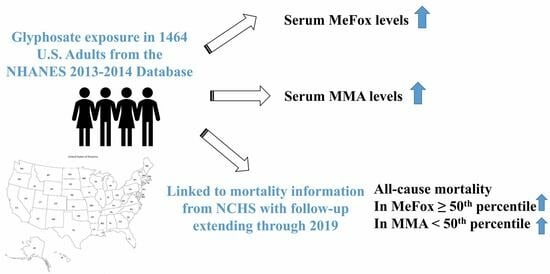
Glyphosate Exposure, Oxidative Stress, Mitochondrial Dysfunction, and Mortality Risk in US Adults: Insights from the National Health and Nutrition Examination Survey
Abstract
1. Introduction
2. Materials and Methods
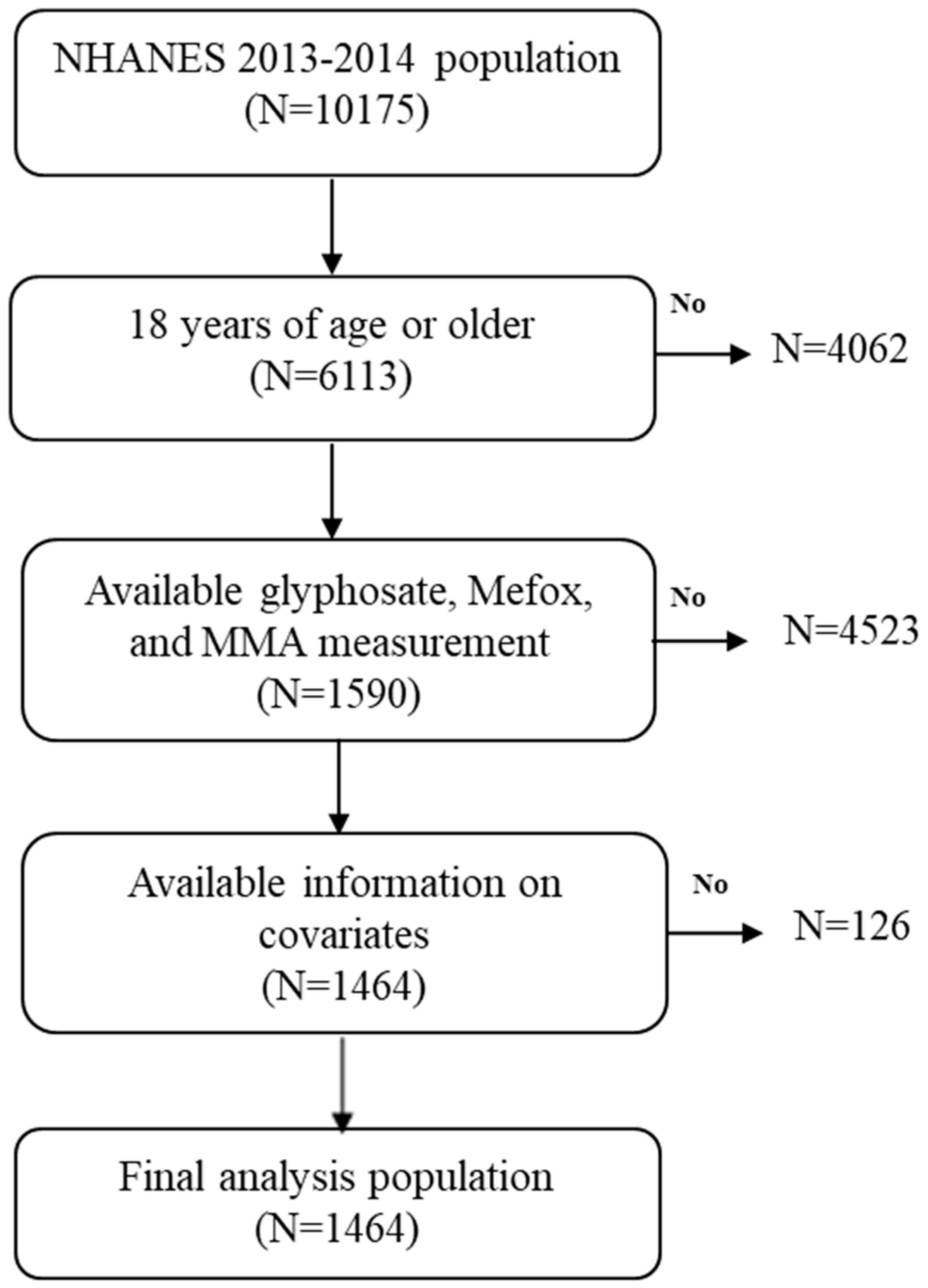
2.1. Study Population
2.2. Measurement of Urinary Glyphosate Levels
2.3. Measurement of Serum MeFox
2.4. Measurement of Serum MMA
2.5. Covariates
2.6. Outcomes
2.7. Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lacroix, R.; Kurrasch, D.M. Glyphosate toxicity: In vivo, in vitro, and epidemiological evidence. Toxicol. Sci. 2023, 192, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest. Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef]
- Rivas-Garcia, T.; Espinosa-Calderón, A.; Hernández-Vázquez, B.; Schwentesius-Rindermann, R. Overview of Environmental and Health Effects Related to Glyphosate Usage. Sustainability 2022, 14, 6868. [Google Scholar] [CrossRef]
- Hsiao, C.C.; Yang, A.M.; Wang, C.; Lin, C.Y. Association between glyphosate exposure and cognitive function, depression, and neurological diseases in a representative sample of US adults: NHANES 2013-2014 analysis. Environ. Res. 2023, 237, 116860. [Google Scholar] [CrossRef]
- Qi, X.; Huang, Q.; Chen, X.; Qiu, L.; Wang, S.; Ouyang, K.; Chen, Y. Associations between urinary glyphosate and diabetes mellitus in the US general adult: A cross-sectional study from NHANES 2013-2016. Environ. Sci. Pollut. Res. Int. 2023, 30, 124195–124203. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.H.; Chu, P.L.; Wang, C.; Lin, C.Y. Association between Glyphosate Exposure and Erythrograms in a Representative Sample of US Adults: NHANES 2013-2014. Environ. Sci. Pollut. Res. Int. 2023, 30, 91207–91215. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.L.; Wang, C.S.; Wang, C.; Lin, C.Y. Association of urinary glyphosate levels with iron homeostasis among a representative sample of US adults: NHANES 2013-2018. Ecotoxicol. Environ. Saf. 2024, 284, 116962. [Google Scholar] [CrossRef]
- Untalan, M.; Ivic-Pavlicic, T.; Taioli, E. Urinary glyphosate levels and association with mortality in the 2013-16 National Health and Nutrition Examination Survey. Carcinogenesis 2024, 45, 163–169. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Huras, B.; Bukowska, B. The effect of metabolites and impurities of glyphosate on human erythrocytes (in vitro). Pestic. Biochem. Physiol. 2014, 109, 34–43. [Google Scholar] [CrossRef]
- Bali, Y.A.; Kaikai, N.E.; Ba-M’hamed, S.; Bennis, M. Learning and memory impairments associated to acetylcholinesterase inhibition and oxidative stress following glyphosate based-herbicide exposure in mice. Toxicology 2019, 415, 18–25. [Google Scholar] [CrossRef]
- Arrigo, E.; Gilardi, S.; Muratori, L.; Raimondo, S.; Mancardi, D. Biological effects of sub-lethal doses of glyphosate and AMPA on cardiac myoblasts. Front. Physiol. 2023, 14, 1165868. [Google Scholar] [CrossRef] [PubMed]
