Associations Between Aromatic Compounds and Hepatorenal Biomarkers Among Coking Workers: Insights from Mediation Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Measurement of Serum HRBs
2.3. Measurement of the Urinary ACs and OSBs for Each Sample
2.4. Assessment of Covariates
2.5. Statistical Analysis
2.6. Mediation Analysis
3. Results
3.1. Descriptive Results
3.2. Associations Between Urinary ACs and HBRs in Multivariable Linear Regression
3.3. AC Exposure on Hepatorenal Biomarkers in BKMR Models
3.4. Mediation Analysis Results
3.5. Subgroup Analysis
4. Discussion
4.1. Association of Urinary AC Metabolites with Kidney Biomarkers
4.2. Association of Urinary AC Metabolites with Liver Biomarkers
4.3. Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACs | Aromatic compounds |
| HRBs | Hepatorenal biomarkers |
| OSBs | Oxidative stress biomarkers |
| MLR | Multiple linear regression |
| BKMR | Bayesian kernel machine regression |
| TBIL | Serum total bilirubin |
| AST/ALT | Serum aspartate aminotransferase/alanine aminotransferase |
| A/G | Serum albumin/globulin |
| UA | Serum uric acid |
| Cr | Serum creatinine |
| UREA | Serum urea |
| 2-OH-Nap | 2-hydroxynaphthalene |
| 1-OH-Nap | 1-hydroxynaphthalene |
| 3-OH-Flu | 3-hydroxyfluorene |
| 2-OH-Flu | 2-hydroxyfluorene |
| 2/3-OH-Phe | 2/3-hydroxyphenanthrene |
| 4-OH-Phe | 4-hydroxyphenanthrene |
| 1/9-OH-Phe | 1/9-hydroxyphenanthrene |
| 1-OH-Pyr | 1-Hydroxy-Pyrene |
| 6-OH-Chr | 6-Hydroxychrysene |
| 3-OH-Bap | 3-Hydroxybenzo(A)Pyrene |
| 2-NapCA | 2-Naphthoic acid |
| 4-OH-NNap | 4-Nitro-1-naphthol |
| 5-OH-iQNL | 5-hydroxyisoquinoline |
| 3-OH-CBZ | 3-hydroxycarbazole |
| 2-OH-DBF | 2-hydroxydibenzofuran |
| 4-CCT | 4-Chlorocatechol |
| 3/4-moCP | 3/4-monochlorophenol |
| PCP | Pentachlorophenol |
| 2/4-NP | 2/4-Nitrophenol |
| 3-NP | 3-Nitrophenol |
| 3-M-4-NP | 3-methyl-4-nitrophenol |
| 8-OHdG | 8-hydroxy-2’-deoxyguanosine |
| 8-iso-PGF2α | 8-iso-prostaglandin-F2α |
| 8-iso-15-PGF2α | 8-iso,15(R)-prostaglandinF2α |
| 15-PGF2α | 15(R)-prostaglandinF2α |
| 4-OH-NMA | 4-hydroxy-nonenal-mercapturic acid |
References
- Kuang, D.; Zhang, W.; Deng, Q.; Zhang, X.; Huang, K.; Guan, L.; Hu, D.; Wu, T.; Guo, H. Dose-Response Relationships of Polycyclic Aromatic Hydrocarbons Exposure and Oxidative Damage to DNA and Lipid in Coke Oven Workers. Environ. Sci. Technol. 2013, 47, 7446–7456. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, B.; Yin, Z.; Jia, L.; Zhou, Y.; Jin, T. Increased Risk of Chronic Obstructive Pulmonary Diseases in Coke Oven Workers: Interaction between Occupational Exposure and Smoking. Thorax 2006, 61, 290–295. [Google Scholar] [CrossRef]
