Microplastic Pollution and Its Ecological Risks in the Xisha Islands, South China Sea
Abstract
1. Introduction
2. Materials and Methods
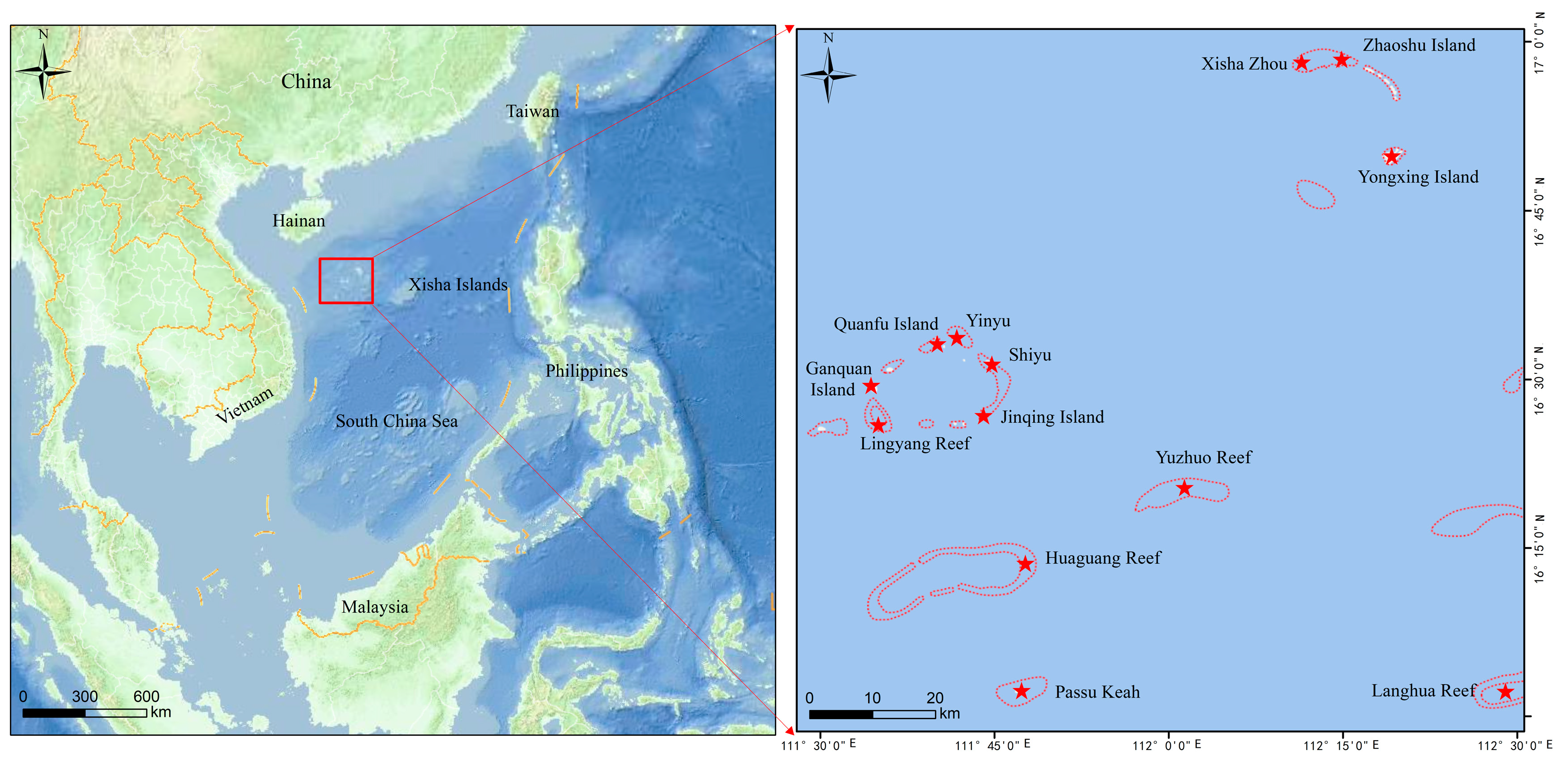
2.1. Sample Collection
2.2. Isolation of Microplastics
2.2.1. Sediment, Soil, and Water Samples
2.2.2. Fish and Bird Samples
2.3. Observation and Identification of Microplastics
2.4. Quality Control of Experiments
2.5. Ecological Risk Assessment
2.6. Data Analysis
3. Results and Discussion
3.1. Abundance of Microplastic Pollution
3.1.1. Abundance of Microplastic in Environmental Media
3.1.2. Abundance of Microplastic in Biological Samples
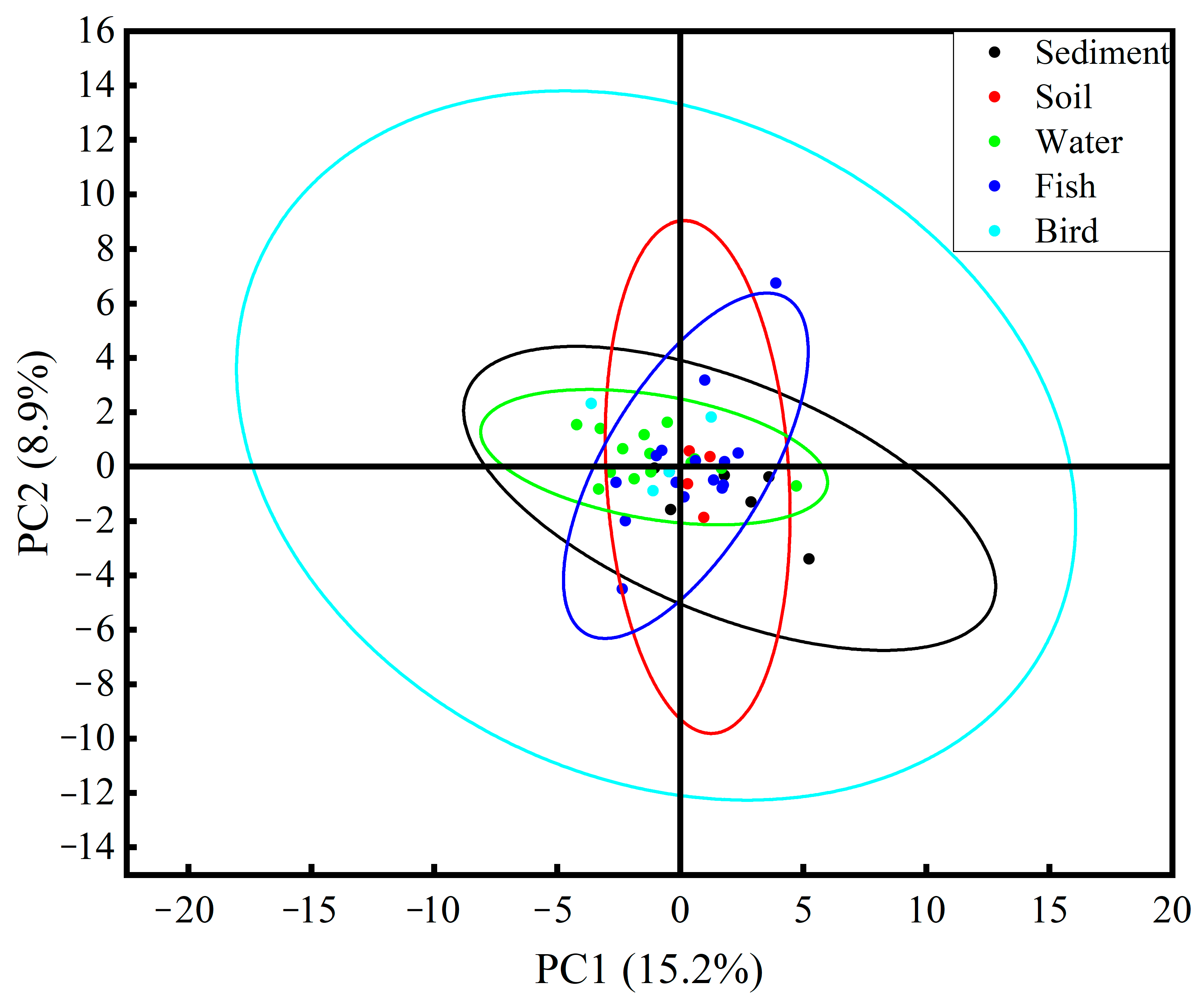
3.2. Characteristics of Microplastics in Environmental Media and Biological Samples
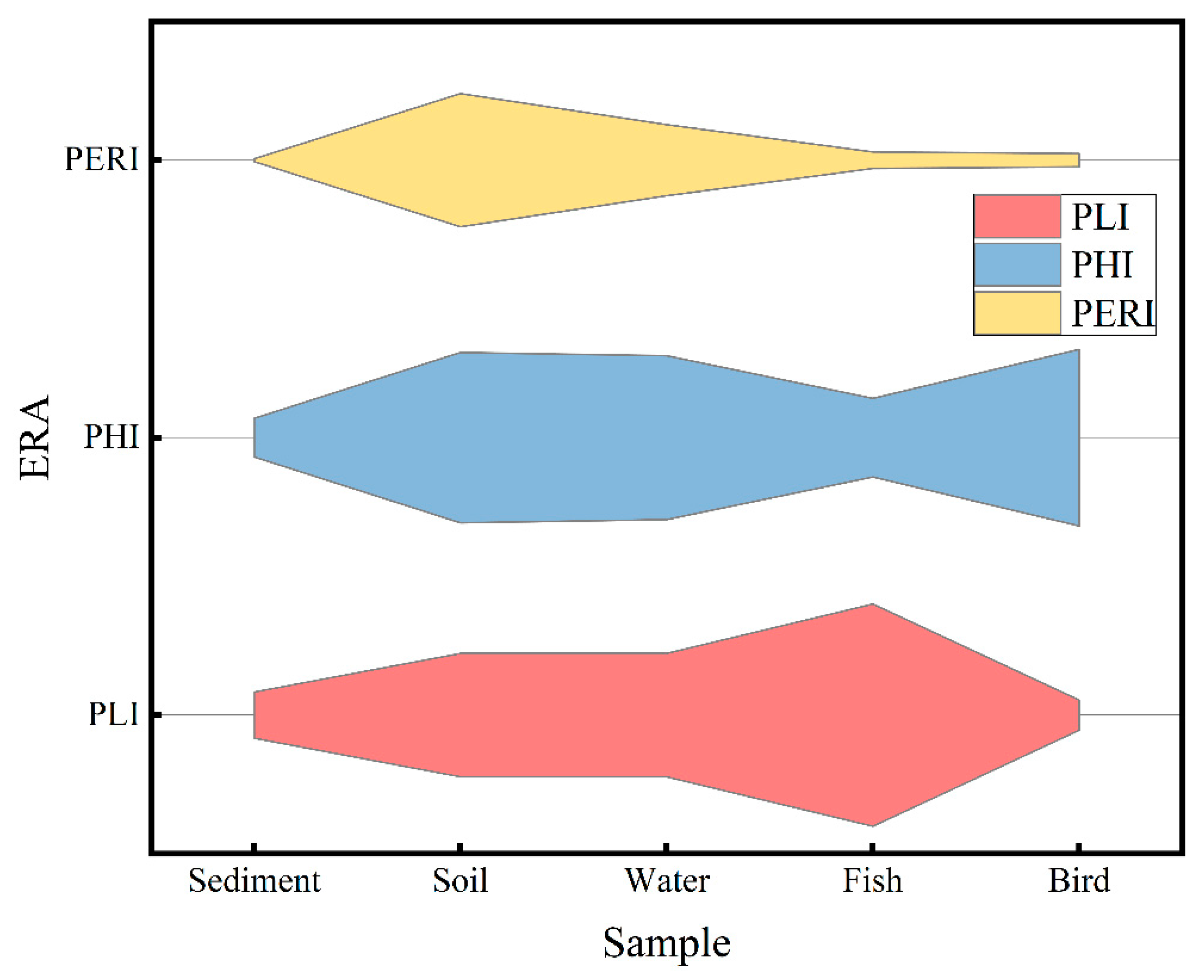
3.3. Ecological Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Jiang, F.; Li, J.; Wang, Z.; Sun, C.; Wang, Z.; Fu, L.; Ding, N.X.; He, C. Microplastics in the coral reef systems from Xisha Islands of south China sea. Environ. Sci. Technol. 2019, 53, 8036–8046. [Google Scholar] [CrossRef] [PubMed]
- Simionov, I.-A.; Călmuc, M.; Iticescu, C.; Călmuc, V.; Georgescu, P.-L.; Faggio, C.; Petrea, Ş.-M. Human health risk assessment of potentially toxic elements and microplastics accumulation in products from the danube river basin fish market. Environ. Toxicol. Pharmacol. 2023, 104, 104307. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. Taahe physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Li, W.C.; Tse, H.F.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016, 566, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Nyadjro, E.S.; Webster, J.A.; Boyer, T.P.; Cebrian, J.; Collazo, L.; Kaltenberger, G.; Larsen, K.; Lau, Y.H.; Mickle, P.; Toft, T. The NOAA NCEI marine microplastics database. Sci. Data 2023, 10, 726. [Google Scholar] [CrossRef]
