Novel Sodium Carbonate Activation for Manufacturing Sludge-Based Biochar and Assessment of Its Organic Adsorption Property in Treating Wool Scouring Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sludge-Based Biochar
2.2. Characterization Methods of Sludge-Based Biochar
2.3. Adsorption Experiments of Sludge-Based Biochar
3. Results and Discussion
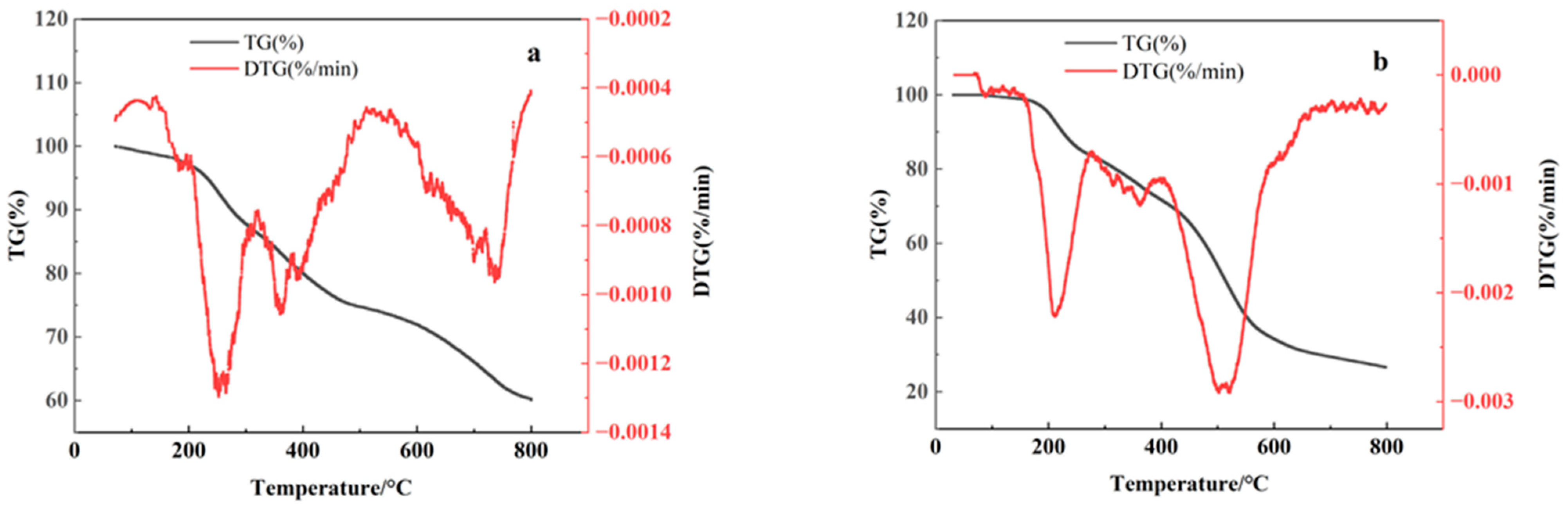
3.1. Optimization of Carbonization Temperature for Sludge-Based Biochar
3.2. Structural Characteristics Analysis
3.2.1. Specific Surface Area and Pore Size Distribution
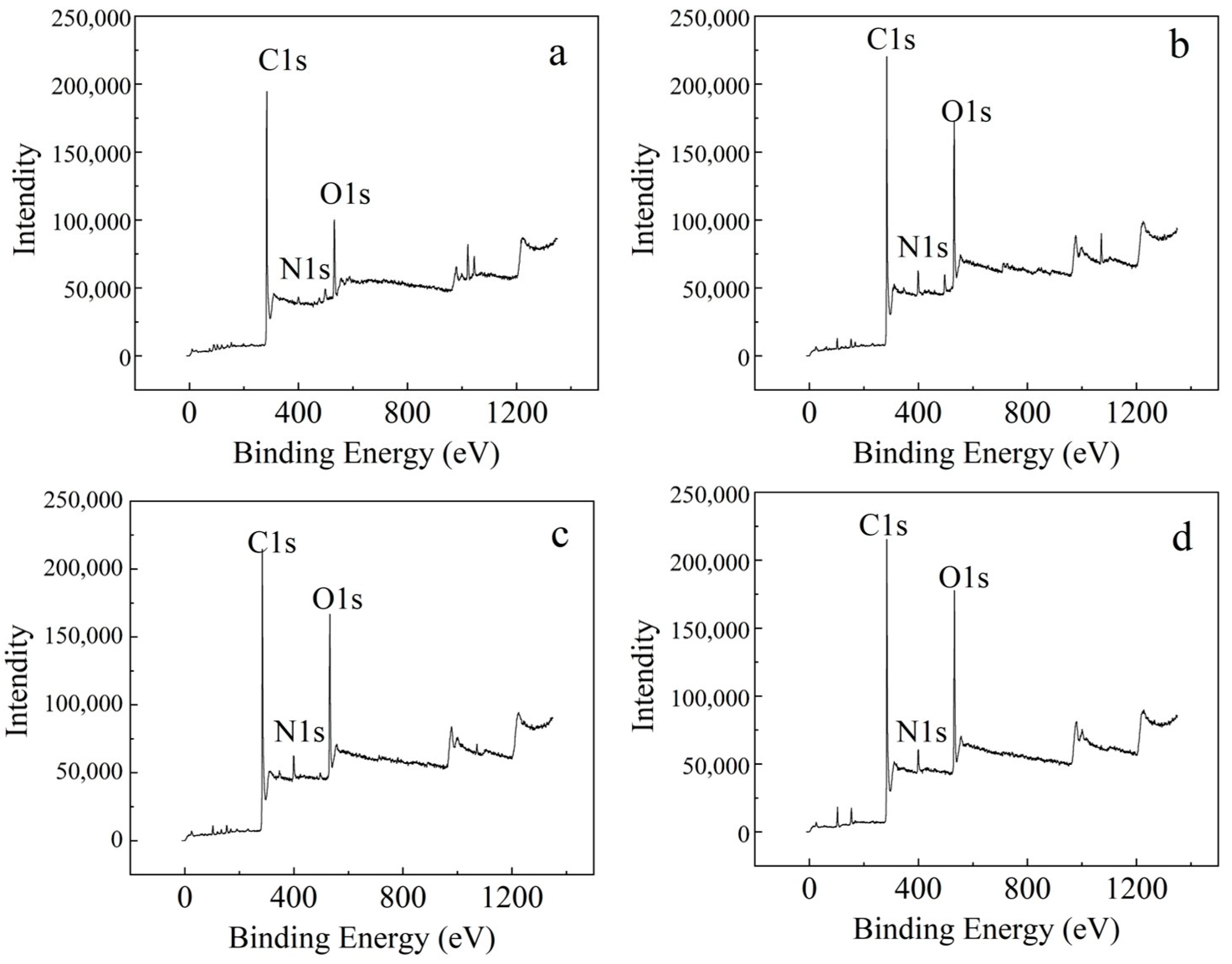
3.2.2. Elemental Composition
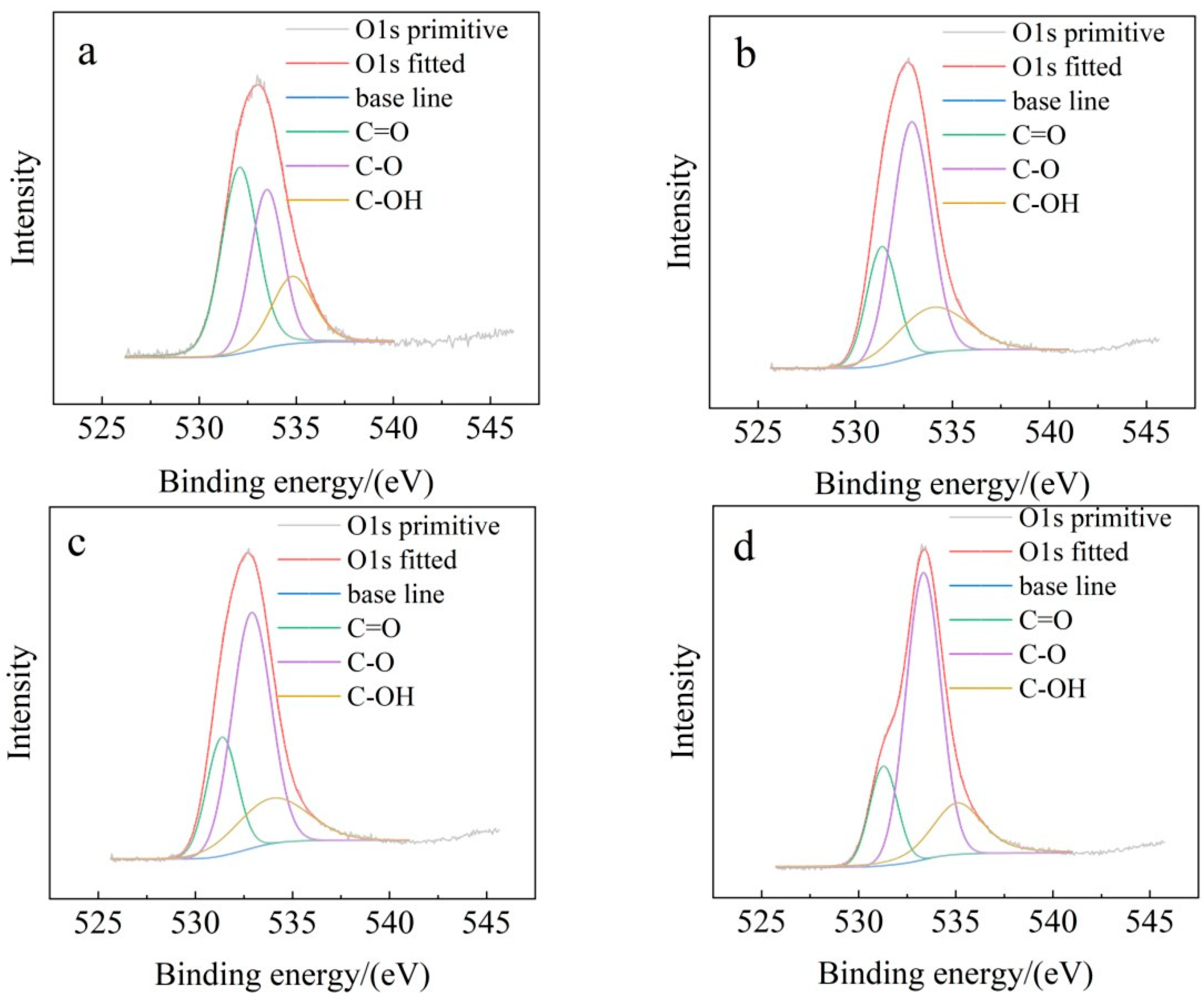
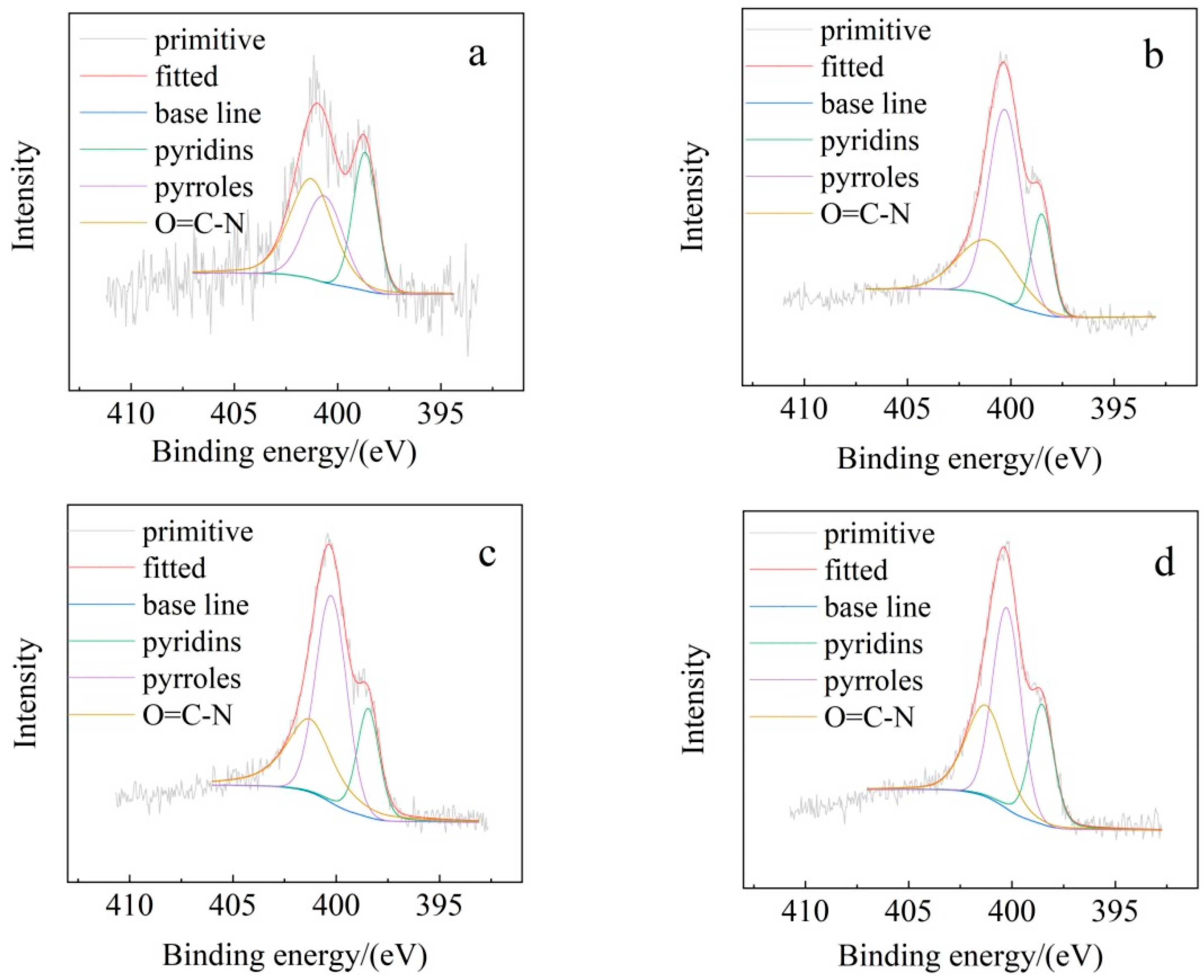
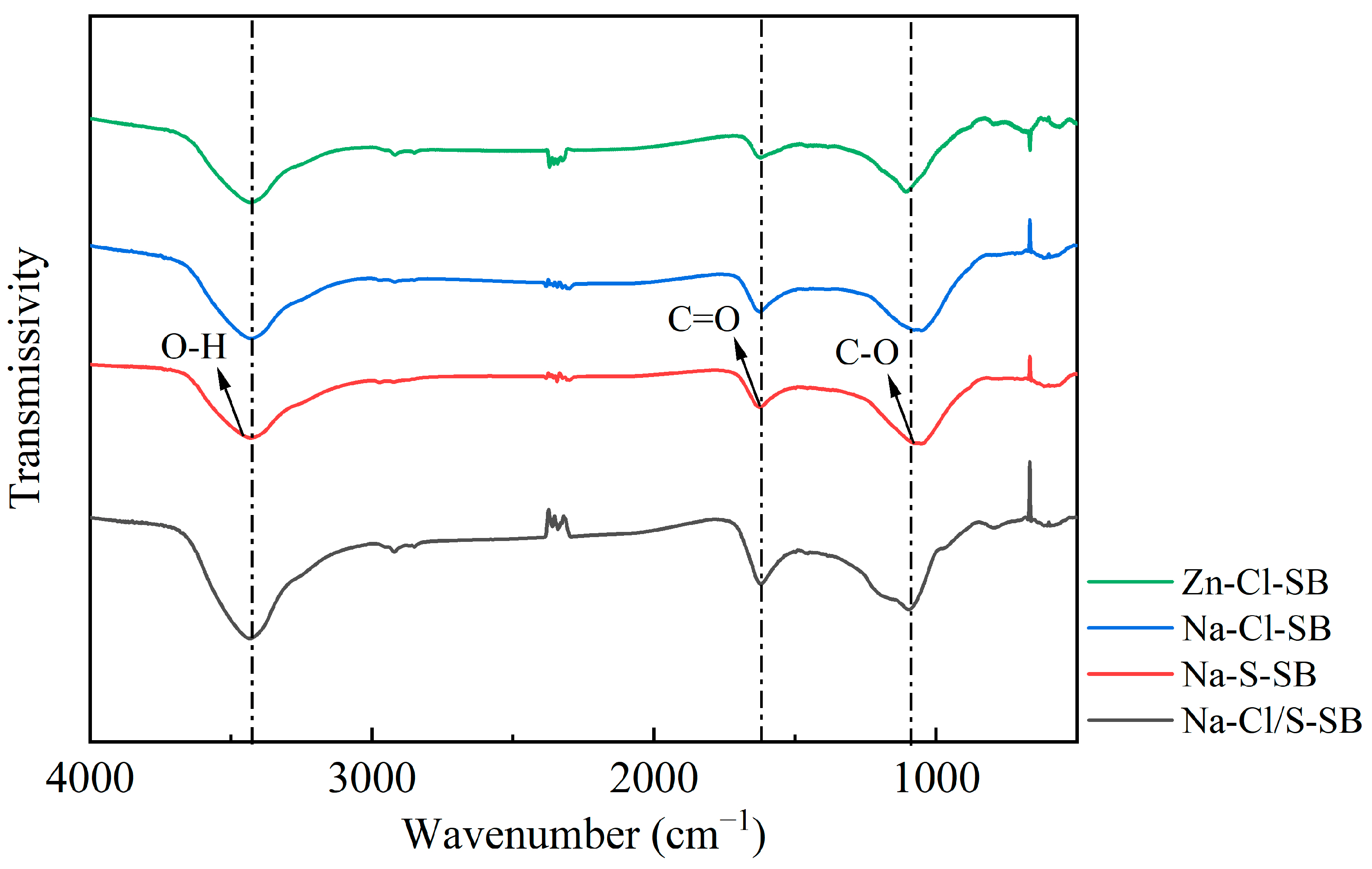
3.2.3. Functional Group
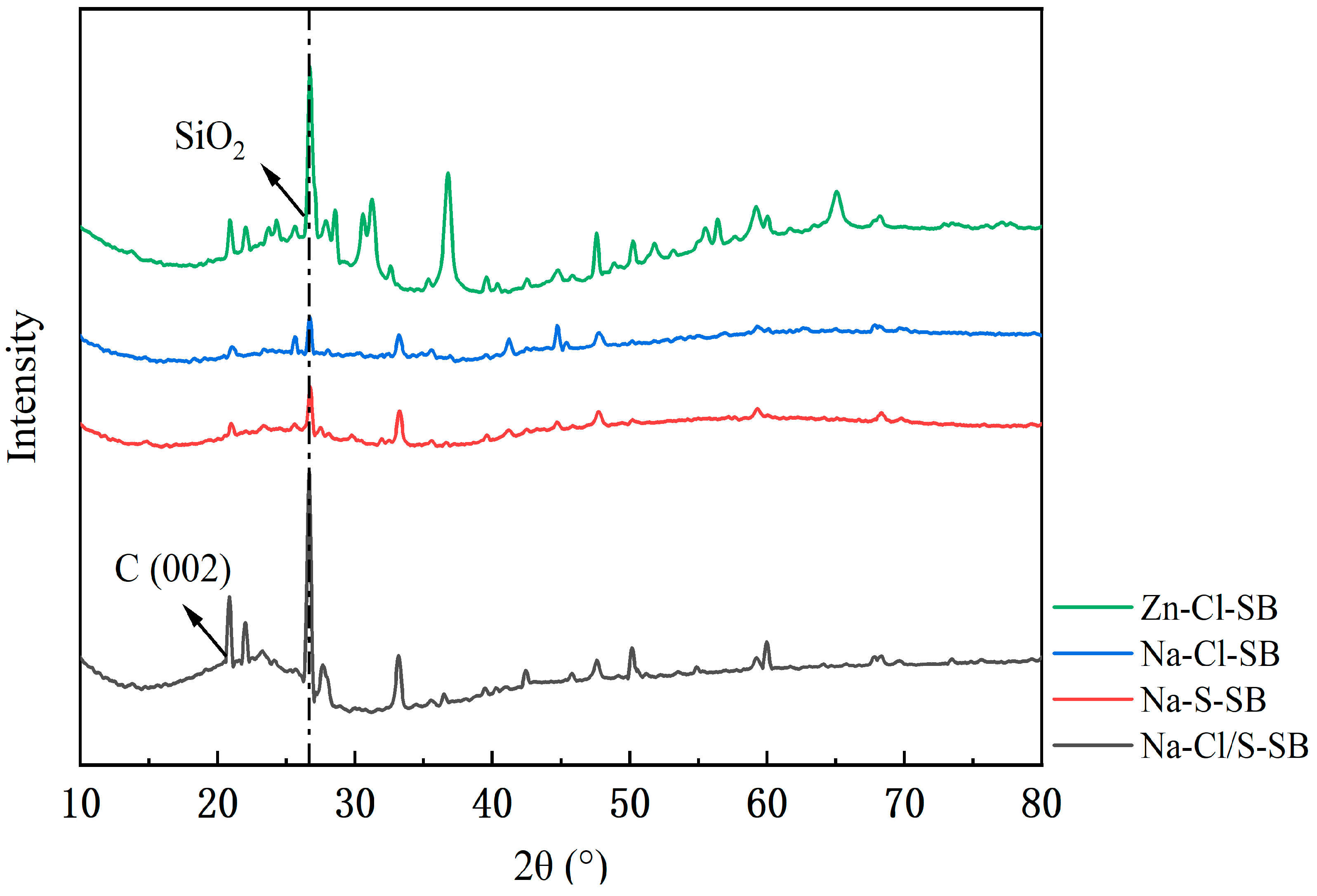
3.2.4. Crystal Structure
3.3. Adsorption Performance for Wool Scouring Wastewater
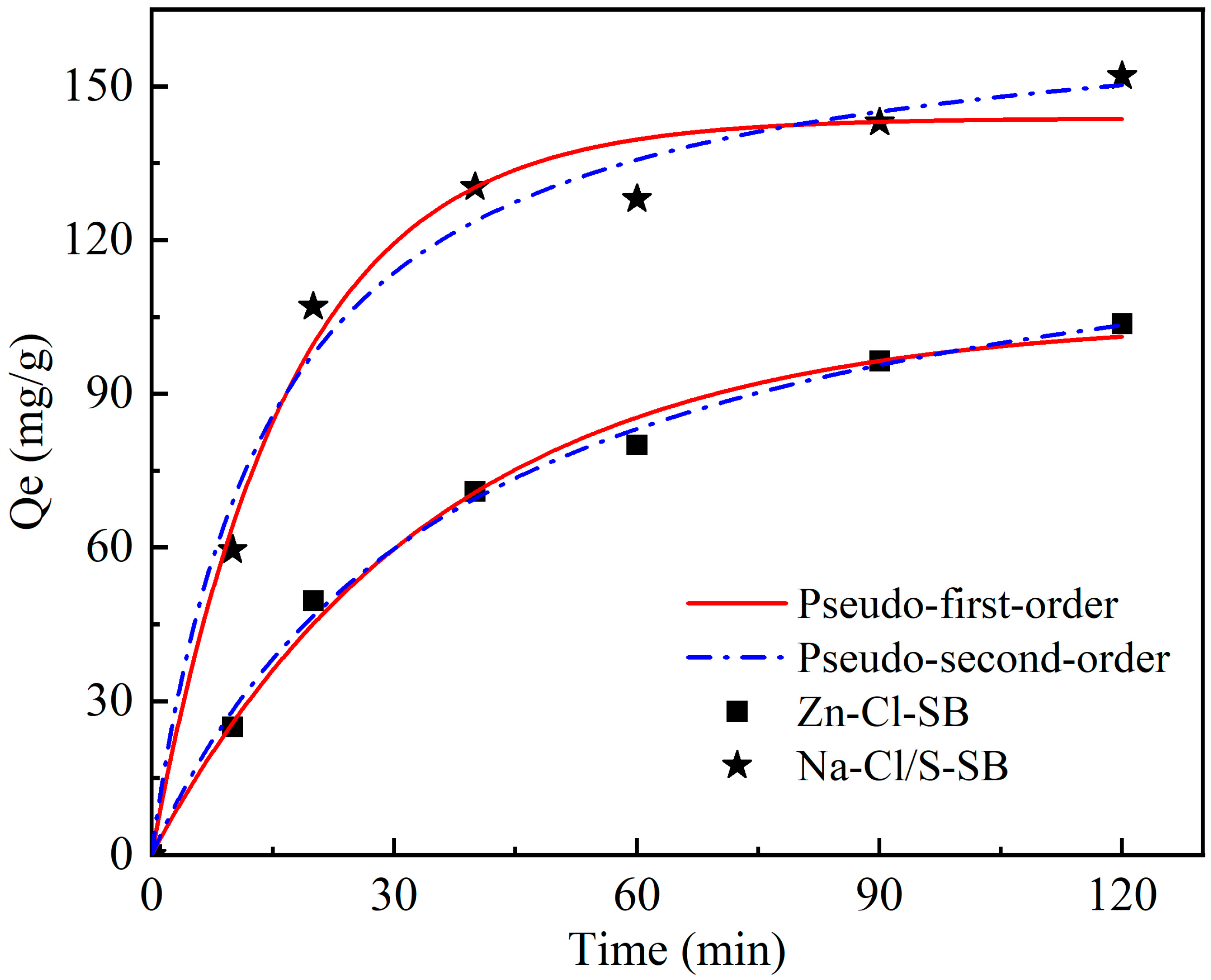
3.3.1. Organic Matter Adsorption Kinetic Analysis
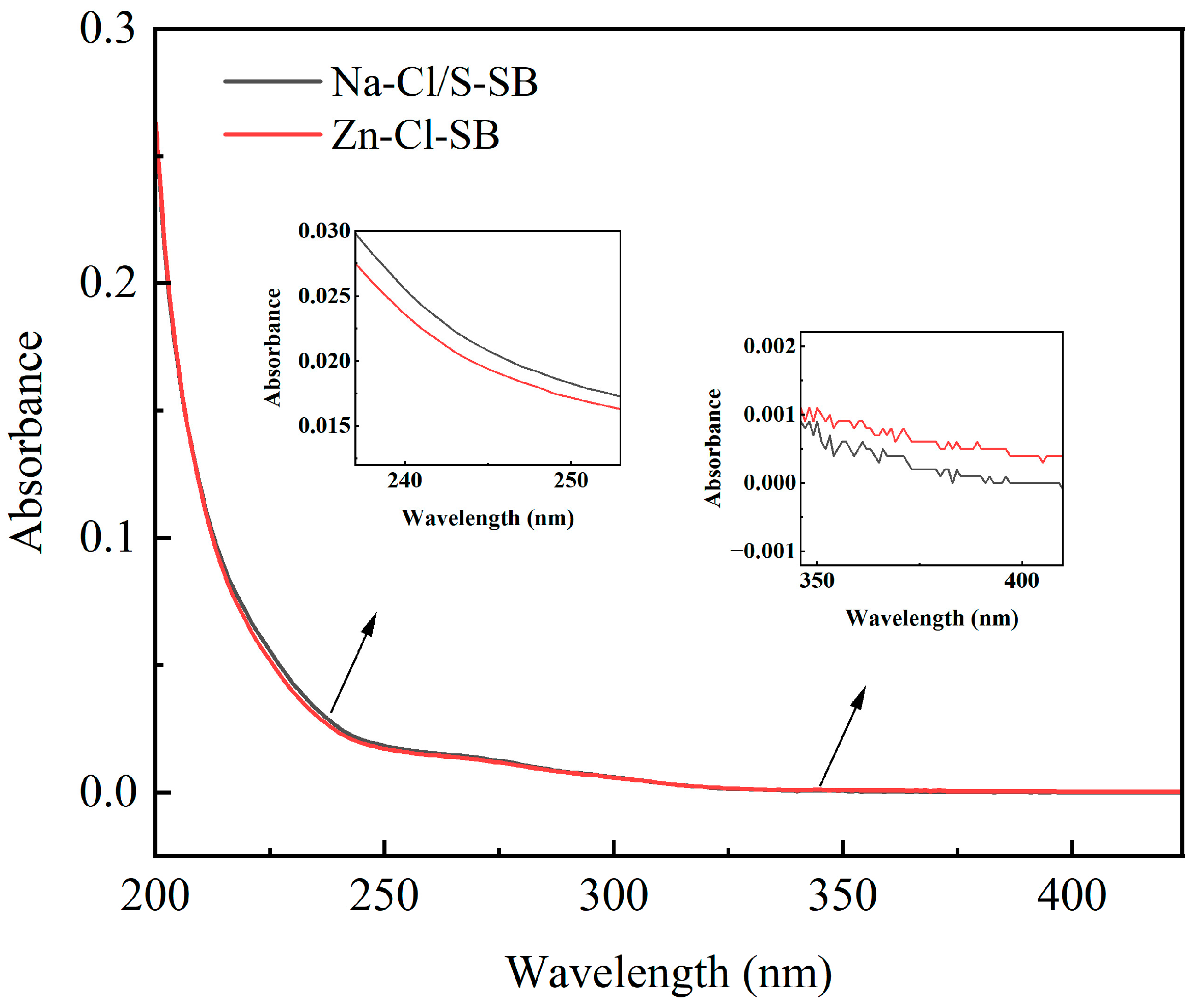
3.3.2. UV-Vis Spectra Analysis
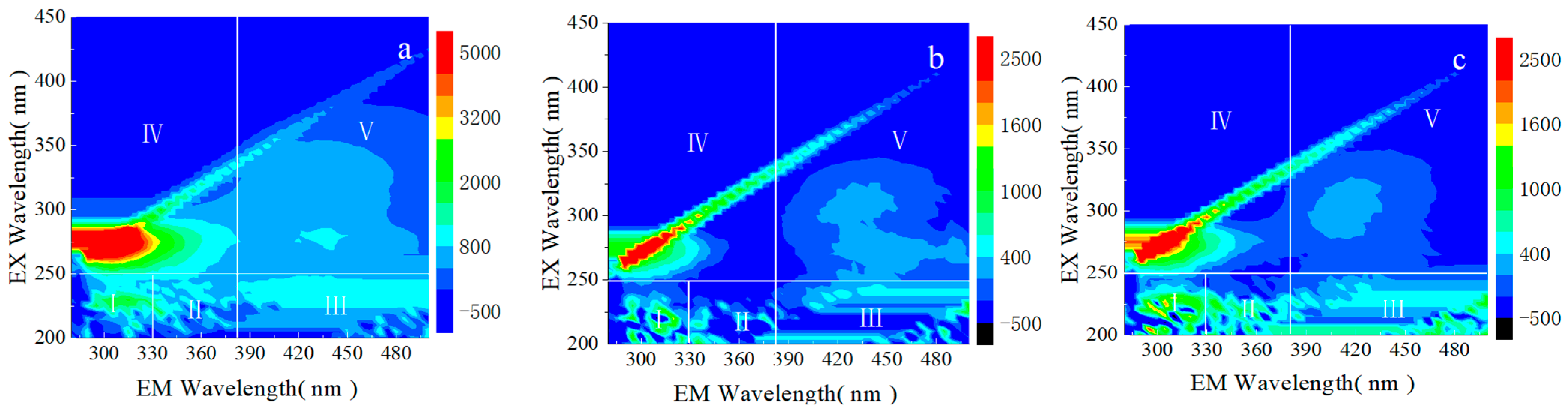
3.3.3. EEM Spectra Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SB | Sludge-based biochar |
| MLSSs | Mixed liquor suspended solids |
| MLVSSs | Mixed liquor volatile suspended solids |
| COD | Chemical oxygen demand |
| NDRC | National development and reform commission |
| PFASs | Perfluoroalkyl and polyfluoroalkyl substances |
| TGA | Thermogravimetric analysis |
| DTG | Derivative thermogravimetry |
| BET | Brunauer–Emmett–Teller |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| FTIR | Fourier-transform infrared spectroscopy |
| EEM | Excitation–emission matrix spectroscopy |
| DOM | Dissolved organic matter |
| PFO | Pseudo-first-order |
| PSO | Pseudo-second-order |
References
