Abstract
Environmental monitoring requires reliable bioindicators to assess the genotoxic effects of pollutants in aquatic ecosystems. In this study, the marine fish Thalassophryne maculosa was evaluated as a bioindicator of genotoxicity through the application of the micronucleus test. Fish were exposed to varying concentrations of mercuric chloride (HgCl2) (0.1, 0.25, and 0.5 µg HgCl2/g body weight) over different time intervals (24, 48, 72, and 96 h). A dose- and time-dependent increase in nuclear abnormalities, including micronuclei, was observed, with significant chromosomal damage detected at 0.25 and 0.5 µg HgCl2/g body weight. These results demonstrate the sensitivity of T. maculosa to mercury exposure, even at concentrations below regulatory safety thresholds, emphasizing its suitability as a bioindicator for detecting genotoxic contamination in coastal ecosystems. This study provides critical insights into the ecological risks posed by mercury and highlights the potential of T. maculosa to enhance environmental monitoring programs, particularly in regions vulnerable to heavy metal pollution.
1. Introduction
Human activities such as deforestation, agriculture, construction, and industrial expansion have drastically elevated pollutant emissions, overwhelming ecosystems’ natural ability to mitigate them. This increase in contamination has had profound consequences, from cellular disruptions to ecosystem-wide impacts on biodiversity and human health [1,2,3,4,5,6]. Among these pollutants, heavy metals such as mercury stand out for their extreme toxicity, environmental persistence, and capacity for bioaccumulation and biomagnification in aquatic food webs, posing serious risks to marine and freshwater ecosystems [7,8,9,10,11].
In aquatic ecosystems, mercury occurs in three main forms: elemental (Hg0), inorganic (Hg2+) and organic (methylmercury, MeHg). While Hg0 is relatively inert, Hg2+ and MeHg are the most toxic due to their strong biological impact [12]. Through microbial methylation, Hg2+ is converted into MeHg, the most dangerous form, as it bioaccumulates in fish tissues and biomagnifies along the food web. As a result, top predators face the highest exposure, with MeHg concentrations up to six orders of magnitude higher than those present in surface water [13]. This prolonged accumulation generates oxidative stress, alters enzymatic defenses, and causes physiological and neurological dysfunctions, affecting the health of exposed organisms. In addition, MeHg accumulates predominantly in muscle tissues, where it accounts for more than 90% of total mercury, increasing its toxicity to aquatic predators [14].
Beyond oxidative stress and enzymatic alterations, mercury exposure has been associated with genotoxic effects, such as chromosomal instability and micronucleus formation, which compromise DNA integrity [15,16]. Likewise, histopathological alterations, such as hyperplasia, inflammation, necrosis, and nuclear abnormalities, reinforce the magnitude of the ecological impact of mercury contamination in aquatic ecosystems [17].
Mercury contamination in aquatic ecosystems, especially near industrial discharge, agricultural runoff and sewage outfalls, represents a serious threat to water quality and marine biodiversity [1,18,19,20]. Fish, as an integral part of the food web, play a key role in environmental assessment due to their ability to bioaccumulate toxins and respond to low concentrations of pollutants, making them ideal bioindicators of the ecological impact of pollution [21,22].
The micronucleus assay is a widely used tool for detecting genotoxicity. Originally developed to assess chromosomal damage in rodents [23,24,25], this method identifies extranuclear bodies formed by chromosomal fragments or complete chromosomes that fail to integrate into daughter nuclei during cell division [26,27]. These micronuclei are reliable indicators of genotoxicity and chromosomal instability. Because of its sensitivity to environmental pollutants, it has been adapted to various organisms, most notably in fish, where it is used to assess the impact of pollution on aquatic ecosystems [22,28,29,30].
Numerous studies have validated the use of fish as bioindicators for genotoxicity caused by contaminants, with the micronucleus assay as one of the most widely used tools. In marine environments, species such as Odontesthes argentinensis, Paralichthys orbignyanus, Micropogonias furnieri, and Mugil platanus have demonstrated sensitivity to genotoxic agents [31]. Similarly, Epinephelus chlorostigma, Scomberomorus commerson [32], Limanda limanda, and Melanogrammus aeglefinus [33] have been used in marine pollution studies. Freshwater species like Andinoacara rivulatus [15], Danio reiro [34,35], Cyprinus carpio, Astyanax eigenmanniorum, Cheirodon interruptus [36], Oreochromis niloticus [37,38,39], Labeo rohita [40], Prochilodus magdalenae, Hoplias malabaricus [41], and Collosoma macropomum [42], among others, have been effective in detecting genotoxicity with this assay.
While most studies have focused on commercially important freshwater and marine species, estuarine benthic fish have been explored little in terms of their genotoxic response to mercury, despite inhabiting ecosystems subject to environmental fluctuations and variable levels of contamination.