- Zhang, G.; Li, J.; Cheng, H.; Li, X.; Xu, W.; Jones, K.C. Distribution of organochlorine pesticides in the Northern South China Sea: Implications for land outflow and air-sea exchange. Environ. Sci. Technol. 2007, 41, 3884–3890. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, S.; Sun, L.; Luo, H.; Yin, X.; Xie, Z.; Wang, Y.; Liu, K.; Wu, X.; Ding, X. Geochemical evidence for the variation of historical seabird population on Dongdao Island of the South China Sea. J. Paleolimnol. 2006, 36, 259–279. [Google Scholar] [CrossRef]
- Fang, Z.; Tan, F.; Yang, H.; Xu, H.; Xu, X.; Li, H. Distribution characteristics of plastic debris and microplastics on the beaches of Ganquan Island and Quanfu Island in Xisha Sea. J. Trop. Oceanogr. 2021, 40, 123–133. [Google Scholar]
- Matsukami, H.; Tue, N.M.; Suzuki, G.; Someya, M.; Viet, P.H.; Takahashi, S.; Tanabe, S.; Takigami, H. Flame retardant emission from e-waste recycling operation in northern Vietnam: Environmental occurrence of emerging organophosphorus esters used as alternatives for PBDEs. Sci. Total Environ. 2015, 514, 492–499. [Google Scholar] [CrossRef]
- Zhao, J.; Ran, W.; Teng, J.; Liu, Y.; Liu, H.; Yin, X.; Cao, R.; Wang, Q. Microplastic pollution in sediments from the Bohai Sea and the Yellow Sea, China. Sci. Total Environ. 2018, 640, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, H.; Fu, C.; Zhou, Y.; Dai, Z.; Li, Y.; Tu, C.; Luo, Y. The distribution and morphology of microplastics in coastal soils adjacent to the Bohai Sea and the Yellow Sea. Geoderma 2018, 322, 201–208. [Google Scholar] [CrossRef]
- Wang, J.; Wang, M.; Ru, S.; Liu, X. High levels of microplastic pollution in the sediments and benthic organisms of the South Yellow Sea, China. Sci. Total Environ. 2019, 651, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Cincinelli, A.; Scopetani, C.; Chelazzi, D.; Martellini, T.; Pogojeva, M.; Slobodnik, J. Microplastics in the Black Sea sediments. Sci. Total Environ. 2021, 760, 143898. [Google Scholar] [CrossRef]
- Wang, T.; Hu, M.; Song, L.; Yu, J.; Liu, R.; Wang, S.; Wang, Z.; Sokolova, I.M.; Huang, W.; Wang, Y. Coastal zone use influences the spatial distribution of microplastics in Hangzhou Bay, China. Environ. Pollut. 2020, 266, 115137. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, X.; Huang, W.; Li, J.; Wang, C.; Zhang, D.; Zhang, C. Microplastic pollution in deep-sea sediments and organisms of the Western Pacific Ocean. Environ. Pollut. 2020, 259, 113948. [Google Scholar] [CrossRef]