- Lin, Y.; Hao, Z.; Liu, J.; Han, J.; Wang, A.; Ouyang, Q.; Fu, F. Molecular Probing of Dissolved Organic Matter and Its Transformation in a Woolen Textile Wastewater Treatment Station. J. Hazard. Mater. 2023, 457, 131807. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tasnady, D.; Skelley, S.; Calderon, B.; Jiang, S. Enhancing Organic Contaminant Removal from Wool Scouring Wastewater Using Chemically Modified Biochars. C-J. Carbon Res. 2024, 10, 6. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, S.; Liu, R.; Chen, M.; Xu, J.; Liao, M.; Tu, W.; Tang, P. Utilization of Waste Sludge: Activation/Modification Methods and Adsorption Applications of Sludge-Based Activated Carbon. J. Water Process Eng. 2022, 49, 103111. [Google Scholar]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for Preparation and Activation of Activated Carbon: A Review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar]
- Yi, H.; Nakabayashi, K.; Yoon, S.H.; Miyawaki, J. Pressurized Physical Activation: A Simple Production Method for Activated Carbon with a Highly Developed Pore Structure. Carbon 2021, 183, 735–742. [Google Scholar] [CrossRef]
- Wang, X.; Liang, X.; Wang, Y.; Wang, X.; Liu, M.; Yin, D.; Xia, S.; Zhao, J.; Zhang, Y. Adsorption of Copper (II) onto Activated Carbons from Sewage Sludge by Microwave-Induced Phosphoric Acid and Zinc Chloride Activation. Desalination 2011, 278, 231–237. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Sun, Y.; Long, H.; Zheng, Z. Improvement of Low-Temperature NH3-SCR Catalytic Activity over Mn-Ce Oxide Catalysts Supported on Sewage Sludge Char Activated with KOH and H3PO4. Korean J. Chem. Eng. 2020, 37, 2152–2160. [Google Scholar] [CrossRef]
- Williams, N.E.; Oba, O.A.; Aydinlik, N.P. Modification, Production, and Methods of KOH-Activated Carbon. ChemBioEng Rev. 2022, 9, 164–189. [Google Scholar]
- Cao, J.; Jiang, Y.; Tan, X.; Li, L.; Cao, S.; Dou, J.; Chen, R.; Hu, X.; Qiu, Z.; Li, M.; et al. Sludge-Based Biochar Preparation: Pyrolysis and Co-Pyrolysis Methods, Improvements, and Environmental Applications. Fuel 2024, 373, 132265. [Google Scholar]
