Serum Concentrations of Fipronil and Metabolites in Japanese Pregnant Women: Relationship with Thyroid Hormone Levels
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
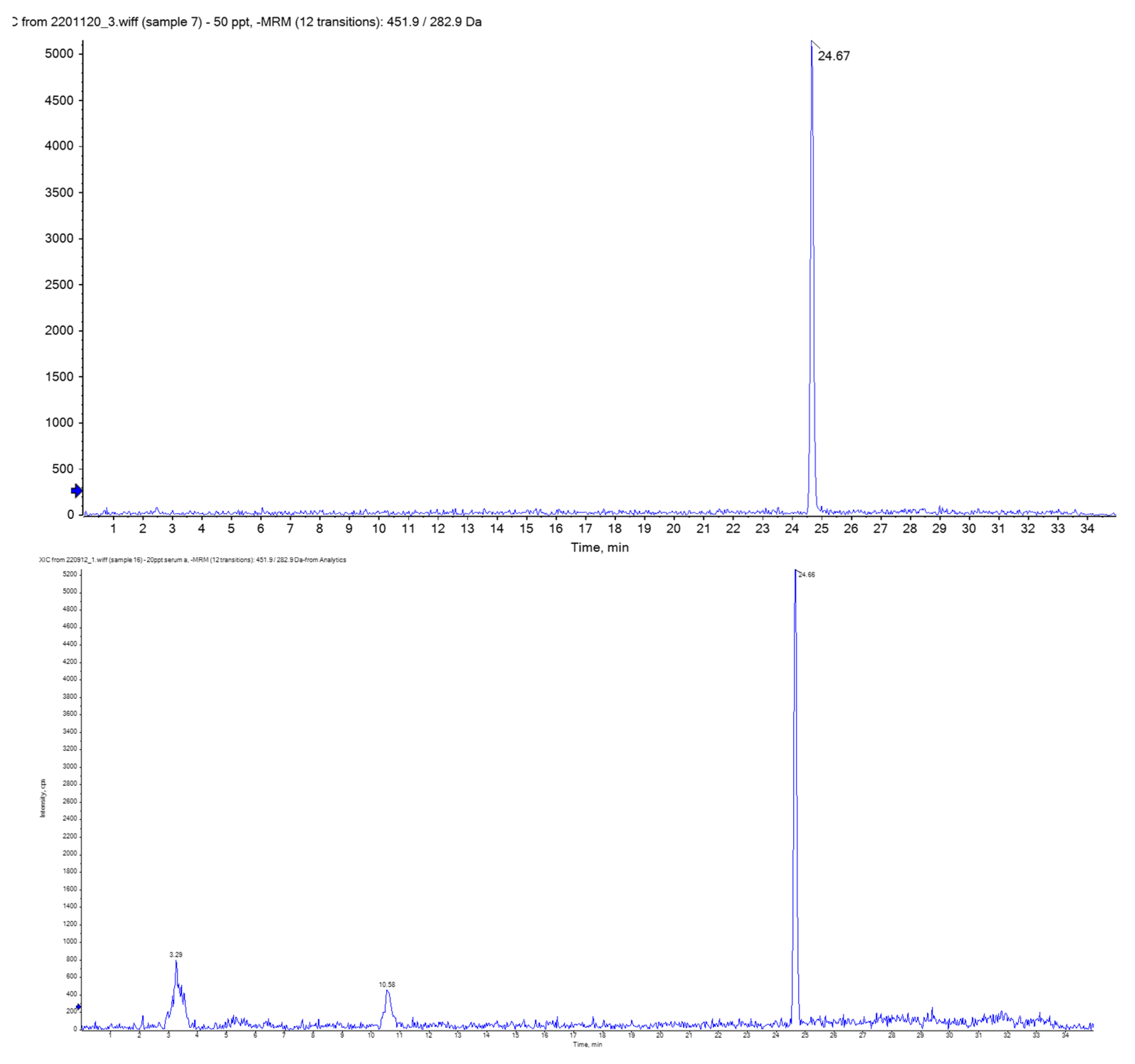
3.1. Analytical Performance
3.2. Serum Concentrations of Fipronils in Japanese Pregnant Women
3.3. Variation of Serum Fipronil Sulfone Due to Attributes and Dietary Habits of Participants
3.4. Association Between the Levels of TH and Fipronil Sulfone
3.5. Limitations of This Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.-X.; Zhao, Z.; Fan, C.-L.; Xu, J.-Z.; Li, H.; Chang, Q.-Y. Fipronil residues and risk assessment of Chinese marketed fruits and vegetables: A long-term investigation over 6 years. Food Control 2019, 106, 106734. [Google Scholar] [CrossRef]
- Mahler, B.J.; Van Metre, P.C.; Wilson, J.T.; Musgrove, M. Fipronil and its degradates in indoor and outdoor dust. Environ. Sci. Technol. 2009, 43, 5665–5670. [Google Scholar] [CrossRef] [PubMed]
- Testa, C.; Salis, S.; Rubattu, N.; Roncada, P.; Miniero, R.; Brambilla, G. Occurrence of fipronil in residential house dust in the presence and absence of pets: A hint for a comprehensive toxicological assessment. J. Environ. Sci. Health B 2019, 54, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Jiang, Y.; Wan, Y.; Huang, J.; Meng, Q.; He, Z.; Xu, S.; Xia, W. Occurrence of the insecticide fipronil and its degradates in indoor dust from South, Central, and North China. Sci. Total Environ. 2020, 741, 140110. [Google Scholar] [CrossRef] [PubMed]
- Hazard, K.; Alkon, A.; Gunier, R.B.; Castoria, R.; Camann, D.; Quarderer, S.; Bradman, A. Predictors of pesticide levels in carpet dust collected from child care centers in Northern California, USA. J. Exp. Sci. Environ. Epidemiol. 2024, 34, 229–240. [Google Scholar] [CrossRef] [PubMed]
- McMahen, R.L.; Strynar, M.J.; Dagnino, S.; Herr, D.W.; Moser, V.C.; Garantziotis, S.; Andersen, E.M.; Freeborn, D.L.; McMillan, L.; Lindstorm, A.B. Identification of fipronil metabolites by time-of-flight mass spectrometry for application in a human exposure study. Environ. Int. 2015, 78, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Yoon, Y.S.; Kim, H.S.; Jeon, S.J.; Cole, E.; Lee, J.; Kho, Y.; Cho, Y.H. Distribution of fipronil in humans, and adverse health outcomes of in utero fipronil sulfone exposure in newborns. Int. J. Hyg. Environ. Health 2019, 222, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wan, Y.; Liu, J.; He, Z.; Xu, S.; Xia, W. Insecticide fipronil and its transformation products in human blood and urine: Assessment of human exposure in general population of China. Sci. Total Environ. 2021, 786, 147342. [Google Scholar] [CrossRef] [PubMed]