- Yang, L.; Yan, K.; Zeng, D.; Lai, X.; Chen, X.; Fang, Q.; Guo, H.; Wu, T.; Zhang, X. Association of Polycyclic Aromatic Hydrocarbons Metabolites and Risk of Diabetes in Coke Oven Workers. Environ. Pollut. 2017, 223, 305–310. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z. Developing a Profile of Urinary PAH Metabolites among Chinese Populations in the 2010s. Sci. Total Environ. 2023, 857, 159449. [Google Scholar] [CrossRef]
- Shi, S.; Qu, Y.; Ma, F.; Zhou, J. Bioremediation of Coking Wastewater Containing Carbazole, Dibenzofuran, Dibenzothiophene and Naphthalene by a Naphthalene-Cultivated Arthrobacter sp. W1. Bioresour. Technol. 2014, 164, 28–33. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, C.; Feng, C.; Ren, Y.; Hu, Y.; Yan, B.; Wu, C. The Occurrence and Fate of Phenolic Compounds in a Coking Wastewater Treatment Plant. Water Sci. Technol. 2013, 68, 433–440. [Google Scholar] [CrossRef]
- Moorthy, B.; Chu, C.; Carlin, D.J. Polycyclic Aromatic Hydrocarbons: From Metabolism to Lung Cancer. Toxicol. Sci. 2015, 145, 5–15. [Google Scholar] [CrossRef]
- Szabo, G.; Petrasek, J. Inflammasome Activation and Function in Liver Disease. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 387–400. [Google Scholar] [CrossRef]
- Hoekstra, L.T.; de Graaf, W.; Nibourg, G.A.A.; Heger, M.; Bennink, R.J.; Stieger, B.; van Gulik, T.M. Physiological and Biochemical Basis of Clinical Liver Function Tests: A Review. Ann. Surg. 2013, 257, 27–36. [Google Scholar] [CrossRef]
- Zhou, S.; Guo, C.; Dai, Y.; Pan, X.; Luo, X.; Qin, P.; Tan, L. Association between Polycyclic Aromatic Hydrocarbon Exposure and Liver Function: The Mediating Roles of Inflammation and Oxidative Stress. Environ. Pollut. 2024, 342, 123068. [Google Scholar] [CrossRef]
- Dai, M.; Luo, L.; Xie, C.; Chen, Z.; Zhang, M.; Xie, Y.; Shang, X.; Shen, X.; Tian, K.; Zhou, Y. Single and Joint Associations of Polycyclic Aromatic Hydrocarbon Exposure with Liver Function during Early Pregnancy. Toxics 2023, 11, 863. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Cramb, R.; Davison, S.M.; Dillon, J.F.; Foulerton, M.; Godfrey, E.M.; Hall, R.; Harrower, U.; Hudson, M.; Langford, A.; et al. Guidelines on the Management of Abnormal Liver Blood Tests. Gut 2018, 67, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Green, R.M.; Flamm, S. AGA Technical Review on the Evaluation of Liver Chemistry Tests. Gastroenterology 2002, 123, 1367–1384. [Google Scholar] [CrossRef] [PubMed]
- Oskay, T.; Keskin, C.; Ozen, M. Antioxidant and Inflammatory Biomarkers in Herpes Zoster. J. Med. Virol. 2022, 94, 3924–3929. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Deng, G.; Zheng, X.; Huang, Y.; Chen, J.; Meng, Z.; Gao, Y.; Qian, Z.; Liu, F.; et al. Association of AST/ALT Ratio with 90-Day Outcomes in Patients with Acute Exacerbation of Chronic Liver Disease: A Prospective Multicenter Cohort Study in China. Front. Med. 2024, 11, 1307901. [Google Scholar] [CrossRef]