- Chianese, T.; Trinchese, G.; Leandri, R.; De Falco, M.; Mollica, M.; Scudiero, R.; Rosati, L. Glyphosate Exposure Induces Cytotoxicity, Mitochondrial Dysfunction and Activation of ERα and ERβ Estrogen Receptors in Human Prostate PNT1A Cells. Int. J. Mol. Sci. 2024, 25, 7039. [Google Scholar] [CrossRef]
- Pereira, A.G.; Jaramillo, M.L.; Remor, A.P.; Latini, A.; Davico, C.E.; da Silva, M.O.L.; Müller, Y.M.R.; Ammar, D.; Nazari, E.M. Low-concentration exposure to glyphosate-based herbicide modulates the complexes of the mitochondrial respiratory chain and induces mitochondrial hyperpolarization in the Danio rerio brain. Chemosphere 2018, 209, 353–362. [Google Scholar] [CrossRef]
- Strilbyska, O.M.; Tsiumpala, S.; Kozachyshyn, I.I.; Strutynska, T.R.; Burdyliuk, N.I.; Lushchak, V.I.; Lushchak, O. The effects of low-toxic herbicide Roundup and glyphosate on mitochondria. EXCLI J. 2022, 21, 183–196. [Google Scholar] [CrossRef]
- Sidthilaw, S.; Sapbamrer, R.; Pothirat, C.; Wunnapuk, K.; Khacha-ananda, S. Effects of exposure to glyphosate on oxidative stress, inflammation, and lung function in maize farmers, Northern Thailand. BMC Public Health 2022, 22, 1343. [Google Scholar] [CrossRef] [PubMed]
- Chang, V.C.; Andreotti, G.; Ospina, M.; Parks, C.G.; Liu, D.; Shearer, J.J.; Rothman, N.; Silverman, D.T.; Sandler, D.P.; Calafat, A.M.; et al. Glyphosate exposure and urinary oxidative stress biomarkers in the Agricultural Health Study. J. Natl. Cancer Inst. 2023, 115, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Eaton, J.L.; Cathey, A.L.; Fernandez, J.A.; Watkins, D.J.; Silver, M.K.; Milne, G.L.; Velez-Vega, C.; Rosario, Z.; Cordero, J.; Alshawabkeh, A.; et al. The association between urinary glyphosate and aminomethyl phosphonic acid with biomarkers of oxidative stress among pregnant women in the PROTECT birth cohort study. Ecotoxicol. Environ. Saf. 2022, 233, 113300. [Google Scholar] [CrossRef]
- Vasseur, C.; Serra, L.; El Balkhi, S.; Lefort, G.; Ramé, C.; Froment, P.; Dupont, J. Glyphosate presence in human sperm: First report and positive correlation with oxidative stress in an infertile French population. Ecotoxicol. Environ. Saf. 2024, 278, 116410. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Zhang, X.; Cai, H.; Liu, J.; Fang, S.; Yu, B. The Regulation and Characterization of Mitochondrial-Derived Methylmalonic Acid in Mitochondrial Dysfunction and Oxidative Stress: From Basic Research to Clinical Practice. Oxid. Med. Cell Longev. 2022, 2022, 7043883. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Chang, C.M.; Yu, C.C.; Lu, H.T.; Chou, Y.F.; Huang, R.F. Folate deprivation promotes mitochondrial oxidative decay: DNA large deletions, cytochrome c oxidase dysfunction, membrane depolarization and superoxide overproduction in rat liver. Br. J. Nutr. 2007, 97, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Ghanavati, M.; Ashtary-Larky, D.; Bagheri, R.; Rezaei Kelishadi, M.; Nazarian, B.; Nordvall, M.; Wong, A.; Dutheil, F.; Suzuki, K.; et al. Effects of Folic Acid Supplementation on Oxidative Stress Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antioxidants 2021, 10, 871. [Google Scholar] [CrossRef]
