Genetic Damage and Multi-Elemental Exposure in Populations in Proximity to Artisanal and Small-Scale Gold (ASGM) Mining Areas in North Colombia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Population
2.3. Blood Sample Collection and Processing
2.4. Hair Sample Collection, Preparation and Processing
2.5. Determination of DNA Damage and Chromosomal Instability Parameters
2.6. Determination of Essential Trace and Toxic Elements in Hair Samples
2.7. Statistical Analysis
3. Results and Discussion
3.1. Sociodemographic Characteristics of the Study Population
3.2. DNA Damage and Chromosomal Instability Parameters in Exposed and Reference Populations
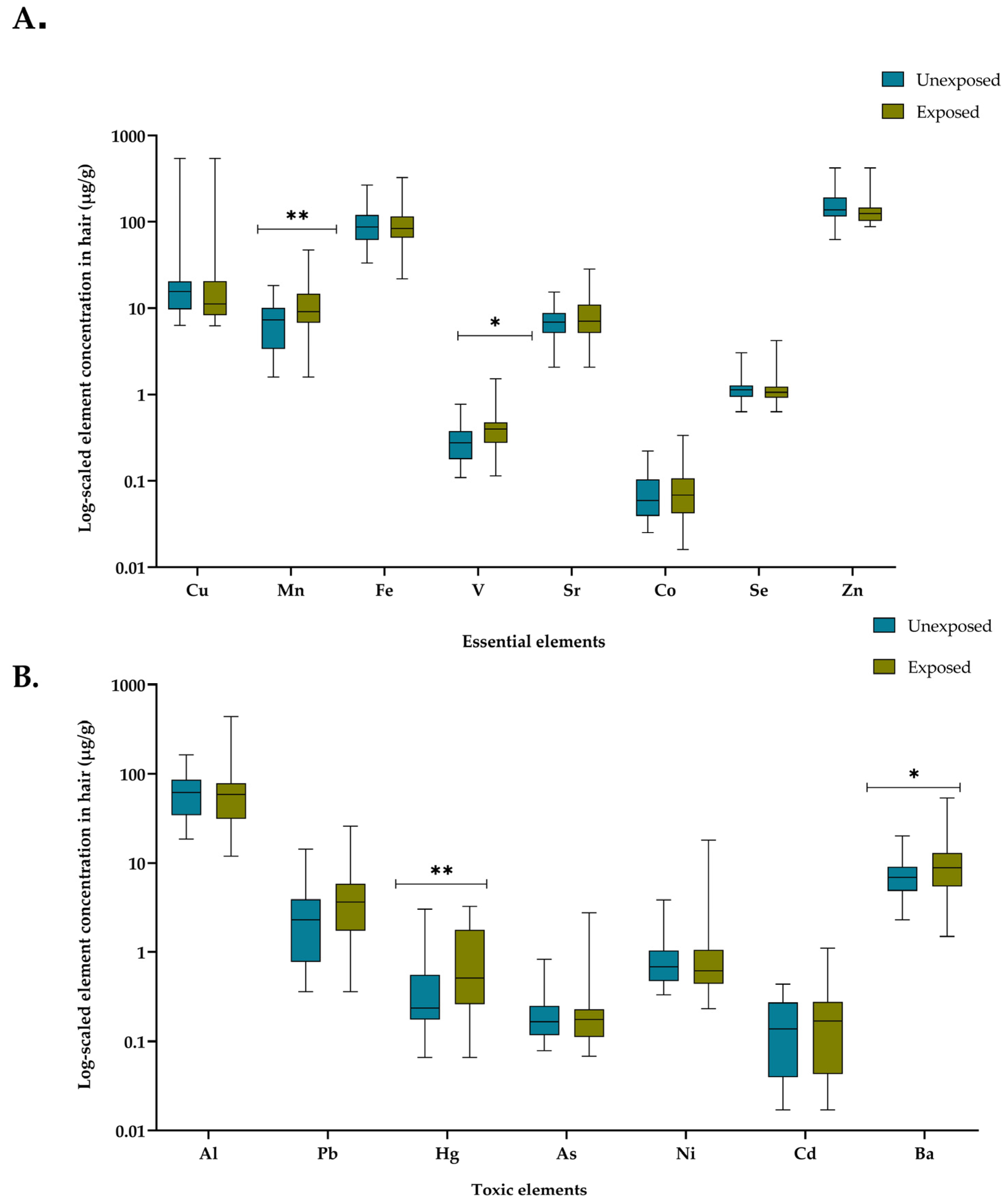
3.3. Concentration of Essential and Toxic Elements in Hair Samples
3.4. Principal Component Analysis (PCA) Loadings
3.4.1. PC1: Soil-Derived Mining-Associated Element
3.4.2. PC2: Mining-Related Heavy Metals
3.4.3. PC3: Agricultural and Mining-Related Contaminant Elements
3.4.4. PC4: Tailing-Associated Elements
3.5. Main Effects of PCs and DNA Damage and Chromosomal Instability Parameters
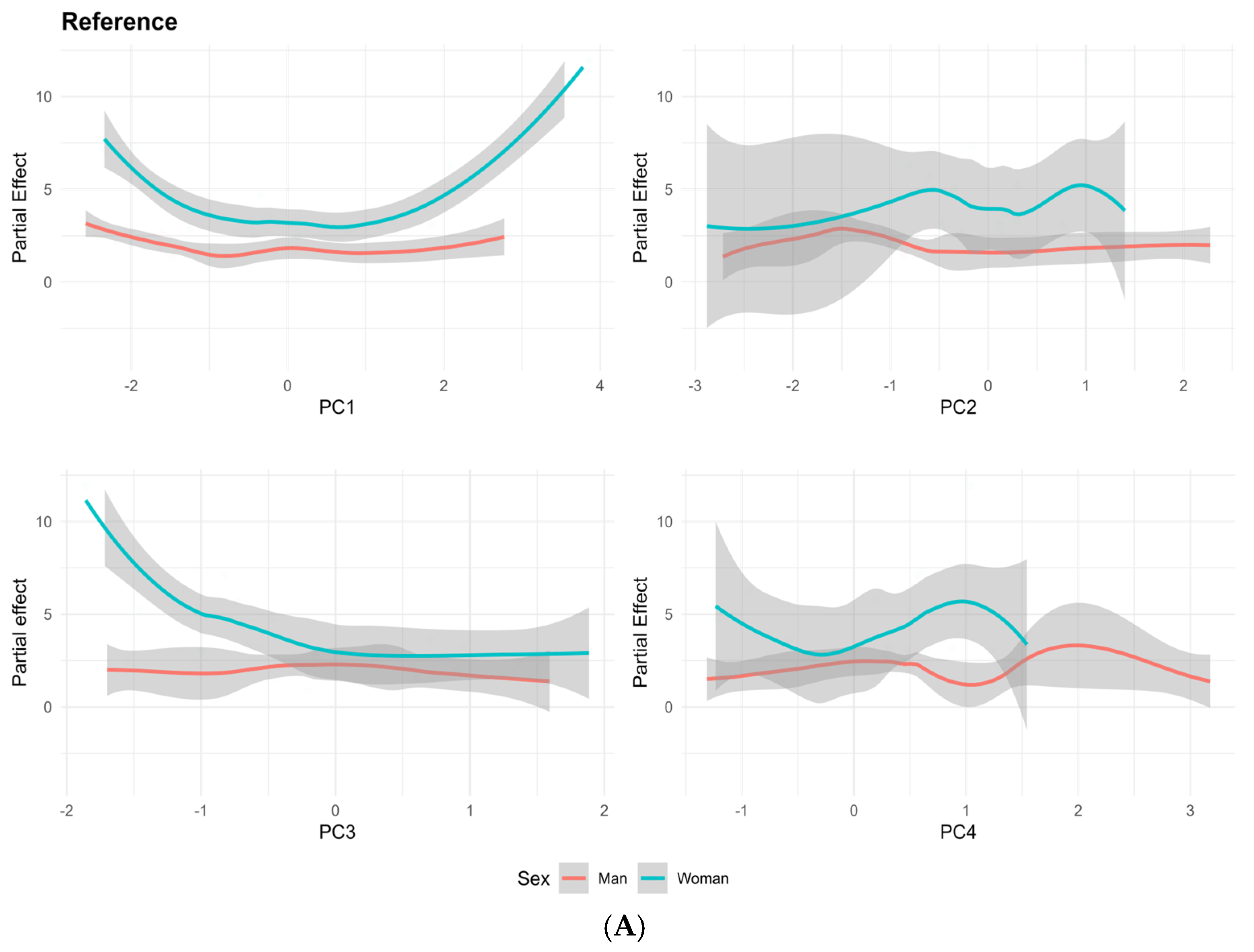
3.6. Exposure-Dependent and Sex-Specific Trends Across PC
3.7. Strengths and Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinilla, A.R.R.; Espana, V.A.A. Mining in Colombia: An Opportunity for Better Management of Environmental Impacts. In Derelict Mines; CRC Press: Boca Raton, FL, USA, 2025; pp. 90–106. [Google Scholar]
- Betancur-Corredor, B.; Loaiza-Usuga, J.C.; Denich, M.; Borgemeister, C. Gold mining as a potential driver of development in Colombia: Challenges and opportunities. J. Clean. Prod. 2018, 199, 538–553. [Google Scholar] [CrossRef]
- Cano-Londoño, N.A.; Capaz, R.S.; Hasenstab, C.; Velásquez, H.I.; McIntyre, N.; Corder, G.D.; Posada, J.A. Life cycle impacts assessment of two gold extraction systems in Colombia: Open-pit and alluvial mining. Int. J. Life Cycle Assess. 2023, 28, 380–397. [Google Scholar] [CrossRef]
- Santos, R.J. Blessing and curse. The gold boom and local development in Colombia. World Dev. 2018, 106, 337–355. [Google Scholar] [CrossRef]
- Ngom, N.M.; Baratoux, D.; Bolay, M.; Dessertine, A.; Abass Saley, A.; Baratoux, L.; Mbaye, M.; Faye, G.; Yao, A.K.; Kouamé, K.J. Artisanal Exploitation of Mineral Resources: Remote Sensing Observations of Environmental Consequences, Social and Ethical Aspects. Surv. Geophys. 2023, 44, 225–247. [Google Scholar] [CrossRef]
- Veiga, M.M.; Marshall, B.G. The Colombian artisanal mining sector: Formalization is a heavy burden. Extr. Ind. Soc. 2019, 6, 223–228. [Google Scholar] [CrossRef]
- Villar, D.; Schaeffer, D.J. Disarmament is the New War, Gold is the New Opium, and Ecohealth is the Historic Victim. Environ. Health Insights 2019, 13, 1178630219862241. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, N.; Danoucaras, N.; McIntyre, N.; Díaz-Martínez, J.C.; Restrepo-Baena, O.J. Review of improving the water management for the informal gold mining in Colombia. Rev. Fac. Ing. Univ. Antioq. 2016, 79, 163–172. [Google Scholar] [CrossRef]
- Acosta, J.A.; Arocena, J.M.; Faz, A. Speciation of arsenic in bulk and rhizosphere soils from artisanal cooperative mines in Bolivia. Chemosphere 2015, 138, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Pavilonis, B.; Grassman, J.; Johnson, G.; Diaz, Y.; Caravanos, J. Characterization and risk of exposure to elements from artisanal gold mining operations in the Bolivian Andes. Environ. Res. 2017, 154, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rajaee, M.; Obiri, S.; Green, A.; Long, R.; Cobbina, S.J.; Nartey, V.; Buck, D.; Antwi, E.; Basu, N. Integrated Assessment of Artisanal and Small-Scale Gold Mining in Ghana—Part 2: Natural Sciences Review. Int. J. Environ. Res. Public Health 2015, 12, 8971–9011. [Google Scholar] [CrossRef]
