 2025, 495, 117186. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Wu, B.N.; Chen, Q.Y.; Pan, J.R.; Wang, Z.Y.; Wang, W. Di-butyl phthalate induces apoptosis in Ctenopharyngodon idellus kidney cells through oxidative stress injury. Fish Shellfish Immunol. 2025, 160, 110207. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Liu, M.T.; Zhao, J.H.; Hu, P.; Gao, L.B.; Tian, S.; Zhang, J.; Liu, H.Y.; Xu, X.X.; He, Z.W. Oxidative stress and dysregulated long noncoding RNAs in the pathogenesis of Parkinson’s disease. Biol. Res. 2025, 58, 7. [Google Scholar] [CrossRef]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef]
- Dong, R.H.; Chen, J.S.; Zheng, J.H.; Zhang, M.R.; Zhang, H.; Wu, M.; Li, S.G.; Chen, B. The role of oxidative stress in cardiometabolic risk related to phthalate exposure in elderly diabetic patients from Shanghai. Environ. Int. 2018, 121, 340–348. [Google Scholar] [CrossRef]
- Babadi, R.S.; Riederer, A.M.; Sampson, P.D.; Sathyanarayana, S.; Kavanagh, T.J.; Krenz, J.E.; Andra, S.S.; Kim-Schulze, S.; Jansen, K.L.; Torres, E.; et al. Associations between repeated measures of urinary phthalate metabolites and biomarkers of oxidative stress in a rural agricultural cohort of children with asthma. Sci. Total Environ. 2022, 848, 157493. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, Y.Q.; Li, Z.X.; Tao, Y.; Yang, Y. Hazards of phthalates (PAEs) exposure: A review of aquatic animal toxicology studies. Sci. Total Environ. 2021, 771, 145418. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Cui, J.G.; Li, X.N.; Li, J.L. DEHP-induced mitophagy and mitochondrial damage in the heart are associated with dysregulated mitochondrial biogenesis. Food Chem. Toxicol. 2022, 161, 112818. [Google Scholar] [CrossRef]
- Macczak, A.; Cyrkler, M.; Bukowska, B.; Michalowicz, J. Bisphenol A, bisphenol S, bisphenol F and bisphenol AF induce different oxidative stress and damage in human red blood cells (in vitro study). Toxicol. Vitr. 2017, 41, 143–149. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Biomarkers of oxidative stress in red blood cells. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2011, 155, 131–136. [Google Scholar] [CrossRef]
- Flohe, L.; Toppo, S.; Orian, L. The glutathione peroxidase family: Discoveries and mechanism. Free Radic. Biol. Med. 2022, 187, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.X.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Yamamoto, A.; Murota, K.; Tsujiuchi, T.; Iwamori, M.; Fukushima, N. Polyunsaturated fatty acids induce ovarian cancer cell death through ROS-dependent MAP kinase activation. Biochem. Biophys. Res. Commun. 2017, 493, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Gawel, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. (Wars. Pol. 1960) 2004, 57, 453–455. [Google Scholar]
- Gordon, D.S.; Rudinsky, A.J.; Guillaumin, J.; Parker, V.J.; Creighton, K.J. Vitamin C in Health and Disease: A Companion Animal Focus. Top. Companion Anim. Med. 2020, 39, 100432. [Google Scholar] [CrossRef]
- Sun, L.M.; Ye, X.L.; Ding, D.F.; Kai, L. Opposite Effects of Vitamin C and Vitamin E on the Antifungal Activity of Honokiol. J. Microbiol. Biotechnol. 2019, 29, 538–547. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Shatnawi, A.F.; Al-Qudah, M.A.; Alfaqih, M.A. Vitamin C attenuates memory loss induced by post-traumatic stress like behavior in a rat model. Behav. Brain Res. 2020, 379, 112350. [Google Scholar] [CrossRef]
- Alpsoy, L.; Yalvac, M.E. Key Roles of Vitamins A, C, and E in Aflatoxin B1-Induced Oxidative Stress. Vitam. Immune Syst. 2011, 86, 287–305. [Google Scholar]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef]
- Davitashvili, D.T.; Museridze, D.P.; Svanidze, I.K.; Pavliashvili, N.S.; Sanikidze, T.V. Correction of oxidative stress in the rat brain cortical cellular culture with vitamines E and C. Georgian Med. News 2010, 180, 56–60. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Gao, X.; Chi, Z. Metabolite Monomethyl Phthalate (MMP) Induces Oxidative Damage in Rat Erythrocytes: Role of Vitamins C and E. Toxics 2025, 13, 379. https://doi.org/10.3390/toxics13050379
Zhang X, Gao X, Chi Z. Metabolite Monomethyl Phthalate (MMP) Induces Oxidative Damage in Rat Erythrocytes: Role of Vitamins C and E. Toxics. 2025; 13(5):379. https://doi.org/10.3390/toxics13050379
Chicago/Turabian StyleZhang, Xuxin, Xu Gao, and Zhenxing Chi. 2025. "Metabolite Monomethyl Phthalate (MMP) Induces Oxidative Damage in Rat Erythrocytes: Role of Vitamins C and E" Toxics 13, no. 5: 379. https://doi.org/10.3390/toxics13050379
APA StyleZhang, X., Gao, X., & Chi, Z. (2025). Metabolite Monomethyl Phthalate (MMP) Induces Oxidative Damage in Rat Erythrocytes: Role of Vitamins C and E. Toxics, 13(5), 379. https://doi.org/10.3390/toxics13050379