Since metabolic and ecological characteristics influence sensitivity to contaminants, it is essential to extend research toward new bioindicator organisms. In this context, Thalassophryne maculosa Günther, 1861, known as ‘cano toadfish’, emerges as a relevant model for assessing the effects of heavy metal pollution. This venomous fish of the family Batrachoididae inhabits marine and brackish waters along the northern coast of South America and surrounding islands [43]. Although it lacks commercial value, it has attracted scientific interest due to its distinctive camouflage, sound production, cytogenetic characteristics and ability to survive in estuarine environments, ecosystems particularly vulnerable to heavy metal pollution [44,45,46]. These characteristics make it an ecologically relevant model for assessing mercury-induced genotoxicity [47,48].
In this study, we applied the micronucleus assay to assess chromosomal damage in T. maculosa individuals exposed to varying concentrations of inorganic mercury. The goal was to establish the species’ potential as a bioindicator for mercury-induced genotoxicity and contribute to a deeper understanding of the ecological risks posed by heavy metal pollution in marine environments.
2. Materials and Methods
2.1. Specimen Collection and Acclimation
Fish specimens for this study were collected on the same day from the entrance of La Restinga Lagoon, Margarita Island, Venezuela (coordinates: 10°58.7′ N, 64°9.7′ W). Through free diving at a depth of 1.2 m, specimens were carefully captured with hand nets to minimize stress and potential injury. Immediately after collection, the fish were transported to the facilities of the Escuela de Ciencias Aplicadas del Mar (E.C.A.M) at the Universidad de Oriente.
Upon arrival, the fish were housed in a concrete holding tank equipped with aeration and a controlled flow of seawater to maintain water quality. They were acclimated under stable environmental conditions for 48 h to allow recovery and adjustment prior to the experimental procedures. During this period, no feeding was provided to standardize physiological conditions among individuals. Environmental parameters, such as temperature, salinity, dissolved oxygen, and pH, were monitored regularly to ensure consistency with the collection site.
2.2. Experimental Setup and Grouping
Following acclimatization, the fish were randomly assigned to 12 experimental groups, each comprising five individuals. The groups were housed in cages measuring 80 cm × 60 cm × 30 cm, constructed with plastic mesh featuring 1-inch openings to allow water flow. These cages were suspended 50 cm below the tidal level at the distal end of the E.C.A.M dock, approximately 65 m offshore, ensuring continuous exposure to high-quality seawater.
While the suspended setup did not fully replicate the benthic conditions typically inhabited by Thalassophryne maculosa, the plastic mesh provided a stable surface for the fish to rest and reduced potential stress by maintaining proximity to their natural aquatic environment. To account for potential mortality, each group initially contained five fish; however, all individuals survived during the study. For subsequent analyses, three fish were randomly selected from each group to ensure consistency. To maintain uniform experimental conditions and avoid confounding variables, the fish were not fed throughout the duration of the study.
2.3. Factorial Design and Treatment Administration
A factorial design was employed to evaluate the effects of HgCl2 concentration and exposure duration on the test specimens. Fish were exposed to four concentrations of mercuric chloride (HgCl2)—0.0, 0.1, 0.25, and 0.5 µg/g body wet weight—with the control group (0 µg/mL) receiving only distilled water. The exposure levels were achieved through intraperitoneal injection at a dose of 1 mL per 100 g of fish weight, ensuring precise and uniform administration while minimizing variability due to differential uptake or metabolic differences.
The exposure duration included four time points (24, 48, 72, and 96 h) to capture both acute and progressive genotoxic effects. This factorial approach allowed for the independent and interactive assessment of HgCl2 concentration and exposure duration, providing a comprehensive evaluation of its genotoxic potential.
2.4. Selection of HgCl2 as the Experimental Compound
Mercury(II) chloride (HgCl2) was chosen for its high solubility in water and relevance to aquatic environments. Inorganic mercury (Hg2+)—including HgCl2—is a predominant form of mercury pollution introduced into aquatic ecosystems through industrial and anthropogenic activities [49,50]. Once released into the environment, HgCl2 remains highly bioavailable and can undergo microbial methylation, transforming into methylmercury and contributing to mercury cycling [12].
Additionally, HgCl2 is widely used in toxicological studies as a representative form of inorganic mercury due to its strong interaction with biological systems. Its use facilitates comparisons with the existing literature on mercury toxicity in fish species [49,50], making it a suitable model for assessing genotoxic effects in aquatic organisms.
2.5. Blood Sampling and Micronucleus Analysis
Blood samples were collected at 24, 48, 72, and 96 h of exposure from fish in each treatment group. Sampling was performed via caudal vein puncture using a heparinized syringe to prevent coagulation. A small volume of blood was spread evenly onto two clean glass slides per fish to ensure sufficient sample representation. The slides were left to air dry at room temperature, and then fixed in absolute methanol for 10 min to preserve cellular structures. Subsequently, the slides were stained with a 5% Giemsa solution (pH 6.8) for 10 min to enhance chromosomal and nuclear visibility.
To achieve reliable quantification, the number of photographs taken per slide was adjusted based on the concentration of erythrocytes in each sample, ensuring that at least 1000 cells per slide were evaluated. Digital images were captured at 1000× magnification using a Motic photomicroscope, with care taken to avoid overlapping or damaged cells during analysis.
