A Review on Flame Retardants in Soils: Occurrence, Environmental Impact, Health Risks, Remediation Strategies, and Future Perspectives
Abstract
1. Introduction
2. Methods
3. Characteristics, Sources, and Occurrence in Soil Environments
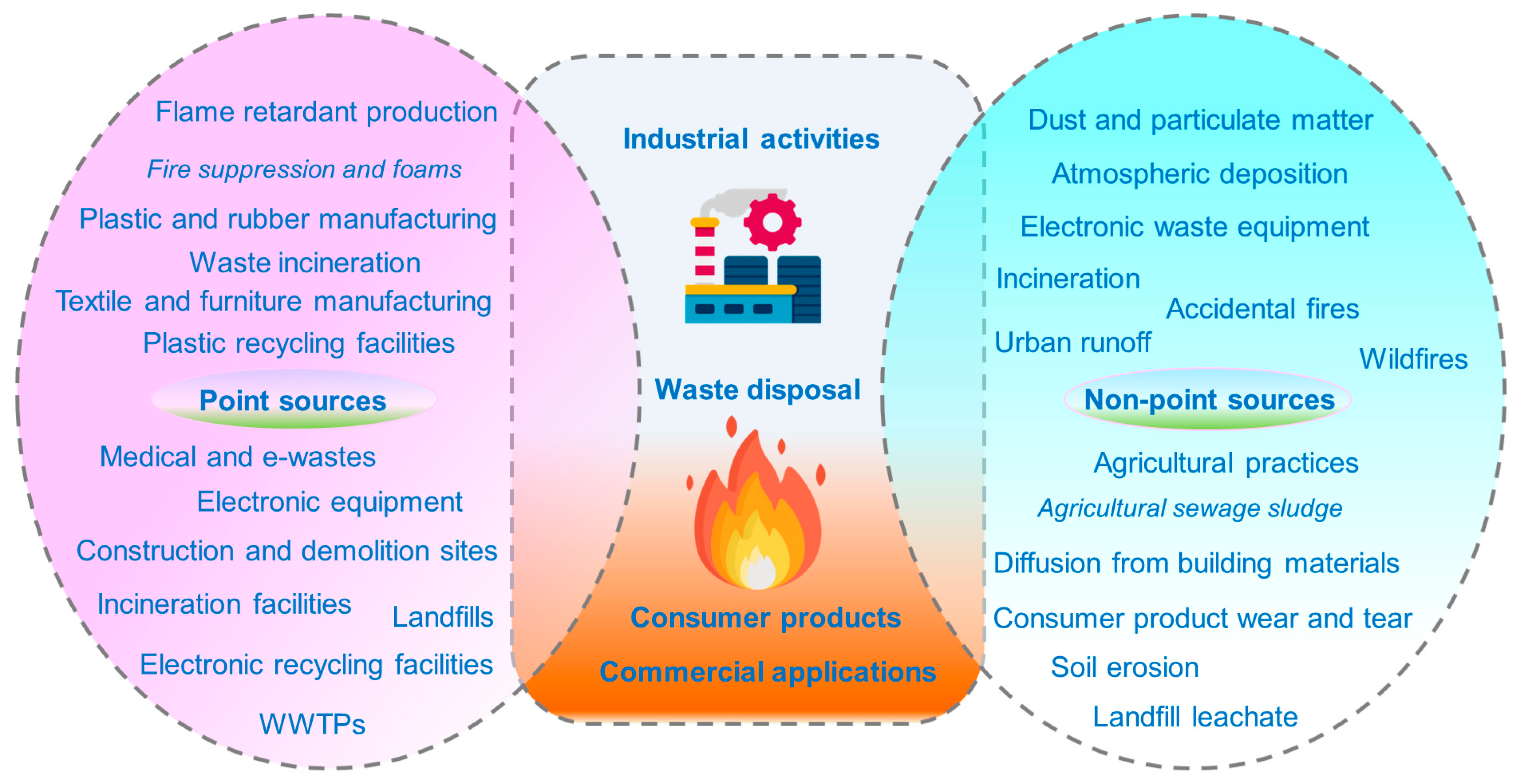
3.1. Characteristics and Sources
3.2. Occurrence and Distribution of FRs in Soils
| Location | Media | Flame Retardants | Levels | Remarks | References |
|---|---|---|---|---|---|
| China | Forest soil | DBDPE | ND—18,122 pg g−1 | Contribution of human activities Gas chromatography–mass spectrometry (GC–MS) | [35] |
| China | Production park and surrounding areas | PBDEs DBDPE | 2.88 × 104 ng g−1 8.46 × 104 ng g−1 | Point source characteristics Originated from human activities Gas chromatography–mass spectrometry (GC–MS) | [36] |
| Australia | Urban soils | NBFRs | ND—385 ng g−1 | E-waste recycling and polymer manufacturing are the main sources Gas chromatography–tandem mass spectrometry (GC–MS/MS) | [37] |
| Nigeria | Dismantling sites E-waste dumpsites | ∑17OPFRs | 0.2–68 μg g−1 (5.5 μg g−1) 0.4–42.3 μg g−1 (9.0 μg g−1) | From e-waste dismantling and dumpsites Liquid chromatography–triple quadrupole mass spectrometry (LC–QQQ) | [32] |
| Himalayas | Soils, mountain valleys | ∑7NBFRs | 4.89–2853 pg g−1 | DBDPE and TPhP were the predominant compounds Gas chromatography–triple quadrupole mass spectrometry | [10] |
| Nepal | Surface soils | ∑HFRs | 9.50–3320 ng g−1 (median, 144 ng g−1) | Long-range atmospheric transport Related to the use of a wide variety of commercial products Gas chromatography–mass spectrometry (GC–MS) | [38] |
| UK | Surface soils | BDE-209 ΣPBDEs | 11 ng g−1 15 ng g−1 | Urban activity as a source of FRs Gas chromatography–electron ionization–mass spectrometry (GC–EI–MS) | [39] |
| Brazil | Soils, landfill site | PBDEs NBFRs OPFRs | 276 (0.73–851) ng g−1 19 (1.1–83) ng g−1 67 (1.8–186) ng g−1 | Mismanagement of waste containing FRs Gas chromatography–triple quadrupole mass spectrometry | [40] |