- Yang, H.; Yu, K.; Zhao, M.; Shi, Q.; Tao, S.; Yan, H.; Chen, T.; Liu, G. Impact on the coral reefs at Yongle Atoll, Xisha Islands, South China Sea from a strong typhoon direct sweep: Wutip, September 2013. J. Asian Earth Sci. 2015, 114, 457–466. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Fang, T.; Xu, P.; Zhu, L.; Li, D. Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Sci. Total Environ. 2019, 675, 462–471. [Google Scholar] [CrossRef]
- Patterson, J.; Jeyasanta, K.I.; Laju, R.; Booth, A.M.; Sathish, N.; Edward, J.P. Microplastic in the coral reef environments of the Gulf of Mannar, India-Characteristics, distributions, sources and ecological risks. Environ. Pollut. 2022, 298, 118848. [Google Scholar] [CrossRef]
- Peng, G.; Xu, P.; Zhu, B.; Bai, M.; Li, D. Microplastics in freshwater river sediments in Shanghai, China: A case study of risk assessment in mega-cities. Environ. Pollut. 2018, 234, 448–456. [Google Scholar] [CrossRef]
- Li, B.; Su, L.; Zhang, H.; Deng, H.; Chen, Q.; Shi, H. Microplastics in fishes and their living environments surrounding a plastic production area. Sci. Total Environ. 2020, 727, 138662. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Wen, S.; Ma, Y.; Ji, R. Quantification of Ingestion of Microplastics by Marine Medaka (Oryzias melastigma) Using Fluorescence and C-14 Isotope Radiotracer. Res. Environ. Sci. 2021, 34, 2571–2578. [Google Scholar]
- Li, C.; Wang, X.; Liu, K.; Zhu, L.; Wei, N.; Zong, C.; Li, D. Pelagic microplastics in surface water of the Eastern Indian Ocean during monsoon transition period: Abundance, distribution, and characteristics. Sci. Total Environ. 2021, 755, 142629. [Google Scholar] [CrossRef]
- Tomlinson, D.; Wilson, J.; Harris, C.; Jeffrey, D. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresunters. 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhou, S.; Zhang, C.; Qin, W.; Lv, C. A framework for systematic microplastic ecological risk assessment at a national scale. Environ. Pollut. 2023, 327, 121631. [Google Scholar] [CrossRef]
