Phytotoxicity of Zero-Valent Iron-Based Nanomaterials in Mung Beans: Seed Germination and Seedling Growth Experiments
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Germination Test
2.1.1. Determination of Seed Germination
2.1.2. Determination of Fresh Dry Weight of Seeds
2.2. Seedling Growth Test
2.2.1. Measurement of Growth Indicators
2.2.2. Measurement of Photosynthetic Pigment Content
2.2.3. Antioxidant Enzyme Activity Assay
2.2.4. Determination of Elemental Fe Content
2.2.5. TEM Analysis
2.3. Data Processing
3. Results and Discussion
3.1. Seed Germination Experiment
3.2. Seedling Growth Experiment
3.2.1. Effect on Growth Index of Mung Bean Seedlings
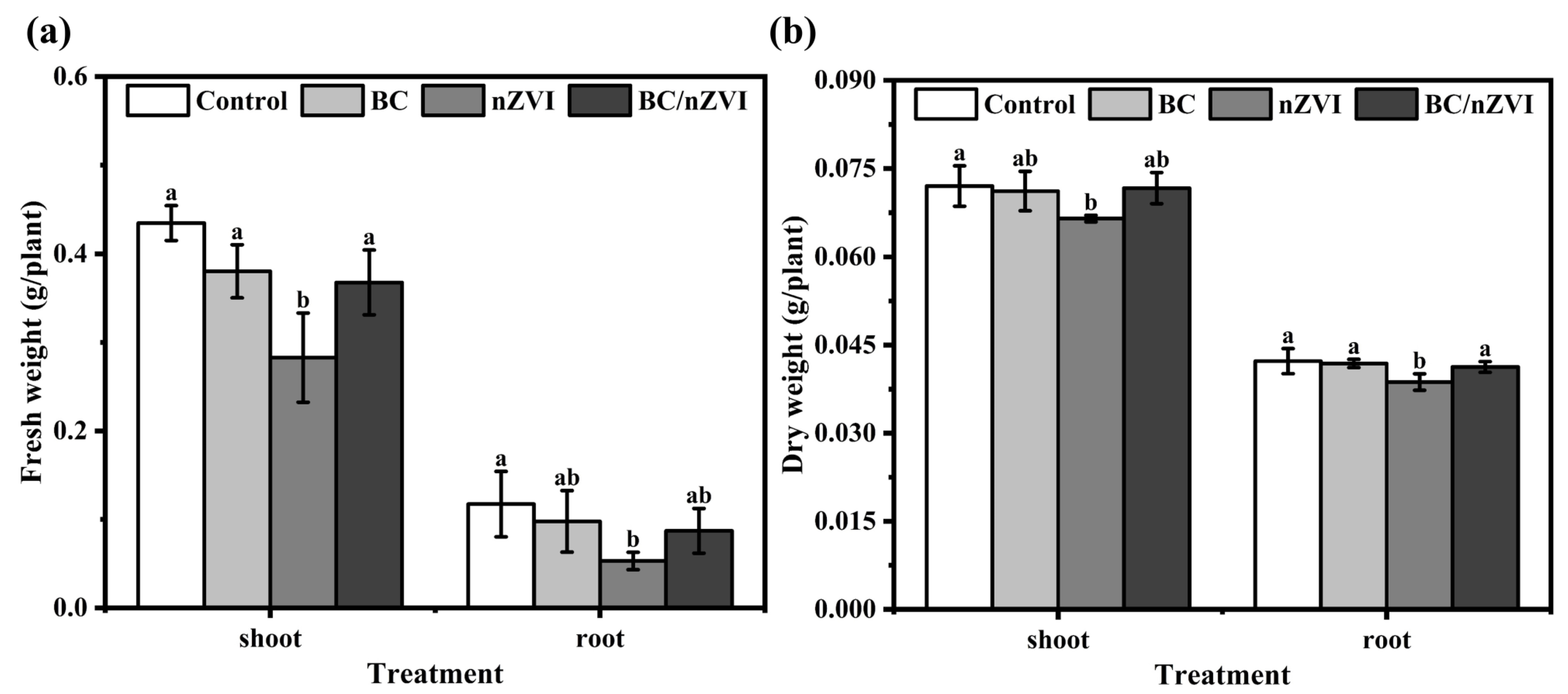
3.2.2. Effects on Mung Bean Seedling Biomass
3.2.3. Photosynthetic Pigment Content
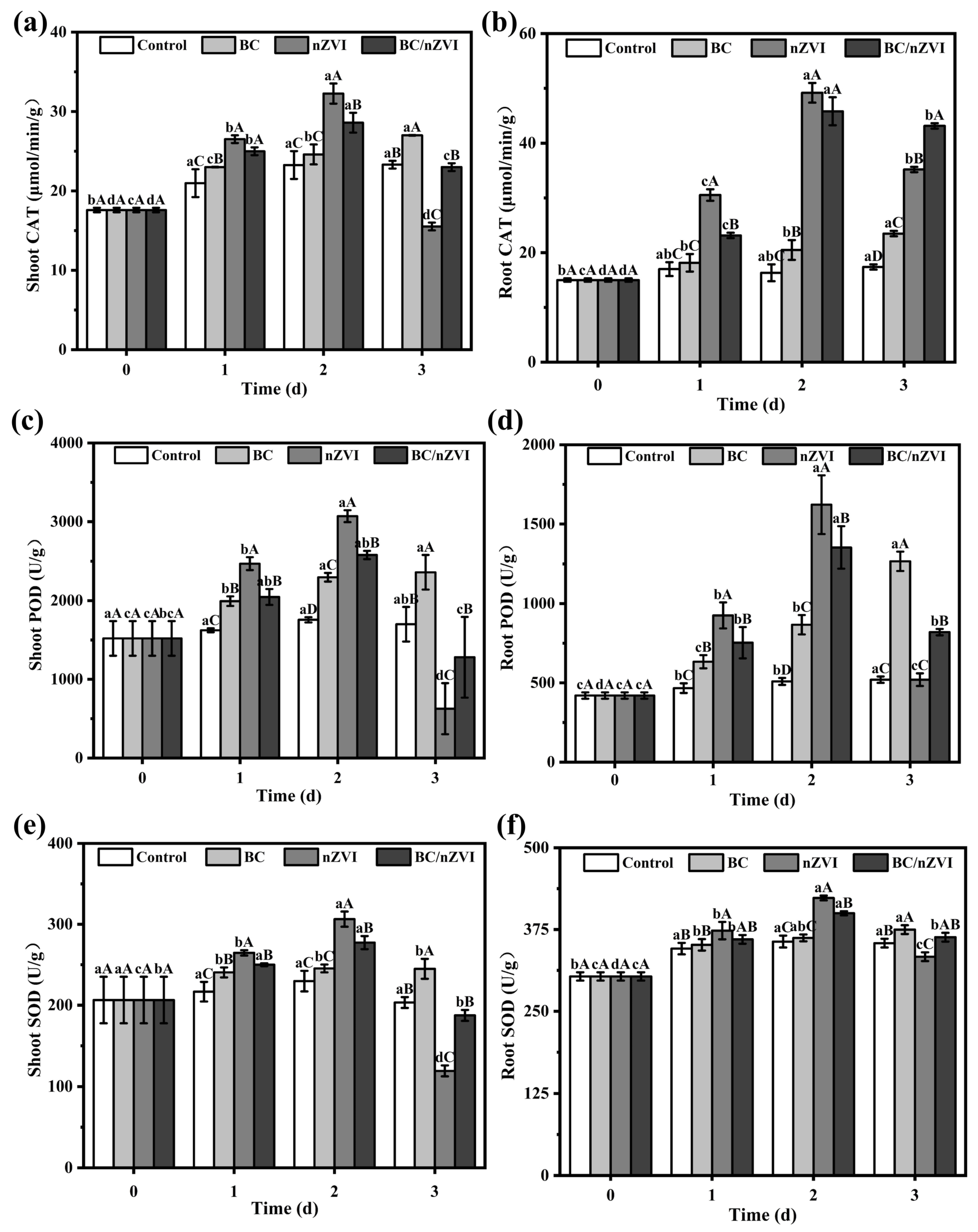
3.2.4. Antioxidant Enzyme System Activity
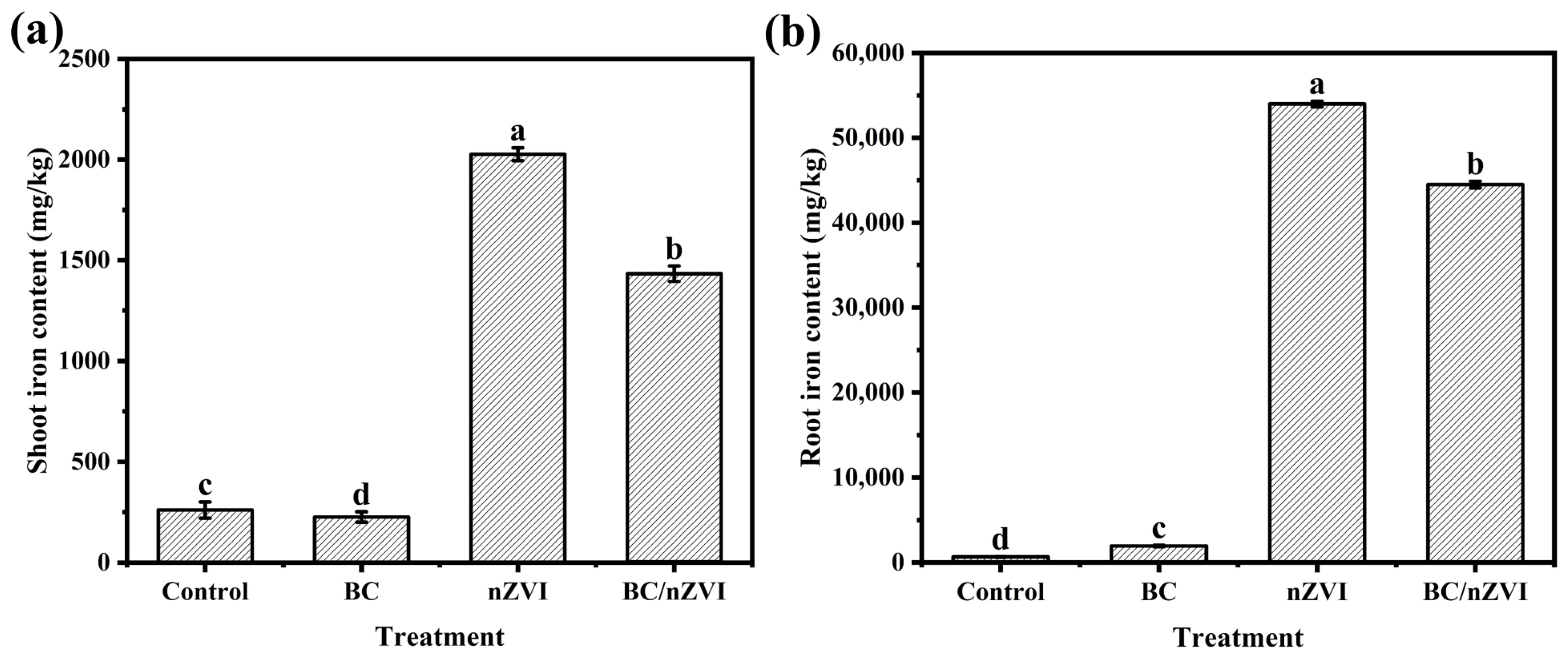
3.2.5. Effect of BC/nZVI on Iron Content of Mung Bean Seedlings
3.2.6. TEM Analysis
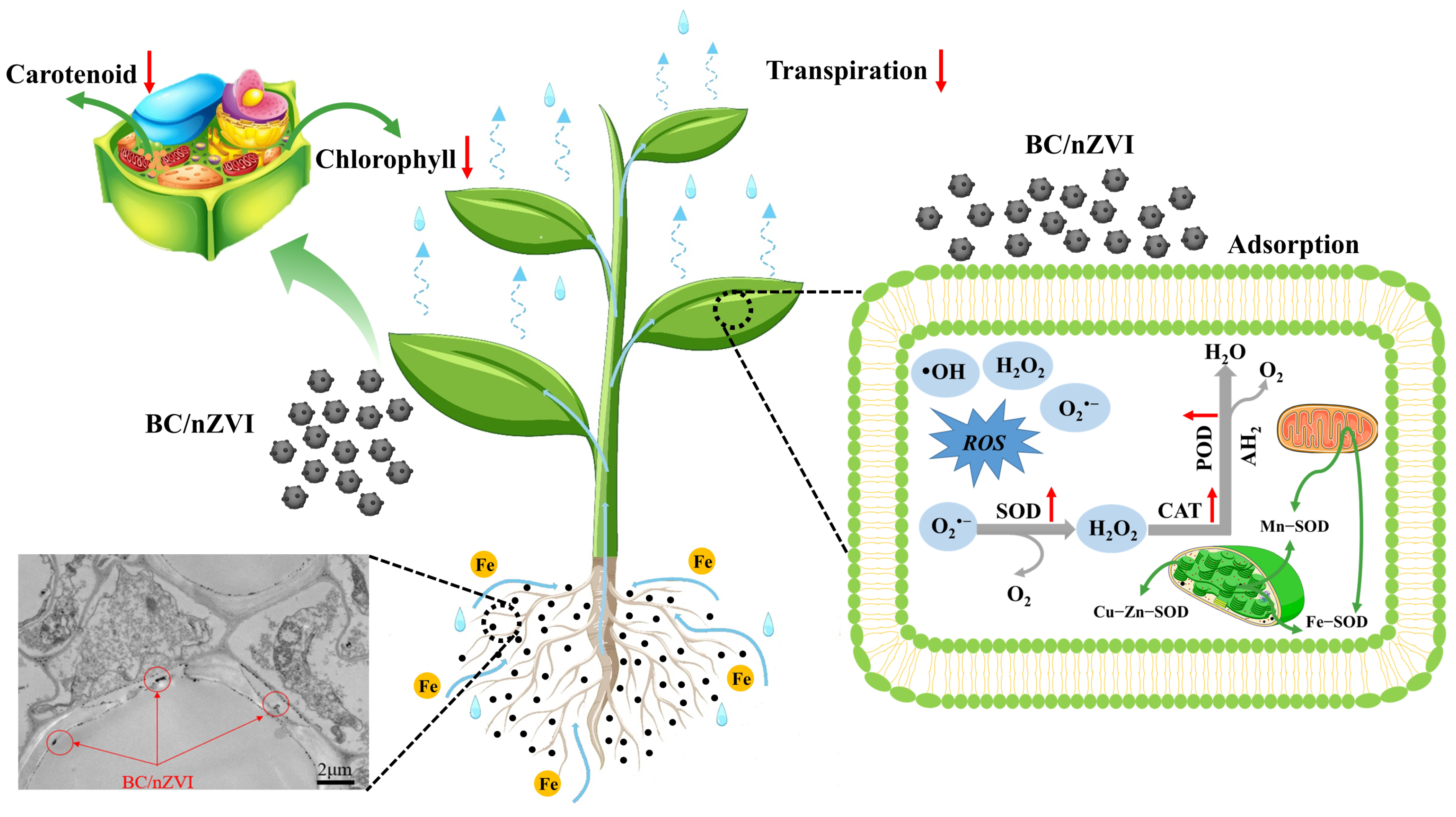
3.3. Analysis of Virulence Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lefevre, E.; Bossa, N.; Wiesner, M.R.; Gunsch, C.K. A review of the environmental implications of in situ remediation by nanoscale zero valent iron (nZVI): Behavior, transport and impacts on microbial communities. Sci. Total Environ. 2016, 565, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Liu, L.; Zhang, Y.; Pan, Y.; Ding, N.; Zhang, Y. In vivo study of chelating agent-modified nano zero-valent iron: Biodistribution and toxicity in mice. Water Res. 2024, 257, 121649. [Google Scholar] [CrossRef]