- Prasetia, H.; Sakakibara, M.; Omori, K.; Laird, J.S.; Sera, K.; Kurniawan, I.A. Mangifera indica as Bioindicator of Mercury Atmospheric Contamination in an ASGM Area in North Gorontalo Regency, Indonesia. Geosciences 2018, 8, 31. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Redwan, M.; Bamousa, A.O. Characterization and environmental impact assessment of gold mine tailings in arid regions: A case study of Barramiya gold mine area, Eastern Desert, Egypt. J. Afr. Earth Sci. 2019, 160, 103644. [Google Scholar] [CrossRef]
- IARC. A Review of Human Carcinogens. Part C: Arsenic, Metals, Fibres, and Dusts/IARC Working Group on the Evaluation of Carcinogenic Risk to Humans; IARC Monographs: Lyon, France, 2012; Volume 100C. [Google Scholar]
- Chen, K.; Liao, Q.L.; Ma, Z.W.; Jin, Y.; Hua, M.; Bi, J.; Huang, L. Association of soil arsenic and nickel exposure with cancer mortality rates, a town-scale ecological study in Suzhou, China. Environ. Sci. Pollut. Res. Int. 2015, 22, 5395–5404. [Google Scholar] [CrossRef] [PubMed]
- Obiri, S.; Yeboah, P.O.; Osae, S.; Adu-Kumi, S.; Cobbina, S.J.; Armah, F.A.; Ason, B.; Antwi, E.; Quansah, R. Human Health Risk Assessment of Artisanal Miners Exposed to Toxic Chemicals in Water and Sediments in the Prestea Huni Valley District of Ghana. Int. J. Environ. Res. Public Health 2016, 13, 139. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Inorganic and Organic Lead Compounds; IARC Monographs: Lyon, France, 2006; Volume 87. [Google Scholar]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Cobalt in Hard Metals and Cobalt Sulfate, Gallium Arsenide, Indium Phosphide and Vanadium Pentoxide; IARC Monographs: Lyon, France, 2006; Volume 86. [Google Scholar]
- Sauni, R.; Oksa, P.; Uitti, J.; Linna, A.; Kerttula, R.; Pukkala, E. Cancer incidence among Finnish male cobalt production workers in 1969–2013: A cohort study. BMC Cancer 2017, 17, 340. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- O’Neal, S.L.; Zheng, W. Manganese Toxicity Upon Overexposure: A Decade in Review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef]
- Schoofs, H.; Schmit, J.; Rink, L. Zinc Toxicity: Understanding the Limits. Molecules 2024, 29, 3130. [Google Scholar] [CrossRef] [PubMed]
- Olivero-Verbel, J.; Caballero-Gallardo, K.; Turizo-Tapia, A. Mercury in the gold mining district of San Martin de Loba, South of Bolivar (Colombia). Environ. Sci. Pollut. Res. Int. 2015, 22, 5895–5907. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Negrete, J.; Olivero-Verbel, J.; Ceballos, E.L.; Benitez, L.N. Total mercury and methylmercury concentrations in fish from the Mojana region of Colombia. Environ. Geochem. Health 2008, 30, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Olivero-Verbel, J.; Johnson-Restrepo, B.; Mendoza-Marín, C.; Paz-Martinez, R.; Olivero-Verbel, R. Mercury in the Aquatic Environment of the Village of Caimito at the Mojana Region, North of Colombia. Water Air Soil Pollut. 2004, 159, 409–420. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Benitez, L.; Olivero-Verbel, J. Distribution of Mercury in Several Environmental Compartments in an Aquatic Ecosystem Impacted by Gold Mining in Northern Colombia. Arch. Environ. Contam. Toxicol. 2008, 55, 305–316. [Google Scholar] [CrossRef]
- Calao, C.R.; Marrugo, J.L. Efectos genotóxicos asociados a metales pesados en una población humana de la región de La Mojana, Colombia, 2013. Biomédica 2015, 35, 139–151. [Google Scholar] [CrossRef]
- Madrid, G.; Gracia, L.; Marrugo, J.L.; Urango, I. Genotoxicidad de metales pesados (Hg, Zn, Cu, Pb y Cd) asociado a explotaciones mineras en pobladores de la cuenca del río San Jorge del departamento de Córdoba, Colombia. Rev. Asoc. Colomb. Cienc. Biol. 2011, 23, 103–111. [Google Scholar]