- Xiu, Y.; Field, M.S. The Roles of Mitochondrial Folate Metabolism in Supporting Mitochondrial DNA Synthesis, Oxidative Phosphorylation, and Cellular Function. Curr. Dev. Nutr. 2020, 4, nzaa153. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, L.; Guan, S.; Jiang, F.; He, D.; Song, H.; Sun, W.; Jiang, S.; Tian, F. Association between serum methylmalonic acid and chronic kidney disease in adults: A cross-sectional study from NHANES 2013–2014. Front Endocrinol 2024, 15, 1434299. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, R.; Torkki, P.; Zheng, W.; Chen, A. Hypertension may lead to cognitive dysfunction in older adults via methylmalonic acid: Evidence from NHANES 2011–2014 population. BMC Geriatr. 2024, 24, 1009. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ding, L.; Tao, M.; Zhu, Y. The association of metabolic profile of folate with diabetic kidney disease: Evidence from 2011–2020 cycles of the NHANES. Ren. Fail. 2024, 46, 2420830. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, C.; Ye, Z.; Liu, M.; Zhang, Z.; He, P.; Zhang, Y.; Li, H.; Liu, C.; Qin, X. Relationship of several serum folate forms with the prevalence of hypertension. Precis. Nutr. 2023, 2, e00058. [Google Scholar] [CrossRef]
- CDC. NHANES 2013–2014. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2013 (accessed on 18 January 2025).
- CDC. 2013–2014 Data Documentation, Codebook, and Frequencies: Glyphosate (GLYP). Available online: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2013/DataFiles/SSGLYP_H.htm (accessed on 28 January 2025).
- CDC. 2013–2014 Data Documentation, Codebook, and Frequencies: Folate Forms—Total & Individual—Serum. Available online: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2013/DataFiles/FOLFMS_H.htm (accessed on 28 January 2025).
- CDC. 2013–2014 Data Documentation, Codebook, and Frequencies: Methylmalonic Acid. Available online: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2013/DataFiles/MMA_H.htm (accessed on 28 January 2025).
- Wang, J.; Wu, Y.; Ning, F.; Zhang, C.; Zhang, D. The Association between Leisure-Time Physical Activity and Risk of Undetected Prediabetes. J. Diabetes Res. 2017, 2017, 4845108. [Google Scholar] [CrossRef]
- Yan, M.T.; Chao, C.T.; Lin, S.H. Chronic Kidney Disease: Strategies to Retard Progression. Int. J. Mol. Sci. 2021, 22, 10084. [Google Scholar] [CrossRef]
- CDC. 2013–2014 Data Documentation, Codebook, and Frequencies: Vitamin B12 (VITB12_H). Available online: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2013/DataFiles/VITB12_H.htm (accessed on 28 January 2025).
- NCHS. NCHS Data Linkage: 2019 Public-Use Linked Mortality Files. Available online: https://www.cdc.gov/nchs/data-linkage/mortality-public.htm (accessed on 4 January 2025).
- CDC. National Health and Nutrition Examination Survey: Analytic Guidelines, 2011–2014 and 2015–2016. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/analyticguidelines/11-16-analytic-guidelines.pdf (accessed on 3 January 2025).