- Ma, A.; Zheng, X.; Gao, L.; Li, K.; Omran, M.; Chen, G. Enhanced Leaching of Zinc from Zinc-Containing Metallurgical Residues via Microwave Calcium Activation Pretreatment. Metals 2021, 11, 1922. [Google Scholar] [CrossRef]
- Wan, Y.; Xin, C.; Ding, W.; Zhang, H.; Yang, H.; Bao, S. Kinetics and Mechanism of Ultrasonic-Enhanced Mixed Acid Leaching of Zinc from Zinc-Bearing Dust. J. Environ. Chem. Eng. 2024, 12, 113246. [Google Scholar] [CrossRef]
- Zheng, X.; Li, J.; Ma, A.; Liu, B. Recovery of Zinc from Metallurgical Slag and Dust by Ammonium Acetate Using Response Surface Methodology. Materials 2023, 16, 5132. [Google Scholar] [CrossRef] [PubMed]
- Arumugham, T.; Yuniarto, A.; Abdullah, N.; Yuzir, A.; Kamyab, H.; Pa, N.F.C.; Rezania, S.; Hatta, M.N.M. Preparation and Characterisation of Porous Activated Carbon Using Potassium Hydroxide Chemical Activation with Ultrasonic Association. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Doczekalska, B.; Bartkowiak, M.; Łopatka, H.; Zborowska, M. Activated Carbon Prepared from Corn Biomass by Chemical Activation with Potassium Hydroxide. Bioresources 2022, 17, 1794–1804. [Google Scholar] [CrossRef]
- Tangsathitkulchai, C.; Naksusuk, S.; Wongkoblap, A.; Phadungbut, P.; Borisut, P. Equilibrium and Kinetics of CO2 Adsorption by Coconut Shell Activated Carbon Impregnated with Sodium Hydroxide. Processes 2021, 9, 201. [Google Scholar] [CrossRef]
- Maulina, S.; Anwari, F.N. Utilization of Oil Palm Fronds in Producing Activated Carbon Using Na2CO3 as an Activator. IOP Conf. Ser. Mater. Sci. Eng. 2018, 309, 012087. [Google Scholar] [CrossRef]
- Paluch, D.; Bazan-Wozniak, A.; Nosal-Wiercińska, A.; Pietrzak, R. Removal of Methylene Blue and Methyl Red from Aqueous Solutions Using Activated Carbons Obtained by Chemical Activation of Caraway Seed. Molecules 2023, 28, 6306. [Google Scholar] [CrossRef]
- Paluch, D.; Bazan-Wozniak, A.; Nosal-Wiercińska, A.; Pietrzak, R. Efficient Dye Removal by Biocarbon Obtained by Chemical Recycling of Waste from the Herbal Industry. Ind. Crops Prod. 2024, 220, 119254. [Google Scholar] [CrossRef]