Micronucleus scoring was conducted by a single, experienced observer following the standardized guidelines of Nirchio et al. [28] to minimize inter-observer variability. Micronuclei were recorded as the number observed per 2000 erythrocytes per individual, providing a precise estimate of micronucleus frequency and minimizing the impact of interindividual variability. This approach is consistent with previous studies in genotoxic biomonitoring in fish, where analyzing a high number of cells per individual compensates for the use of a moderate sample size, ensuring the detectability of significant differences between treatments. Furthermore, the selection of an optimized sample size adheres to the ethical principle of reduction in animal experimentation, in accordance with international guidelines for aquatic toxicology studies.
Other nuclear abnormalities were noted but not included in the primary micronucleus frequency analysis.
2.6. Statistical Analyses
Initial statistical evaluations revealed that the data did not meet the assumptions of normality (Shapiro–Wilk test) or homoscedasticity (Levene test). To address these violations, an inverse square root transformation [1/√(Micronuclei/2000 cells)] was applied, enabling the use of parametric analysis of variance (ANOVA). The transformed data were analyzed to assess the independent effects of mercuric chloride (HgCl2) concentration and exposure time, as well as their interaction. Post hoc comparisons were performed using the Least Significant Difference (LSD) test at a 95% confidence level, identifying statistically significant differences (p < 0.05) across treatments [51,52].
Statistical analyses were conducted using StatGraphics Centurion XVI software. Version 16.1.18. In order to provide a comprehensive visual representation overview of the dose- and time-dependent effects of HgCl2 on chromosomal damage, a three-dimensional plot was generated in Python (version 3.8) using the Matplotlib library (version 3.5.1) [53]. The plot_trisurf function was employed to create a 3D surface illustrating the relationship between HgCl2 concentration, exposure time, and micronucleated cell frequency. The ‘viridis’ colormap was applied, with lighter tones representing higher frequencies of micronuclei.
3. Results
Salinity in the collection area ranged between 36.1 and 37.8 g/L, with water temperature between 21.8 and 27.4 °C, pH between 7.4 and 7.8, and dissolved oxygen concentrations between 4.9 and 7.3 mg/L. Experimental conditions remained stable, with salinity (35.6–38.0 g/L), temperature (22.1–28.5 °C), pH (7.3–7.7), and dissolved oxygen (5.5–7.0 mg/L) falling within expected ranges.
Microscopic analysis of blood smears revealed a variety of abnormalities, including micronucleated mature erythrocytes (MMEs), micronucleated immature erythrocytes (MIEs), binucleated immature erythrocytes (BIEs), binucleated immature erythrocytes with a cytoplasmic bridge (BIECBs), and erythrocytes exhibiting vacuoles or cytoplasmic loss (VLCs) (Figure 1). Normal erythrocytes were characterized by an oval or ellipsoidal shape, an elliptical nucleus, and cytoplasm free of nuclear aggregates
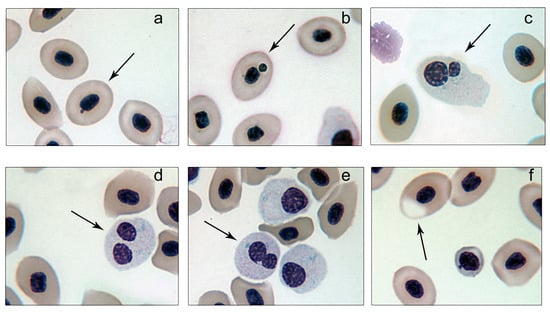
Figure 1.
Nuclear abnormalities in peripheral blood of Thalassophryne maculosa. (a,b) Mature micronucleated erythrocytes (MMEs) with micronuclei of varying sizes; (c) immature binucleated erythrocytes (BIEs); (d) mature binucleated erythrocytes (MBEs); (e) immature binucleated erythrocytes with a cytoplasmic bridge (BIECBs); (f) erythrocytes with vacuoles or cytoplasmic loss (VLCs).
The ANOVA results (Table 1) demonstrate that both HgCl2 concentration and exposure time had highly significant effects on micronucleus frequency (p < 0.01). The interaction between these two factors, however, was not significant (F = 0.26, p = 0.9819), indicating that their effects were additive rather than synergistic.

Table 1.
Analysis of variance for 1/(√(Micronuclei/2000 cells2)).
Post hoc analysis revealed that the frequency of micronucleated cells significantly increased with higher HgCl2 concentrations and longer exposure times (Table 2). Treatments were categorized into homogeneous subsets, confirming distinct thresholds for genotoxic effects.

Table 2.
Post hoc LSD test results for the effects of HgCl2 concentration and exposure time on micronucleus frequency in Thalassophryne maculosa.
The micronucleus frequency showed a clear dose- and time-dependent pattern (Figure 2). At lower concentrations (0 and 0.1 µg/g body weight), micronucleated cells remained relatively stable over time, with a frequency ranging between 1 and 5 MN/2000 cells. This indicates minimal genotoxicity at these exposure levels. In contrast, higher concentrations (0.25 and 0.5 µg/g body weight) led to a pronounced increase in micronucleated cells, particularly with extended exposure.