- Leghait, J.; Gayrard, V.; Picard-Hagen, N.; Camp, M.; Perdu, E.; Toutain, P.L.; Viguié, C. Fipronil-induced disruption of thyroid function in rats is mediated by increased total and free thyroxine clearances concomitantly to increased activity of hepatic enzymes. Toxicology 2009, 255, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Moser, V.C.; Stewart, N.; Freeborn, D.L.; Crooks, J.; MacMillan, D.K.; Hedge, J.M.; Wood, C.E.; McMahen, R.C.; Strynar, M.J.; Herr, D.W. Assessment of serum biomarkers in rats after exposure to pesticides of different chemical classes. Toxicol. Appl. Pharmacol. 2015, 282, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Roques, B.B.; Lacroix, M.Z.; Puel, S.; Gayard, V.; Picard-Hagen, N.; Jouanin, I.; Perdu, E.; Martin, P.G.; Viguié, C. CYP450-dependent biotransformation of the insecticide fipronil into fipronil sulfone can mediate fipronil-induced thyroid disruption in rats. Toxicol. Sci. 2012, 127, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Korevaar, T.M.I.; Muetzel, R.; Medici, M.; Chaker, L.; Jaddoe, V.W.V.; de Rijke, Y.B.; Steegers, E.A.P.; Visser, T.J.; White, T.; Tiemeier, H.; et al. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: A population based prospective cohort study. Lancet Diabetes Endocrinol. 2016, 4, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-N.; Tsai, T.-H. Preclinical transplacental transfer and pharmacokinetics of fipronil in rats. Drug Metab. Dispos. 2020, 48, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Hisada, A.; Shimodaira, K.; Okai, T.; Watanabe, K.; Takemori, H.; Takasuga, T.; Noda, Y.; Shirakawa, M.; Kato, N.; Yoshinaga, J. Serum levels of hydroxylated PCBs, PCBs and thyroid hormone measures of Japanese pregnant women. Environ. Health Prev. Med. 2013, 18, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hisada, A.; Yoshinaga, J.; Shiraishi, H.; Shimodaira, K.; Okai, T.; Noda, Y.; Shirakawa, M.; Kato, N. Exposure to pyrethroids insecticides and serum levels of thyroid-related measures in pregnant women. Environ. Res. 2013, 127, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Cravedi, J.P.; Delous, G.; Zalko, D.; Viguié, C.; Debrauwer, L. Disposition of fipronil in rats. Chemosphere 2013, 93, 2276–2283. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Environmental Studies, Chemical Information Database Webkis Plus. Available online: https://www.nies.go.jp/kisplus/dtl/chem/NT300103 (accessed on 26 February 2025).