- Hanum, F.; Bani, O.; Izdiharo, A.M. Characterization of Sodium Carbonate (Na2CO3) Treated Rice Husk Activated Carbon and Adsorption of Lead from Car Battery Wastewater. IOP Conf. Ser. Mater. Sci. Eng. 2017, 180, 012149. [Google Scholar] [CrossRef]
- Pereira, L.; Martín-Lara, M.Á.; Garcia-Garcia, G.; Calvo, C.; Robledo, T.; Solís, R.R.; Calero, M. Plastic Waste to Carbon Adsorbent: Activation with Sodium Carbonate and Functionalization with Citric Acid. Appl. Sci. 2025, 15, 1634. [Google Scholar] [CrossRef]
- Shao, F.; Zhang, X.; Sun, X.; Shang, J. Antibiotic Removal by Activated Biochar: Performance, Isotherm, and Kinetic Studies. J. Dispers. Sci. Technol. 2021, 42, 1274–1285. [Google Scholar] [CrossRef]
- Niu, Y.; Huang, Z.; Huang, J.; Qin, D.; Tang, L.; Hu, W.; Dong, K.; Wang, D. Highly Effective Removal of Sulfamethoxazole by Na2CO3-Modified Biochar Derived from Sorghum Straw and Sewage Sludge. Environ. Sci. Water Res. Technol. 2023, 9, 2355–2367. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A. Influence of Activation Conditions on the Physicochemical Properties of Activated Biochar: A Review. Biomass Convers. Biorefin. 2020, 12, 925–947. [Google Scholar] [CrossRef]
- Ihsanullah, I.; Khan, M.T.; Zubair, M.; Bilal, M.; Sajid, M. Removal of Pharmaceuticals from Water Using Sewage Sludge-Derived Biochar: A Review. Chemosphere 2022, 289, 133196. [Google Scholar] [CrossRef]
- Srivastava, A.; Gupta, B.; Majumder, A.; Gupta, A.K.; Nimbhorkar, S.K. A Comprehensive Review on the Synthesis, Performance, Modifications, and Regeneration of Activated Carbon for the Adsorptive Removal of Various Water Pollutants. J. Environ. Chem. Eng. 2021, 9, 106177. [Google Scholar] [CrossRef]
- Zendelska, A.; Pancevska, V.; Golomeova, M.; Golomeov, B.; Mirakovski, D.; Hadzi-Nikolova, M.; Doneva, N. Characterization and Isothermal Studies of Cd Removal from Aqueous Solutions Using Sludge-Based Activated Carbon. Desalin.Water Treat. 2022, 276, 142–149. [Google Scholar] [CrossRef]