| Antarctica | Soil | NBFRs | 61.2–225 pg g−1 | DBDPE was the dominant NBFR Gas chromatograph coupled with an electron capture negative ionization mass spectrometer (GC–NCI–MS) | [18] |
| Italy | Woodland soils | OPFRs and BFRs | 0.09–15 ng g−1 | Environmental contaminants Gas chromatography–triple quadrupole mass spectrometry | [41] |
4. Environmental Impact and Health Risk
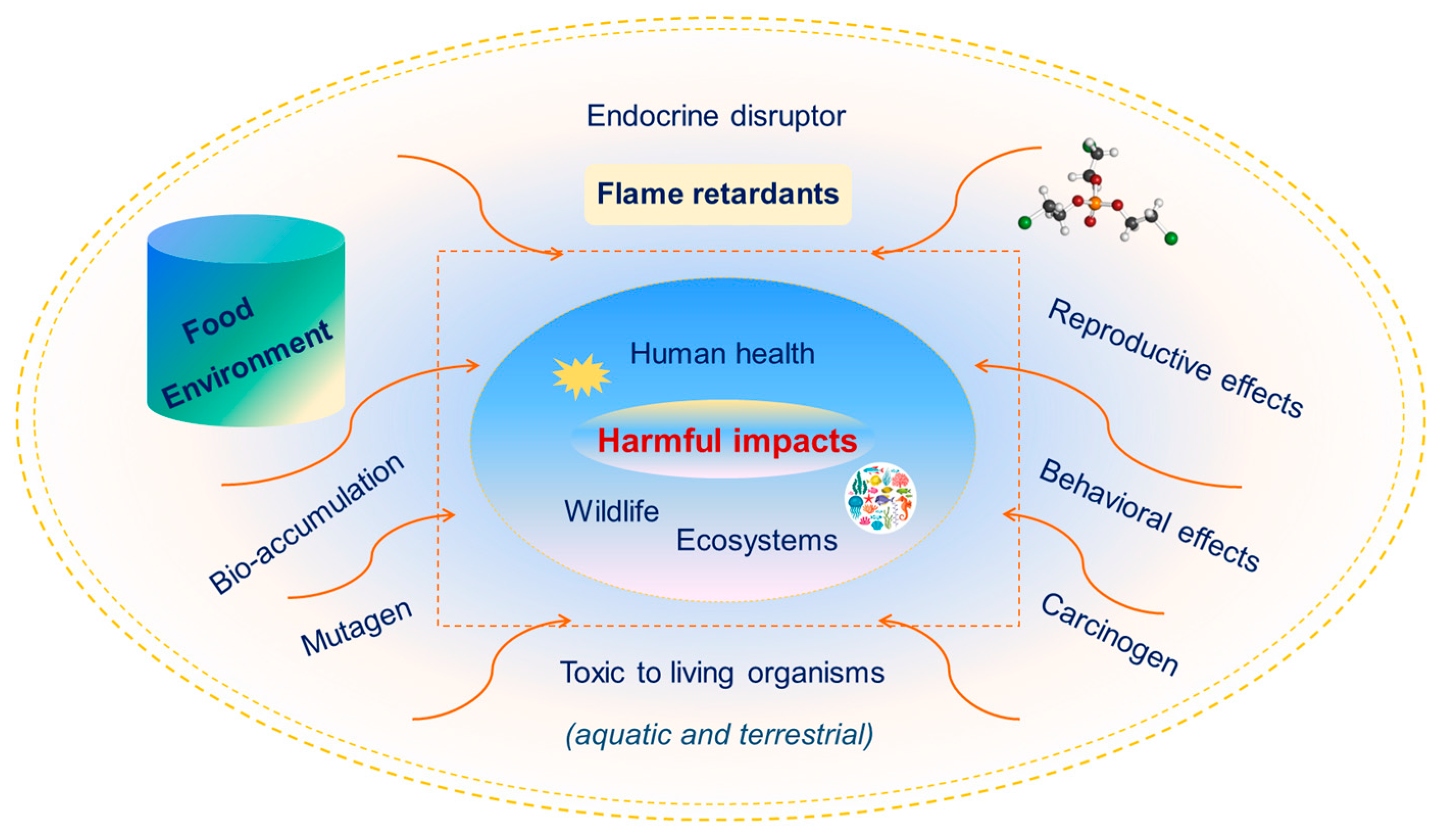
4.1. Ecological and Environmental Impacts
| Flame Retardants | Objects | Toxicity Effects | References |
|---|---|---|---|
| TCEP, TCIPP, TDCIPP | Wheat (Triticum aestivum L.) | Oxidative stress Disrupting photosynthesis | [48] |
| TCIPP | Pakchoi (Brassica chinensis L.) | Oxidative stress Growth inhibition Changing chlorophyll and proline content | [49] |
| EHTBB, TBPH | American kestrels (Falco sparverius) | Oxidative stress Thyroid disruption | [50] |
| TCP | Chicken embryos | Embryonic deformities Impacted growth Altered mRNA expression levels of genes | [51] |
| TCEP | Rats | Neurotoxicity Memory impairment | [52] |
| TPhP | Zebrafish (Danio rerio) | Development disorders Disrupted neurotransmitter system | [53] |
| RDP | Zebrafish (Danio rerio) | Neurotoxicity | [54] |
| TCEP, TCP | Earthworm (Eisenia fetida) | Neurotoxicity Intestinal damage Oxidative damage DNA damage | [55] |
| TCP | Brevibacillus brevis | Oxidative stress Enhanced cell membrane permeability Disrupted cell membrane | [56] |
| PBDEs | Human serum | Toxicological concerns | [57] |
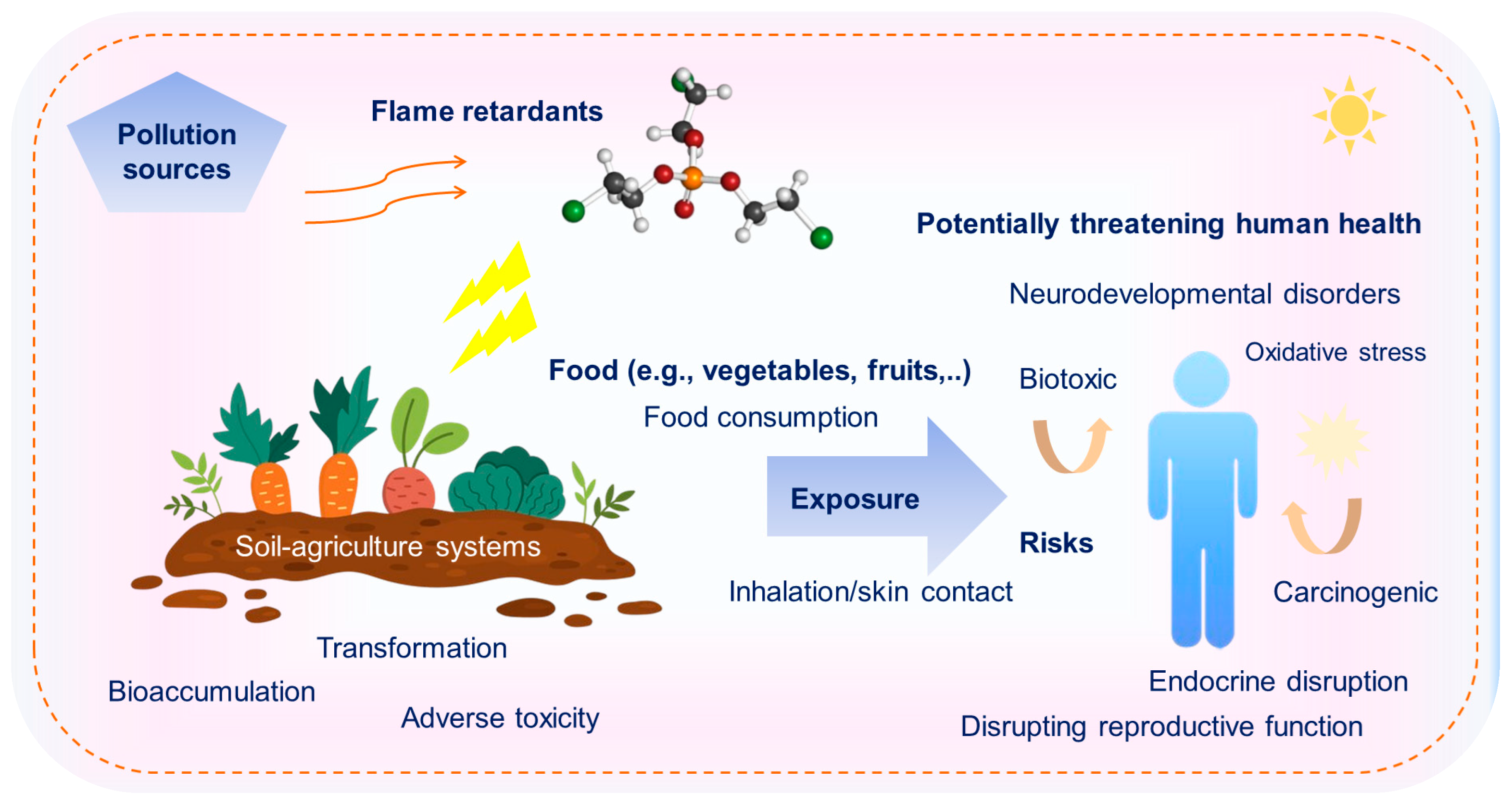
4.2. Health Risk
5. Strategies for Remediation of Flame Retardants
6. Challenges and Future Perspectives
- 1