- Rubini, M.; Di Minno, G.; Ferrazzi, E. Is there a multidisciplinary role for 5-methyltetrahydrofolate? The obstetric evidence in perspective. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 3934–3945. [Google Scholar] [CrossRef]
- Ringling, C.; Rychlik, M. Simulation of Food Folate Digestion and Bioavailability of an Oxidation Product of 5-Methyltetrahydrofolate. Nutrients 2017, 9, 969. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhou, C.; Zhang, Z.; Li, Q.; He, P.; Zhang, Y.; Li, H.; Liu, C.; Fan Hou, F.; Qin, X. Relationship of several serum folate forms with kidney function and albuminuria: Cross-sectional data from the National Health and Nutrition Examination Surveys (NHANES) 2011–2018. Br. J. Nutr. 2022, 127, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Z.; Zhou, C.; Li, Q.; He, P.; Zhang, Y.; Li, H.; Liu, C.; Liang, M.; Wang, X.; et al. Relationship of several serum folate forms with the risk of mortality: A prospective cohort study. Clin. Nutr. 2021, 40, 4255–4262. [Google Scholar] [CrossRef]
- Takahashi-Iñiguez, T.; García-Hernandez, E.; Arreguín-Espinosa, R.; Flores, M.E. Role of vitamin B12 on methylmalonyl-CoA mutase activity. J. Zhejiang Univ. Sci. B 2012, 13, 423–437. [Google Scholar] [CrossRef]
- Chandler, R.J.; Zerfas, P.M.; Shanske, S.; Sloan, J.; Hoffmann, V.; DiMauro, S.; Venditti, C.P. Mitochondrial dysfunction in mut methylmalonic acidemia. Faseb J. 2009, 23, 1252–1261. [Google Scholar] [CrossRef]
- Luciani, A.; Schumann, A.; Berquez, M.; Chen, Z.; Nieri, D.; Failli, M.; Debaix, H.; Festa, B.P.; Tokonami, N.; Raimondi, A.; et al. Impaired mitophagy links mitochondrial disease to epithelial stress in methylmalonyl-CoA mutase deficiency. Nat. Commun. 2020, 11, 970. [Google Scholar] [CrossRef]
- Wu, L.; Chang, D.-Y.; Zhao, M.-H.; Tang, S.C.W.; Chen, M. Association between blood methylmalonic acid and chronic kidney disease in the general US population: Insights from multi-cycle National Health and Nutrition Examination Survey (NHANES). Ann. Transl. Med. 2024, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Liu, J.-B.; Wang, J.-Q.; Lian, C.-Y.; Wang, Z.-Y.; Wang, L. Glyphosate-induced mitochondrial reactive oxygen species overproduction activates parkin-dependent mitophagy to inhibit testosterone synthesis in mouse leydig cells. Environ. Pollut. 2022, 314, 120314. [Google Scholar] [CrossRef]