- Ciardullo, S.; Oltolini, A.; Cannistraci, R.; Muraca, E.; Perseghin, G. Sex-Related Association of Nonalcoholic Fatty Liver Disease and Liver Fibrosis with Body Fat Distribution in the General US Population. Am. J. Clin. Nutr. 2022, 115, 1528–1534. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Tain, Y.-L. Adverse Impact of Environmental Chemicals on Developmental Origins of Kidney Disease and Hypertension. Front. Endocrinol. 2021, 12, 745716. [Google Scholar] [CrossRef]
- Diaz de Leon-Martinez, L.; Ortega-Romero, M.S.; Barbier, O.C.; Perez-Herrera, N.; May-Euan, F.; Perera-Rios, J.; Rodriguez-Aguilar, M.; Flores-Ramirez, R. Evaluation of Hydroxylated Metabolites of Polycyclic Aromatic Hydrocarbons and Biomarkers of Early Kidney Damage in Indigenous Children from Ticul, Yucatan, Mexico. Environ. Sci. Pollut. Res. 2021, 28, 52001–52013. [Google Scholar] [CrossRef]
- Kataria, A.; Trasande, L.; Trachtman, H. The Effects of Environmental Chemicals on Renal Function. Nat. Rev. Nephrol. 2015, 11, 610–625. [Google Scholar] [CrossRef]
- Sun, S.; Mao, W.; Tao, S.; Zou, X.; Tian, S.; Qian, S.; Yao, C.; Zhang, G.; Chen, M. Polycyclic Aromatic Hydrocarbons and the Risk of Kidney Stones in US Adults: An Exposure-Response Analysis of NHANES 2007-2012. Int. J. Gen. Med. 2021, 14, 2665–2676. [Google Scholar] [CrossRef]
- Ruan, F.; Wu, L.; Yin, H.; Fang, L.; Tang, C.; Huang, S.; Fang, L.; Zuo, Z.; He, C.; Huang, J. Long-Term Exposure to Environmental Level of Phenanthrene Causes Adaptive Immune Response and Fibrosis in Mouse Kidneys. Environ. Pollut. 2021, 283, 117028. [Google Scholar] [CrossRef] [PubMed]
- Kovalcikova, A.; Jansakova, K.; Gyuraszova, M.; Podracka, L.; Sebekova, K.; Celec, P.; Tothova, L. Salivary Creatinine and Urea Are Higher in an Experimental Model of Acute but Not Chronic Renal Disease. PLoS ONE 2018, 13, e0200391. [Google Scholar] [CrossRef] [PubMed]
- Kashani, K.; Rosner, M.H.; Ostermann, M. Creatinine: From Physiology to Clinical Application. Eur. J. Intern. Med. 2020, 72, 9–14. [Google Scholar] [CrossRef]
- Nashar, K.; Fried, L.F. Hyperuricemia and the Progression of Chronic Kidney Disease: Is Uric Acid a Marker or an Independent Risk Factor? Adv. Chronic Kidney Dis. 2012, 19, 386–391. [Google Scholar] [CrossRef]
- Idowu, O.; Semple, K.T.; Ramadass, K.; O’Connor, W.; Hansbro, P.; Thavamani, P. Beyond the Obvious: Environmental Health Implications of Polar Polycyclic Aromatic Hydrocarbons. Environ. Int. 2019, 123, 543–557. [Google Scholar] [CrossRef]
- Wang, W.; Jariyasopit, N.; Schrlau, J.; Jia, Y.; Tao, S.; Yu, T.-W.; Dashwood, R.H.; Zhang, W.; Wang, X.; Simonich, S.L.M. Concentration and Photochemistry of PAHs, NPAHs, and OPAHs and Toxicity of PM 2.5 during the Beijing Olympic Games. Environ. Sci. Technol. 2011, 45, 6887–6895. [Google Scholar] [CrossRef]