- Kumari, N.; Arya, S.; Behera, M.; Seth, C.S.; Singh, R. Chitosan anchored nZVI bionanocomposites for treatment of textile wastewater: Optimization, mechanism, and phytotoxic assessment. Environ. Res. 2024, 245, 118041. [Google Scholar] [CrossRef] [PubMed]
- Barberon, M.; Dubeaux, G.; Kolb, C.; Isono, E.; Zelazny, E.; Vert, G. Polarization of IRON-REGULATED TRANSPORTER 1 (IRT1) to the plant-soil interface plays crucial role in metal homeostasis. Proc. Natl. Acad. Sci. USA 2014, 111, 8293–8298. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Guo, X.; Kobayashi, T.; Kakei, Y.; Nakanishi, H.; Nozoye, T.; Zhang, L.; Shen, H.; Qiu, W.; Nishizawa, N.K.; et al. Expression of peanut Iron Regulated Transporter 1 in tobacco and rice plants confers improved iron nutrition. Plant Physiol. Biochem. 2014, 80, 83–89. [Google Scholar] [CrossRef]
- Yang, Y.M.; Zhu, Y.; Naseer, M.; Wang, Q.; Li, G.; Tao, H.Y.; Zhu, S.G.; Wang, B.Z.; Wang, W.; Xiong, Y.C. Rhizosphere effect of nanoscale zero-valent iron on mycorrhiza-dependent maize assimilation. Plant Cell Environ. 2023, 46, 251–267. [Google Scholar] [CrossRef]
- Guha, T.; Gopal, G.; Chatterjee, R.; Mukherjee, A.; Kundu, R. Differential growth and metabolic responses induced by nano-scale zero valent iron in germinating seeds and seedlings of Oryza sativa L. cv. Swarna. Ecotoxicol. Environ. Saf. 2020, 204, 111104. [Google Scholar] [CrossRef]
- Brasili, E.; Bavasso, I.; Petruccelli, V.; Vilardi, G.; Valletta, A.; Dal Bosco, C.; Gentili, A.; Pasqua, G.; Di Palma, L. Remediation of hexavalent chromium contaminated water through zero-valent iron nanoparticles and effects on tomato plant growth performance. Sci. Rep. 2020, 10, 1920. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Gao, B.; Zhang, M. Stimulation of peanut seedling development and growth by zero-valent iron nanoparticles at low concentrations. PLoS ONE 2015, 10, e0122884. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, Y.; Kim, E.-J.; Gu, S.; Sohn, E.J.; Seo, Y.S.; An, H.J.; Chang, Y.-S. Exposure of iron nanoparticles to Arabidopsis thaliana enhances root elongation by triggering cell wall loosening. Environ. Sci. Technol. 2014, 48, 3477–3485. [Google Scholar] [CrossRef]
- Teodoro, M.; Clemente, R.; Ferrer-Bustins, E.; Martínez-Fernández, D.; Pilar Bernal, M.; Vítková, M.; Vítek, P.; Komárek, M. Nanoscale zero-valent iron has minimum toxicological risk on the germination and early growth of two grass species with potential for phytostabilization. Nanomaterials 2020, 10, 1537. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, W.; Zheng, F.; Zhang, S.; Wang, F.; Liu, S. Phytotoxicity of iron-based materials in mung bean: Seed germination tests. Chemosphere 2020, 251, 126432. [Google Scholar] [CrossRef] [PubMed]
- Ken, D.S.; Sinha, A. Recent developments in surface modification of nano zero-valent iron (nZVI): Remediation, toxicity and environmental impacts. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100344. [Google Scholar] [CrossRef]
- Ma, X.; Gurung, A.; Deng, Y. Phytotoxicity and uptake of nanoscale zero-valent iron (nZVI) by two plant species. Sci. Total Environ. 2013, 443, 844–849. [Google Scholar] [CrossRef]
- Joo, S.H.; Feitz, A.J.; Sedlak, D.L.; Waite, T.D. Quantification of the oxidizing capacity of nanoparticulate zero-valent iron. Environ. Sci. Technol. 2005, 39, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Stefaniuk, M.; Oleszczuk, P.; Ok, Y.S. Review on nano zerovalent iron (nZVI): From synthesis to environmental applications. Chem. Eng. J. 2016, 287, 618–632. [Google Scholar] [CrossRef]