- Anifandis, G.; Amiridis, G.; Dafopoulos, K.; Daponte, A.; Dovolou, E.; Gavriil, E.; Gorgogietas, V.; Kachpani, E.; Mamuris, Z.; Messini, C.I.; et al. The In Vitro Impact of the Herbicide Roundup on Human Sperm Motility and Sperm Mitochondria. Toxics 2017, 6, 2. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Coelho-Junior, H.J.; Landi, F.; Bernabei, R.; Marzetti, E. Mitochondrial Dysfunction, Oxidative Stress, and Neuroinflammation: Intertwined Roads to Neurodegeneration. Antioxidants 2020, 9, 647. [Google Scholar] [CrossRef]
- Caballero, M.; Amiri, S.; Denney, J.T.; Monsivais, P.; Hystad, P.; Amram, O. Estimated Residential Exposure to Agricultural Chemicals and Premature Mortality by Parkinson’s Disease in Washington State. Int. J. Environ. Res. Public Health 2018, 15, 2885. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.A.; Maturano, E.; Etchegoyen, A.; Difilippo, F.S.; MacLean, B. Association between Cancer and Environmental Exposure to Glyphosate. Int. J. Clin. Med. 2017, 8, 73–85. [Google Scholar] [CrossRef]
- Chu, P.L.; Hsiao, C.C.; Su, T.C.; Wang, C.; Lin, C.Y. Urinary glyphosate, selenium status, and their impact on mortality: Evidence from NHANES 2013-2018. Ecotoxicol. Environ. Saf. 2025, 292, 117989. [Google Scholar] [CrossRef] [PubMed]
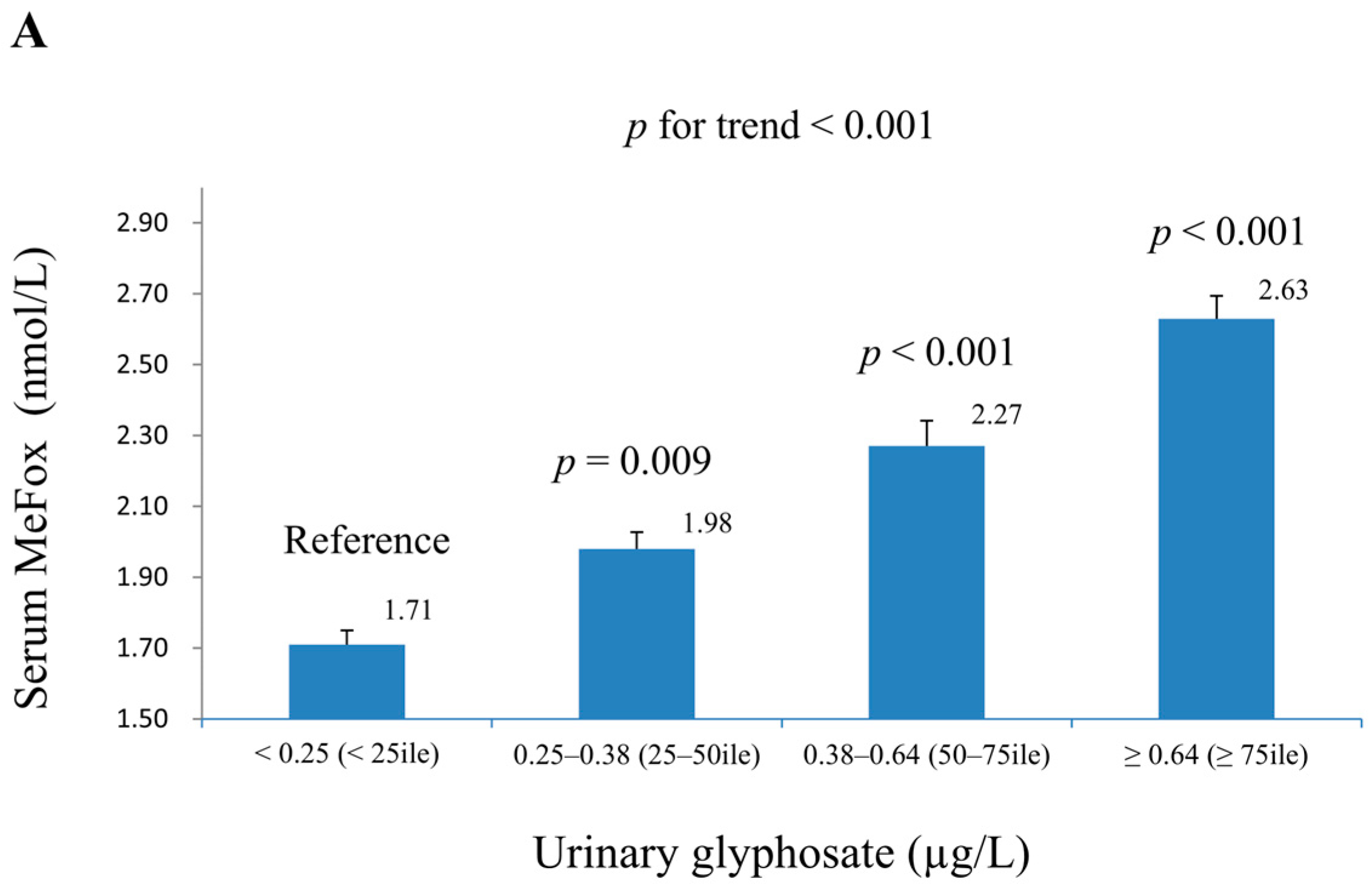
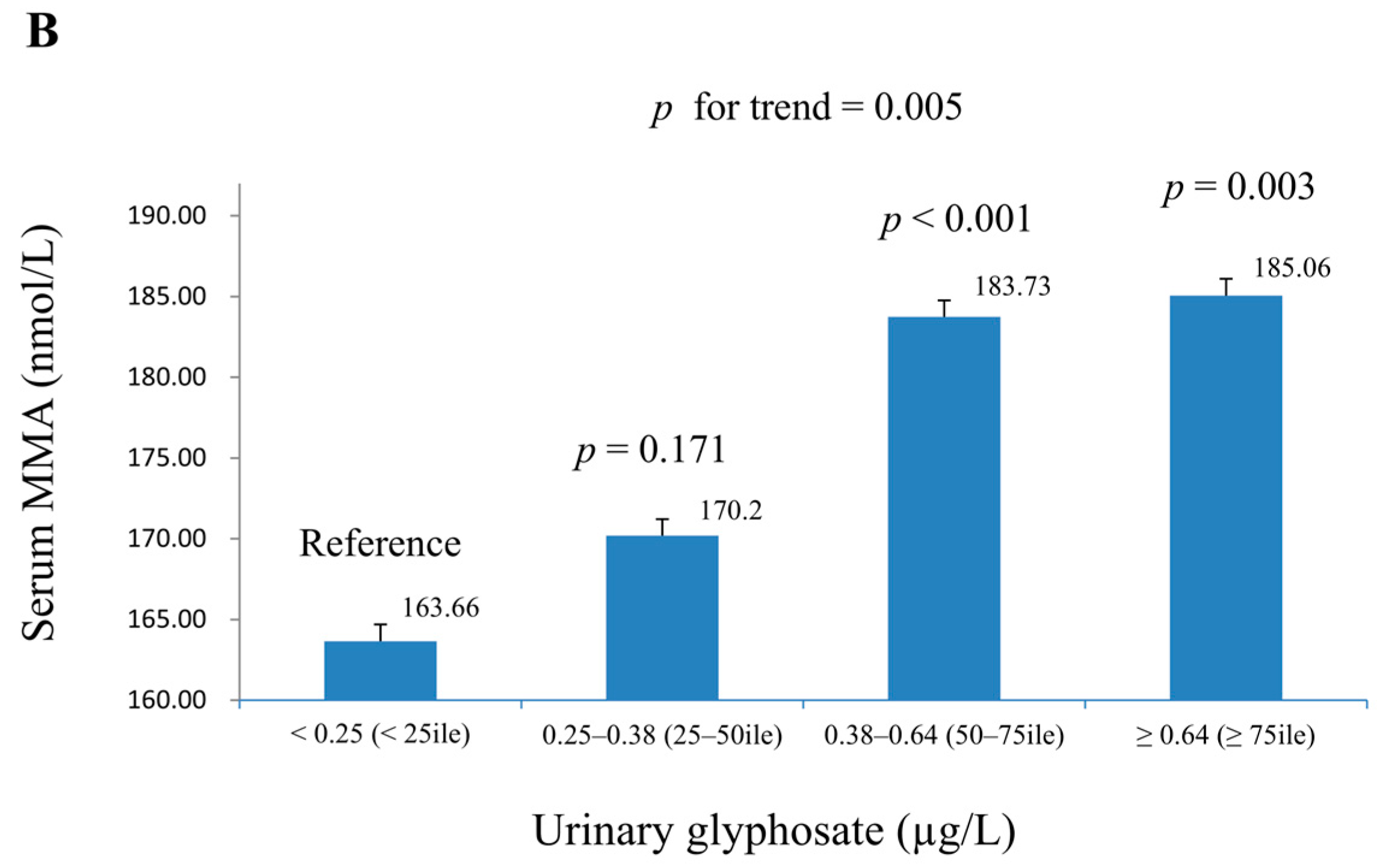
| Glyphosate (μg/g Creatinine) | MeFox (nmol/L) | MMA (nmol/L) | |||||
|---|---|---|---|---|---|---|---|
| N | Geometric Means (SE) | p Value | Geometric Means (SE) | p Value | Geometric Means (SE) | p Value | |
| Total | 1464 | 0.43 (1.01) | 1.61 (1.02) | 151.15 (1.01) | |||
| Sex | <0.001 | 0.155 | 0.942 | ||||
| Males | 707 | 0.37 (1.03) | 1.62 (1.02) | 153.96 (1.03) | |||
| Females | 757 | 0.48 (1.03) | 1.61 (1.02) | 148.58 (1.03) | |||
| Age (years) | <0.001 | <0.001 | <0.001 | ||||
| 19–39 | 498 | 0.35 (1.03) | 1.39 (1.03) | 129.76 (1.02) | |||
| 40–59 | 512 | 0.43 (1.03) | 1.52 (1.03) | 147.88 (1.02) | |||
| ≥ 60 | 454 | 0.54 (1.04) | 2.03 (1.04) | 183.16 (1.02) | |||
| Ethnicity | <0.001 | <0.001 | <0.001 | ||||
| Mexican-American | 187 | 0.38 (1.06) | 1.30 (1.05) | 134.60 (1.04) | |||
| Other Hispanic | 128 | 0.42 (1.07) | 1.45 (1.06) | 142.74 (1.05) | |||