- Chen, D.; Li, J.; Zhao, Y.; Wu, Y. Human exposure of fipronil insecticide and the associated health risk. J. Agric. Food Chem. 2021, 70, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, D.; Lyu, B.; Li, J.; Zhao, Y.; Wu, Y. Exposure to fipronil insecticide in the sixth total diet study—China, 2016–2019. China CDC Wkly. 2022, 4, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Herin, F.; Boutet-Robinet, E.; Levant, A.; Dulaurent, S.; Manika, M.; Galatry-Bouju, F.; Caron, P.; Soulat, J.-M. Thyroid function tests in persons with occupational exposure to fipronil. Thyroid 2011, 21, 701–706. [Google Scholar] [CrossRef] [PubMed]


| LC-MS/MS Parameter | ||||||
|---|---|---|---|---|---|---|
| Instrument | Triple Quad 5500+ QTRAP Ready (SCIEX) | |||||
| Ionization mode | ESI, negative mode | |||||
| Ionization splay voltage (V) | −4500 | |||||
| Ion source temperature (°C) | 300 | |||||
| Monitored ion and MS voltage setting | ||||||
| Ion (m/z) | Voltage setting (V) | |||||
| Precursor | Product | Qualifier | Declustering potential | Collision energy | Collision cell exit | |
| Fipronil | 435.7 | 250.9 | 330.5 | −105 | −40 | −25 |
| Fipronil sulfone | 451.9 | 282.9 | 415.8 | −105 | −40 | −13 |
| Fipronil sulfide | 419.9 | 262.9 | 383.8 | −90 | −40 | −19 |
| 13C-fipronil | 441.9 | 336.8 | 252.8 | −100 | −24 | −33 |
| 13C-fipronil sulfone | 457.9 | 421.8 | 288.8 | −110 | −24 | −33 |
| 13C-fipronil sulfide | 425.9 | 389.8 | 265.2 | −95 | −20 | −19 |
| Chromatographic conditions | ||||||
| Column | InertSustain C18, 5 µm, 150 × 2.1 mm ID (GL Science) | |||||
| Column temperature (°C) | 40 | |||||
| Mobile phase gradient A: Methanol B: 0.05 mM Ammonium fluoride | 0–1 min A 20% B 80% 1–25 min A 20–90% B 80–10% 25–30 min A 90% B 10% 30–35 min A 20% B 80% | |||||
| Mobile phase flow rate (mL/min) | 0.2 | |||||
| Injection (µL) | 8.0 | |||||
| Unit | N | Mean (Standard Deviation) | Min–Max | |
|---|---|---|---|---|
| Age | Yrs | 128 | 34.1 (4.8) | 22–48 |
| Pre-pregnancy BMI | kg/m2 | 129 | 21.1(2.7) | 17.1–35.0 |
| Urinary iodine | μg/g cre | 121 | 390 (2.7) * | 47–6159 |
| fT4 | ng/dL | 131 | 1.24 (1.21) * | 0.83–3.41 |
| TSH | μIU/mL | 116 | 0.651 (4.56) *# | <0.005–27.4 |
| Parity | Primiparous 65 (50%) Multiparous 64 (50%) | |||
| Smoking by the time of pregnancy diagnosis | Yes 11 (8.8%) No 114 (91.2%) | |||
| Current passive smoking | Yes 25 (22%) No 89 (78%) | |||
| Detection Frequency (%) | Mean (SD) | Geometric Mean (SD) | Median | Min–Max | |
|---|---|---|---|---|---|
| Fipronil | 0 | <0.005 | <0.005 | <0.005 | |