- Titaley, I.A.; Chlebowski, A.; Truong, L.; Tanguay, R.L.; Massey Simonich, S.L. Identification and Toxicological Evaluation of Unsubstituted PAHs and Novel PAH Derivatives in Pavement Sealcoat Products. Environ. Sci. Technol. Lett. 2016, 3, 234–242. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Martinez, M.P.; Kannan, K. Simultaneous Analysis of Seven Biomarkers of Oxidative Damage to Lipids, Proteins, and DNA in Urine. Environ. Sci. Technol. 2018, 52, 6647–6655. [Google Scholar] [CrossRef]
- Kuang, H.; Li, Y.; Li, L.; Ma, S.; An, T.; Fan, R. Four-Year Population Exposure Study: Implications for the Effectiveness of e-Waste Control and Biomarkers of e-Waste Pollution. Sci. Total Environ. 2022, 842, 156595. [Google Scholar] [CrossRef]
- Chiou, C.C.; Chang, P.Y.; Chan, E.C.; Wu, T.L.; Tsao, K.C.; Wu, J.T. Urinary 8-Hydroxydeoxyguano Sine and Its Analogs as DNA Marker of Oxidative Stress: Development of an ELISA and Measurement in Both Bladder and Prostate Cancers. Clin. Chim. Acta 2003, 334, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, S.-T.; Peng, K.-H.; Cheng, T.-J.; Wu, K.-Y. Concurrent Quantification of Multiple Biomarkers Indicative of Oxidative Stress Status Using Liquid Chromatography-Tandem Mass Spectrometry. Anal. Biochem. 2016, 512, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-W.; Chen, M.-L.; Huang, L.-W.; Yang, W.; Wu, K.-Y.; Huang, Y.-F. Prenatal Nonylphenol Exposure, Oxidative and Nitrative Stress, and Birth Outcomes: A Cohort Study in Taiwan. Environ. Pollut. 2015, 207, 145–151. [Google Scholar] [CrossRef]
- Il’yasova, D.; Scarbrough, P.; Spasojevic, I. Urinary Biomarkers of Oxidative Status. Clin. Chim. Acta 2012, 413, 1446–1453. [Google Scholar] [CrossRef]
- Montuschi, P.; Barnes, P.J.; Roberts, L.J. Isoprostanes: Markers and Mediators of Oxidative Stress. Faseb J. 2004, 18, 1791–1800. [Google Scholar] [CrossRef]
- Ai, Q.; Gao, L.; Huang, D.; Yang, J.; Fu, Q.; Zheng, X.; Liu, Y.; Qiao, L.; Weng, J.; Zheng, M. Non-Target and Target Analysis to Identify and Characterize Thiophenes in Soil from an Abandoned Coking Plant. J. Hazard. Mater. 2023, 460, 132444. [Google Scholar] [CrossRef]
- Guan, X.; Fu, W.; Wei, W.; Li, G.; Wu, X.; Bai, Y.; Feng, Y.; Meng, H.; Li, H.; Li, M.; et al. Mediation of the Association between Polycyclic Aromatic Hydrocarbons Exposure and Telomere Attrition by Oxidative Stress: A Prospective Cohort Study. J. Hazard. Mater. 2020, 399, 123058. [Google Scholar] [CrossRef]
- Farzan, S.F.; Chen, Y.; Trachtman, H.; Trasande, L. Urinary Polycyclic Aromatic Hydrocarbons and Measures of Oxidative Stress, Inflammation and Renal Function in Adolescents: NHANES 2003-2008. Environ. Res. 2016, 144, 149–157. [Google Scholar] [CrossRef]