| Non-Hispanic White | 681 | 0.47 (1.03) | 1.87 (1.03) | 170.33 (1.02) | |||
| Non-Hispanic Black | 261 | 0.36 (1.05) | 1.30 (1.04) | 132.80 (1.03) | |||
| Non-Hispanic Asian | 164 | 0.45 (1.06) | 1.64 (1.06) | 134.15 (1.04) | |||
| Other ethnicity | 43 | 0.36 (1.12) | 2.02 (1.10) | 154.82 (1.08) | |||
| Smoking status | 0.011 | 0.010 | 0.337 | ||||
| Non-smoker | 893 | 0.45 (1.03) | 1.69 (1.02) | 151.50 (1.02) | |||
| ETS | 210 | 0.40 (1.06) | 1.52 (1.05) | 144.96 (1.03) | |||
| Current smoker | 361 | 0.40 (1.04) | 1.50 (1.04) | 154.00 (1.02) | |||
| BMI | 0.343 | <0.001 | 0.864 | ||||
| <25 | 440 | 0.45 (1.04) | 1.50 (1.03) | 151.33 (1.02) | |||
| 25–29 | 464 | 0.43 (1.04) | 1.51 (1.03) | 152.41 (1.02) | |||
| ≥ 30 | 560 | 0.42 (1.03) | 1.80 (1.03) | 149.98 (1.02) | |||
| Hypertension | <0.001 | <0.001 | <0.001 | ||||
| No | 848 | 0.40 (1.03) | 1.45 (1.01) | 140.34 (1.02) | |||
| Yes | 616 | 0.46 (1.03) | 1.87 (1.02) | 167.41 (1.03) | |||
| Diabetes Mellitus | <0.001 | <0.001 | <0.001 | ||||
| No | 1228 | 0.41 (1.02) | 1.53 (1.01) | 249.15 (1.02) | |||
| Yes | 236 | 0.52 (1.05) | 2.14 (1.04) | 167.77 (1.05) | |||
| Chronic kidney disease | 0.121 | <0.001 | <0.001 | ||||
| No | 1377 | 0.42 (1.02) | 1.54 (1.01) | 145.99 (1.02) | |||
| Yes | 87 | 0.48 (1.09) | 3.44 (1.06) | 261.91 (1.08) | |||
| Hypercholesterolemia | 0.015 | <0.001 | <0.001 | ||||
| No | 600 | 0.40 (1.03) | 1.48 (1.02) | 141.50 (1.03) | |||
| Yes | 864 | 0.45 (1.03) | 1.72 (1.02) | 158.24 (1.02) | |||
| Ln-MMA (nmol/L) | Ln-MeFox (nmol/L) | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| Continuous Variables | Adjusted β (SE) | p Value | Adjusted β (SE) | p Value | Adjusted β (SE) | p Value | Adjusted β (SE) | p Value |
| Urinary glyphosate (Unweighted number/population size = 1464/210267786) | ||||||||
| Ln-glyphosate (µg/L) | 0.063 (0.016) | <0.001 | 0.061 (0.014) | <0.001 | 0.221 (0.017) | <0.001 | 0.215 (0.018) | <0.001 |
| Age (years) | 0.008 (0.001) | <0.001 | 0.007 (0.001) | <0.001 | 0.005 (0.001) | 0.003 | 0.002 (0.002) | 0.143 |
| Poverty–income ratio | −0.032 (0.007) | 0.001 | −0.029 (0.007) | 0.001 | −0.008 (0.012) | 0.525 | −0.002 (0.012) | 0.881 |
| Ln-urinary creatinine (g/L) | −0.012 (0.020) | 0.555 | −0.021 (0.018) | 0.266 | −0.113 (0.030) | 0.002 | −0.121 (0.032) | 0.002 |