- Sanz-Santos, E.; Álvarez-Torrellas, S.; Ceballos, L.; Larriba, M.; Águeda, V.I.; García, J. Application of Sludge-Based Activated Carbons for the Effective Adsorption of Neonicotinoid Pesticides. Appl. Sci. 2021, 11, 3087. [Google Scholar] [CrossRef]
- Minaei, S.; Zoroufchi Benis, K.; McPhedran, K.N.; Soltan, J. Evaluation of a ZnCl2-Modified Biochar Derived from Activated Sludge Biomass for Adsorption of Sulfamethoxazole. Chem. Eng. Res. Des. 2023, 190, 407–420. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Li, L.Y.; Hamid, H.; Jeronimo, M. Sludge-Based Activated Carbon and Its Application in the Removal of Perfluoroalkyl Substances: A Feasible Approach towards a Circular Economy. Chemosphere 2022, 294, 133707. [Google Scholar] [CrossRef]
- Xia, Y.; Li, W.; He, X.; Liu, D.; Sun, Y.; Chang, J.; Liu, J. Efficient Removal of Organic Matter from Biotreated Coking Wastewater by Coagulation Combined with Sludge-Based Activated Carbon Adsorption. Water 2022, 14, 2446. [Google Scholar] [CrossRef]
- Mian, M.M.; Ao, W.; Deng, S. Sludge-Based Biochar Adsorbent: Pore Tuning Mechanisms, Challenges, and Role in Carbon Sequestration. Biochar 2023, 5, 83. [Google Scholar]
- Ferraz, F.M.; Yuan, Q. Performance of Oat Hulls Activated Carbon for COD and Color Removal from Landfill Leachate. J. Water Process Eng. 2020, 33, 101040. [Google Scholar] [CrossRef]
- Sun, L.; Chen, D.; Wan, S.; Yu, Z. Performance, Kinetics, and Equilibrium of Methylene Blue Adsorption on Biochar Derived from Eucalyptus Saw Dust Modified with Citric, Tartaric, and Acetic Acids. Bioresour. Technol. 2015, 198, 300–308. [Google Scholar] [CrossRef]
- Wu, C.; Li, L.; Zhou, H.; Ai, J.; Zhang, H.; Tao, J.; Wang, D.; Zhang, W. Effects of Chemical Modification on Physicochemical Properties and Adsorption Behavior of Sludge-Based Activated Carbon. J. Environ. Sci. 2021, 100, 340–352. [Google Scholar] [CrossRef]