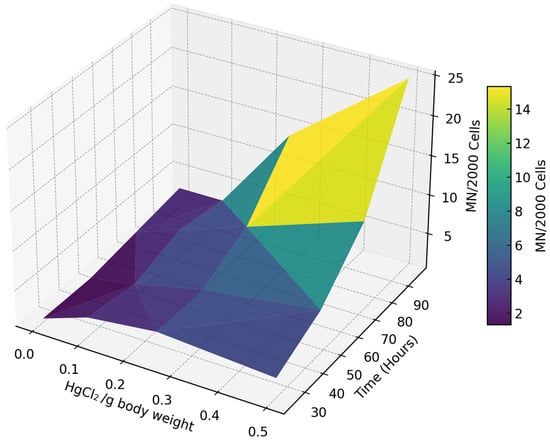
Figure 2.
A three-dimensional representation of the variation in micronucleated cells (MN/2000 cells) in response to HgCl2 concentration (µg/body weight) and exposure time (hours). Darker colors (blue/green) indicate fewer micronucleated cells, while lighter colors (yellow) denote higher frequencies.
Exposure to 0.5 µg/g body weight for 96 h resulted in a mean frequency of up to 25 MN/2000 cells, highlighting the intensified genotoxic effects of mercury with both increasing concentration and prolonged exposure.
4. Discussion
The dose- and time-dependent increase in nuclear abnormalities observed in Thalassophryne maculosa reinforces the well-documented genotoxic potential of mercury in aquatic organisms [15,54,55,56,57].
Exposure to higher concentrations of HgCl2 significantly elevated the micronucleus frequency, providing strong evidence of mercury’s ability to induce chromosomal damage in fish erythrocytes. The low baseline frequency of micronucleated cells in the control group aligns with spontaneous micronuclei levels reported in other fish species, such as Astyanax bimaculatus, Colossoma macropomum, and Oreochromis niloticus [39,42,58].
To ensure ecological relevance, this study was conducted in situ using cages placed in the sea. This setup allowed fish to be exposed to a natural environment while ensuring controlled mercury exposure conditions. Throughout the study, key water parameters—including salinity, temperature, dissolved oxygen, and pH—remained stable, minimizing potential confounding effects. Although conditions remained stable during the experiment, seasonal variations in salinity and temperature may influence mercury bioaccumulation and toxicity under natural conditions. Future studies should incorporate these variables to better understand their role in T. maculosa’s response to mercury contamination.
T. maculosa exhibited heightened sensitivity to HgCl2, showing significant increases in micronuclei, even at concentrations below regulatory safety thresholds. The tested exposure levels (0.1–0.5 µg/g) represent environmentally relevant contamination scenarios, with the highest concentration corresponding to the maximum allowable mercury level in fish and seafood for human consumption [59]. These findings suggest that T. maculosa is a promising bioindicator for the early detection of mercury contamination in estuarine and coastal environments.
The genotoxic effects observed in T. maculosa align with findings in other teleost species, including Danio rerio, O. niloticus, and Prochilodus magdalenae, where similar dose- and time-dependent increases in nuclear abnormalities have been reported [39,60]. However, the higher micronucleus frequencies and nuclear abnormalities detected at lower HgCl2 concentrations in T. maculosa suggest greater sensitivity compared to some commercially important species, such as O. niloticus and Cyprinus carpio [61]. This variability in genotoxic responses may be attributed to differences in DNA repair efficiency, antioxidant defense mechanisms, and metabolic adaptations. Understanding these interspecific differences is crucial for selecting species in mercury contamination monitoring programs. A distinct threshold for genotoxicity was identified in T. maculosa, with minimal effects at 0.1 µg/g but a significant increase in micronucleated cells at 0.25–0.5 µg/g. This pattern is consistent with findings in P. magdalenae and O. niloticus, which also exhibit dose-dependent responses but varying species-specific sensitivities [39,60]. Notably, statistical analysis confirmed that the effects of HgCl2 concentration and exposure time were additive rather than synergistic, mirroring findings in Clarias gariepinus [32].
The genotoxic effects observed in T. maculosa are consistent with mercury-induced oxidative stress and chromosomal instability. In species like C. carpio, HgCl2 inhibits key antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT), leading to reactive oxygen species (ROS) accumulation and cellular damage [61]. While oxidative stress likely plays a role in T. maculosa, biochemical markers such as antioxidant enzyme activity or lipid peroxidation were not assessed in this study. Future research should incorporate these biomarkers to clarify mercury’s mechanistic effects in this species.
Additionally, the formation of micronuclei and other nuclear abnormalities may be linked to clastogenic and aneugenic mechanisms. Mercury’s strong affinity for sulfhydryl groups in proteins can interfere with DNA repair pathways and mitotic spindle function, leading to chromosomal instability and cell cycle errors [61,62]. However, additional cytogenetic assays are needed to differentiate between these mechanisms in T. maculosa.
Overall, these findings establish T. maculosa as a highly sensitive species for detecting genotoxic contamination in coastal ecosystems. Its ability to detect mercury-induced genotoxicity, even at low concentrations, underscores its potential as a valuable tool for environmental monitoring and risk assessment.
