Alpha-Lipoic Acid Alleviates Lead-Induced Testicular Damage in Roosters by Reducing Oxidative Stress and Modulating Key Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds and Treatments
2.2. Semen Evaluation
2.3. Pb Content Analysis
2.4. Determination of Serum Testosterone and Testicular Oxidative Status
2.5. Testicular Morphology Analysis and Immunohistochemistry
2.6. RNA Sequencing
2.7. Bioinformatic Analysis
2.8. Quantitative Real-Time PCR (qRT-PCR)
2.9. Statistical Analysis
3. Results
3.1. Effect of ALA on Testicular Weights and Sperm Quality in Pb-Exposed Roosters
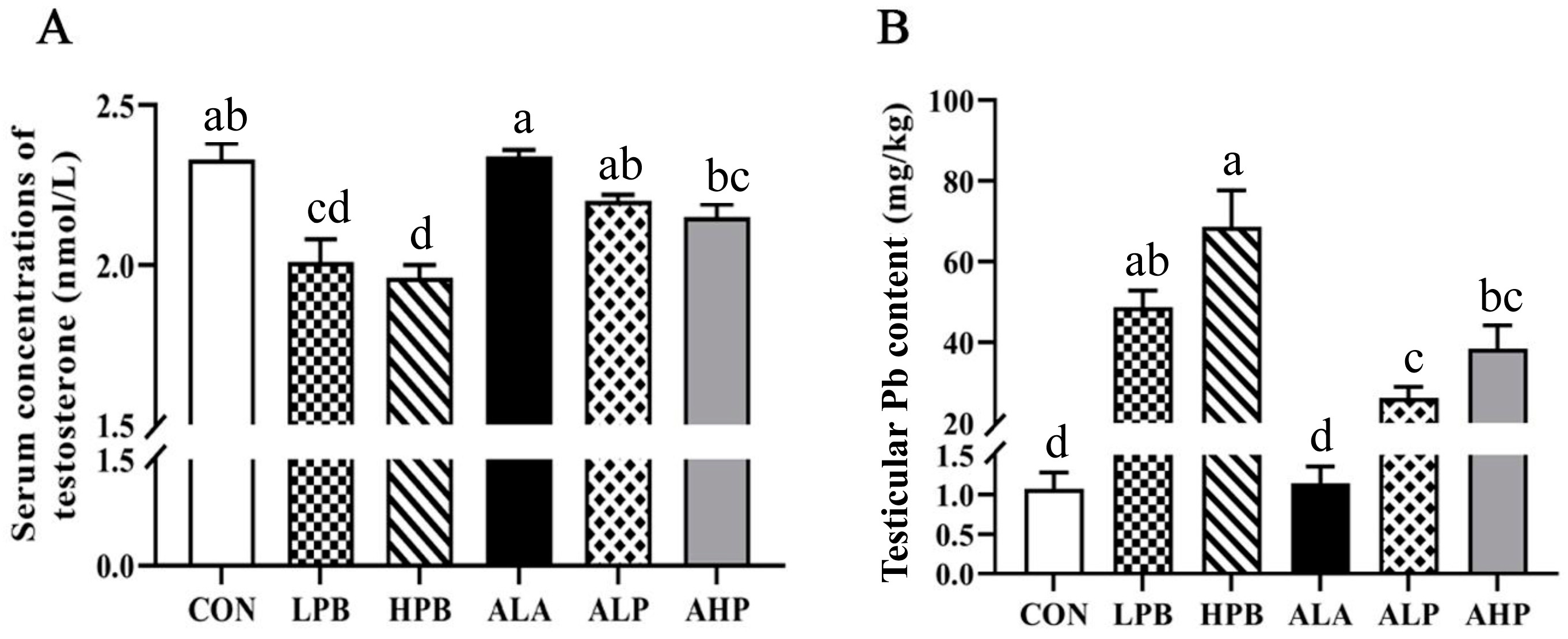
3.2. Effect of ALA on Serum Testosterone Levels and Testicular Pb Content in Pb-Exposed Roosters
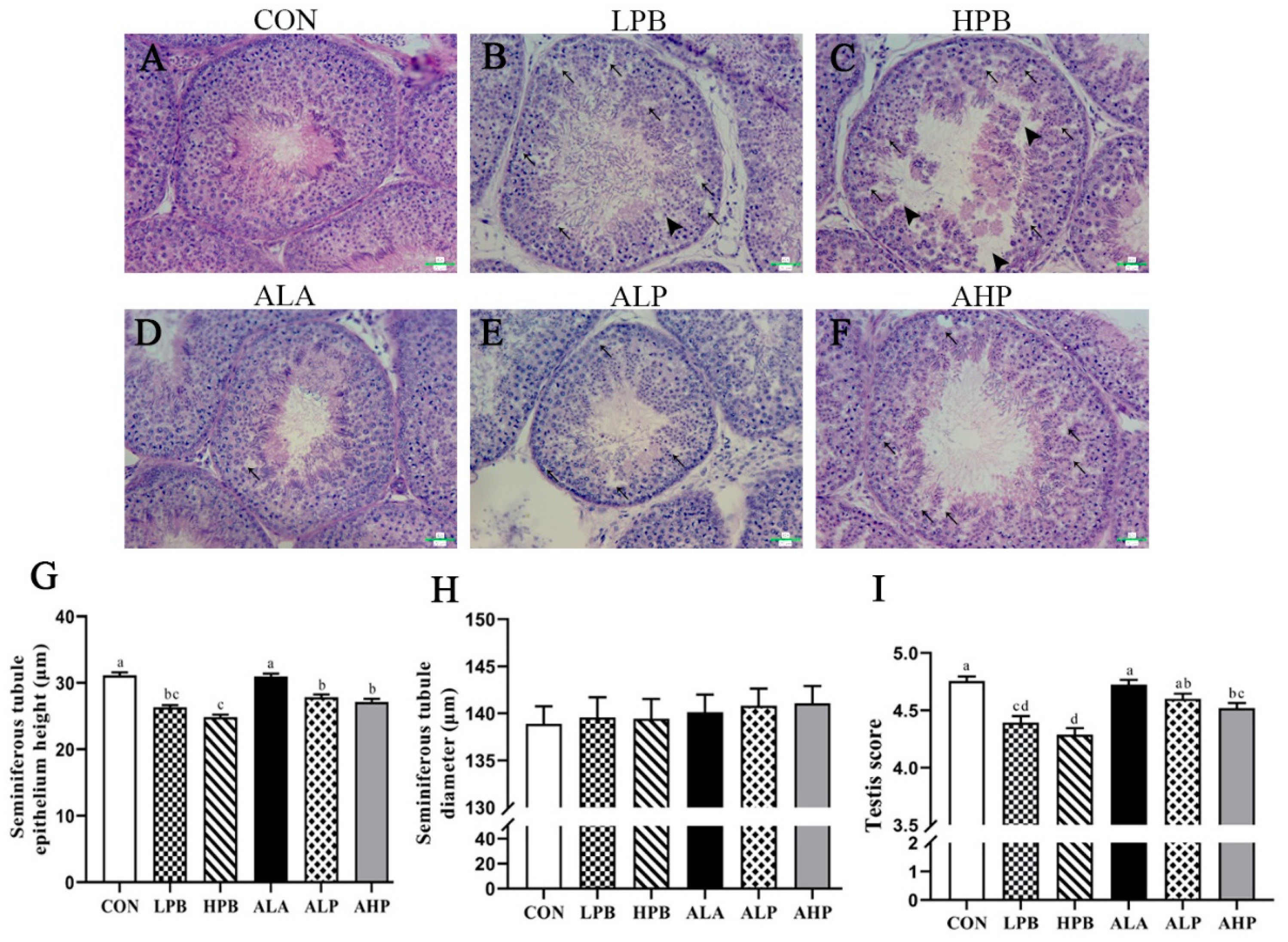
3.3. Effect of ALA on Testicular Morphology in Pb-Exposed Roosters
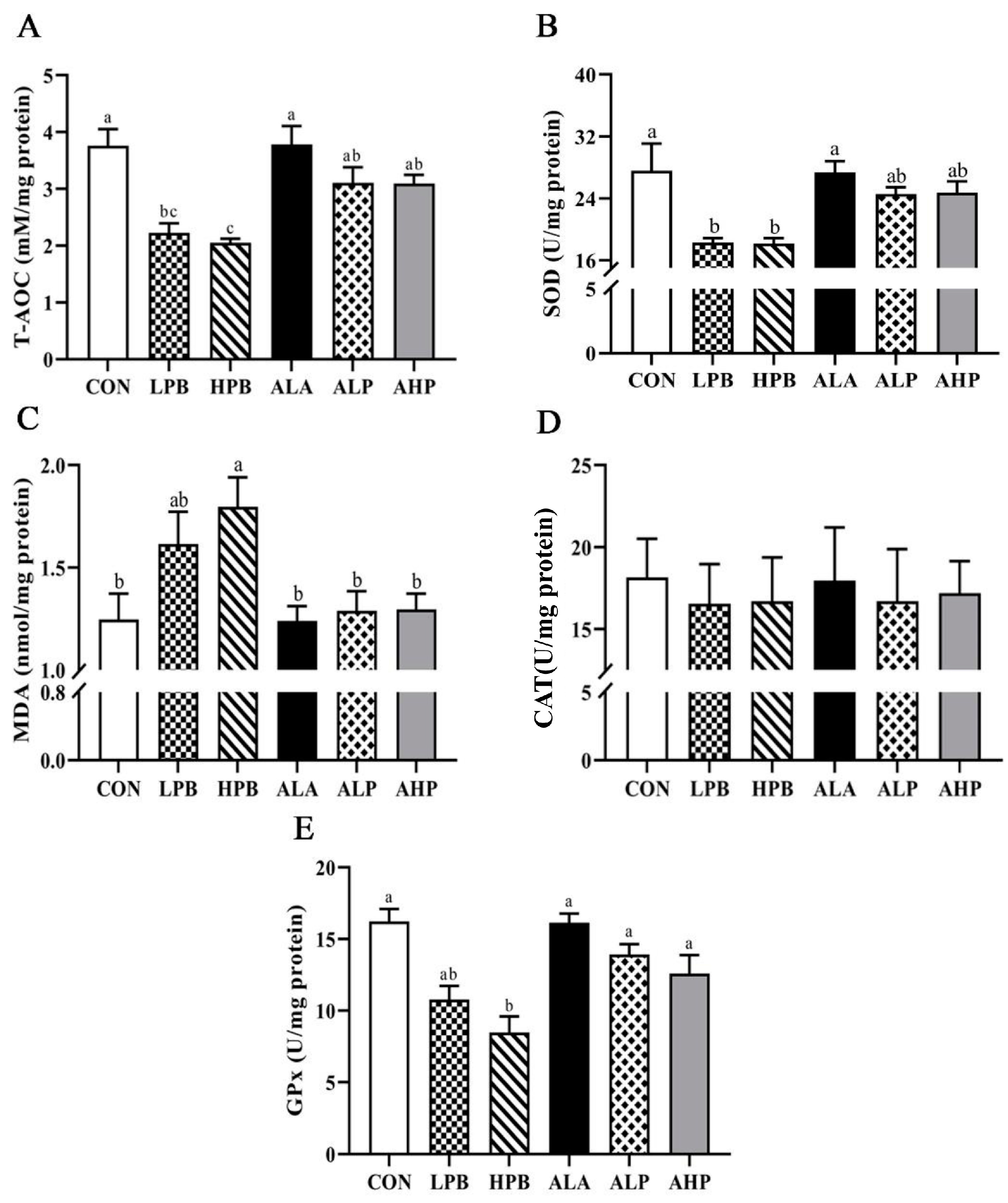
3.4. Effect of ALA on Testicular Oxidative Status in Pb-Exposed Roosters