- To better understand their impact on public health, next-phase research is needed to determine the potential health effects linked with chronic and long-term exposure to OPFRs. Future studies should concentrate on investigating the transport pathways of NBFRs between soil and other environmental compartments, as well as on evaluating the cumulative effects of NBFRs on organisms at higher trophic levels [14]. A prospective examination needs to assess the prolonged survival and impacts on organisms exposed to these toxic chemicals.
- 2
- The analysis and monitoring of FRs in soils face numerous challenges, from detection sensitivity and matrix effects to regulatory gaps and long-term environmental variability. Despite progress in research and analytical methods, significant gaps remain in understanding the fate and bioavailability of these pollutants, as well as in developing cost-effective and sustainable remediation technologies. Addressing these challenges will require ongoing interdisciplinary research, improved standardization of analytical methods, and greater collaboration between researchers, regulators, and industry to ensure more effective management of FR contamination in soils.
- 3
- Acute toxicity tests in living forms could not fully elucidate the metabolism and transformation of NBFRs within tissues. Therefore, researchers should focus on the external and internal exposure threats to plants, animals, and humans. To gain a more complete understanding of these chemicals’ behavior and their potential risks, researchers must broaden their focus. Important external exposure can occur through environmental contamination, such as in air, water, and soil, while internal exposure arises from the uptake of these chemicals into the body through ingestion, inhalation, or dermal contact. For example, research should focus on how these chemicals may accumulate in the food chain and impact ecosystems. Understanding these pathways and their subsequent effects on different biological systems is essential for evaluating the full extent of the threat posed by NBFRs.