- Xue, W.; Shi, X.; Guo, J.; Wen, S.; Lin, W.; He, Q.; Gao, Y.; Wang, R.; Xu, Y. Affecting factors and mechanism of removing antibiotics and antibiotic resistance genes by nano zero-valent iron (nZVI) and modified nZVI: A critical review. Water Res. 2024, 253, 121309. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y.; Zhang, Y.; Zhao, K.; Feng, X. Selective removal of Cr (VI) by tannic acid and polyethyleneimine modified zero-valent iron particles with air stability. J. Hazard. Mater. 2023, 458, 132018. [Google Scholar] [CrossRef]
- Wang, B.; Deng, C.; Ma, W.; Sun, Y. Modified nanoscale zero-valent iron in persulfate activation for organic pollution remediation: A review. Environ. Sci. Pollut. Res. 2021, 28, 34229–34247. [Google Scholar] [CrossRef]
- Phenrat, T.; Long, T.C.; Lowry, G.V.; Veronesi, B. Partial oxidation (“aging”) and surface modification decrease the toxicity of nanosized zerovalent iron. Environ. Sci. Technol. 2009, 43, 195–200. [Google Scholar] [CrossRef]
- Yoon, H.; Pangging, M.; Jang, M.-H.; Hwang, Y.S.; Chang, Y.-S. Impact of surface modification on the toxicity of zerovalent iron nanoparticles in aquatic and terrestrial organisms. Ecotoxicol. Environ. Saf. 2018, 163, 436–443. [Google Scholar] [CrossRef]
- Zhou, L.; Le Thanh, T.; Gong, J.; Kim, J.-H.; Kim, E.-J.; Chang, Y.-S. Carboxymethyl cellulose coating decreases toxicity and oxidizing capacity of nanoscale zerovalent iron. Chemosphere 2014, 104, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Kadar, E.; Tarran, G.A.; Jha, A.N.; Al-Subiai, S.N. Stabilization of engineered zero-valent nanoiron with Na-acrylic copolymer enhances spermiotoxicity. Environ. Sci. Technol. 