- Marrugo-Negrete, J.; Benítez, L.N.; Olivero-Verbel, J.; Lans, E.; Gutierrez, F.V. Spatial and seasonal mercury distribution in the Ayapel Marsh, Mojana region, Colombia. Int. J. Environ. Health Res. 2010, 20, 451–459. [Google Scholar] [CrossRef]
- Bonassi, S.; El-Zein, R.; Bolognesi, C.; Fenech, M. Micronuclei frequency in peripheral blood lymphocytes and cancer risk: Evidence from human studies. Mutagenesis 2011, 26, 93–100. [Google Scholar] [CrossRef]
- Fenech, M.; Kirsch-Volders, M.; Natarajan, A.T.; Surralles, J.; Crott, J.W.; Parry, J.; Norppa, H.; Eastmond, D.A.; Tucker, J.D.; Thomas, P. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis 2011, 26, 125–132. [Google Scholar] [CrossRef]
- Amorim, M.I.; Mergler, D.; Bahia, M.O.; Dubeau, H.; Miranda, D.; Lebel, J.; Burbano, R.R.; Lucotte, M. Cytogenetic damage related to low levels of methyl mercury contamination in the Brazilian Amazon. An. Da Acad. Bras. De Cienc. 2000, 72, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Franchi, E.; Loprieno, G.; Ballardin, M.; Petrozzi, L.; Migliore, L. Cytogenetic monitoring of fishermen with environmental mercury exposure. Mutat. Res. Genet. Toxicol. 1994, 320, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Knasmueller, S.; Bolognesi, C.; Bonassi, S.; Holland, N.; Migliore, L.; Palitti, F.; Natarajan, A.T.; Kirsch-Volders, M. Molecular mechanisms by which in vivo exposure to exogenous chemical genotoxic agents can lead to micronucleus formation in lymphocytes in vivo and ex vivo in humans. Mutat. Res./Rev. Mutat. Res. 2016, 770, 12–25. [Google Scholar] [CrossRef]
- El-Zein, R.; Vral, A.; Etzel, C.J. Cytokinesis-blocked micronucleus assay and cancer risk assessment. Mutagenesis 2011, 26, 101–106. [Google Scholar] [CrossRef]
- Fenech, M. Chromosomal biomarkers of genomic instability relevant to cancer. Drug Discov. Today 2002, 7, 1128–1137. [Google Scholar] [CrossRef]
- Fenech, M.; Knasmueller, S.; Knudsen, L.E.; Kirsch-Volders, M.; Deo, P.; Franzke, B.; Stopper, H.; Andreassi, M.-G.; Bolognesi, C.; Dhillon, V.S.; et al. “Micronuclei and Disease” special issue: Aims, scope, and synthesis of outcomes. Mutat. Res./Rev. Mutat. Res. 2021, 788, 108384. [Google Scholar] [CrossRef]
- Thomas, P.; Umegaki, K.; Fenech, M. Nucleoplasmic bridges are a sensitive measure of chromosome rearrangement in the cytokinesis-block micronucleus assay. Mutagenesis 2003, 18, 187–194. [Google Scholar] [CrossRef]
- Galeano-Páez, C.; Espitia-Pérez, P.; Jimenez-Vidal, L.; Pastor-Sierra, K.; Salcedo-Arteaga, S.; Hoyos-Giraldo, L.S.; Gioda, A.; Saint’Pierre, T.D.; García, S.C.; Brango, H. Dietary exposure to mercury and its relation to cytogenetic instability in populations from “La Mojana” region, northern Colombia. Chemosphere 2021, 265, 129066. [Google Scholar] [CrossRef]
- Pastor-Sierra, K.; Espitia-Pérez, L.; Espitia-Pérez, P.; Peñata-Taborda, A.; Brango, H.; Galeano-Páez, C.; Bru-Cordero, O.E.; Palma-Parra, M.; Díaz, S.M.; Trillos, C.; et al. Micronuclei frequency and exposure to chemical mixtures in three Colombian mining populations. Sci. Total Environ. 2023, 901, 165789. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Pinedo-Hernández, J.; Paternina-Uribe, R.; Quiroz-Aguas, L.; Pacheco-Florez, S. Spatial distribution and evaluation of environmental pollution by mercury in the Mojana region, Colombia. Rev. MVZ Córdoba 2018, 23, 7062–7075. [Google Scholar]
- Diaz, S.M.; Palma, R.M.; Muñoz, M.N.; Becerra-Arias, C.; Fernández Niño, J.A. Factors associated with high mercury levels in women and girls from the Mojana Region, Colombia, 2013–2015. Int. J. Environ. Res. Public Health 2020, 17, 1827. [Google Scholar] [CrossRef] [PubMed]