Author Contributions
Conceptualization, M.N.T.; methodology, M.N.T. and N.N.C.; validation, M.N.T., J.A.G. and N.N.C.; formal analysis, M.N.T.; J.I.G.M., N.N.C. and J.A.G.; investigation, N.N.C. and J.I.G.M.; resources, M.N.T.; data curation, N.N.C. and M.N.T.; writing—original draft preparation, N.N.C.; writing—review and editing, M.N.T., J.A.G., J.I.G.M. and N.N.C.; supervision, M.N.T.; project administration, M.N.T.; funding acquisition, M.N.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad de Panamá, Project VIP-01-04-10-2024-16 and Universidad Técnica de Machala, project 2024/UTMACH-PR-GEN-278.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee on Animal Experimentation of Universidad Técnica de Machala (Approval Code: 003-2024) on 2 August 2024.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data generated in this study are not publicly available but can be obtained upon reasonable request from the corresponding author.
Acknowledgments
We sincerely thank the Universidad Técnica de Machala for funding this research and covering the publication costs. Additionally, we extend our gratitude to the authorities of the Escuela de Ciencias Aplicadas del Mar at the Universidad de Oriente for their logistical support, including access to facilities and security personnel, which greatly facilitated the execution of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bashir, I.; Lone, F.A.; Bhat, R.A.; Mir, S.A.; Dar, Z.A.; Dar, S.A. Concerns and Threats of Contamination on Aquatic Ecosystems. In Bioremediation and Biotechnology: Sustainable Approaches to Pollution Degradation; Hakeem, K.R., Bhat, R.A., Qadri, H., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–26. ISBN 9783030356910. [Google Scholar]
- Edo, G.I.; Itoje-akpokiniovo, L.O.; Obasohan, P.; Ikpekoro, V.O.; Samuel, P.O.; Jikah, A.N.; Nosu, L.C.; Ekokotu, H.A.; Ugbune, U.; Oghroro, E.E.A.; et al. Impact of Environmental Pollution from Human Activities on Water, Air Quality and Climate Change. Ecol. Front. 2024, 44, 874–889. [Google Scholar] [CrossRef]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- De Marco, A.; Proietti, C.; Anav, A.; Ciancarella, L.; D’Elia, I.; Fares, S.; Fornasier, M.F.; Fusaro, L.; Gualtieri, M.; Manes, F.; et al. Impacts of Air Pollution on Human and Ecosystem Health, and Implications for the National Emission Ceilings Directive: Insights from Italy. Environ. Int. 2019, 125, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Verma, A.K.; Prakash, S. The Web of Life: Role of Pollution in Biodiversity Decline. Int. J. Fauna Biol. Stud. 2023, 10, 49–52. [Google Scholar] [CrossRef]
- Ogidi, O.I.; Akpan, U.M. Aquatic Biodiversity Loss: Impacts of Pollution and Anthropogenic Activities and Strategies for Conservation. In Sustainable Development and Biodiversity; Springer Nature Singapore: Singapore, 2022; pp. 421–448. ISBN 9789811933257. [Google Scholar]
- Berzas Nevado, J.J.; García Bermejo, L.F.; Rodríguez Martín-Doimeadios, R.C. Distribution of Mercury in the Aquatic Environment at Almadén, Spain. Environ. Pollut. 2003, 122, 261–271. [Google Scholar] [CrossRef]
- Wu, C.; Wu, B.; Qu, Y.; Fu, H.; Chen, Y.; Lu, Y.; Ji, S.; Ding, L.; Li, Z.; Sun, Q.; et al. Blood Mercury Mediates the Associations between Fish Consumption and Serum Uric Acid Levels among Chinese Adults: A Nationally Representative Study. Environ. Res. 2024, 260, 119612. [Google Scholar] [CrossRef]
- Al-Sulaiti, M.M.; Soubra, L.; Al-Ghouti, M.A. The Causes and Effects of Mercury and Methylmercury Contamination in the Marine Environment: A Review. Curr. Pollut. Rep. 2022, 8, 249–272. [Google Scholar] [CrossRef]
- de Almeida Rodrigues, P.; Ferrari, R.G.; Dos Santos, L.N.; Conte Junior, C.A. Mercury in Aquatic Fauna Contamination: A Systematic Review on Its Dynamics and Potential Health Risks. J. Environ. Sci. 2019, 84, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Das, B.K.; Kumari, K.; Kumar, S.; Kush, A. Impacts of Mercury Toxicity in Aquatic Ecosistem: A Review. Eur. Chem. Bull. 2023, 12, 1476–1482. [Google Scholar] [CrossRef]