| Fipronil sulfone | 100 | 24 (15) | 21 (1.7) | 21 | 6.8–89 |
| Fipronil sulfide | 0 | <0.001 | <0.001 | <0.001 |
| Dependent Variable: Serum Free T4 * | |||
|---|---|---|---|
| Independent Variable | β (95% CI) | Adjusted β | p |
| Fipronil sulfone * | 0.010 (−0.033–0.052) | 0.039 | 0.65 |
| Age | −0.001 (−0.005–0.004) | −0.020 | 0.81 |
| BMI | 0.001 (−0.009–0.010) | 0.014 | 0.87 |
| Parity | −0.014 (−0.062–0.034) | −0.049 | 0.58 |
| Urinary iodine * | 0.005 (−0.019–0.030) | 0.037 | 0.66 |
| Smoking until pregnancy | −0.001 (−0.090–0.089) | −0.002 | 0.99 |
| Passive smoking | −0.015 (−0.079–0.049) | −0.044 | 0.64 |
| TSH * | −0.089 (−0.107–0.071) | −0.716 | <0.001 |
| Constant | 0.133 (−0.209–0.475) | 0.44 | |
| Model F = 8.282 p < 0.001 Adjusted R2: 0.380 | |||
| Dependent Variable: Serum TSH * | |||
|---|---|---|---|
| Independent Variable | β (95% CI) | Adjusted β | p |
| Fipronil sulfone * | 0.088 (−0.290–0.467) | 0.039 | 0.64 |
| Age | 0.014 (−0.028–0.057) | 0.055 | 0.50 |
| BMI | −0.063 (−0.147–0.021) | −0.124 | 0.14 |
| Urinary iodine * | 0.047 (−0.172–0.266) | 0.036 | 0.67 |
| Parity | −0.056 (−0.487–0.375) | −0.022 | 0.80 |
| Smoking until pregnancy | −0.115 (−0.917–0.688) | −0.025 | 0.78 |
| Passive smoking | 0.226 (−0.344–0.795) | 0.072 | 0.43 |
| Serum free T4 * | −5.688 (−7.171–4.214) | −0.623 | <0.001 |
| Constant | 1.184 (−1.879–4.247) | 0.44 | |
| Model F = 9.033 p < 0.001 Adjusted R2: 0.495 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, K.; Hisada, A.; Otake, T.; Omagari, R.; Nakajima, D.; Kato, N.; Yoshinaga, J. Serum Concentrations of Fipronil and Metabolites in Japanese Pregnant Women: Relationship with Thyroid Hormone Levels. Toxics 2025, 13, 213. https://doi.org/10.3390/toxics13030213
Ikeda K, Hisada A, Otake T, Omagari R, Nakajima D, Kato N, Yoshinaga J. Serum Concentrations of Fipronil and Metabolites in Japanese Pregnant Women: Relationship with Thyroid Hormone Levels. Toxics. 2025; 13(3):213. https://doi.org/10.3390/toxics13030213
Chicago/Turabian StyleIkeda, Kunishige, Aya Hisada, Takamitsu Otake, Ryo Omagari, Daisuke Nakajima, Nobumasa Kato, and Jun Yoshinaga. 2025. "Serum Concentrations of Fipronil and Metabolites in Japanese Pregnant Women: Relationship with Thyroid Hormone Levels" Toxics 13, no. 3: 213. https://doi.org/10.3390/toxics13030213
APA StyleIkeda, K., Hisada, A., Otake, T., Omagari, R., Nakajima, D., Kato, N., & Yoshinaga, J. (2025). Serum Concentrations of Fipronil and Metabolites in Japanese Pregnant Women: Relationship with Thyroid Hormone Levels. Toxics, 13(3), 213. https://doi.org/10.3390/toxics13030213