- Li, H.; Yao, C.; He, C.; Yu, H.; Yue, C.; Zhang, S.; Li, G.; Ma, S.; Zhang, X.; Cao, Z.; et al. Coking-Produced Aromatic Compounds in Urine of Exposed and Nonexposed Populations: Exposure Levels, Source Identification, and Model-Based Health Implications. Environ. Sci. Technol. 2023, 57, 15379–15391. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.; Yao, C.; He, R.; Lu, P.; Li, G.; Liu, R.; Ma, S.; Zhang, X.; Cao, Z.; et al. Nontarget Screening of Novel Urinary Biomarkers for Occupational Exposure to Toxic Chemicals from Coking Industry Using HPLC-QTOF-MS. Environ. Sci. Technol. 2023, 57, 13004–13014. [Google Scholar] [CrossRef]
- Bobb, J.F.; Valeri, L.; Claus Henn, B.; Christiani, D.C.; Wright, R.O.; Mazumdar, M.; Godleski, J.J.; Coull, B.A. Bayesian Kernel Machine Regression for Estimating the Health Effects of Multi-Pollutant Mixtures. Biostatistics 2015, 16, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Bobb, J.F.; Claus Henn, B.; Valeri, L.; Coull, B.A. Statistical Software for Analyzing the Health Effects of Multiple Concurrent Exposures via Bayesian Kernel Machine Regression. Environ. Health 2018, 17, 67. [Google Scholar] [CrossRef]
- MacKinnon, D.P.; Lockwood, C.M.; Hoffman, J.M.; West, S.G. A Comparison of Methods to Test Mediation and Other Intervening Variable Effects. Psychol Methods 2002, 7, 83–104. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, D.P.; Warsi, G.; Dwyer, J.H. A Simulation Study of Mediated Effect Measures. Multivar. Behav. Res. 1995, 30, 41–62. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D.R. Sex Differences in Pharmacokinetics and Pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Zeweil, M.M.; Khafaga, A.F.; Mahmoud, S.F.; Wasef, L.; Saleh, H.; Abd Elrehim, A.M.; Bassuoni, N.F.; Alwaili, M.A.; Saeedi, N.H.; Ghoneim, H.A. Annona Muricata L. Extract Restores Renal Function, Oxidative Stress, Immunohistochemical Structure, and Gene Expression of TNF-α, IL-Β1, and CYP2E1 in the Kidney of DMBA-Intoxicated Rats. Front. Pharmacol. 2024, 15, 1348145. [Google Scholar] [CrossRef]
- Yuan, T.-H.; Ke, D.-Y.; Wang, J.E.-H.; Chan, C.-C. Associations between Renal Functions and Exposure of Arsenic and Polycyclic Aromatic Hydrocarbon in Adults Living near a Petrochemical Complex. Environ. Pollut. 2020, 256, 113457. [Google Scholar] [CrossRef]
- Liao, Q.; Huang, L.; Cai, F.; Luo, W.; Li, M.; Yang, J.; Tang, B.; Xiao, X.; Yan, X.; Zheng, J. Metabolomics Perspectives into the Co-Exposure Effect of Polycyclic Aromatic Hydrocarbons and Metals on Renal Function: A Meet-in-the-Middle Approach. Sci. Total Environ. 2024, 921, 170975. [Google Scholar] [CrossRef]
- Peng, S.; Lu, T.; Liu, Y.; Li, Z.; Liu, F.; Sun, J.; Chen, M.; Wang, H.; Xiang, H. Short-Term Exposure to Fine Particulate Matter and Its Constituents May Affect Renal Function via Oxidative Stress: A Longitudinal Panel Study. Chemosphere 2022, 293, 133570. [Google Scholar] [CrossRef]