- 4
- Several techniques, including adsorption, thermal and hydrothermal methods, photocatalytic degradation, reductive debromination, biological degradation, and advanced oxidation processes, have been applied for the elimination of PBDEs [23]. Photocatalysis is among the most commonly reported approaches for PBDE remediation. Nanomaterials, with their unique properties, have proven to be among the most effective approaches for removing BFRs through photocatalysis. Additionally, combining TiO2 with other materials such as graphene oxide (GO) or carbon nanotubes (CNTs) can enhance charge carrier mobility and reduce recombination of electron–hole pairs, which significantly improves photocatalytic efficiency. These solutions are widely recognized as cost-effective, rapid, and highly efficient [28]. To enhance the use of these combined processes, further research is needed to simplify their operation and improve the design of integrated approaches. Moreover, biodegradation, particularly microbial degradation, holds promising potential for the remediation of soil contaminated with OPEs [29]. Biological degradation is considered one of the most significant solutions for the removal of PBDEs due to its environmentally friendly nature and low cost [23]. Therefore, to further improve the remediation efficiency, future research could explore combining photocatalysis with biological methods to create a powerful, integrated solution for remediating difficult-to-degrade pollutants such as PBDEs. This hybrid approach would improve pollutant degradation rates, reduce the toxicity of byproducts, and enhance the overall sustainability of the remediation process.
- 5
- The rapid growth of the global population, urbanization, and the impact of the COVID-19 outbreak have made plastic pollution and medical equipment-related waste discharge a substantial global concern [26,73,74]. Some medical devices, protective equipment (e.g., masks and gowns), and electronic medical tools contain BFRs for fire resistance, and their improper disposal during the COVID-19 pandemic likely contributed to BFR pollution in healthcare waste streams. Additionally, incinerating medical plastic waste with BFRs can release toxic brominated compounds into the air, posing environmental and health risks. Recycling is required, and circular economy approaches can help reduce reliance on BFR-containing plastics and mitigate environmental contamination.
- 6
- Comprehending the mechanism of pollutant transfer across phase boundaries is more crucial than simply measuring transfer within a single phase. The root–soil boundary, serving as a transition zone between biotic and abiotic components, represents the primary entry pathway for pollutants into food chains [14]. Further investigation should focus on strengthening the understanding of the mechanisms behind the multiphase transport of NBFRs, particularly the uptake and transfer of FRs from the soil environment by crop roots.
- 7
- Lastly, and equally important, in designing alternative chemicals, one strategy is to prioritize reducing the emission of organic contaminants into the soil and their volatilization into the air. This approach aims to mitigate the environmental and health risks associated with the persistence and spread of harmful substances. An example of a chemical design strategy that reduces the emission of organic contaminants into the soil and their volatilization into the air is the development of bio-based FRs as alternatives to traditional halogenated FRs [75,76]. For future development, efforts should on developing more environmentally friendly and sustainable FR materials.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | biochar |
| BDE-209 | decabromodiphenyl ether |
| BFRs | brominated flame retardants |
| BTBPE | 1,2-bis(2,4,6-tribromophenoxy)ethane |
| CNTs | carbon nanotubes |
| DBCDD | dibromocyclododecadiene |
| DBDPE | decabromodiphenyl ethane |
| deca-BDE | decabromodiphenyl ether |
| EHTBB | 2-ethylhexyl-2,3,4,5-tetrabromobenzoate |
| FRs | flame retardants |
| GC-MS | gas chromatography–mass spectrometry |
| GO | graphene oxide |
| HBB | hexabromobenzene |
| HBCD | hexabromocyclododecane |
| HFRs | halogenated flame retardants |
| NBFRs | novel brominated flame retardants |
| nZVI | nano zerovalent iron |
| octa-BDE | octabromodiphenyl ether |
| OPEs | organophosphate esters |
| OPFRs | organophosphate flame retardants |
| PBBs | polybrominated biphenyls |
| PBBzs | polybromobenzenes |
| PBDEs | polybrominated diphenyl ethers |
| PBEB | pentabromoethylbenzene |
| PCBs | polychlorinated biphenyls |
| penta-BDE | pentabromodiphenyl ether |
| POPs | persistent organic pollutants |
| RDP | resorcinol bis(diphenylphosphate) |
| TBB | 2-ethylhexyl-2,3,4,5-tetrabromobenzoate |
| TBBPA | tetrabromobisphenol A |
| TBCD | tetrabromocyclododecene |
| TBPH | bis-(2-ethylhexyl) tetrabromophthalate |
| TCEP | tris(2-chloroethyl) phosphate |
| TCIPP | tris(1-chloro-2-propyl) phosphate |
| TCP | tricresyl phosphate |
| TDCIPP | tris(1,3-dichloro-2-propyl) phosphate |
| TPhP | triphenyl phosphate |
| WWTPs | wastewater treatment plants |
References