2011, 45, 3245–3251. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, C.; Kong, D.; Lian, L.; Liu, Y. Review on application of algae-based biochars in environmental remediation: Progress, challenge and perspectives. J. Environ. Chem. Eng. 2023, 11, 111263. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, C.; Wu, J. One-pot synthesis of magnetic algal carbon/sulfidated nanoscale zerovalent iron composites for removal of bromated disinfection by-product. Chemosphere 2020, 250, 126257. [Google Scholar] [CrossRef]
- Zhu, L.; Tong, L.; Zhao, N.; Li, J.; Lv, Y. Coupling interaction between porous biochar and nano zero valent iron/nano alpha-hydroxyl iron oxide improves the remediation efficiency of cadmium in aqueous solution. Chemosphere 2019, 219, 493–503. [Google Scholar] [CrossRef]
- Sahu, U.K.; Ji, W.; Liang, Y.; Ma, H.; Pu, S. Mechanism enhanced active biochar support magnetic nano zero-valent iron for efficient removal of Cr(VI) from simulated polluted water. J. Environ. Chem. Eng. 2022, 10, 107077. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Mosa, A.; Natasha; Abdelrahman, H.; Niazi, N.K.; Antoniadis, V.; Shahid, M.; Song, H.; Kwon, E.E.; Rinklebe, J. Removal of toxic elements from aqueous environments using nano zero-valent iron- and iron oxide-modified biochar: A review. Biochar 2022, 4, 24. [Google Scholar] [CrossRef]
- Zeng, G.; Wang, F.; Liang, D.; Zhao, H.; Zhang, J.; He, X.; Wang, Q.; Zhang, M.; Yu, C.; Sun, D. Application of biochar-loaded nano-zero-valent iron in the treatment of cyanobacterial blooms: A review. Fresenius Environ. Bull. 2023, 32, 5–14. [Google Scholar]