- Guo, C.; Liu, X.; Liao, X.; Wu, H.; Zhang, Z.; Wu, D.; Ma, R.; Huang, Y.; Zhao, N.; Xiao, Y.; et al. Associations of Co-Exposure to Polycyclic Aromatic Hydrocarbons and Metals with Hyperuricemia Risk in Chinese Coke Oven Workers: Mediating Roles of Oxidative Damage*. Environ. Pollut. 2023, 318, 120891. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Q.; Liang, J.; Weng, Z.; Xu, J.; Jiang, Z.; Gu, A. Urinary Biomarkers of Polycyclic Aromatic Hydrocarbons and Their Associations with Liver Function in Adolescents. Environ. Pollut. 2021, 278, 116842. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ma, J.; Chen, D.; Gao, Y.; Li, G.; An, T. Associations between Inhalation of Typical Volatile and Semi-Volatile Organic Compounds in e-Waste Dismantling Workers with Liver Function Damage. J. Hazard. Mater. 2024, 464, 133004. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Cheng, J.; Song, J.; Pan, R.; Liang, Y.; Sun, X.; Li, Y.; Wu, Y.; Yan, S.; Jin, X.; et al. Associations of Polycyclic Aromatic Hydrocarbons, Water-Soluble Ions and Metals in PM2.5 with Liver Function: Evidence from Schizophrenia Cohort. Sci. Total Environ. 2023, 868, 161624. [Google Scholar] [CrossRef]
- van Meteren, N.; Lagadic-Gossmann, D.; Podechard, N.; Gobart, D.; Gallais, I.; Chevanne, M.; Collin, A.; Burel, A.; Dupont, A.; Rault, L.; et al. Extracellular Vesicles Released by Polycyclic Aromatic Hydrocarbons-Treated Hepatocytes Trigger Oxidative Stress in Recipient Hepatocytes by Delivering Iron. Free Radic. Biol. Med. 2020, 160, 246–262. [Google Scholar] [CrossRef]
- Tao, L.-P.; Li, X.; Zhao, M.-Z.; Shi, J.-R.; Ji, S.-Q.; Jiang, W.-Y.; Liang, Q.-J.; Lei, Y.-H.; Zhou, Y.-Y.; Cheng, R.; et al. Chrysene, a Four-Ring Polycyclic Aromatic Hydrocarbon, Induces Hepatotoxicity in Mice by Activation of the Aryl Hydrocarbon Receptor (AhR). Chemosphere 2021, 276, 130108. [Google Scholar] [CrossRef]
- Hua, L.; Gao, Y.; Guo, S.; Zhu, H.; Yao, Y.; Wang, B.; Fang, J.; Sun, H.; Xu, F.; Zhao, H. Urinary Metabolites of Polycyclic Aromatic Hydrocarbons of Rural Population in Northwestern China: Oxidative Stress and Health Risk Assessment. Environ. Sci. Technol. 2024, 58, 7758–7769. [Google Scholar] [CrossRef]
- Khan, F.; Jaoui, M.; Rudzinski, K.; Kwapiszewska, K.; Martinez-Romero, A.; Gil-Casanova, D.; Lewandowski, M.; Kleindienst, T.E.; Offenberg, J.H.; Krug, J.D.; et al. Cytotoxicity and Oxidative Stress Induced by Atmospheric Mono-Nitrophenols in Human Lung Cells. Environ. Pollut. 2022, 301, 119010. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxidative Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef]