- Hou, R.; Lin, L.; Li, H.; Liu, S.; Xu, X.; Xu, Y.; Jin, X.; Yuan, Y.; Wang, Z. Occurrence, bioaccumulation, fate, and risk assessment of novel brominated flame retardants (NBFRs) in aquatic environments—A critical review. Water Res. 2021, 198, 117168. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elwafa Abdallah, M.; Harrad, S. Dermal uptake of chlorinated organophosphate flame retardants via contact with furniture fabrics; implications for human exposure. Environ. Res. 2022, 209, 112847. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.A.-E.; Harrad, S. Dermal contact with furniture fabrics is a significant pathway of human exposure to brominated flame retardants. Environ. Int. 2018, 118, 26–33. [Google Scholar] [PubMed]
- Yao, C.; Yang, H.; Li, Y. A review on organophosphate flame retardants in the environment: Occurrence, accumulation, metabolism and toxicity. Sci. Total Environ. 2021, 795, 148837. [Google Scholar]
- Saeed, A.; Altarawneh, M.; Siddique, K.; Conesa, J.A.; Ortuño, N.; Dlugogorski, B.Z. Photodecomposition properties of brominated flame retardants (BFRs). Ecotoxicol. Environ. Saf. 2020, 192, 110272. [Google Scholar]
- Chen, S.-J.; Ma, Y.-J.; Wang, J.; Chen, D.; Luo, X.-J.; Mai, B.-X. Brominated Flame Retardants in Children’s Toys: Concentration, Composition, and Children’s Exposure and Risk Assessment. Environ. Sci. Technol. 2009, 43, 4200–4206. [Google Scholar]
- De Wit, C.A. An overview of brominated flame retardants in the environment. Chemosphere 2002, 46, 583–624. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.-N.; Zhang, L.-H.; Meng, B.; Li, Y.-F.; Xiao, H.; Egorovich, K.V.; Nikolaevna, P.N.; Zhang, Z.-F.; Tang, Z.-H. Brominated flame retardants in road dust and green belt soil from Harbin, China: Contamination characteristics, sources and health risks. Environ. Chem. Ecotoxicol. 2024, 6, 229–235. [Google Scholar]
- Legler, J.; Brouwer, A. Are brominated flame retardants endocrine disruptors? Environ. Int. 2003, 29, 879–885. [Google Scholar]
- Chen, Y.; Xian, H.; Zhu, C.; Li, Y.; Pei, Z.; Yang, R.; Zhang, Q.; Jiang, G. The transport and distribution of novel brominated flame retardants (NBFRs) and organophosphate esters (OPEs) in soils and moss along mountain valleys in the Himalayas. J. Hazard. Mater. 2024, 465, 133044. [Google Scholar]
- Dong, L.; Wang, S.; Qu, J.; You, H.; Liu, D. New understanding of novel brominated flame retardants (NBFRs): Neuro (endocrine) toxicity. Ecotoxicol. Environ. Saf. 2021, 208, 111570. [Google Scholar] [CrossRef]
- Nava, A.; Sarma, H. Persistence, Toxicity, and Strategies for Remediation of Brominated Flame Retardants in Soil and Sedimentation in Aquatic Matrices Under Aerobic and Anaerobic Conditions. In Land Remediation and Management: Bioengineering Strategies; Sarma, H., Joshi, S., Eds.; Springer Nature Singapore: Singapore, 2023; pp. 103–125. [Google Scholar]
- Qiao, Z.; Lu, C.; Han, Y.; Luo, K.; Fu, M.; Zhou, S.; Peng, C.; Zhang, W. Enrichment and removal of five brominated flame retardants in the presence of co-exposure in a soil-earthworm system. Environ. Pollut. 2022, 310, 119877. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Cheng, H.; Bian, Z. Global occurrence and environmental behavior of novel brominated flame retardants in soils: Current knowledge and future perspectives. J. Hazard. Mater. 2024, 480, 136298. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Shang, P.; Mei, Z.; Lu, Z.; Gong, Y.; Zhang, H.; Xu, S.; Zhang, X. Environmental safety perspectives on organophosphate flame retardants: Current strategies and advancements in their environmental removal and detection. J. Environ. Chem. Eng. 2025, 13, 115340. [Google Scholar]
- Wang, Y.; Sun, H.; Zhu, H.; Yao, Y.; Chen, H.; Ren, C.; Wu, F.; Kannan, K. Occurrence and distribution of organophosphate flame retardants (OPFRs) in soil and outdoor settled dust from a multi-waste recycling area in China. Sci. Total Environ. 2018, 625, 1056–1064. [Google Scholar] [CrossRef]
- Page, J.; Whaley, P.; Bellingham, M.; Birnbaum, L.S.; Cavoski, A.; Fetherston Dilke, D.; Garside, R.; Harrad, S.; Kelly, F.; Kortenkamp, A.; et al. A new consensus on reconciling fire safety with environmental & health impacts of chemical flame retardants. Environ. Int. 2023, 173, 107782. [Google Scholar]
- Xiong, S.; Hao, Y.; Li, Y.; Yang, R.; Pei, Z.; Zhang, Q.; Jiang, G. Accumulation and influencing factors of novel brominated flame retardants in soil and vegetation from Fildes Peninsula, Antarctica. Sci. Total Environ. 2021, 756, 144088. [Google Scholar] [CrossRef]
- Turner, A. PBDEs in the marine environment: Sources, pathways and the role of microplastics. Environ. Pollut. 2022, 301, 118943. [Google Scholar]
- Chen, Y.; Li, J.; Liu, L.; Zhao, N. Polybrominated diphenyl ethers fate in China: A review with an emphasis on environmental contamination levels, human exposure and regulation. J. Environ. Manag. 2012, 113, 22–30. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, L.; Lin, Q.; Ma, S.; Yu, Y. Polybrominated diphenyl ethers in the environment and human external and internal exposure in China: A review. Sci. Total Environ. 2019, 696, 133902. [Google Scholar] [CrossRef]