- Enamorado-Montes, G.; Reino-Causil, B.; Urango-Cardenas, I.; Marrugo-Madrid, S.; Marrugo-Negrete, J. Mercury accumulation in commercial varieties of Oryza sativa L. cultivated in soils of La Mojana region, Colombia. Toxics 2021, 9, 304. [Google Scholar] [CrossRef]
- De la Ossa, C.A.; Ramírez-Giraldo, A.F.; Arroyo-Alvis, K.; Marrugo-Negrete, J.; Díez, S. Neuropsychological effects and cognitive deficits associated with exposure to mercury and arsenic in children and adolescents of the Mojana region, Colombia. Environ. Res. 2023, 216, 114467. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.M.; Muñoz-Guerrero, M.N.; Palma-Parra, M.; Becerra-Arias, C.; Fernández-Niño, J.A. Exposure to mercury in workers and the population surrounding gold mining areas in the Mojana region, Colombia. Int. J. Environ. Res. Public Health 2018, 15, 2337. [Google Scholar] [CrossRef] [PubMed]
- Baleta-Anaya, W.; Garay-Román, Y.; Consuegra-Solorzano, A.; Vidal-Durango, J.; Buelvas-Soto, J.; Marrugo-Negrete, J. Methylmercury (MeHg) in the most consumed fish in a municipality of La Mojana, Colombia. Rev. UDCA Actual. Divulg. Cient. 2022, 25, 2. [Google Scholar] [CrossRef]
- Duarte, B. Análisis Comparado de las Dinámicas Hídricas de la Cuenca Baja del río Sinú con los Cambios de Coberturas en el Complejo de la Ciénaga Grande de Lorica; Trabajo de pregrado para optar por el título de ecóloga; Pontificia Universidad Javeriana: Bogotá, Colombia, 2005. [Google Scholar]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef]
- Fenech, M.; Chang, W.P.; Kirsch-Volders, M.; Holland, N.; Bonassi, S.; Zeiger, E. HUMN project: Detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2003, 534, 65–75. [Google Scholar] [CrossRef]
- Rigby, R.A.; Stasinopoulos, D.M. Generalized additive models for location, scale and shape. J. R. Stat. Soc. Ser. C Appl. Stat. 2005, 54, 507–554. [Google Scholar] [CrossRef]
- Ek-Huchim, J.P.; Árcega-Cabrera, F.; May-Tec, A.L.; Améndola-Pimenta, M.; Ceja-Moreno, V.; Rodríguez-Canul, R. Red Blood Cell Cytotoxicity Associated to Heavy Metals and Hydrocarbons Exposure in Flounder Fish from Two Regions of the Gulf of Mexico. Bull. Environ. Contam. Toxicol. 2022, 108, 78–84. [Google Scholar] [CrossRef]
- Fendrich, A.N.; Van Eynde, E.; Stasinopoulos, D.M.; Rigby, R.A.; Mezquita, F.Y.; Panagos, P. Modeling arsenic in European topsoils with a coupled semiparametric (GAMLSS-RF) model for censored data. Environ. Int. 2024, 185, 108544. [Google Scholar] [CrossRef]
- Ye, Z.; Hong, S.; He, C.; Zhang, Y.; Wang, Y.; Zhu, H.; Hou, H. Evaluation of different factors on metal leaching from nickel tailings using generalized additive model (GAM). Ecotoxicol. Environ. Saf. 2022, 236, 113488. [Google Scholar] [CrossRef] [PubMed]
- Kirsch-Volders, M.; Bonassi, S.; Herceg, Z.; Hirvonen, A.; Möller, L.; Phillips, D. Gender-related differences in response to mutagens and carcinogens. Mutagenesis 2010, 25, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Nefic, H.; Handzic, I. The effect of age, sex, and lifestyle factors on micronucleus frequency in peripheral blood lymphocytes of the Bosnian population. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2013, 753, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jursa, T.; Stein, C.R.; Smith, D.R. Determinants of Hair Manganese, Lead, Cadmium and Arsenic Levels in Environmentally Exposed Children. Toxics 2018, 6, 19. [Google Scholar] [CrossRef]
- Quarm, J.A.; Anning, A.K.; Fei-Baffoe, B.; Siaw, V.F.; Amuah, E.E.Y. Perception of the environmental, socio-economic and health impacts of artisanal gold mining in the Amansie West District, Ghana. Environ. Chall. 2022, 9, 100653. [Google Scholar] [CrossRef]
- Nurcholis, M.; Yudiantoro, D.F.; Haryanto, D.; Mirzam, A. Heavy Metals Distribution in the Artisanal Gold Mining Area in Wonogiri. Indones. J. Geogr. 2017, 49, 133–144. [Google Scholar] [CrossRef]