| BMI (kg/m2) | −0.003 (0.002) | 0.239 | −0.003 (0.002) | 0.207 | 0.013 (0.002) | <0.001 | 0.011 (0.003) | 0.001 |
| Ln-vitamin B12 (pg/mL) | −0.273 (0.021) | <0.001 | −0.284 (0.019) | <0.001 | 0.050 (0.042) | 0.255 | 0.036 (0.038) | 0.355 |
| Serum MeFox * (Unweighted number/population size = 1464/63262637) | ||||||||
| Ln-MeFox (nmol/L) | 0.152 (0.018) | <0.001 | 0.131 (0.017) | <0.001 | ||||
| Age (years) | 0.008 (0.001) | <0.001 | 0.007 (0.001) | <0.001 | ||||
| Poverty–income ratio | −0.032 (0.007) | <0.001 | −0.030 (0.007) | 0.001 | ||||
| BMI (kg/m2) | −0.004 (0.002) | 0.047 | −0.004 (0.002) | 0.049 | ||||
| Ln-vitamin B12 (pg/mL) | −0.275 (0.019) | <0.001 | −0.283 (0.017) | <0.001 | ||||
| Ln-MeFox (nmol/L) | Ln-MMA (nmol/L) | ||||||
|---|---|---|---|---|---|---|---|
| N | Adjusted β (SE) | p Value | p for Interaction | Adjusted β (SE) | p Value | p for Interaction | |
| Age, years | 0.258 | 0.174 | |||||
| 19–49 | 764 | 0.203 (0.030) | <0.001 | 0.078 (0.016) | <0.001 | ||
| ≥ 50 | 700 | 0.237 (0.033) | <0.001 | 0.048 (0.021) | 0.040 | ||
| Gender | 0.501 | 0.430 | |||||
| Male | 707 | 0.228 (0.035) | <0.001 | 0.068 (0.016) | 0.001 | ||
| Female | 757 | 0.194 (0.035) | <0.001 | 0.056 (0.019) | 0.010 | ||
| Ethnicity | 0.560 | 0.010 | |||||
| Non-Hispanic White | 681 | 0.231 (0.027) | <0.001 | 0.053 (0.025) | 0.048 | ||
| Other | 783 | 0.191 (0.027) | <0.001 | 0.077 (0.017) | 0.001 | ||
| BMI, kg/m2 | 0.550 | 0.652 | |||||
| < 27.9 (50%ile) | 737 | 0.239 (0.029) | <0.001 | 0.093 (0.032) | 0.010 | ||
| ≥ 27.9 (50%ile) | 727 | 0.197 (0.031) | <0.001 | 0.036 (0.020) | 0.094 | ||
| Hypertension | 0.797 | 0.931 | |||||
| No | 848 | 0.208 (0.035) | <0.001 | 0.068 (0.020) | 0.004 | ||
| Yes | 616 | 0.232 (0.043) | <0.001 | 0.051 (0.021) | 0.029 | ||
| Diabetes Mellitus | 0.494 | 0.061 | |||||
| No | 1228 | 0.199 (0.025) | <0.001 | 0.071 (0.015) | <0.001 | ||
| Yes | 236 | 0.325 (0.063) | <0.001 | 0.026 (0.032) | 0.431 | ||
| Chronic kidney disease | 0.543 | 0.183 | |||||
| No | 1377 | 0.213 (0.018) | <0.001 | 0.063 (0.016) | <0.001 | ||
| Yes | 86 | 0.232 (0.071) | 0.006 | 0.023 (0.043) | 0.591 | ||
| Hypercholesterolemia | 0.575 | 0.784 | |||||
| No | 600 | 0.187 (0.043) | 0.001 | 0.071 (0.030) | 0.031 | ||
| Yes | 864 | 0.241 (0.037) | <0.001 | 0.055 (0.016) | 0.003 | ||