- Zhu, H.; Sun, H.; Yao, Y.; Gan, Z.; Wang, Y.; Kannan, K. Legacy and alternative brominated flame retardants in outdoor dust and pine needles in mainland China: Spatial trends, dust-plant partitioning and human exposure. Environ. Pollut. 2018, 243, 758–765. [Google Scholar] [PubMed]
- Yao, B.; Luo, Z.; Zhi, D.; Hou, D.; Luo, L.; Du, S.; Zhou, Y. Current progress in degradation and removal methods of polybrominated diphenyl ethers from water and soil: A review. J. Hazard. Mater. 2021, 403, 123674. [Google Scholar]
- Wu, Z.; Han, W.; Yang, X.; Li, Y.; Wang, Y. The occurrence of polybrominated diphenyl ether (PBDE) contamination in soil, water/sediment, and air. Environ. Sci. Pollut. Res. 2019, 26, 23219–23241. [Google Scholar]
- Malkoske, T.; Tang, Y.; Xu, W.; Yu, S.; Wang, H. A review of the environmental distribution, fate, and control of tetrabromobisphenol A released from sources. Sci. Total Environ. 2016, 569–570, 1608–1617. [Google Scholar]
- Le, V.-G.; Nguyen, M.-K.; Lin, C.; Nguyen, H.-L.; Nguyen, T.Q.H.; Hue, N.K.; Truong, Q.-M.; Chang, S.W.; Nguyen, X.H.; Nguyen, D.D. Review on personal protective equipment: Emerging concerns in micro(nano)plastic pollution and strategies for addressing environmental challenges. Environ. Res. 2024, 257, 119345. [Google Scholar]
- Nguyen, M.-K.; Lin, C.; Nguyen, H.-L.; Le, V.-R.; Kl, P.; Singh, J.; Chang, S.W.; Um, M.-J.; Nguyen, D.D. Emergence of microplastics in the aquatic ecosystem and their potential effects on health risks: The insights into Vietnam. J. Environ. Manag. 2023, 344, 118499. [Google Scholar]
- Rani, M.; Keshu; Meenu; Sillanpää, M.; Shanker, U. An updated review on environmental occurrence, scientific assessment and removal of brominated flame retardants by engineered nanomaterials. J. Environ. Manag. 2022, 321, 115998. [Google Scholar]
- Tian, Y.X.; Chen, H.Y.; Ma, J.; Liu, Q.Y.; Qu, Y.J.; Zhao, W.H. A critical review on sources and environmental behavior of organophosphorus flame retardants in the soil: Current knowledge and future perspectives. J. Hazard. Mater. 2023, 452, 131161. [Google Scholar]
- Ge, X.; Ma, S.; Zhang, X.; Yang, Y.; Li, G.; Yu, Y. Halogenated and organophosphorous flame retardants in surface soils from an e-waste dismantling park and its surrounding area: Distributions, sources, and human health risks. Environ. Int. 2020, 139, 105741. [Google Scholar]
- Wang, N.; Lai, C.; Xu, F.; Huang, D.; Zhang, M.; Zhou, X.; Xu, M.; Li, Y.; Li, L.; Liu, S.; et al. A review of polybrominated diphenyl ethers and novel brominated flame retardants in Chinese aquatic environment: Source, occurrence, distribution, and ecological risk assessment. Sci. Total Environ. 2023, 904, 166180. [Google Scholar]
- Folarin, B.T.; Poma, G.; Yin, S.; Altamirano, J.C.; Cleys, P.; Oluseyi, T.; Covaci, A. Source identification and human exposure assessment of organophosphate flame retardants and plasticisers in soil and outdoor dust from Nigerian e-waste dismantling and dumpsites. Environ. Pollut. 2024, 362, 124998. [Google Scholar] [PubMed]
- Matsukami, H.; Suzuki, G.; Someya, M.; Uchida, N.; Tue, N.M.; Viet, P.H.; Takahashi, S.; Tanabe, S.; Takigami, H. Concentrations of polybrominated diphenyl ethers and alternative flame retardants in surface soils and river sediments from an electronic waste-processing area in northern Vietnam, 2012–2014. Chemosphere 2017, 167, 291–299. [Google Scholar]
- Xian, H.; Hao, Y.; Lv, J.; Wang, C.; Zuo, P.; Pei, Z.; Li, Y.; Yang, R.; Zhang, Q.; Jiang, G. Novel brominated flame retardants (NBFRs) in soil and moss in Mt. Shergyla, southeast Tibetan Plateau: Occurrence, distribution and influencing factors. Environ. Pollut. 2021, 291, 118252. [Google Scholar]
- Liu, J.; Kuang, J.; Chen, X.; Huang, L.; Shi, Z. Potential bacterial resources for bioremediation of organochlorine pesticides and flame retardants recognized from forest soil across China. J. Hazard. Mater. 2025, 486, 137027. [Google Scholar] [PubMed]
- Bai, C.; Ge, X.; Huang, Z.; Qi, Z.; Ren, H.; Yu, Y.; An, T. Polybrominated diphenyl ethers and their alternatives in soil cores from a typical flame-retardant production park: Vertical distribution and potential influencing factors. Environ. Pollut. 2024, 359, 124597. [Google Scholar]
- McGrath, T.J.; Morrison, P.D.; Ball, A.S.; Clarke, B.O. Detection of novel brominated flame retardants (NBFRs) in the urban soils of Melbourne, Australia. Emerg. Contam. 2017, 3, 23–31. [Google Scholar]
- Yadav, I.C.; Devi, N.L.; Li, J.; Zhang, G. Environmental concentration and atmospheric deposition of halogenated flame retardants in soil from Nepal: Source apportionment and soil-air partitioning. Environ. Pollut. 2018, 233, 642–654. [Google Scholar]
- Drage, D.S.; Newton, S.; de Wit, C.A.; Harrad, S. Concentrations of legacy and emerging flame retardants in air and soil on a transect in the UK West Midlands. Chemosphere 2016, 148, 195–203. [Google Scholar]
- Cristale, J.; Aragão Belé, T.G.; Lacorte, S.; de Marchi, M.R.R. Occurrence of flame retardants in landfills: A case study in Brazil. Environ. Res. 2019, 168, 420–427. [Google Scholar]
- Vecchiato, M.; Bonato, T.; Barbante, C.; Gambaro, A.; Piazza, R. Organic pollutants in protected plain areas: The occurrence of PAHs, musks, UV-filters, flame retardants and hydrocarbons in woodland soils. Sci. Total Environ. 2021, 796, 149003. [Google Scholar]