- Zeng, G.; He, Y.; Fan, X.; Lei, X.; Wang, Q.; Wei, H.; Sun, D. Mechanisms of action of chlorella biochar-loaded nano zero-valent iron systems on Escherichia coli: Insights from cell biology and untargeted metabolomics. Environ. Technol. Innov. 2024, 36, 103906. [Google Scholar] [CrossRef]
- Vahtmäe, E.; Kotta, J.; Orav-Kotta, H.; Kotta, I.; Pärnoja, M.; Kutser, T. Predicting macroalgal pigments (chlorophylla, chlorophyllb, chlorophylla + b, carotenoids) in various environmental conditions using high-resolution hyperspectral spectroradiometers. Int. J. Remote Sens. 2017, 39, 5716–5738. [Google Scholar] [CrossRef]
- Lopez-Millan, A.F.; Grusak, M.A.; Abadia, A.; Abadia, J. Iron deficiency in plants: An insight from proteomic approaches. Front. Plant Sci. 2013, 4, 254. [Google Scholar] [CrossRef] [PubMed]
- Rout, G.R.; Sahoo, S. Role of Iron in Plant Growth and Metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Chen, L.; Ding, C.; Zhao, X.; Xu, J.; Mohammad, A.A.; Wang, S.; Ding, Y. Differential regulation of proteins in rice (Oryza sativa L.) under iron deficiency. Plant Cell Rep. 2015, 34, 83–96. [Google Scholar] [CrossRef]
- Moradbeygi, H.; Jamei, R.; Heidari, R.; Darvishzadeh, R. Investigating the enzymatic and non-enzymatic antioxidant defense by applying iron oxide nanoparticles in Dracocephalum moldavica L. plant under salinity stress. Sci. Hortic. 2020, 272, 109537. [Google Scholar] [CrossRef]
- Liu, D.-l.; Zhang, S.-p.; Chen, Z.; Qiu, W.-w. Soil Cadmium Regulates Antioxidases in Sorghum. Agric. Sci. China 2010, 9, 1475–1480. [Google Scholar] [CrossRef]