- Yang, H.; Shi, Z.; Wang, X.-X.; Cheng, R.; Lu, M.; Zh, J.; Deng, W.; Zeng, Y.; Zhao, L.-Y.; Zhang, S.-Y. Phenanthrene, but Not Its Isomer Anthracene, Effectively Activates Both Human and Mouse Nuclear Receptor Constitutive Androstane Receptor (CAR) and Induces Hepatotoxicity in Mice. Toxicol. Appl. Pharmacol. 2019, 378, 114618. [Google Scholar] [CrossRef]
- Lam, S.H.; Ung, C.Y.; Hlaing, M.M.; Hu, J.; Li, Z.-H.; Mathavan, S.; Gong, Z. Molecular Insights into 4-Nitrophenol-Induced Hepatotoxicity in Zebrafish: Transcriptomic, Histological and Targeted Gene Expression Analyses. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 4778–4789. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, Y.; Ruan, J.; Huang, D.; Xiao, J.; Zhao, X.; Li, J.; Qu, J.; Wang, X. The Association Between Brominated Flame Retardants Exposure and Liver-Related Biomarkers in US Adults. Toxics 2024, 12, 852. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.W.; Joubert, B.R.; Braun, J.M.; Dilworth, C.; Gennings, C.; Hauser, R.; Heindel, J.J.; Rider, C.V.; Webster, T.F.; Carlin, D.J. Statistical Approaches for Assessing Health Effects of Environmental Chemical Mixtures in Epidemiology: Lessons from an Innovative Workshop. Environ. Health Perspect. 2016, 124, A227–A229. [Google Scholar] [CrossRef] [PubMed]
- Brüggenwirth, I.M.A.; Lantinga, V.A.; Lascaris, B.; Thorne, A.M.; Meerdink, M.; de Kleine, R.H.; Blokzijl, H.; van den Berg, A.P.; Reyntjens, K.M.; Lisman, T.; et al. Prolonged Hypothermic Machine Perfusion Enables Daytime Liver Transplantation—An IDEAL Stage 2 Prospective Clinical Trial. eClinicalMedicine 2024, 68, 102411. [Google Scholar] [CrossRef]

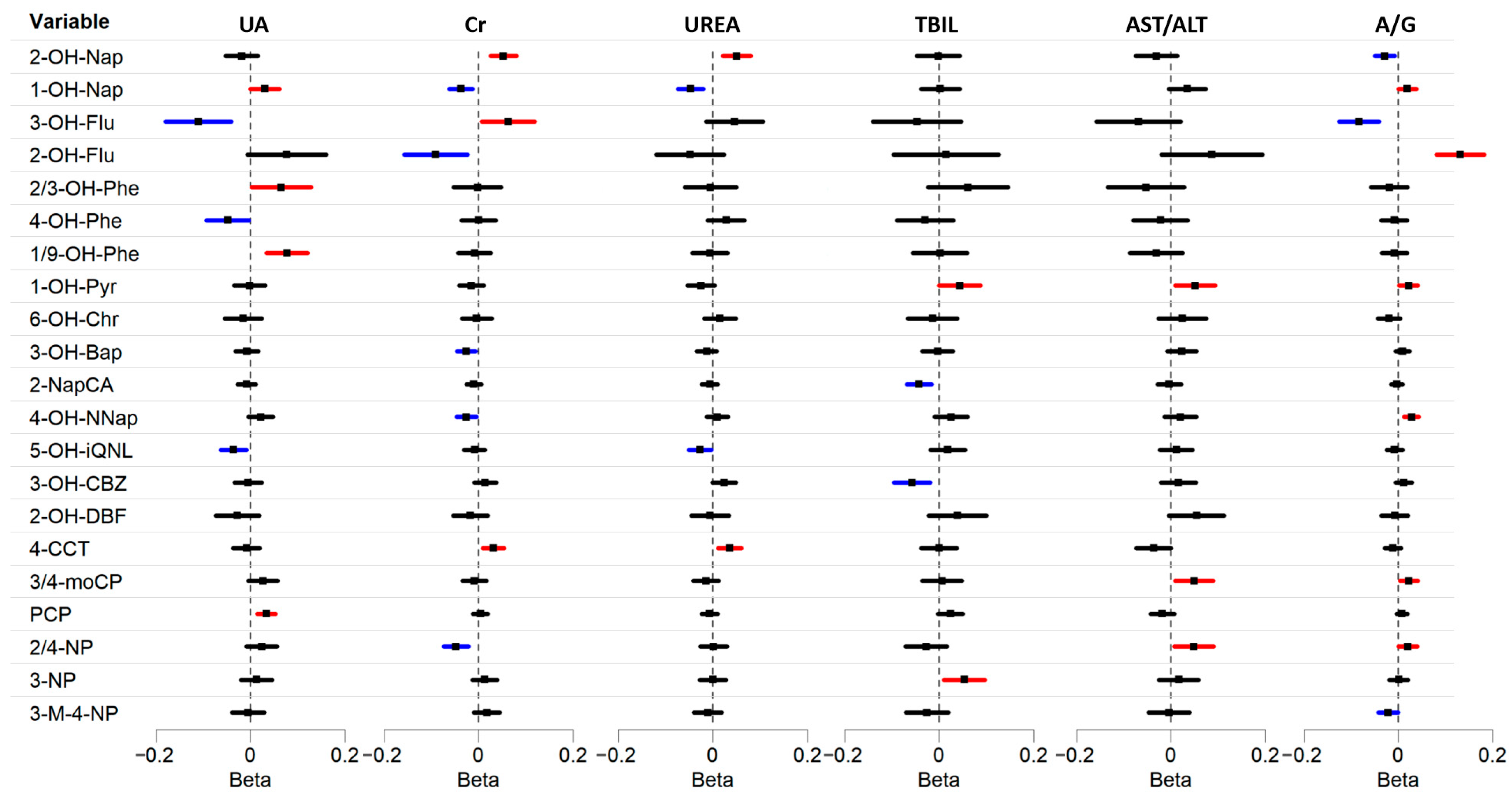

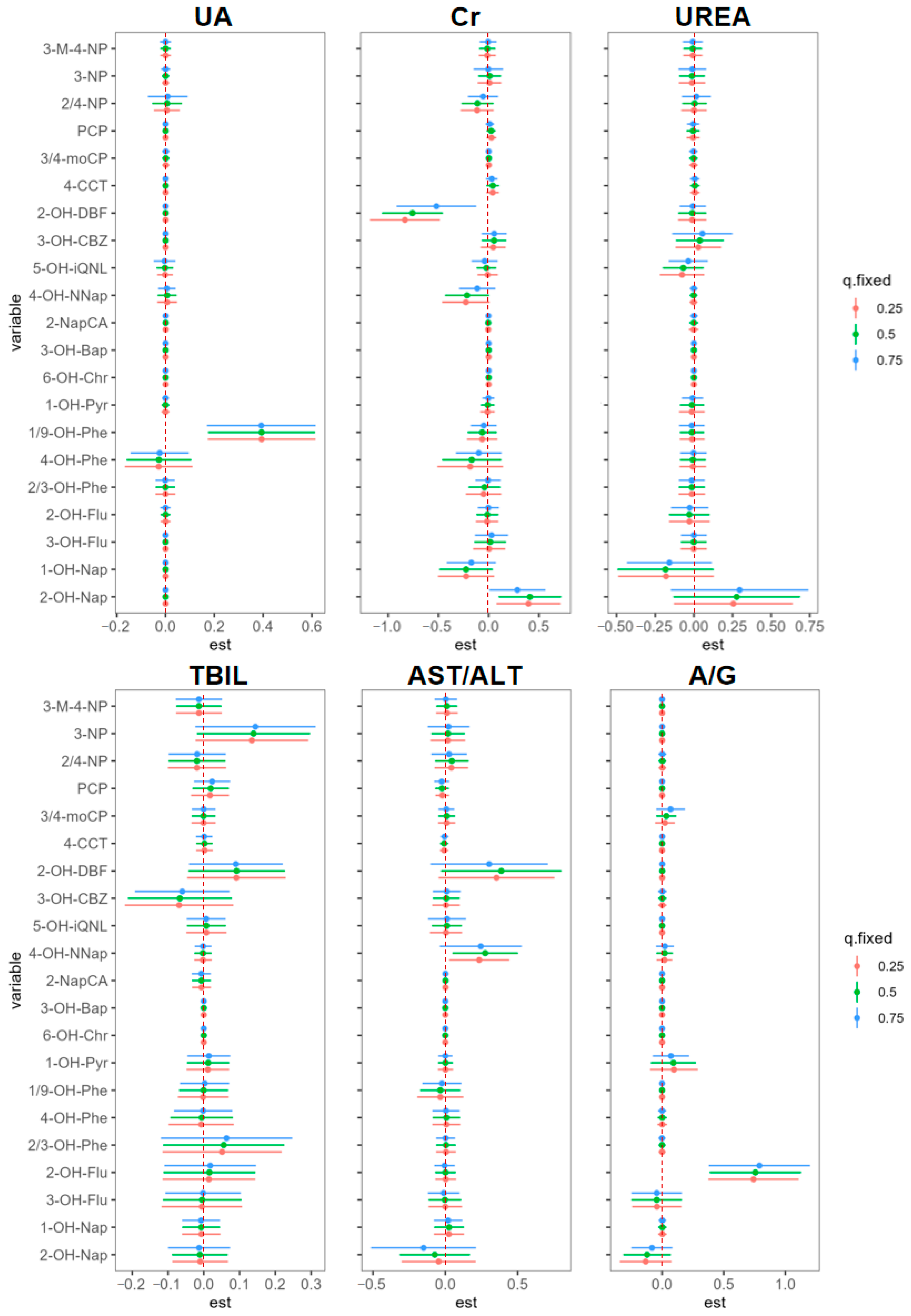
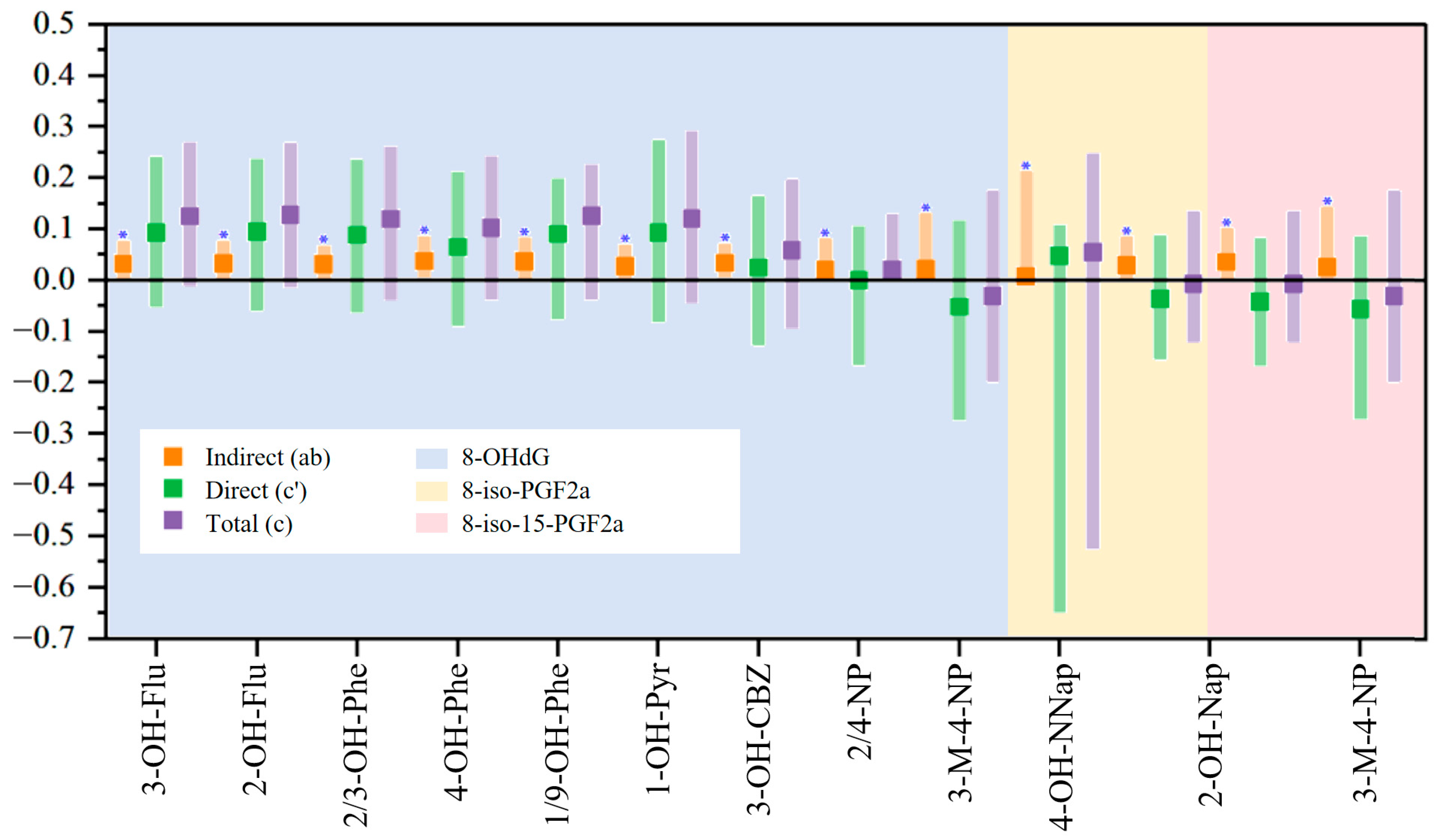
| Characteristic | Worker (n = 226) | Resident (n = 163) | Control (n = 248) |
|---|---|---|---|
| Age | 45.88 [35.71, 51.15] | 57.38 [49.7, 64.59] | 51 [39.25, 58] |
| Sex (%) | |||
| Male | 174 (77) | 46 (28.2) | 77 (31) |
| Female | 52 (23) | 117 (71.8) | 171 (69) |
| Smoking Status (%) | |||
| Current smokers | 87 (38.5) | 125 (76.7) | 190 (76.6) |
| Other | 139 (61.5) | 38 (23.3) | 58 (23.4) |
| Drinking Status (%) | |||
| Current drinkers | 102 (45.1) | 133 (81.6) | 219 (88.3) |
| Other | 124 (54.9) | 30 (18.4) | 29 (11.7) |
| Kidney function biomarkers | |||
| Cr (μmol/L) | 64.43 [56.78, 72.15] | 53.25 [48.09, 60.14] | 84.11 [73.37, 93.35] |
| UREA (mmol/L) | 5.07 [4.43, 5.89] | 4.91 [4.19, 5.73] | 5.5 [4.6, 6.7] |
| UA (μmol/L) | 337.5 [278, 407.33] | 275 [221.75, 324.25] | 260.75 [213.73, 311.53] |
| Liver function biomarkers | |||
| TBIL (μmol/L) | 15.05 [11, 13.6, 17.3] | 12.48 [9.64, 14.28] | 13.14 [9, 15] |
| AST/ALT (%) | 1 [0.73, 1.16] | 1.18 [0.92, 1.4] | 0.74 [0.61, 0.82] |
| A/G (%) | 1.69 [1.53, 1.84] | 1.46 [1.35, 1.59] | 1.23 [1.11, 1.31] |
| Urine AC concentration (ng/mL) | |||