- Slavković, L.; Škrbić, B.; Miljević, N.; Onjia, A. Principal component analysis of trace elements in industrial soils. Environ. Chem. Lett. 2004, 2, 105–108. [Google Scholar] [CrossRef]
- Souza, A.S.; Bezerra, M.A.; Cerqueira, U.M.F.M.; Rodrigues, C.J.O.; Santos, B.C.; Novaes, C.G.; Almeida, E.R.V. An introductory review on the application of principal component analysis in the data exploration of the chemical analysis of food samples. Food Sci. Biotechnol. 2024, 33, 1323–1336. [Google Scholar] [CrossRef]
- Danala Danga, S.; Ekengele Nga, L.; Makhubela, T.; Ibrahim, B.; Bitom, D.; Kramers, J. Assessing soil contamination by potentially toxic elements in artisanal and small-scale gold mining sites of the Adamawa Region, Cameroon. Geochem. Explor. Environ. Anal. 2024, 24, geochem2023-055. [Google Scholar] [CrossRef]
- Montalván-Olivares, D.; Santana, C.; Velasco, F.; Luzardo, F.; Andrade, S.; Ticianelli, R.; Armelin, M.; Genezini, F. Multi-element contamination in soils from major mining areas in Northeastern of Brazil. Environ. Geochem. Health 2021, 43, 4553–4576. [Google Scholar] [CrossRef]
- Ondayo, M.A.; Watts, M.J.; Humphrey, O.S.; Osano, O. Public health assessment of Kenyan ASGM communities using multi-element biomonitoring, dietary and environmental evaluation. Ecotoxicol. Environ. Saf. 2024, 277, 116323. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Peng, C.; Wang, H.; Chen, W. Health risk assessment of trace metals in various environmental media, crops and human hair from a mining affected area. Int. J. Environ. Res. Public Health 2017, 14, 1595. [Google Scholar] [CrossRef] [PubMed]
- Assem, F.L.; Levy, L.S. A review of current toxicological concerns on vanadium pentoxide and other vanadium compounds: Gaps in knowledge and directions for future research. J. Toxicol. Environ. Health Part B 2009, 12, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Lashari, A.; Kazi, T.G.; Afridi, H.I.; Baig, J.A.; Arain, M.B.; Lashari, A.A. Evaluate the Work-Related Exposure of Vanadium on Scalp Hair Samples of Outdoor and Administrative Workers of Oil Drilling Field: Related Health Risks. Biol. Trace Elem. Res. 2024, 202, 5366–5372. [Google Scholar] [CrossRef]
- Varrica, D.; Tamburo, E.; Alaimo, M.G. Levels of trace elements in human hair samples of adolescents living near petrochemical plants. Environ. Geochem. Health 2022, 44, 3779–3797. [Google Scholar] [CrossRef]
- Mantey, J.; Nyarko, K.B.; Owusu-Nimo, F.; Awua, K.A.; Bempah, C.K.; Amankwah, R.K.; Akatu, W.E.; Appiah-Effah, E. Influence of illegal artisanal small-scale gold mining operations (galamsey) on oil and grease (O/G) concentrations in three hotspot assemblies of Western Region, Ghana. Environ. Pollut. 2020, 263, 114251. [Google Scholar] [CrossRef]
- Indriati Arifin, Y.; Sakakibara, M.; Sera, K. Heavy metals concentrations in scalp hairs of ASGM miners and inhabitants of the Gorontalo Utara regency. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Kuala Lumpur, Malaysia, 24–26 April 2017; p. 012028. [Google Scholar]
- Liu, W.; Xin, Y.; Li, Q.; Shang, Y.; Ping, Z.; Min, J.; Cahill, C.M.; Rogers, J.T.; Wang, F. Biomarkers of environmental manganese exposure and associations with childhood neurodevelopment: A systematic review and meta-analysis. Environ. Health 2020, 19, 104. [Google Scholar] [CrossRef]
- Dórea, J.G. Neurodevelopment and exposure to neurotoxic metal (loid)s in environments polluted by mining, metal scrapping and smelters, and e-waste recycling in low and middle-income countries. Environ. Res. 2021, 197, 111124. [Google Scholar] [CrossRef]
- Saim, A.K. Mercury (Hg) use and pollution assessment of ASGM in Ghana: Challenges and strategies towards Hg reduction. Environ. Sci. Pollut. Res. 2021, 28, 61919–61928. [Google Scholar] [CrossRef]