- Römheld, V.; Marschner, H. Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol. 1986, 80, 175–180. [Google Scholar] [CrossRef]
- Lucena, J.J.; Hernandez-Apaolaza, L. Iron nutrition in plants: An overview. Plant Soil 2017, 418, 1–4. [Google Scholar] [CrossRef]
- dos Santos, L.R.; Paula, L.d.S.; Pereira, Y.C.; da Silva, B.R.S.; Batista, B.L.; Alsahli, A.A.; Lobato, A.K.d.S. Brassinosteroids-Mediated Amelioration of Iron Deficiency in Soybean Plants: Beneficial Effects on the Nutritional Status, Photosynthetic Pigments and Chlorophyll Fluorescence. J. Plant Growth Regul. 2020, 40, 1803–1823. [Google Scholar] [CrossRef]
- Jeong, J.; Connolly, E.L. Iron uptake mechanisms in plants: Functions of the FRO family of ferric reductases. Plant Sci. 2009, 176, 709–714. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Q.; Wu, Y.; Fu, J.; Wang, T.; Jiang, G. Effects of waterborne nano-iron on medaka (Oryzias latipes): Antioxidant enzymatic activity, lipid peroxidation and histopathology. Ecotoxicol. Environ. Saf. 2009, 72, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yin, J.J.; Wamer, W.G.; Zeng, M.; Lo, Y.M. Reactive oxygen species-related activities of nano-iron metal and nano-iron oxides. J. Food Drug Anal. 2014, 22, 86–94. [Google Scholar] [CrossRef]
- Majerus, V.; Bertin, P.; Lutts, S. Effects of iron toxicity on osmotic potential, osmolytes and polyamines concentrations in the African rice (Oryza glaberrima Steud.). Plant Sci. 2007, 173, 96–105. [Google Scholar] [CrossRef]