| 2-OH-Nap | 20.16 [8.52, 46.3] | 6.97 [3.33, 13.72] | 4.34 [2.06, 9.98] |
| 1-OH-Nap | 18.53 [8.47, 33.97] | 7.39 [3.13, 15.19] | 2.6 [1.53, 5.92] |
| 3-OH-Flu | 3.89 [2.39, 8.32] | 1.23 [0.74, 2.02] | 0.7 [0.46, 1.24] |
| 2-OH-Flu | 8.41 [4.65, 16.78] | 2.11 [1.27, 3.74] | 0.87 [0.52, 1.75] |
| 2/3-OH-Phe | 1.16 [0.67, 2.59] | 0.52 [0.35, 0.9] | 0.23 [0.15, 0.4] |
| 4-OH-Phe | 1.31 [0.77, 2.55] | 0.63 [0.38, 1.17] | 0.32 [0.19, 0.54] |
| 1/9-OH-Phe | 0.27 [0.15, 0.48] | 0.11 [0.07, 0.21] | 0.07 [0.03, 0.11] |
| 1-OH-Pyr | 4.56 [2.12, 11.9] | 0.9 [0.48, 1.55] | 0.39 [0.21, 0.73] |
| 6-OH-Chr | 0 [0, 0.03] | 0 [0, 0.01] | 0 [0, 0] |
| 3-OH-Bap | 0.01 [0, 0.12] | 0.01 [0, 0.07] | 0 [0, 0.01] |
| 2-NapCA | 1.43 [0.65, 3.77] | 0.66 [0.26, 2.34] | 0.3 [0.13, 0.78] |
| 4-OH-NNap | 0.08 [0.05, 0.15] | 0.06 [0.03, 0.1] | 0.02 [0.01, 0.04] |
| 5-OH-iQNL | 7.91 [5.62, 12.37] | 6.94 [3.53, 12.84] | 5.37 [2.94, 9.67] |
| 3-OH-CBZ | 0.69 [0.36, 1.54] | 0.2 [0.11, 0.4] | 0.12 [0.06, 0.31] |
| 2-OH-DBF | 9.94 [5.78, 18.72] | 2.6 [1.67, 5.01] | 1.32 [0.81, 2.35] |
| 4-CCT | 0.62 [0.39, 1.05] | 0.52 [0.3, 0.83] | 0.44 [0.28, 0.81] |
| 3/4-moCP | 0.05 [0.03, 0.09] | 0.03 [0.02, 0.05] | 0.03 [0, 0.04] |
| PCP | 0.09 [0.04, 0.28] | 0.02 [0.01, 0.08] | 0.03 [0, 0.07] |
| 2/4-NP | 1.63 [1, 2.29] | 1.39 [0.82, 2.3] | 0.68 [0.39, 1.14] |
| 3-NP | 0.05 [0.03, 0.07] | 0.03 [0.02, 0.05] | 0.02 [0, 0.04] |
| 3-M-4-NP | 0.12 [0.07, 0.21] | 0.1 [0.06, 0.23] | 0.04 [0, 0.09] |
| Total | 102 [21.2–1150] | 57.0 [11.5–523] | 30.7 [7.72–798] |
| OSB concentration (ng/mL) | |||
| 8-OHdG | 0.81 [0.54, 1.08] | --- | --- |
| 4-OH-NMA | 45.26 [11.03, 129.23] | --- | --- |
| 8-iso-PGF2α | 0.61 [0.46, 0.9] | --- | --- |
| 8-iso-15-PGF2α | 0.35 [0.24, 0.58] | --- | --- |
| 15-PGF2α | 1.13 [0.79, 1.63] | --- | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Yu, H.; Li, H.; Li, G.; An, T. Associations Between Aromatic Compounds and Hepatorenal Biomarkers Among Coking Workers: Insights from Mediation Analysis. Toxics 2025, 13, 298. https://doi.org/10.3390/toxics13040298
Chen D, Yu H, Li H, Li G, An T. Associations Between Aromatic Compounds and Hepatorenal Biomarkers Among Coking Workers: Insights from Mediation Analysis. Toxics. 2025; 13(4):298. https://doi.org/10.3390/toxics13040298
Chicago/Turabian StyleChen, Dongming, Hang Yu, Hailing Li, Guiying Li, and Taicheng An. 2025. "Associations Between Aromatic Compounds and Hepatorenal Biomarkers Among Coking Workers: Insights from Mediation Analysis" Toxics 13, no. 4: 298. https://doi.org/10.3390/toxics13040298
APA StyleChen, D., Yu, H., Li, H., Li, G., & An, T. (2025). Associations Between Aromatic Compounds and Hepatorenal Biomarkers Among Coking Workers: Insights from Mediation Analysis. Toxics, 13(4), 298. https://doi.org/10.3390/toxics13040298