- Xu, P.; Tao, B.; Zhou, Z.; Fan, S.; Zhang, T.; Liu, A.; Dong, S.; Yuan, J.; Li, H.; Chen, J. Occurrence, composition, source, and regional distribution of halogenated flame retardants and polybrominated dibenzo-p-dioxin/dibenzofuran in the soils of Guiyu, China. Environ. Pollut. 2017, 228, 61–71. [Google Scholar] [PubMed]
- Jones, L.B.; Arnold, K.E.; Allchin, O. Evidence on the effects of flame retardant substances at ecologically relevant endpoints: A systematic map protocol. Evid.-Based Toxicol. 2024, 2, 2375113. [Google Scholar]
- Tran, C.M.; Lee, H.; Lee, B.; Ra, J.-S.; Kim, K.-T. Effects of the chorion on the developmental toxicity of organophosphate esters in zebrafish embryos. J. Hazard. Mater. 2021, 401, 123389. [Google Scholar]
- Sutha, J.; Anila, P.A.; Umamaheswari, S.; Ramesh, M.; Narayanasamy, A.; Poopal, R.-K.; Ren, Z. Biochemical responses of a freshwater fish Cirrhinus mrigala exposed to tris(2-chloroethyl) phosphate (TCEP). Environ. Sci. Pollut. Res. 2020, 27, 34369–34387. [Google Scholar]
- Ernest, S.R.; Wade, M.G.; Lalancette, C.; Ma, Y.-Q.; Berger, R.G.; Robaire, B.; Hales, B.F. Effects of Chronic Exposure to an Environmentally Relevant Mixture of Brominated Flame Retardants on the Reproductive and Thyroid System in Adult Male Rats. Toxicol. Sci. 2012, 127, 496–507. [Google Scholar]
- Cao, Y.; Gao, Y.; Hu, X.; Zeng, Y.; Luo, X.; Li, G.; An, T.; Mai, B. Insight into phototransformation mechanism and toxicity evolution of novel and legacy brominated flame retardants in water: A comparative analysis. Water Res. 2022, 211, 118041. [Google Scholar]
- Liu, Q.; Liu, M.; Wu, S.; Xiao, B.; Wang, X.; Sun, B.; Zhu, L. Metabolomics reveals antioxidant stress responses of wheat (Triticum aestivum L.) exposed to chlorinated organophosphate esters. J. Agric. Food Chem. 2020, 68, 6520–6529. [Google Scholar]
- Luo, Q.; Li, Y.; Wu, Z.; Wang, X.; Wang, C.; Shan, Y.; Sun, L. Phytotoxicity of tris-(1-chloro-2-propyl) phosphate in soil and its uptake and accumulation by pakchoi (Brassica chinensis L. cv. SuZhou). Chemosphere 2021, 277, 130347. [Google Scholar]
- Goodchild, C.; Karouna-Renier, N.K.; Henry, P.F.P.; Letcher, R.J.; Schultz, S.L.; Maddox, C.M.; Bean, T.G.; Peters, L.E.; Palace, V.; Fernie, K.J. Thyroid disruption and oxidative stress in American kestrels following embryonic exposure to the alternative flame retardants, EHTBB and TBPH. Environ. Int. 2021, 157, 106826. [Google Scholar]
- Crump, D.; Porter, E.; Egloff, C.; Williams, K.L.; Letcher, R.J.; Gauthier, L.T.; Kennedy, S.W. 1, 2-Dibromo-4-(1, 2-dibromoethyl)-cyclohexane and tris (methylphenyl) phosphate cause significant effects on development, mRNA expression, and circulating bile acid concentrations in chicken embryos. Toxicol. Appl. Pharmacol. 2014, 277, 279–287. [Google Scholar]
- Yang, W.; Zhao, F.; Fang, Y.; Li, L.; Li, C.; Ta, N. 1H-nuclear magnetic resonance metabolomics revealing the intrinsic relationships between neurochemical alterations and neurobehavioral and neuropathological abnormalities in rats exposed to tris (2-chloroethyl) phosphate. Chemosphere 2018, 200, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, R.; Zheng, S.; Luo, C.; Huang, W.; Shi, X.; Wu, K. Exposure of male adult zebrafish (Danio rerio) to triphenyl phosphate (TPhP) induces eye development disorders and disrupts neurotransmitter system-mediated abnormal locomotor behavior in larval offspring. J. Hazard. Mater. 2024, 465, 133332. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Lei, Y.; Li, W.; Jiang, X.; Li, M. Coupled digital visualization and multi-omics uncover neurobehavioral dysfunction in zebrafish induced by resorcinol bis(diphenylphosphate). Environ. Int. 2024, 192, 109023. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xiao, Y.; Chang, Y.; Cui, Y.; Klobučar, G.; Li, M. Intestinal damage, neurotoxicity and biochemical responses caused by tris (2-chloroethyl) phosphate and tricresyl phosphate on earthworm. Ecotoxicol. Environ. Saf. 2018, 158, 78–86. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, H.; Wei, K.; Peng, H.; Lu, G.; Dang, Z. Biodegradation of tricresyl phosphate isomers by Brevibacillus brevis: Degradation pathway and metabolic mechanism. Chemosphere 2019, 232, 195–203. [Google Scholar] [CrossRef]
- Brasseur, C.; Pirard, C.; Scholl, G.; De Pauw, E.; Viel, J.-F.; Shen, L.; Reiner, E.J.; Focant, J.-F. Levels of dechloranes and polybrominated diphenyl ethers (PBDEs) in human serum from France. Environ. Int. 2014, 65, 33–40. [Google Scholar] [CrossRef]
- Liu, B.; Ding, L.; Lv, L.; Yu, Y.; Dong, W. Organophosphate esters (OPEs) and novel brominated flame retardants (NBFRs) in indoor dust: A systematic review on concentration, spatial distribution, sources, and human exposure. Chemosphere 2023, 345, 140560. [Google Scholar] [CrossRef]