- Lithner, D.; Larsson, Å.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef] [PubMed]
- Everaert, G.; Van Cauwenberghe, L.; De Rijcke, M.; Koelmans, A.A.; Mees, J.; Vandegehuchte, M.; Janssen, C.R. Risk assessment of microplastics in the ocean: Modelling approach and first conclusions. Environ. Pollut. 2018, 242, 1930–1938. [Google Scholar] [CrossRef]
- Xie, C.; Long, C.; Li, D.; Zhu, C.; Peng, X.; Sun, Y.; Luo, X.; Zhang, L.; Mai, B. Distribution of Microplastics and Halogenated Flame Retardants in Soils from Yongxing Island and East Island, South China Sea. Ecol. Environ. 2022, 31, 1008. [Google Scholar]
- Xu, L. Plastic Pollution Status in the Environmental Media and Common Fish of the Bohai Sea. Ph.D. Thesis, University of Chinese Academy of Sciences, Qingdao, China, 2021. [Google Scholar]
- Zheng, Y.; Li, J.; Cao, W.; Liu, X.; Jiang, F.; Ding, J.; Yin, X.; Sun, C. Distribution characteristics of microplastics in the seawater and sediment: A case study in Jiaozhou Bay, China. Sci. Total Environ. 2019, 674, 27–35. [Google Scholar] [CrossRef]
- Cai, M.; He, H.; Liu, M.; Li, S.; Tang, G.; Wang, W.; Huang, P.; Wei, G.; Lin, Y.; Chen, B. Lost but can’t be neglected: Huge quantities of small microplastics hide in the South China Sea. Sci. Total Environ. 2018, 633, 1206–1216. [Google Scholar] [CrossRef]
- Sun, X.; Li, Q.; Zhu, M.; Liang, J.; Zheng, S.; Zhao, Y. Ingestion of microplastics by natural zooplankton groups in the northern South China Sea. Mar. Pollut. Bull. 2017, 115, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Cheng, H.; Wu, S.; Li, H.; Chen, H.; Li, Z.; Yao, X.; Zhang, Y.; Chen, Y.; Chen, S. Microplastics footprint in nature reserves—A case study on the microplastics in the guano from Yancheng Wetland Rare Birds National Nature Reserve, China. Environ. Res. 2024, 256, 119252. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Distribution and Transfer of Microplastics in Black-Billed Gulls’ Habitat and Major Foraging Food Chains. Master’s Thesis, Qufu Normal University, Qufu, China, 2021. [Google Scholar]