| Ln-glyphosate (µg/L) | Unweighted No./Population Size | HR | 95% CI | p Value | p for Interaction |
|---|---|---|---|---|---|
| All-cause mortality (Adjusted for model 3) | 0.773 | ||||
| Total | 1463/210,214,570 | 1.342 | 0.942—1.912 | 0.097 | |
| MeFox < 50%ile | 734/102,979,608 | 1.529 | 0.785—2.978 | 0.195 | |
| MeFox ≥ 50%ile | 729/107,234,961 | 1.395 | 1.044—1.864 | 0.027 | |
| Cardiovascular mortality * (Adjusted for model 3) | 0.933 | ||||
| Total | 1463/210,214,570 | 1.207 | 0.689—2.114 | 0.486 | |
| MeFox < 50%ile | 734/102,979,608 | 1.410 | 0.809—2.457 | 0.208 | |
| MeFox ≥ 50%ile | 729/107,234,961 | 1.367 | 0.998—1.872 | 0.051 | |
| All-cause mortality (Adjusted for model 4) | 0.002 | ||||
| Total | 1463/210,214,570 | 1.204 | 0.838—1.729 | 0.293 | |
| MMA < 50%ile | 741/101,870,296 | 2.679 | 1.603—4.475 | 0.001 | |
| MMA ≥ 50%ile | 722/108,344,274 | 0.919 | 0.645—1.309 | 0.617 | |
| Cardiovascular mortality * (Adjusted for model 4) | 0.038 | ||||
| Total | 1463/210,214,570 | 1.213 | 0.583—2.526 | 0.583 | |
| MMA < 50%ile | 741/101,870,296 | 1.689 | 0.461—6.195 | 0.403 | |
| MMA ≥ 50%ile | 722/108,344,274 | 1.199 | 0.709—2.030 | 0.473 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Y.-W.; Lin, H.-C.; Wang, C.; Lin, C.-Y. Glyphosate Exposure, Oxidative Stress, Mitochondrial Dysfunction, and Mortality Risk in US Adults: Insights from the National Health and Nutrition Examination Survey. Toxics 2025, 13, 373. https://doi.org/10.3390/toxics13050373
Fang Y-W, Lin H-C, Wang C, Lin C-Y. Glyphosate Exposure, Oxidative Stress, Mitochondrial Dysfunction, and Mortality Risk in US Adults: Insights from the National Health and Nutrition Examination Survey. Toxics. 2025; 13(5):373. https://doi.org/10.3390/toxics13050373
Chicago/Turabian StyleFang, Yu-Wei, Hsuan-Cheng Lin, Chikang Wang, and Chien-Yu Lin. 2025. "Glyphosate Exposure, Oxidative Stress, Mitochondrial Dysfunction, and Mortality Risk in US Adults: Insights from the National Health and Nutrition Examination Survey" Toxics 13, no. 5: 373. https://doi.org/10.3390/toxics13050373
APA StyleFang, Y.-W., Lin, H.-C., Wang, C., & Lin, C.-Y. (2025). Glyphosate Exposure, Oxidative Stress, Mitochondrial Dysfunction, and Mortality Risk in US Adults: Insights from the National Health and Nutrition Examination Survey. Toxics, 13(5), 373. https://doi.org/10.3390/toxics13050373