- Siqueira-Silva, A.I.; Rios, C.O.; Pereira, E.G. Iron toxicity resistance strategies in tropical grasses: The role of apoplastic radicular barriers. J. Environ. Sci. 2019, 78, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.B.; Ueda, Y.; Lai, S.K.; Frei, M. Shoot tolerance mechanisms to iron toxicity in rice (Oryza sativa L.). Plant Cell Environ. 2016, 40, 570–584. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Wu, M.; Wang, Y.; Yan, Y.; Mao, Q.; Ren, J.; Ma, R.; Liu, A.; Chen, S. Melatonin alleviates iron stress by improving iron homeostasis, antioxidant defense and secondary metabolism in cucumber. Sci. Hortic. 2020, 265, 109205. [Google Scholar] [CrossRef]
- Li, K.; Hu, G.; Yu, S.; Tang, Q.; Liu, J. Effect of the iron biofortification on enzymes activities and antioxidant properties in germinated brown rice. J. Food Meas. Charact 2018, 12, 789–799. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Li, S.; He, Y.; Zhou, B.; Zeng, G.; Huang, Y.; Sun, D. Phytotoxicity of Zero-Valent Iron-Based Nanomaterials in Mung Beans: Seed Germination and Seedling Growth Experiments. Toxics 2025, 13, 250. https://doi.org/10.3390/toxics13040250
Wu H, Li S, He Y, Zhou B, Zeng G, Huang Y, Sun D. Phytotoxicity of Zero-Valent Iron-Based Nanomaterials in Mung Beans: Seed Germination and Seedling Growth Experiments. Toxics. 2025; 13(4):250. https://doi.org/10.3390/toxics13040250
Chicago/Turabian StyleWu, Huan, Sha Li, Yu He, Bin Zhou, Guoming Zeng, Yuanyuan Huang, and Da Sun. 2025. "Phytotoxicity of Zero-Valent Iron-Based Nanomaterials in Mung Beans: Seed Germination and Seedling Growth Experiments" Toxics 13, no. 4: 250. https://doi.org/10.3390/toxics13040250
APA StyleWu, H., Li, S., He, Y., Zhou, B., Zeng, G., Huang, Y., & Sun, D. (2025). Phytotoxicity of Zero-Valent Iron-Based Nanomaterials in Mung Beans: Seed Germination and Seedling Growth Experiments. Toxics, 13(4), 250. https://doi.org/10.3390/toxics13040250