- Aldous, A.R.; Tear, T.; Fernandez, L.E. The global challenge of reducing mercury contamination from artisanal and small-scale gold mining (ASGM): Evaluating solutions using generic theories of change. Ecotoxicology 2024, 33, 506–517. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Silwamba, M.; Paglinawan, F.C.; Mondejar, A.J.S.; Duc, H.G.; Resabal, V.J.; Opiso, E.M.; Igarashi, T.; Tomiyama, S.; Ito, M.; et al. Solid-phase partitioning and release-retention mechanisms of copper, lead, zinc and arsenic in soils impacted by artisanal and small-scale gold mining (ASGM) activities. Chemosphere 2020, 260, 127574. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.L.; Urango-Cardenas, I.D.; Núñez, S.M.B.; Díez, S. Atmospheric deposition of heavy metals in the mining area of the San Jorge river basin, Colombia. Air Qual. Atmos. Health 2014, 7, 577–588. [Google Scholar] [CrossRef]
- Arifin, Y.I.; Sakakibara, M.; Sera, K. Impacts of Artisanal and Small-Scale Gold Mining (ASGM) on Environment and Human Health of Gorontalo Utara Regency, Gorontalo Province, Indonesia. Geosciences 2015, 5, 160–176. [Google Scholar] [CrossRef]
- Skalny, A.V.; Skalnaya, M.G.; Nikonorov, A.A.; Tinkov, A.A. Selenium Antagonism with Mercury and Arsenic: From Chemistry to Population Health and Demography. In Selenium: Its Molecular Biology and Role in Human Health; Hatfield, D.L., Schweizer, U., Tsuji, P.A., Gladyshev, V.N., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 401–412. [Google Scholar]
- Berry, M.J.; Ralston, N.V.C. Mercury Toxicity and the Mitigating Role of Selenium. EcoHealth 2008, 5, 456–459. [Google Scholar] [CrossRef]
- Tinggi, U.; Perkins, A.V. Selenium Status: Its Interactions with Dietary Mercury Exposure and Implications in Human Health. Nutrients 2022, 14, 5308. [Google Scholar] [CrossRef]
- Arnaud, J.; van Dael, P. Selenium Interactions with Other Trace Elements, with Nutrients (and Drugs) in Humans. In Selenium; Michalke, B., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 413–447. [Google Scholar]
- Marrugo-Negrete, J.; Pinedo-Hernández, J.; Díez, S. Assessment of heavy metal pollution, spatial distribution and origin in agricultural soils along the Sinú River Basin, Colombia. Environ. Res. 2017, 154, 380–388. [Google Scholar] [CrossRef]
- Lans, E.; Marrugo Negrete, J.L.; Díaz, B. Study of Contamination by Organochlorine Pesticides in the Cienaga Grande Waters of the Low Sinú River Valley; University of Cordoba: Montería, Colombia, 2008. [Google Scholar]
- Marrugo, L. Determinación de Los Niveles de Pesticidas en Los Ríos Sinú, San Jorge y Canalete, Con el Fín de Darle Soporte al Diagnostico Del Plan de Ordenamiento Territorial de Las Cuencas; CVS-Universidad de Córdoba: Córdoba, Spain, 2005. [Google Scholar]
- Abedi, T.; Gavanji, S.; Mojiri, A. Lead and Zinc Uptake and Toxicity in Maize and Their Management. Plants 2022, 11, 1922. [Google Scholar] [CrossRef]
- Karaca, O.; Cameselle, C.; Reddy, K.R. Mine tailing disposal sites: Contamination problems, remedial options and phytocaps for sustainable remediation. Rev. Environ. Sci. Bio Technol. 2018, 17, 205–228. [Google Scholar] [CrossRef]
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- Durand, A.; Goux, X.; Lopez, S.; Leglize, P.; Benizri, E. Soil nickel contamination levels entail changes in the bacterial communities associated to the rhizosphere and endosphere of Odontarrhena chalcidica. Plant Soil 2023, 493, 17–43. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Y.; Liao, Y.; Tang, C.; Wen, Z.; Fazal, A.; Yang, R.; Qi, J.; Hong, Z.; Li, Y.; et al. Excess copper promotes catabolic activity of gram-positive bacteria and resistance of gram-negative bacteria but inhibits fungal community in soil. Environ. Sci. Pollut. Res. 2022, 29, 22602–22612. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Yadav, A.; Ramanathan, A.L. Arsenic Contamination in Environment, Ecotoxicological and Health Effects, and Bioremediation Strategies for Its Detoxification. In Bioremediation of Industrial Waste for Environmental Safety: Volume I: Industrial Waste and Its Management; Saxena, G., Bharagava, R.N., Eds.; Springer: Singapore, 2020; pp. 245–264. [Google Scholar]