- Qadafi, M.; Wulan, D.R.; Rosmalina, R.T.; Wulandari, R.; Prayogo, W.; Utami, R.R.; Maulana, Y.E.; Notodarmojo, S.; Zevi, Y. Using UV–Vis Differential Absorbance Spectra of Tropical Peat Water DOM Fraction to Determine Trihalomethanes Formation Potential and Its Estimated Cytotoxicity. Water Cycle 2023, 4, 207–215. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Guo, S.; Xiong, X.; Che, D.; Liu, H.; Sun, B. Effects of Sludge Pyrolysis Temperature and Atmosphere on Characteristics of Biochar and Gaseous Products. Korean J. Chem. Eng. 2021, 38, 55–63. [Google Scholar] [CrossRef]
- Huang, Y.; Chu, H.; Wang, D.; Hui, S. Performance and Mechanism of Benzene Adsorption on ZnCl2 One-Step Modified Corn Cob Biochar. Environ. Sci. Pollut. Res. 2024, 31, 15209–15222. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Q.; Hao, M.; Zhang, Y.; Ren, X.; Cao, F.; Zhang, L.; Sun, Q.; Wennersten, R. Effect of Functional Groups on VOCs Adsorption by Activated Carbon: DFT Study. Surf. Sci. 2023, 736, 122352. [Google Scholar] [CrossRef]
- Kotsis, K.; Staemmler, V. Ab Initio Calculations of the O1s XPS Spectra of ZnO and Zn Oxo Compounds. Phys. Chem. Chem. Phys. 2006, 8, 1490–1498. [Google Scholar] [CrossRef]
- Su, C.; Guo, Y.; Chen, H.; Zou, J.; Zeng, Z.; Li, L. VOCs Adsorption of Resin-Based Activated Carbon and Bamboo Char: Porous Characterization and Nitrogen-Doped Effect. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 124983. [Google Scholar] [CrossRef]
- Cunha, M.R.; Lima, E.C.; Cimirro, N.F.G.M.; Thue, P.S.; Dias, S.L.P.; Gelesky, M.A.; Dotto, G.L.; dos Reis, G.S.; Pavan, F.A. Conversion of Eragrostis Plana Nees Leaves to Activated Carbon by Microwave-Assisted Pyrolysis for the Removal of Organic Emerging Contaminants from Aqueous Solutions. Environ. Sci. Pollut. Res. 2018, 25, 23315–23327. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, M.H.L.; de Oliveira, S.B.; Scalize, P.S. Production of Activated Carbon from Exhausted Coffee Grounds Chemically Modified with Natural Eucalyptus Ash Lye and Its Use in the Fluoride Adsorption Process. Environ. Sci. Pollut. Res. 2023, 30, 91276–91291. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, G.; Ahmed, Z.; Usman, M. Feedstock Type, Pyrolysis Temperature and Acid Modification Effects on Physiochemical Attributes of Biochar and Soil Quality. Arab. J. Geosci. 2022, 15, 305. [Google Scholar] [CrossRef]
- Li, J.; Hao, X.; Gan, W.; van Loosdrecht, M.C.M.; Wu, Y. Recovery of Extracellular Biopolymers from Conventional Activated Sludge: Potential, Characteristics and Limitation. Water Res. 2021, 205, 117706. [Google Scholar] [CrossRef]
- Xiao, K.; Shen, Y.; Liang, S.; Tan, J.; Wang, X.; Liang, P.; Huang, X. Characteristic Regions of the Fluorescence Excitation-Emission Matrix (EEM) to Identify Hydrophobic/Hydrophilic Contents of Organic Matter in Membrane Bioreactors. Environ. Sci. Technol. 2018, 52, 11251–11258. [Google Scholar] [CrossRef]
| Sample | BET Specific Surface Area/m2·g−1 | Specific Surface Area of Micropores/m2·g−1 | Total Pore Volume /cm3·g−1 | Micropore Volume/cm3·g−1 | Average Pore Size/nm | Average Mesopore Size/nm | Micropore Volume /% |
|---|---|---|---|---|---|---|---|
| Zn-Cl-SB | 445.0 | 102.4 | 0.40 | 0.054 | 3.551 | 5.225 | 13.5 |
| Na-Cl-SB | 340.0 | 113.0 | 0.40 | 0.061 | 4.669 | 7.349 | 15.3 |
| Na-S-SB | 431.8 | 142.1 | 0.47 | 0.076 | 4.337 | 9.396 | 16.2 |
| Na-Cl/S-SB | 509.3 | 184.1 | 0.51 | 0.099 | 3.978 | 7.896 | 19.4 |
| Activator | Material | BET Specific Surface Area/m2·g−1 | Organic Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|---|
| H2SO4 | Biochar | 339.2 | 36.9 | [2] |
| KMnO4 | Biochar | 238.7 | 29.7 | [2] |
| Na2CO3 (Na-Cl/S-SB) | Sludge-based biochar | 509.3 | 168.3 | This study |
| Sample | O1s Content of Elements % | C1s Content of Elements % | N1s Content of Elements % | S2 and Cl2s Content of Elements % |
|---|---|---|---|---|
| Zn-Cl-SB | 14.86 | 82.03 | 2.47 | 0.64 |
| Na-Cl-SB | 20.24 | 74.47 | 4.91 | 0.38 |