- Gworek, B.; Bemowska-Kałabun, O.; Kijeńska, M.; Wrzosek-Jakubowska, J. Mercury in Marine and Oceanic Waters—A Review. Water Air Soil Pollut. 2016, 227, 371. [Google Scholar] [CrossRef]
- Wu, P.; Kainz, M.J.; Bravo, A.G.; Åkerblom, S.; Sonesten, L.; Bishop, K. The Importance of Bioconcentration into the Pelagic Food Web Base for Methylmercury Biomagnification: A Meta-Analysis. Sci. Total Environ. 2019, 646, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, W.; Leermakers, M.; Papina, T.; Saprykin, A.; Brion, N.; Noyen, J.; De Gieter, M.; Elskens, M.; Goeyens, L. Bioconcentration and Biomagnification of Mercury and Methylmercury in North Sea and Scheldt Estuary Fish. Arch. Environ. Contam. Toxicol. 2003, 45, 498–508. [Google Scholar] [CrossRef]
- Nirchio, M.; Ventimilla, O.J.C.; Cordero, P.F.Q.; Hernández, J.G.; Oliveira, C. Genotoxic Effects of Mercury Chloride on the Neotropical Fish Andinoacara rivulatus (Cichlidae: Cichlasomatini). Rev. Biol. Trop. 2019, 67, 745–754. [Google Scholar] [CrossRef]
- Porto, J.I.R.; Araujo, C.S.O.; Feldberg, E. Mutagenic Effects of Mercury Pollution as Revealed by Micronucleus Test on Three Amazonian Fish Species. Environ. Res. 2005, 97, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Zulkipli, S.Z.; Liew, H.J.; Ando, M.; Lim, L.S.; Wang, M.; Sung, Y.Y.; Mok, W.J. A Review of Mercury Pathological Effects on Organs Specific of Fishes. Environ. Pollut. Bioavailab. 2021, 33, 76–87. [Google Scholar] [CrossRef]
- Fei, Y.; Hu, Y.H. Recent Progress in Removal of Heavy Metals from Wastewater: A Comprehensive Review. Chemosphere 2023, 335, 139077. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; González, J.A.; Lorenzo, J.M.; Thorne-Bazarra, T.; Hardisson, A.; Rubio, C.; González-Weller, D.; Paz, S.; Gutiérrez, Á.J. Metal Concentration in Palaemon Elegans along the Coastal Areas of Gran Canaria (Canary Islands): Potential Bioindicator of Pollution. Diversity 2023, 15, 1151. [Google Scholar] [CrossRef]
- Saravanan, P.; Saravanan, V.; Rajeshkannan, R.; Arnica, G.; Rajasimman, M.; Baskar, G.; Pugazhendhi, A. Comprehensive Review on Toxic Heavy Metals in the Aquatic System: Sources, Identification, Treatment Strategies, and Health Risk Assessment. Environ. Res. 2024, 258, 119440. [Google Scholar] [CrossRef]
- Carvan, M.J., 3rd; Gallagher, E.P.; Goksøyr, A.; Hahn, M.E.; Larsson, D.G.J. Fish Models in Toxicology. Zebrafish 2007, 4, 9–20. [Google Scholar] [CrossRef]
- Salunke, A.; Pandya, P.; Upadhyay, A.; Parikh, P. Chapter 4—Fish Biomarkers in Environmental Biomonitoring: An Insight into Water Pollution. In Biomarkers in Environmental and Human Health Biomonitoring; Mishra, R., Madhav, S., Dhaka, R.K., Garg, P., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 65–79. ISBN 9780443138607. [Google Scholar]
- Heddle, J.A.; Cimino, M.C.; Hayashi, M.; Romagna, F.; Shelby, M.D.; Tucker, J.D.; Vanparys, P.; MacGregor, J.T. Micronuclei as an Index of Cytogenetic Damage: Past, Present, and Future. Environ. Mol. Mutagen. 1991, 18, 277–291. [Google Scholar] [CrossRef]
- Schmid, W. The Micronucleus Test for Cytogenetic Analysis. In Chemical Mutagens: Principles and Methods for Their Detection; Hollaender, A., Ed.; Springer: Boston, MA, USA, 1976; pp. 31–53. ISBN 9781468408928. [Google Scholar]
- Schmid, W. The Micronucleus Test. Mutat. Res. 1975, 31, 9–15. [Google Scholar] [CrossRef]
- Hayashi, M.; Ueda, T.; Uyeno, K.; Wada, K.; Kinae, N.; Saotome, K.; Tanaka, N.; Takai, A.; Sasaki, Y.F.; Asano, N.; et al. Development of Genotoxicity Assay Systems That Use Aquatic Organisms. Mutat. Res. 1998, 399, 125–133. [Google Scholar] [CrossRef]
- Minissi, S.; Ciccotti, E.; Rizzoni, M. Micronucleus Test in Erythrocytes of Barbus plebejus (Teleostei, Pisces) from Two Natural Environments: A Bioassay for the in Situ Detection of Mutagens in Freshwater. Mutat. Res. 1996, 367, 245–251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mustafa, S.A.; Al-Rudainy, A.J.; Salman, N.M. Effect of Environmental Pollutants on Fish Health: An Overview. Egypt. J. Aquat. Res. 2024, 50, 225–233. [Google Scholar] [CrossRef]