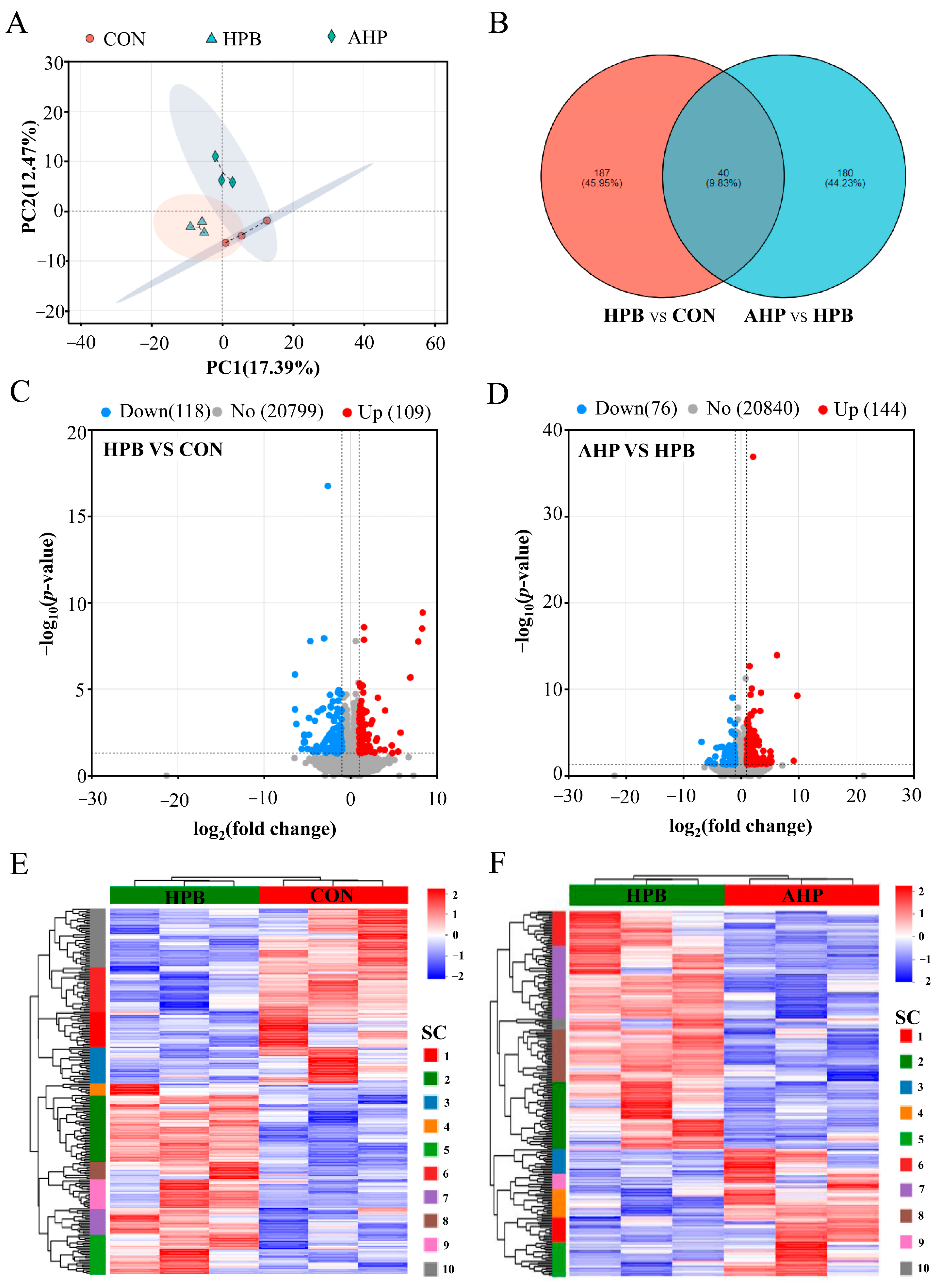
3.5. Effect of ALA on DEGs Expression in Testes in Pb-Exposed Roosters
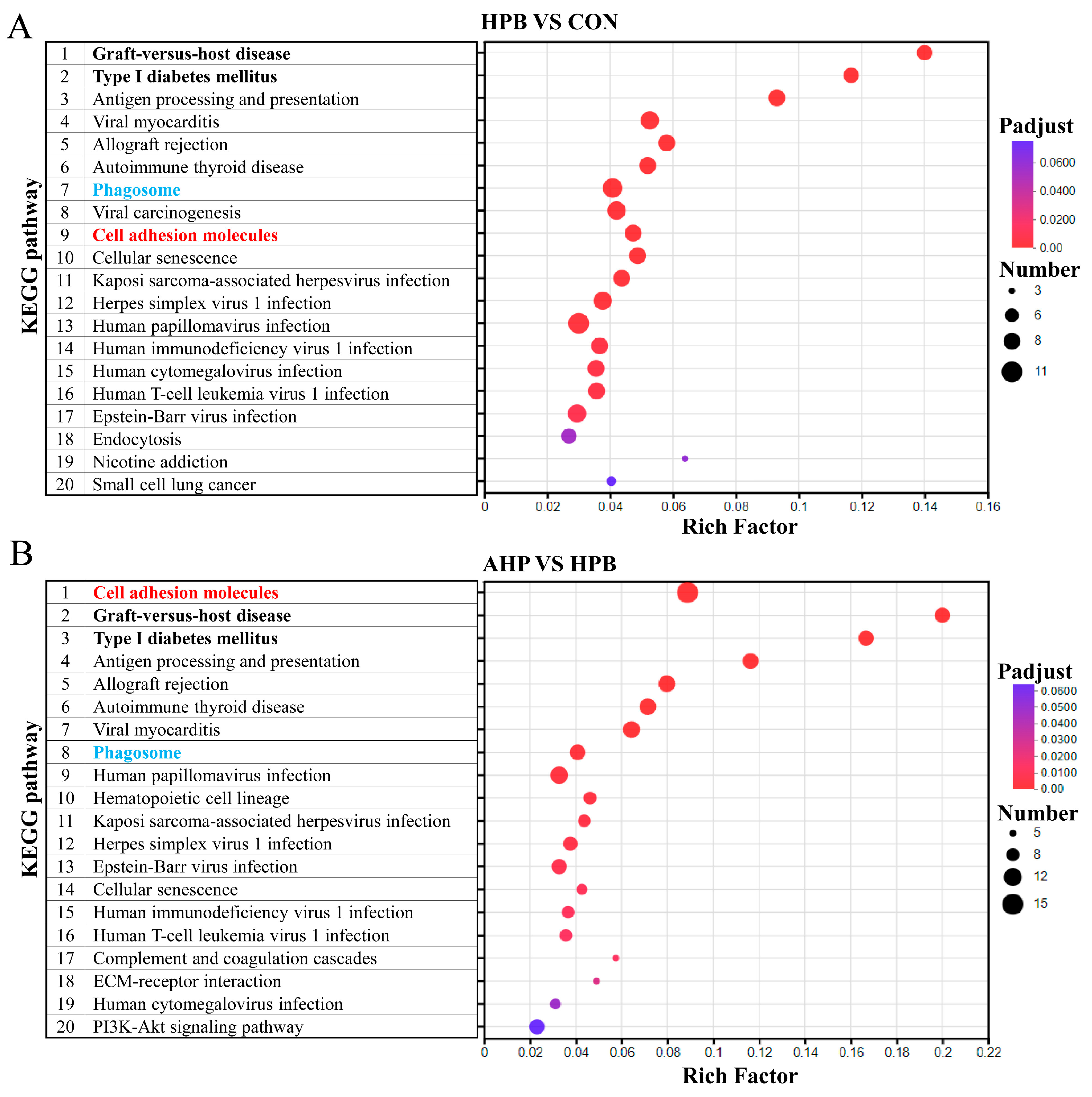
3.6. KEGG Enrichment Analysis of DEGs Between Groups of Rooster Testes
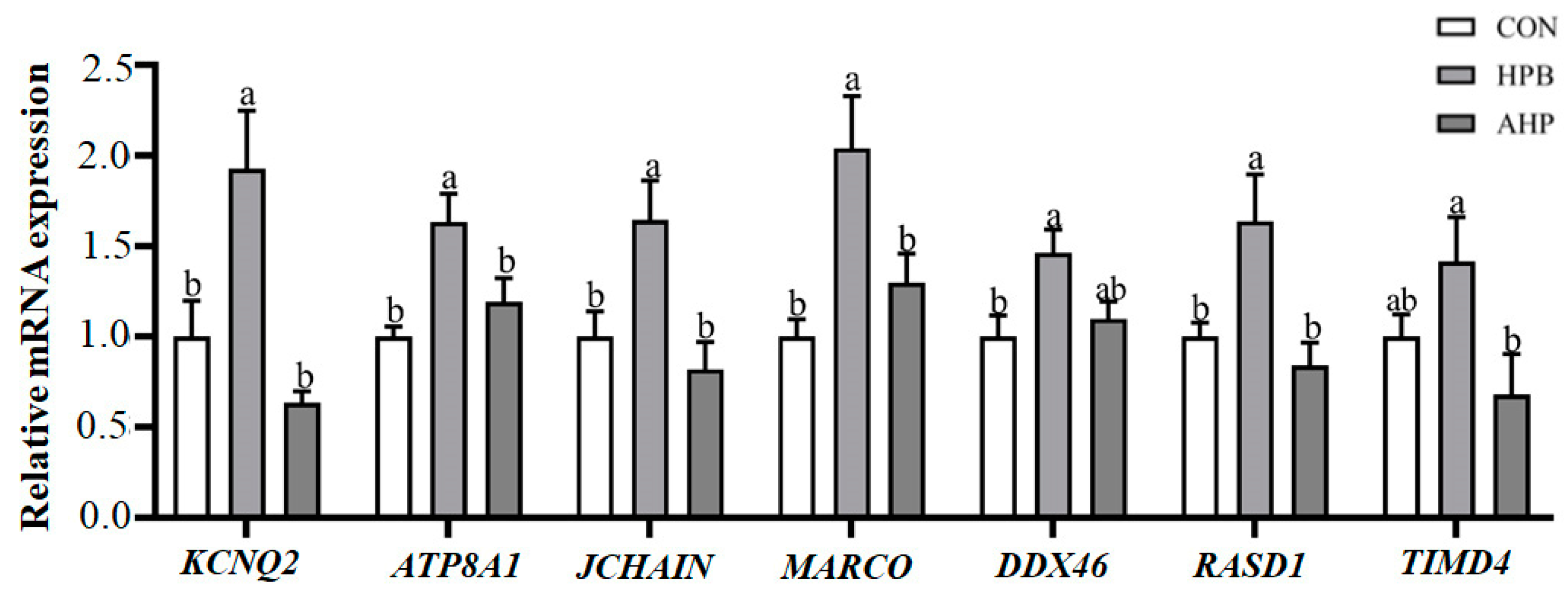
3.7. Detection of DEGs by qRT-PCR
4. Discussion
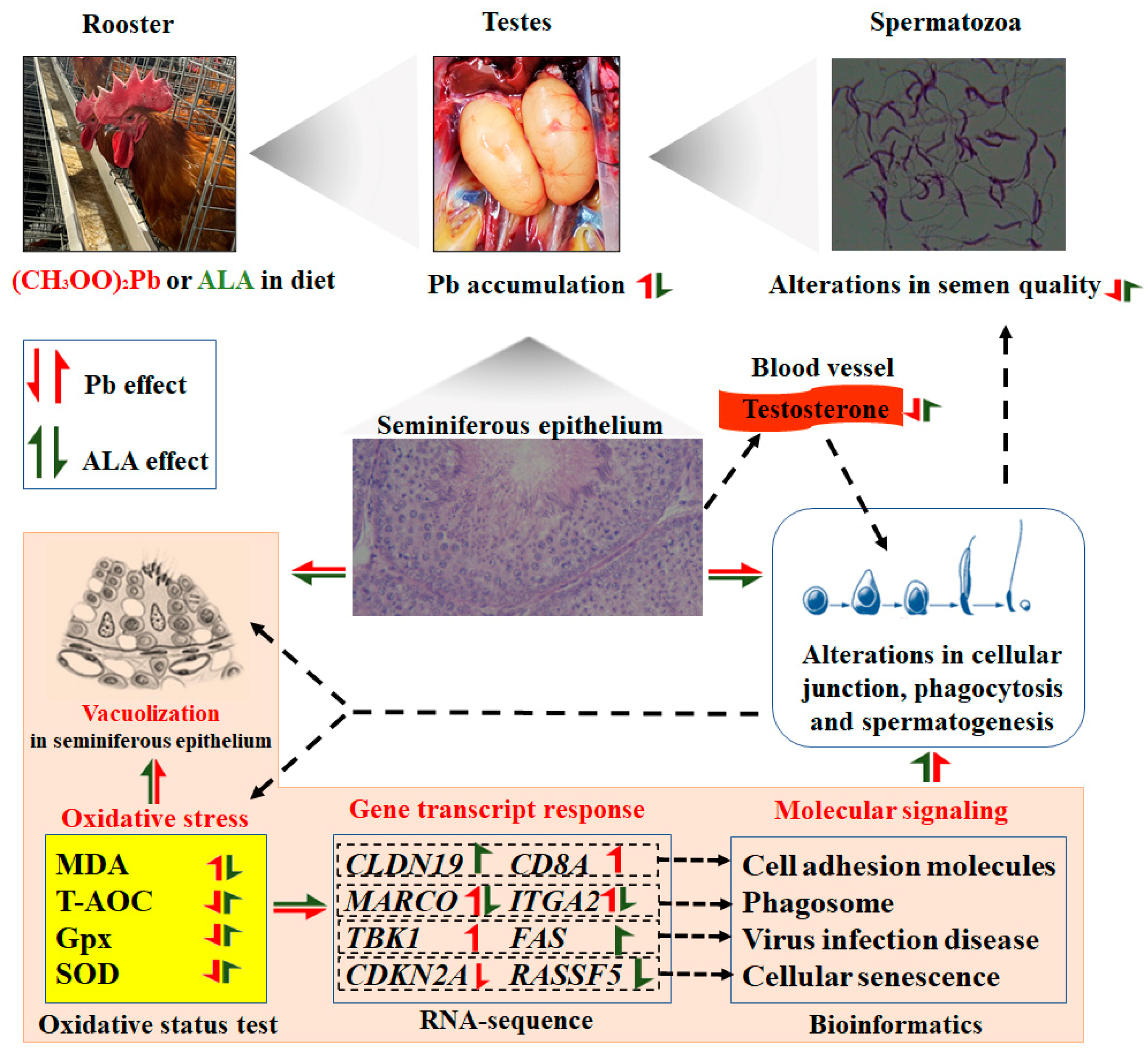
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boskabady, M.; Marefati, N.; Farkhondeh, T.; Shakeri, F.; Farshbaf, A.; Boskabady, M.H. The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environ. Int. 2018, 120, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Wirth, J.J.; Mijal, R.S. Adverse effects of low level heavy metal exposure on male reproductive function. Syst. Biol. Reprod. Med. 2010, 56, 147–167. [Google Scholar] [CrossRef]