- Carlin, J.; Craig, C.; Little, S.; Donnelly, M.; Fox, D.; Zhai, L.; Walters, L. Microplastic accumulation in the gastrointestinal tracts in birds of prey in central Florida, USA. Environ. Pollut. 2020, 264, 114633. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, X.; Liang, T.; Mu, T.; Ding, Y.; Liu, Y.; Liu, X. Varying abundance of microplastics in tissues associates with different foraging strategies of coastal shorebirds in the Yellow Sea. Sci. Total Environ. 2023, 866, 161417. [Google Scholar] [CrossRef]
- Remy, F.; Collard, F.; Gilbert, B.; Compère, P.; Eppe, G.; Lepoint, G. When microplastic is not plastic: The ingestion of artificial cellulose fibers by macrofauna living in seagrass macrophytodetritus. Environ. Sci. Technol. 2015, 49, 11158–11166. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Y.; Leung, J.Y.; Huang, W.; Zeng, J.; Tang, Y.; Chen, J.; Shi, A.; Yu, X.; Xu, X. Accumulation of microplastics in typical commercial aquatic species: A case study at a productive aquaculture site in China. Sci. Total Environ. 2020, 708, 135432. [Google Scholar] [CrossRef]
- Park, C.H.; Kang, Y.K.; Im, S.S. Biodegradability of cellulose fabrics. J. Appl. Polym. Sci. 2004, 94, 248–253. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, L.; Li, D.; Wang, J.; Liu, Y.; Li, R.; Wu, S.; Shi, H. Microplastic pollution at Qilianyu, the largest green sea turtle nesting grounds in the northern South China Sea. PeerJ 2022, 10, e13536. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Chen, M.; Jin, M.; Tao, P.; Wang, Z.; Xie, W.; Yu, X.; Wang, K. Assessment of microplastics derived from mariculture in Xiangshan Bay, China. Environ. Pollut. 2018, 242, 1146–1156. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T. Ingestion of microplastics by commercial fish off the Portuguese coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef]