- Tejeda-Benitez, L.; Flegal, R.; Odigie, K.; Olivero-Verbel, J. Pollution by metals and toxicity assessment using Caenorhabditis elegans in sediments from the Magdalena River, Colombia. Environ. Pollut. 2016, 212, 238–250. [Google Scholar] [CrossRef]
- Álvarez-Barrera, L.; Rodríguez-Mercado, J.J.; Mateos-Nava, R.A.; Acosta-San Juan, A.; Altamirano-Lozano, M.A. Cytogenetic damage by vanadium(IV) and vanadium(III) on the bone marrow of mice. Drug Chem. Toxicol. 2024, 47, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Ciranni, R.; Antonetti, M.; Migliore, L. Vanadium salts induce cytogenetic effects in in vivo treated mice. Mutat. Res. Genet. Toxicol. 1995, 343, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, P.; Jiang, H.; Mao, Y.; Ahmed, N.; Dalal, N. Vanadium(IV) causes 2′-deoxyguanosine hydroxylation and deoxyribonucleic acid damage via free radical reactions. Ann. Clin. Lab. Sci. 1996, 26, 39–49. [Google Scholar]
- Rodríguez-Mercado, J.J.; Álvarez-Barrera, L.; Altamirano-Lozano, M.A. Chromosomal damage induced by vanadium oxides in human peripheral lymphocytes. Drug Chem. Toxicol. 2010, 33, 97–102. [Google Scholar] [CrossRef]
- Hengstler, J.G.; Bolm-Audorff, U.; Faldum, A.; Janssen, K.; Reifenrath, M.; Götte, W.; Jung, D.; Mayer-Popken, O.; Fuchs, J.; Gebhard, S.; et al. Occupational exposure to heavy metals: DNA damage induction and DNA repair inhibition prove co-exposures to cadmium, cobalt and lead as more dangerous than hitherto expected. Carcinogenesis 2003, 24, 63–73. [Google Scholar] [CrossRef]
- Wan, R.; Mo, Y.; Zhang, Z.; Jiang, M.; Tang, S.; Zhang, Q. Cobalt nanoparticles induce lung injury, DNA damage and mutations in mice. Part. Fibre Toxicol. 2017, 14, 38. [Google Scholar] [CrossRef]
- Tenan, M.R.; Nicolle, A.; Moralli, D.; Verbouwe, E.; Jankowska, J.D.; Durin, M.A.; Green, C.M.; Mandriota, S.J.; Sappino, A.P. Aluminum Enters Mammalian Cells and Destabilizes Chromosome Structure and Number. Int. J. Mol. Sci. 2021, 22, 9515. [Google Scholar] [CrossRef]
- Harischandra, D.S.; Ghaisas, S.; Zenitsky, G.; Jin, H.; Kanthasamy, A.; Anantharam, V.; Kanthasamy, A.G. Manganese-Induced Neurotoxicity: New Insights Into the Triad of Protein Misfolding, Mitochondrial Impairment, and Neuroinflammation. Front. Neurosci. 2019, 13, 654. [Google Scholar] [CrossRef]
- Nersesyan, A.; Kundi, M.; Mišík, M.; Wultsch, G.; Knasmueller, S. Heavy metals–lead, mercury and cadmium and their impact on DNA damage measured by the micronucleus assay. In The Micronucleus Assay in Toxicology; Royal Society of Chemistry: London, UK, 2019. [Google Scholar]
- Moura, D.J.; Péres, V.F.; Jacques, R.A.; Saffi, J. Heavy Metal Toxicity: Oxidative Stress Parameters and DNA Repair. In Metal Toxicity in Plants: Perception, Signaling and Remediation; Gupta, D.K., Sandalio, L.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 187–205. [Google Scholar]
- Matuszczak, M.; Kiljańczyk, A.; Marciniak, W.; Derkacz, R.; Stempa, K.; Baszuk, P.; Bryśkiewicz, M.; Cybulski, C.; Dębniak, T.; Gronwald, J.; et al. Antioxidant Properties of Zinc and Copper—Blood Zinc-to Copper-Ratio as a Marker of Cancer Risk BRCA1 Mutation Carriers. Antioxidants 2024, 13, 841. [Google Scholar] [CrossRef] [PubMed]