- Massanyi, P.; Massanyi, M.; Madeddu, R.; Stawarz, R.; Lukac, N. Effects of cadmium, lead, and mercury on the structure and function of reproductive organs. Toxics 2020, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Godinez-Solis, Y.; Solis-Heredia, M.J.; Roa-Espitia, A.; Parra-Forero, L.Y.; Hernandez-Gonzalez, E.O.; Hernandez-Ochoa, I.; Quintanilla-Vega, B. Low concentrations of lead decrease the sperm fertilization ability by altering the acrosome reaction in mice. Toxicol. Appl. Pharmacol. 2019, 380, 114694. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.J.D. Reactive oxygen species (ROS) as pleiotropic physiological signaling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Yadegari, P.; Imani, A.; Taghdir, M. Vitamin D3 protects against lead-induced testicular toxicity by modulating Nrf2 and NF-kappaB genes expression in rat. Reprod. Toxicol. 2021, 103, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Al-Megrin, W.A.; Alomar, S.; Alkhuriji, A.F.; Metwally, D.M.; Mohamed, S.K.; Kassab, R.B.; Abdel-Moneim, A.E.; EI-Khadragy, M.F. Luteolin protects against testicular injury induced by lead acetate by activating the Nrf2/HO-1 pathway. IUBMB Life 2020, 72, 1787–1798. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef]
- Shanaida, M.; Lysiuk, R.; Mykhailenko, O.; Hudz, N.; Abdulsalam, A.; Gontova, T.; Oleshchuk, O.; Ivankiv, Y.; Shanaida, V.; Lytkin, D.; et al. Alpha-lipoic acid: An antioxidant with anti-aging properties for disease therapy. Curr. Med. Chem. 2025, 32, 23–54. [Google Scholar] [CrossRef]
- Superti, F.; Russo, R. Alpha-lipoic acid: Biological mechanisms and health benefits. Antioxidants 2024, 13, 1288. [Google Scholar] [CrossRef]
- Ye, N.; Lv, Z.; Huang, Z.; Cheng, Y.; Wei, Q.; Shi, F. Dietary folic acid supplementation improves semen quality and spermatogenesis through altering autophagy and histone methylation in the testis of aged broiler breeder roosters. Theriogenology 2022, 181, 8–15. [Google Scholar] [CrossRef] [PubMed]
- El-Maraghy, S.A.; Nassar, N.N. Modulatory effects of lipoic acid and selenium against cadmium-induced biochemical alterations in testicular steroidogenesis. J. Biochem. Mol. Toxicol. 2011, 25, 15–25. [Google Scholar] [CrossRef]
- Huang, H.; Wang, M.; Hou, L.; Lin, X.; Pan, S.; Zheng, P.; Zhao, Q. A potential mechanism associated with lead-induced spermatogonia and Leydig cell toxicity and mitigative effect of selenium in chicken. Ecotox. Environ. Saf. 2021, 209, 111671. [Google Scholar] [CrossRef]
- Dolati, P.; Khodabandeh, Z.; Zamiri, M.J.; Jamhiri, I.; Mehrabani, D. The effect of lead acetate and quercetin on the tight and gap junctions in the mouse testis. Biol. Trace Elem. Res. 2020, 198, 535–543. [Google Scholar] [CrossRef] [PubMed]
- GB 13078-2017; National Criterion of China. Hygienical Standard for Feeds. Standards Press: Beijing, China, 2005.