- Lu, X.; Deng, D.-F.; Huang, F.; Casu, F.; Kraco, E.; Newton, R.J.; Zohn, M.; Teh, S.J.; Watson, A.M.; Shepherd, B. Chronic exposure to high-density polyethylene microplastic through feeding alters the nutrient metabolism of juvenile yellow perch (Perca flavescens). Anim. Nutr. 2022, 9, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017, 1, 0116. [Google Scholar] [CrossRef]
- Georgescu, P.-L.; Moldovanu, S.; Iticescu, C.; Calmuc, M.; Calmuc, V.; Topa, C.; Moraru, L. Assessing and forecasting water quality in the Danube River by using neural network approaches. Sci. Total Environ. 2023, 879, 162998. [Google Scholar] [CrossRef] [PubMed]
- Prarat, P.; Hongsawat, P.; Chouychai, B. Microplastic occurrence in surface sediments from coastal mangroves in Eastern Thailand: Abundance, characteristics, and ecological risk implications. Reg. Stud. Mar. Sci. 2024, 71, 103389. [Google Scholar] [CrossRef]
- Jung, J.-W.; Park, J.-W.; Eo, S.; Choi, J.; Song, Y.K.; Cho, Y.; Hong, S.H.; Shim, W.J. Ecological risk assessment of microplastics in coastal, shelf, and deep sea waters with a consideration of environmentally relevant size and shape. Environ. Pollut. 2021, 270, 116217. [Google Scholar] [CrossRef]

| Ingredient | Quantity Contained |
|---|---|
| Tris-HCl | 400 mmol/L |
| EDTA-2Na | 60 mmol/L |
| NaCl | 105 mmol/L |
| SDS | 10 g |
| H2O | 1 L |
| Sediment | Soil | Seawater | |||
|---|---|---|---|---|---|
| Sampling Point * | Abundance (items/kg) | Sampling Point * | Abundance (items/kg) | Sampling Point * | Abundance (items/L) |
| Se1 | 23.68 ± 8.19 | S1 | 288.21 ± 170.65 | W1 | 7 |
| Se2 | 53.06 ± 41.39 | S2 | 23.79 ± 29.71 | W2 | 0.99 |
| Se3 | 49.71 ± 0.26 | S3 | 41.64 ± 14.41 | W3 | 1.11 |
| Se4 | 23.60 ± 8.19 | S4 | 33.31 ± 21.82 | W4 | 2.94 |
| Se5 | 20.47 ± 10.15 | S5 | 77.74 ± 19.19 | W5 | 2.97 |
| Se6 | 70.55 ± 29.16 | W6 | 4.81 | ||
| Se7 | 49.86 ± 33.01 | W7 | 3.77 | ||
| W8 | 4.69 | ||||
| W9 | 4.32 | ||||
| W10 | 3.21 | ||||
| W11 | 0.46 | ||||
| W12 | 0.41 | ||||
| W13 | 2.11 | ||||
| W14 | 1.62 | ||||
| Fish | Birds | ||
|---|---|---|---|
| Fish Species | Abundance (items/ind) | Bird Species | Abundance (items/ind) |