| Na-S-SB | 19.18 | 74.62 | 5.6 | 0.6 |
| Na-Cl/S-SB | 21.64 | 73.26 | 4.86 | 0.25 |
| Kinetic Models | Parameters | Na-Cl/S-SB | Zn-Cl-SB |
|---|---|---|---|
| Pseudo-First-Order | q1 (mg g−1) | 143.8 ± 4.59 | 104.7 ± 3.55 |
| k1 (min−1) | 0.028 | 0.059 | |
| R2 | 0.98434 | 0.99351 | |
| R2Adj | 0.98121 | 0.99221 | |
| Pseudo-Second-Order | q2 (mg g−1) | 168.3 ± 8.21 | 134.1 ± 5.28 |
| k2 (g mg−1 min−1) | 0.00041 | 0.00019 | |
| R2 | 0.98411 | 0.99617 | |
| R2Adj | 0.98978 | 0.9954 |
| Index of | Wastewater from Raw Wool Spinning | After Zn-Cl-SB Adsorption | After Na-Cl/S-SB Adsorption |
|---|---|---|---|
| E2/E3 | 1. 77 | 2.46 | 6.10 |
| E2/E4 | 233 | 236 | 255 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Huang, H.; Cao, Z.; Kang, S.; Shi, X.; Ma, W.; Ratnaweera, H. Novel Sodium Carbonate Activation for Manufacturing Sludge-Based Biochar and Assessment of Its Organic Adsorption Property in Treating Wool Scouring Wastewater. Toxics 2025, 13, 256. https://doi.org/10.3390/toxics13040256
Zhang W, Huang H, Cao Z, Kang S, Shi X, Ma W, Ratnaweera H. Novel Sodium Carbonate Activation for Manufacturing Sludge-Based Biochar and Assessment of Its Organic Adsorption Property in Treating Wool Scouring Wastewater. Toxics. 2025; 13(4):256. https://doi.org/10.3390/toxics13040256
Chicago/Turabian StyleZhang, Wanru, Hongrong Huang, Zhen Cao, Shuyu Kang, Xueqing Shi, Weiwei Ma, and Harsha Ratnaweera. 2025. "Novel Sodium Carbonate Activation for Manufacturing Sludge-Based Biochar and Assessment of Its Organic Adsorption Property in Treating Wool Scouring Wastewater" Toxics 13, no. 4: 256. https://doi.org/10.3390/toxics13040256
APA StyleZhang, W., Huang, H., Cao, Z., Kang, S., Shi, X., Ma, W., & Ratnaweera, H. (2025). Novel Sodium Carbonate Activation for Manufacturing Sludge-Based Biochar and Assessment of Its Organic Adsorption Property in Treating Wool Scouring Wastewater. Toxics, 13(4), 256. https://doi.org/10.3390/toxics13040256