- Jin, X.; Liu, C.P.; Teng, X.H.; Fu, J. Effects of dietary selenium against lead toxicity are related to the ion profile in chicken muscle. Biol. Trace Elem. Res. 2016, 172, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xu, Z.; Zhao, X.; Chen, M.; Xu, S. The antagonistic effect of selenium on lead-induced apoptosis via mitochondrial dynamics pathway in the chicken kidney. Chemosphere 2017, 180, 259–266. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Hao, X.; Gang, S.; Xu, S. The antagonistic effect of selenium on lead-induced necroptosis via MAPK/NF-kappaB pathway and HSPs activation in the chicken spleen. Ecotoxicol. Environ. Saf. 2020, 204, 111049. [Google Scholar] [CrossRef]
- Wu, H.; Ye, N.; Huang, Z.; Lei, K.; Shi, F.; Wei, Q. Dietary curcumin supplementation relieves hydrogen peroxide-induced testicular injury by antioxidant and anti-apoptotic effects in roosters. Theriogenology 2023, 197, 46–56. [Google Scholar] [CrossRef]
- Vafaee, F.; Derakhshani, M.; Rahbardar, M.G.; Hosseinzadeh, H. Alpha-lipoic acid, as an effective agent against toxic elements: A review. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 398, 3345–3372. [Google Scholar] [CrossRef]
- Long, C.; Zhao, Z.X.; Willing, B.P.; Sheng, X.H.; Wang, X.G.; Xiao, L.F.; Qi, X.L. Alpha-linolenic acid supplementation improves testosterone production in an aged breeder rooster model: Role of mitochondrial modulation and SIRT1 activation. Mol. Nutr. Food Res. 2024, 68, e2400522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Yu, J.; Xie, J.; Liu, D.Y.; Fan, Y.S.; Ma, H.T.; Wang, C.; Hong, Z. Improvement roles of zinc supplementation in low dose lead induced testicular damage and glycolytic inhibition in mice. Toxicology 2021, 462, 152933. [Google Scholar] [CrossRef] [PubMed]
- Kahalerras, L.; Otmani, I.; Abdennour, C. Wild garlic allium triquetrum L. alleviates lead acetate-induced testicular injuries in rats. Biol. Trace Elem. Res. 2022, 200, 2205–2222. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Ali, H.A.; Mutar, T.F. Protective effects of olive leaf extract against reproductive toxicity of the lead acetate in rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 63102–63110. [Google Scholar] [CrossRef] [PubMed]
- Biewenga, G.P.; Haenen, G.R.M.M.; Bast, A. The pharmacology of the antioxidant lipoic acid. Gen. Pharmacol. 1997, 29, 315–331. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K.; Bishen, S.M.; Mishra, H.; Khurana, A. Molecular and therapeutic insights of alpha-lipoic acid as a potential molecule for disease prevention. Rev. Bras. Farmacogn. 2023, 33, 272–287. [Google Scholar] [CrossRef]
- Li, M.; Kong, Y.; Wu, X.; Yin, Z.; Niu, X.; Wang, G. Dietary α-lipoic acid can alleviate the bioaccumulation, oxidative stress, cell apoptosis, and inflammation induced by lead (Pb) in Channa argus. Fish Shellfish Immunol. 2021, 119, 249–261. [Google Scholar] [CrossRef]
- El-fakharany, Y.M.; Mohamed, E.M.; Etewa, R.L.; Hamid, O.I.A. Selenium nanoparticles alleviate lead acetate-induced toxicological and morphological changes in rat testes through modulation of calmodulin-related genes expression. J. Biochem. Mol. Toxic. 2022, 36, e23017. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, N.A.H.; Omer, N.A.; Yi-Ru, W.; Mei-Qian, K.; Ilyas, A.; Abdurahim, Y.; Wang, G.L. Protective effect of betaine against lead-induced testicular toxicity in male mice. Andrologia 2020, 52, e13600. [Google Scholar] [CrossRef]
- Lettieri, G.; Notariale, R.; Ambrosino, A.; Di Bonito, A.; Giarra, A.; Trifuoggi, M.; Manna, C.; Piscopo, M. Spermatozoa transcriptional response and alterations in PL proteins properties after exposure of Mytilus galloprovincialis to mercury. Int. J. Mol. Sci. 2021, 22, 1618. [Google Scholar] [CrossRef]