| Epinephelus chlorostigma | 2.44 ± 2.60 | Ardea intermedia | 1.31 ± 1.47 |
| Amphilophus | 4.25 ± 0.96 | Egretta garzetta | 3.5 |
| Lethrinus ornatus | 2.80 ± 1.92 | Ardea cinerea | 2 |
| Datnioides microlepis | 7 | Ardea purpurea | 0 |
| Acanthurus triostegus | 2 ± 2.65 | ||
| Acanthurus nigrofuscus | 1.6 ± 1.52 | ||
| Scarus psittacus | 2 ± 1.41 | ||
| Scarus dimidiatus | 2 ± 1.41 | ||
| Coris gaimard | 0 | ||
| Upeneus sulphureus | 5 ± 2.83 | ||
| Pterocaesio tile | 5 ± 1.41 | ||
| Parupeneustrifasciatus | 3 ± 2.55 | ||
| Cephalopholis urodeta | 2 ± 2.35 | ||
| Sufflamen fraenatus | 1 | ||
| Cheilinus rhodochrous Gunther | 2 ± 1.41 | ||
| Malacanthus brevirostris | 2.4 ± 1.67 | ||
| Abalistes stellatus | 3.67 ± 3.06 | ||
| Branchiostegus japonicus | 0 | ||
| Sparus fasciatus | 2 | ||
| Sufflamen chrysopterus | 2.33 ± 0.58 | ||
| Neoniphon opercularis | 0 | ||
| Hologymnosus doliatus | 3 | ||
| Hapalogenys mucronatus | 2.67 ± 2.52 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, W.; Zhang, Y.; Wang, L.; Xing, Q.; Xiang, J.; Zhang, Y.; Peng, Q.; Chen, Y.; Hu, Y.; Ma, Y.; et al. Microplastic Pollution and Its Ecological Risks in the Xisha Islands, South China Sea. Toxics 2025, 13, 205. https://doi.org/10.3390/toxics13030205
Wei W, Zhang Y, Wang L, Xing Q, Xiang J, Zhang Y, Peng Q, Chen Y, Hu Y, Ma Y, et al. Microplastic Pollution and Its Ecological Risks in the Xisha Islands, South China Sea. Toxics. 2025; 13(3):205. https://doi.org/10.3390/toxics13030205
Chicago/Turabian StyleWei, Wenchao, Yun Zhang, Licheng Wang, Qiao Xing, Jun Xiang, Yuquan Zhang, Qifei Peng, Yongfu Chen, Yufeng Hu, Yini Ma, and et al. 2025. "Microplastic Pollution and Its Ecological Risks in the Xisha Islands, South China Sea" Toxics 13, no. 3: 205. https://doi.org/10.3390/toxics13030205
APA StyleWei, W., Zhang, Y., Wang, L., Xing, Q., Xiang, J., Zhang, Y., Peng, Q., Chen, Y., Hu, Y., Ma, Y., & Mo, L. (2025). Microplastic Pollution and Its Ecological Risks in the Xisha Islands, South China Sea. Toxics, 13(3), 205. https://doi.org/10.3390/toxics13030205