- Čechová, E.; Vojta, Š.; Kukučka, P.; Kočan, A.; Trnovec, T.; Murínová, Ľ.P.; De Cock, M.; Van de Bor, M.; Askevold, J.; Eggesbø, M. Legacy and alternative halogenated flame retardants in human milk in Europe: Implications for children’s health. Environ. Int. 2017, 108, 137–145. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Lan, Y.; Zhao, Y.; Lu, A.; Li, C.; Lei, R.; Xue, J.; Liu, W. A nationwide survey of 20 legacy brominated flame retardants in indoor dust from China: Continuing occurrence, national distribution, and implication for human exposure. Environ. Sci. Pollut. Res. 2022, 29, 58828–58842. [Google Scholar] [CrossRef]
- Sun, J.; Wu, Y.; Jiang, P.; Zheng, L.; Zhang, A.; Qi, H. Concentration, uptake and human dietary intake of novel brominated flame retardants in greenhouse and conventional vegetables. Environ. Int. 2019, 123, 436–443. [Google Scholar] [CrossRef]
- Xiong, S.; Fu, J.; Dong, C.; Pei, Z.; Yang, R.; Li, Y.; Zhang, Q.; Jiang, G. Bioaccumulation and Trophodynamics of Novel Brominated Flame Retardants (NBFRs) in Marine Food Webs from the Arctic and Antarctic Regions. Environ. Sci. Technol. 2024, 58, 6804–6813. [Google Scholar] [CrossRef] [PubMed]
- Vonderheide, A.P.; Mueller-Spitz, S.R.; Meija, J.; Welsh, G.L.; Mueller, K.E.; Kinkle, B.K.; Shann, J.R.; Caruso, J.A. Rapid breakdown of brominated flame retardants by soil microorganisms. J. Anal. At. Spectrom. 2006, 21, 1232–1239. [Google Scholar]
- Wu, C.D.; Yao, Y.F.; Xie, Q.J. Synergy remediation of PBDEs contaminated soil by electric-magnetic method. Adv. Mater. Res. 2013, 726, 2338–2341. [Google Scholar]
- Xie, Y.; Fang, Z.; Cheng, W.; Tsang, P.E.; Zhao, D. Remediation of polybrominated diphenyl ethers in soil using Ni/Fe bimetallic nanoparticles: Influencing factors, kinetics and mechanism. Sci. Total Environ. 2014, 485, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, C.; Huang, M.; Zhang, J.; Cheng, B.; Zheng, Y.; Chen, S.; Xiang, M.; Li, Y.; Bedia, J.; et al. A review of the present methods used to remediate soil and water contaminated with organophosphate esters and developmental directions. J. Hazard. Mater. 2024, 475, 134834. [Google Scholar] [CrossRef]
- Yan, W.; Yan, L.; Duan, J.; Jing, C. Sorption of organophosphate esters by carbon nanotubes. J. Hazard. Mater. 2014, 273, 53–60. [Google Scholar] [CrossRef]
- Lu, C.; Wan, J.; Chen, X.; Zhang, W.; Lin, K.; Peng, C. Removal of decabromodiphenyl ethane (DBDPE) by BC/nZVI in the soil: Kinetics, pathways and mechanisms. J. Environ. Chem. Eng. 2022, 10, 107004. [Google Scholar] [CrossRef]
- Li, A.; Tai, C.; Zhao, Z.; Wang, Y.; Zhang, Q.; Jiang, G.; Hu, J. Debromination of Decabrominated Diphenyl Ether by Resin-Bound Iron Nanoparticles. Environ. Sci. Technol. 2007, 41, 6841–6846. [Google Scholar]
- Huang, L.; Wang, W.; Shah, S.B.; Hu, H.; Xu, P.; Tang, H. The HBCDs biodegradation using a Pseudomonas strain and its application in soil phytoremediation. J. Hazard. Mater. 2019, 380, 120833. [Google Scholar] [CrossRef]
- Shah, S.B.; Ali, F.; Huang, L.; Wang, W.; Xu, P.; Tang, H. Complete genome sequence of Bacillus sp. HBCD-sjtu, an efficient HBCD-degrading bacterium. 3 Biotech 2018, 8, 1–4. [Google Scholar] [CrossRef]
- Nguyen, M.-K.; Rakib, M.R.J.; Hwangbo, M.; Kim, J. Microplastic accumulation in soils: Unlocking the mechanism and biodegradation pathway. J. Hazard. Mater. Adv. 2025, 17, 100629. [Google Scholar] [CrossRef]
- Le, V.-G.; Nguyen, M.-K.; Ngo, H.H.; Barceló, D.; Nguyen, H.-L.; Um, M.J.; Nguyen, D.D. Microplastics in aquaculture environments: Current occurrence, adverse effects, ecological risk, and nature-based mitigation solutions. Mar. Pollut. Bull. 2024, 209, 117168. [Google Scholar] [CrossRef] [PubMed]
- Pivnenko, K.; Granby, K.; Eriksson, E.; Astrup, T.F. Recycling of plastic waste: Screening for brominated flame retardants (BFRs). Waste Manag. 2017, 69, 101–109. [Google Scholar] [PubMed]
- Wang, M.; Yin, G.-Z.; Yang, Y.; Fu, W.; Díaz Palencia, J.L.; Zhao, J.; Wang, N.; Jiang, Y.; Wang, D.-Y. Bio-based flame retardants to polymers: A review. Adv. Ind. Eng. Polym. Res. 2023, 6, 132–155. [Google Scholar] [CrossRef]
- Li, J.; Cao, Q.; Zhao, Y.; Gu, C.; Liu, B.; Fan, Q.; Zhang, C.; Huang, Y.; Jiang, S.; Jian, X.; et al. Bio-based flame retardant for manufacturing fire safety, strong yet tough versatile epoxy resin. Compos. Part B Eng. 2024, 276, 111362. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thuy, T.L.; Hoang, T.-D.; Hoang, V.-H.; Nguyen, M.-K. A Review on Flame Retardants in Soils: Occurrence, Environmental Impact, Health Risks, Remediation Strategies, and Future Perspectives. Toxics 2025, 13, 228. https://doi.org/10.3390/toxics13030228
Thuy TL, Hoang T-D, Hoang V-H, Nguyen M-K. A Review on Flame Retardants in Soils: Occurrence, Environmental Impact, Health Risks, Remediation Strategies, and Future Perspectives. Toxics. 2025; 13(3):228. https://doi.org/10.3390/toxics13030228
Chicago/Turabian StyleThuy, Trang Le, Tuan-Dung Hoang, Van-Hiep Hoang, and Minh-Ky Nguyen. 2025. "A Review on Flame Retardants in Soils: Occurrence, Environmental Impact, Health Risks, Remediation Strategies, and Future Perspectives" Toxics 13, no. 3: 228. https://doi.org/10.3390/toxics13030228
APA StyleThuy, T. L., Hoang, T.-D., Hoang, V.-H., & Nguyen, M.-K. (2025). A Review on Flame Retardants in Soils: Occurrence, Environmental Impact, Health Risks, Remediation Strategies, and Future Perspectives. Toxics, 13(3), 228. https://doi.org/10.3390/toxics13030228