- Marinaro, C.; Lettieri, G.; Chianese, T.; Bianchi, A.R.; Zarrelli, A.; Palatucci, D.; Scudiero, R.; Rosati, L.; De Maio, A.; Piscopo, M. Exploring the molecular and toxicological mechanism associated with interactions between heavy metals and the reproductive system of Mytilus galloprovincialis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2024, 275, 109778. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Mruk, D.D.; Cheng, C.Y. Drug transporters, the blood-testis barrier, and spermatogenesis. J. Endocrinol. 2011, 208, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Tang, X.L.; Zhou, Y.; Liu, B.; Shen, L.J.; Long, C.L.; Tao, L.; He, D.; Wu, S. DEHP exposure destroys blood-testis barrier (BTB) integrity of immature testes through excessive ROS-mediated autophagy. Genes Dis. 2018, 5, 263–274. [Google Scholar] [CrossRef]
- Shen, Y.F.; You, Y.D.; Zhu, K.; Li, G.S.; Huang, X.P.; Chen, D.; Yang, F.; Dong, L.; Li, J.; Yu, X. The traditional Chinese medicine Qiangjing tablet prevents blood-testis barrier injury induced by CdCl through the PI3K/Akt/Rictor signaling pathway. Environ. Toxicol. 2023, 38, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Mruk, D.D.; Cheng, C.Y. Intercellular adhesion molecules (ICAMs) and spermatogenesis. Hum. Reprod. Update 2013, 19, 167–186. [Google Scholar] [CrossRef]
- Kim, S.E.; Overholtzer, M. Autophagy proteins regulate cell engulfment mechanisms that participate in cancer. Semin. Cancer Biol. 2013, 23, 329–336. [Google Scholar] [CrossRef]
- Mossadegh-Keller, N.; Gentek, R.; Gimenez, G.; Bigot, S.; Mailfert, S.; Sieweke, M.H. Developmental origin and maintenance of distinct testicular macrophage populations. J. Exp. Med. 2017, 214, 2829–2841. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Meng, C.; Sun, Y.; Safdar, A.; Pasha, R.H.; Munir, M.; Ding, C. Oxidative stress in poultry: Lessons from the viral infections. Oxid. Med. Cell. Longev. 2018, 2018, 5123147. [Google Scholar] [CrossRef]
- Cecerska-Heryć, E.; Surowska, O.; Heryć, R.; Serwin, N.; Napiontek-Balińska, S.; Dołęgowska, B. Are antioxidant enzymes essential markers in the diagnosis and monitoring of cancer patients—A review. Clin. Biochem. 2021, 93, 1–8. [Google Scholar] [CrossRef]
- Fang, F.C. Antimicrobial actions of reactive oxygen species. mBio 2011, 2, e00141-11. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Bartosch, B.; Isaguliants, M.G. Oxidative stress in infection and consequent disease. Oxid. Med. Cell. Longev. 2017, 2017, 3496043. [Google Scholar] [CrossRef] [PubMed]
- Faraonio, R. Oxidative stress and cell senescence process. Antioxidants 2022, 11, 1718. [Google Scholar] [CrossRef]
- Loyo-Celis, V.; Sanghvi, S.K.; Raut, S.; Ponnalagu, D.; Singh, H. Chloride intracellular channels regulate spermatogenesis. Biophys. J. 2024, 123, 443a–444a. [Google Scholar] [CrossRef]
- Lin, Q.; Jin, H.J.; Zhang, D.; Gao, L. DDX46 silencing inhibits cell proliferation by activating apoptosis and autophagy in cutaneous squamous cell carcinoma. Mol. Med. Rep. 2020, 22, 4236–4242. [Google Scholar] [CrossRef] [PubMed]
- Yap, Y.T.; Li, Y.H.; Li, W.; Banerjee, P.; Zhang, Z.B. ATP8a1, an IFT27 binding partner, is dispensable for spermatogenesis and male fertility. Mol. Reprod. Dev. 2021, 88, 371–375. [Google Scholar] [CrossRef]
- Yin, X.Q.; Ma, T.; Han, R.T.; Ding, J.; Zhang, H.; Han, X.D.; Li, D. MiR-301b-3p/3584-5p enhances low-dose mono-n-butyl phthalate (MBP) induced proliferation by targeting Rasd1 in Sertoli cells. Toxicol. In Vitro 2018, 11, 79–88. [Google Scholar] [CrossRef]
| Gene | Primer Sequence (5′→3′) | Amplicon Size (bp) | Accession Number |
|---|---|---|---|
| KCNQ2 | forward: GGCAGAACTCCGAAGAAGCA reverse: CTGACTTTCAGTCCTGGCGT | 109 | XM_015296640.4 |
| ATP8A1 | forward: TGCTGACACTGTACTGCTCT reverse: ATGTTAGGGGCAAACCCTGTC | 113 | XM_025149963.3 |
| JCHAIN | forward: TTCGTCCTTGTGGCAGGTTATC reverse: TGTCTTTGGAGGGGACGAAC | 109 | NM_205064.1 |
| MARCO | forward: TGCTGGCGTACAAAGTGTTT reverse: TCTCTTCGGCATGGAAAGCA | 78 | NM_204736.3 |