- Sommer, S.; Buraczewska, I.; Kruszewski, M. Micronucleus Assay: The State of Art, and Future Directions. Int. J. Mol. Sci. 2020, 21, 1534. [Google Scholar] [CrossRef]
- van Treeck, R.; Van Wichelen, J.; Wolter, C. Fish Species Sensitivity Classification for Environmental Impact Assessment, Conservation and Restoration Planning. Sci. Total Environ. 2020, 708, 135173. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Villar, S.; Acuña Plavan, A. Micronucleus Test in Fishes as Indicators of Environmental Quality in Subestuaries of the Río de La Plata (Uruguay). Mar. Pollut. Bull. 2015, 91, 518–523. [Google Scholar] [CrossRef]
- Mahboob, S.; Ahmed, Z.; Farooq Khan, M.; Saho, C.; Virik, P.; Al-Mulhm, N.; Baabbad, A.A.A. Ecogenotoxicological Studies for an Early Toxicity Screening and Monitoring in Epinephalus chlorostigma and Scamberomorus commerson. Saudi J. Biol. Sci. 2022, 29, 2719–2726. [Google Scholar] [CrossRef]
- Baršienė, J.; Rybakovas, A.; Lang, T.; Andreikėnaitė, L.; Michailovas, A. Environmental Genotoxicity and Cytotoxicity Levels in Fish from the North Sea Offshore Region and Atlantic Coastal Waters. Mar. Pollut. Bull. 2013, 68, 106–116. [Google Scholar] [CrossRef]
- Canedo, A.; de Jesus, L.W.O.; Bailão, E.F.L.C.; Rocha, T.L. Micronucleus Test and Nuclear Abnormality Assay in Zebrafish (Danio rerio): Past, Present, and Future Trends. Environ. Pollut. 2021, 290, 118019. [Google Scholar] [CrossRef]
- Canedo, A.; Rocha, T.L. Zebrafish (Danio Rerio) Using as Model for Genotoxicity and DNA Repair Assessments: Historical Review, Current Status and Trends. Sci. Total Environ. 2021, 762, 144084. [Google Scholar] [CrossRef]
- Pollo, F.E.; Salas, N.; Mancini, M.; Martino, A.L. Estudio Comparativo de La Frecuencia de Micronúcleos Y Anormalidades Nucleares En Eritrocitos de Tres Especies ícticas. Acta Toxicol. Argent. 2012, 20, 62–67. [Google Scholar]
- El-Sappah, A.H.; Seif, M.M.; Abdel-Kader, H.H.; Soaud, S.A.; Elhamid, M.A.A.; Abdelghaffar, A.M.; El-Sappah, H.H.; Sarwar, H.; Yadav, V.; Maitra, P.; et al. Genotoxicity and Trace Elements Contents Analysis in Nile Tilapia (Oreochromis niloticus) Indicated the Levels of Aquatic Contamination at Three Egyptian Areas. Front. Vet. Sci. 2022, 9, 818866. [Google Scholar] [CrossRef]
- Ergene, S.; Cavaş, T.; Celik, A.; Köleli, N.; Aymak, C. Evaluation of River Water Genotoxicity Using the Piscine Micronucleus Test. Environ. Mol. Mutagen. 2007, 48, 421–429. [Google Scholar] [CrossRef]
- Francia-Quiroz, J.C.; Contreras-Luya, C.F.; Fernández-Celedonio, V. Genotoxicidad de cloruro de mercurio (II) en alevines de Oreochromis niloticus (Pisces, Cichlidae) expuestos a diferentes temperaturas. Rev. Investig. Vet. Peru 2023, 34, e23451. [Google Scholar] [CrossRef]
- Hussain, B.; Sultana, T.; Sultana, S.; Masoud, M.S.; Ahmed, Z.; Mahboob, S. Fish Eco-Genotoxicology: Comet and Micronucleus Assay in Fish Erythrocytes as in Situ Biomarker of Freshwater Pollution. Saudi J. Biol. Sci. 2018, 25, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Esquivel, Á.; Díez, S.; Marrugo-Negrete, J.L. Genotoxicity Effects in Freshwater Fish Species Associated with Gold Mining Activities in Tropical Aquatic Ecosystems. Ecotoxicol. Environ. Saf. 2023, 253, 114670. [Google Scholar] [CrossRef]
- da Rocha, C.A.M.; da Cunha, L.A.; da Silva Pinheiro, R.H.; de Oliveira Bahia, M.; Burbano, R.M.R. Studies of Micronuclei and Other Nuclear Abnormalities in Red Blood Cells of Colossoma macropomum Exposed to Methylmercury. Genet. Mol. Biol. 2011, 34, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Collette, B.B. A Review of the Venomous Toadfishes, Subfamily Thalassophryninae. Copeia 1966, 1966, 846–864. [Google Scholar] [CrossRef]
- Lopes-Ferreira, M.; Sosa-Rosales, I.; Silva Junior, P.I.; Conceicao, K.; Maleski, A.L.A.; Balan-Lima, L.; Disner, G.R.; Lima, C. Molecular Characterization and Functional Analysis of the Nattectin-like Toxin from the Venomous Fish Thalassophryne maculosa. Toxins 2021, 14, 2. [Google Scholar] [CrossRef]