| DDX46 | forward: CGGGAGTCCAGGCACTATC reverse: GCGTCTGTCCTCACGTTTTC | 105 | NM_001389392.2 |
| RASD1 | forward: GAGGACTTCCACCGCAAGTT reverse: GAGGATGAAAACGTCACCTGT | 132 | NM_001044636.2 |
| TIMD4 | forward: GGAACAGGGTGACGTTCAGA reverse: CATCAATGGAGGTGCTTCGAG | 197 | NM_001006149.2 |
| GAPDH | forward: TGATGCCCCCATGTTTGTGA reverse: TGGCATGGACAGTGGTCATA | 164 | NM_204305.1 |
| Items | Treatments | |||||
|---|---|---|---|---|---|---|
| CON | LPB | HPB | ALA | ALP | AHP | |
| Initial body weight (kg) | 2.83 ± 0.03 | 2.81 ± 0.05 | 2.81 ± 0.08 | 2.80 ± 0.05 | 2.80 ± 0.09 | 2.81 ± 0.06 |
| End body weight (kg) | 3.38 ± 0.06 | 3.33 ± 0.08 | 3.26 ± 0.12 | 3.31 ± 0.08 | 3.26 ± 0.16 | 3.34 ± 0.09 |
| Body weight gain (kg) | 0.55 ± 0.04 | 0.52 ± 0.04 | 0.45 ± 0.06 | 0.51 ± 0.04 | 0.47 ± 0.08 | 0.53 ± 0.03 |
| Testis weight (g) | 39.70 ± 2.94 | 38.12 ± 2.84 | 35.36 ± 1.73 | 38.05 ± 2.10 | 37.87 ± 2.72 | 39.46 ± 4.49 |
| Testis index (%) | 1.17 ± 0.08 | 1.16 ± 0.10 | 1.10 ± 0.08 | 1.16 ± 0.08 | 1.15 ± 0.04 | 1.18 ± 0.14 |
| Items | Treatments | |||||
|---|---|---|---|---|---|---|
| CON | LPB | HPB | ALA | ALP | AHP | |
| Ejaculate volume (mL) | 0.84 ± 0.07 | 0.59 ± 0.06 | 0.70 ± 0.05 | 0.85 ± 0.07 | 0.78 ± 0.09 | 0.75 ± 0.07 |
| Sperm concentration (109 cells/mL) | 3.18 ± 0.20 a | 2.14 ± 0.17 bc | 2.04 ± 0.20 c | 3.11 ± 0.22 a | 2.84 ± 0.11 ab | 2.81 ± 0.14 ab |
| Abnormal sperm (%) | 5.25 ± 0.21 a | 5.75 ± 0.25 ab | 6.25 ± 0.25 b | 5.31 ± 0.23 ab | 5.50 ± 0.19 ab | 5.88 ± 0.25 ab |
| Sperm viability (%) | 84.06 ± 0.46 ab | 81.88 ± 0.53 bc | 80.78 ± 0.82 c | 84.53 ± 0.62 a | 83.44 ± 0.57 ab | 82.99 ± 0.40 ab |
| pH | 7.58 ± 0.41 | 7.51 ± 0.48 | 7.50 ± 0.42 | 7.58 ± 0.42 | 7.59 ± 0.48 | 7.49 ± 0.40 |
| Membrane functional integrity (%) | 94.81 ± 0.17 a | 93.68 ± 0.38 bc | 93.37 ± 0.30 c | 94.77 ± 0.13 a | 94.50 ± 0.25 ab | 94.41 ± 0.11 ab |
| Sample | Clean Reads | Clean Bases | Q20 | Q30 | GC Content | Total Mapped | Uniquely Mapped |
|---|---|---|---|---|---|---|---|
| CON1 | 50,015,498 | 7,372,621,148 | 98.22% | 94.91% | 52.22% | 92.17% | 89.04% |
| CON2 | 56,785,098 | 8,398,140,920 | 98.18% | 94.78% | 52.13% | 91.45% | 88.63% |
| CON3 | 51,271,270 | 7,567,391,561 | 98.25% | 94.90% | 51.35% | 91.71% | 88.96% |
| HPB1 | 57,456,806 | 8,470,844,569 | 98.24% | 94.92% | 51.22% | 91.8% | 89.03% |
| HPB2 | 60,906,272 | 8,983,684,655 | 98.29% | 95.01% | 51.69% | 91.89% | 89.15% |
| HPB3 | 61,928,874 | 9,158,784,735 | 98.24% | 94.89% | 51.72% | 91.68% | 88.81% |
| AHP1 | 71,146,770 | 10,521,995,825 | 98.27% | 94.99% | 51.74% | 91.57% | 88.56% |
| AHP2 | 54,704,400 | 8,120,147,760 | 98.28% | 95.01% | 51.66% | 91.46% | 88.46% |
| AHP3 | 58,582,606 | 8,674,540,804 | 98.31% | 95.09% | 52.13% | 91.97% | 89.14% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Malyar, R.M.; Ye, N.; Wang, Y.; Wei, Q.; Shi, F.; Li, Y. Alpha-Lipoic Acid Alleviates Lead-Induced Testicular Damage in Roosters by Reducing Oxidative Stress and Modulating Key Pathways. Toxics 2025, 13, 341. https://doi.org/10.3390/toxics13050341
Sun J, Malyar RM, Ye N, Wang Y, Wei Q, Shi F, Li Y. Alpha-Lipoic Acid Alleviates Lead-Induced Testicular Damage in Roosters by Reducing Oxidative Stress and Modulating Key Pathways. Toxics. 2025; 13(5):341. https://doi.org/10.3390/toxics13050341
Chicago/Turabian StyleSun, Jiahao, Rahmani Mohammad Malyar, Nanwei Ye, Yueyue Wang, Quanwei Wei, Fangxiong Shi, and Yansen Li. 2025. "Alpha-Lipoic Acid Alleviates Lead-Induced Testicular Damage in Roosters by Reducing Oxidative Stress and Modulating Key Pathways" Toxics 13, no. 5: 341. https://doi.org/10.3390/toxics13050341
APA StyleSun, J., Malyar, R. M., Ye, N., Wang, Y., Wei, Q., Shi, F., & Li, Y. (2025). Alpha-Lipoic Acid Alleviates Lead-Induced Testicular Damage in Roosters by Reducing Oxidative Stress and Modulating Key Pathways. Toxics, 13(5), 341. https://doi.org/10.3390/toxics13050341