- Pareja-Santos, A.; Oliveira Souza, V.M.; Bruni, F.M.; Sosa-Rosales, J.I.; Lopes-Ferreira, M.; Lima, C. Delayed Polymorphonuclear Leukocyte Infiltration Is an Important Component of Thalassophryne maculosa Venom Pathogenesis. Toxicon 2008, 52, 106–114. [Google Scholar] [CrossRef]
- Sosa-Rosales, J.I.; Piran-Soares, A.A.; Farsky, S.H.P.; Takehara, H.A.; Lima, C.; Lopes-Ferreira, M. Important Biological Activities Induced by Thalassophryne maculosa Fish Venom. Toxicon 2005, 45, 155–161. [Google Scholar] [CrossRef]
- Burgeot, T.; Gagné, F. Contaminant Exposure and Ecotoxicological Impacts in Estuaries. Environ. Sci. Pollut. Res. Int. 2013, 20, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.; Soares, J.; Neuparth, T.; Barreiro, A.F.; Xavier, C.; Antunes, C.; Santos, M.M. Prioritizing the Effects of Emerging Contaminants on Estuarine Production under Global Warming Scenarios. Toxics 2022, 10, 46. [Google Scholar] [CrossRef]
- Zheng, N.; Wang, S.; Dong, W.; Hua, X.; Li, Y.; Song, X.; Chu, Q.; Hou, S.; Li, Y. The Toxicological Effects of Mercury Exposure in Marine Fish. Bull. Environ. Contam. Toxicol. 2019, 102, 714–720. [Google Scholar] [CrossRef]
- Wang, Q.; Kim, D.; Dionysiou, D.D.; Sorial, G.A.; Timberlake, D. Sources and Remediation for Mercury Contamination in Aquatic Systems—A Literature Review. Environ. Pollut. 2004, 131, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Sokal, R.R.; James Rohlf, F. Biometry, 3rd ed.; W. H. Freeman: New York, NY, USA, 1995; ISBN 978-0-7167-2411-7. [Google Scholar]
- Sheskin, D.J. Handbook of Parametric and Nonparametric Statistical Procedures, 4th ed.; Chapman & Hall/CRC: Boca Raton, FL, USA, 2007; ISBN 9781584888154. [Google Scholar]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Animasaun, D.A.; Adedibu, P.A.; Afolabi, S.O.; Abdulkareem, K.A.; Ibrahim, S.; Krishnamurthy, R. Hazard Assessment and Cytogenotoxic Effect of Different Concentrations of Mercury Chloride Sterilant Using the Allium cepa Assay. Discov. Toxicol. 2024, 1, 2. [Google Scholar] [CrossRef]
- Dasharathy, S.; Arjunan, S.; Maliyur Basavaraju, A.; Murugasen, V.; Ramachandran, S.; Keshav, R.; Murugan, R. Mutagenic, Carcinogenic, and Teratogenic Effect of Heavy Metals. Evid. Based Complement. Altern. Med. 2022, 2022, 8011953. [Google Scholar] [CrossRef]
- Dourado, P.L.R.; da Rocha, M.P.; Roveda, L.M.; Raposo, J.L., Jr.; Cândido, L.S.; Cardoso, C.A.L.; Morales, M.A.M.; de Oliveira, K.M.P.; Grisolia, A.B. Genotoxic and Mutagenic Effects of Polluted Surface Water in the Midwestern Region of Brazil Using Animal and Plant Bioassays. Genet. Mol. Biol. 2017, 40, 123–133. [Google Scholar] [CrossRef]
- Thier, R.; Bonacker, D.; Stoiber, T.; Böhm, K.J.; Wang, M.; Unger, E.; Bolt, H.M.; Degen, G. Interaction of Metal Salts with Cytoskeletal Motor Protein Systems. Toxicol. Lett. 2003, 140–141, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Corredor-Santamaría, W.; Mora-Romero, C.C.; Escobar-Buitrago, P.S.; Cruz-Casallas, P.E.; Velasco-Santamaría, Y.M. Inducción de Micronúcleos Y Otras Anormalidades Nucleares En Astyanax gr. bimaculatus (Pisces: Characidae) Expuestas a Fenantreno. Orinoquia 2012, 16, 237. [Google Scholar] [CrossRef]
- Brodziak-Dopierała, B.; Fischer, A. Analysis of the Mercury Content in Fish for Human Consumption in Poland. Toxics 2023, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Peñaloza, M.; Camargo, M.; Palacio, J. Genotoxicidad Del Cloruro de Mercurio En Dos Especies ícticas (Prochilodus magdalenae Y Oreochromis sp.). Actual. Biol. 2017, 25, 105–111. [Google Scholar] [CrossRef]
- García-Medina, S.; Galar-Martínez, M.; Gómez-Oliván, L.M.; Ruiz-Lara, K.; Islas-Flores, H.; Gasca-Pérez, E. Relationship between Genotoxicity and Oxidative Stress Induced by Mercury on Common Carp (Cyprinus carpio) Tissues. Aquat. Toxicol. 2017, 192, 207–215. [Google Scholar] [CrossRef]
- Obiakor, M.O.; Okonkwo, J.C.; Ezeonyejiaku, C.D. Genotoxicity of Freshwater Ecosystem Shows DNA Damage in Preponderant Fish as Validated by in Vivo Micronucleus Induction in Gill and Kidney Erythrocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 775–776, 20–30. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).