- Pottier, G.; Viau, M.; Ricoul, M.; Shim, G.; Bellamy, M.; Cuceu, C.; Hempel, W.M.; Sabatier, L. Lead exposure induces telomere instability in human cells. PLoS ONE 2013, 8, e67501. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, R.; Kalahasthi, R.; Balachandar, R.; Bagepally, B.S. Association between lead exposure and DNA damage (genotoxicity): Systematic review and meta-analysis. Arch. Toxicol. 2022, 96, 2899–2911. [Google Scholar] [CrossRef]
- Scanlon, S.E.; Scanlon, C.D.; Hegan, D.C.; Sulkowski, P.L.; Glazer, P.M. Nickel induces transcriptional down-regulation of DNA repair pathways in tumorigenic and non-tumorigenic lung cells. Carcinogenesis 2017, 38, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Haddad, F.; Shahrokhabadi, K. Nickel Increases Chromosomal Abnormalities by Interfering With the Initiation of DNA Repair Pathways. Iran. J. Toxicol. 2022, 16, 229–236. [Google Scholar] [CrossRef]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuča, K.; Musílek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef]
- Cobine, P.A.; Moore, S.A.; Leary, S.C. Getting out what you put in: Copper in mitochondria and its impacts on human disease. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118867. [Google Scholar] [CrossRef]
- Pham, A.N.; Xing, G.; Miller, C.J.; Waite, T.D. Fenton-like copper redox chemistry revisited: Hydrogen peroxide and superoxide mediation of copper-catalyzed oxidant production. J. Catal. 2013, 301, 54–64. [Google Scholar] [CrossRef]
- Wróblewski, M.; Wróblewska, W.; Sobiesiak, M. The Role of Selected Elements in Oxidative Stress Protection: Key to Healthy Fertility and Reproduction. Int. J. Mol. Sci. 2024, 25, 9409. [Google Scholar] [CrossRef]
- Lou, Q.M.; Lai, F.F.; Li, J.W.; Mao, K.J.; Wan, H.T.; He, Y. Mechanisms of cuproptosis and its relevance to distinct diseases. Apoptosis 2024, 29, 981–1006. [Google Scholar] [CrossRef]
- Mandal, P. Molecular insight of arsenic-induced carcinogenesis and its prevention. Naunyn Schmiedeberg’s Arch. Pharmacol. 2017, 390, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Esquivel, Á.; Marrugo-Negrete, J.; Calao-Ramos, C. Genetic damage in human populations at mining sites in the upper basin of the San Jorge River, Colombia. Environ. Sci. Pollut. Res. 2019, 26, 10961–10971. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Li, Y.; Deng, J.; Zhang, Z. PARP-1 inhibitor sensitizes arsenic trioxide in hepatocellular carcinoma cells via abrogation of G2/M checkpoint and suppression of DNA damage repair. Chem. Biol. Interact. 2015, 226, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Mollet, I.G.; Patel, D.; Govani, F.S.; Giess, A.; Paschalaki, K.; Periyasamy, M.; Lidington, E.C.; Mason, J.C.; Jones, M.D.; Game, L.; et al. Low Dose Iron Treatments Induce a DNA Damage Response in Human Endothelial Cells within Minutes. PLoS ONE 2016, 11, e0147990. [Google Scholar] [CrossRef]
- Li, X.; Abdel-Moneim, A.-M.E.; Yang, B. Gene Expression in Bronchial Epithelial Cell Responses to Vanadium Exposure. Biol. Trace Elem. Res. 2023, 201, 3774–3790. [Google Scholar] [CrossRef]
- Stößer, S.; Lumpp, T.; Fischer, F.; Gunesch, S.; Schumacher, P.; Hartwig, A. Effect of Long-Term Low-Dose Arsenic Exposure on DNA Methylation and Gene Expression in Human Liver Cells. Int. J. Mol. Sci. 2023, 24, 15238. [Google Scholar] [CrossRef]
- Wojda, A.; Zietkiewicz, E.; Witt, M. Effects of age and gender on micronucleus and chromosome nondisjunction frequencies in centenarians and younger subjects. Mutagenesis 2007, 22, 195–200. [Google Scholar] [CrossRef][Green Version]
- Kopjar, N.; Kasuba, V.; Milić, M.; Rozgaj, R.; Zeljezić, D.; Gajski, G.; Mladinić, M.; Garaj-Vrhovac, V. Normal and cut-off values of the cytokinesis-block micronucleus assay on peripheral blood lymphocytes in the Croatian general population. Arh. Hig. Rada Toksikol. 2010, 61, 219–234. [Google Scholar] [CrossRef]
- Cavalieri, E.; Frenkel, K.; Liehr, J.G.; Rogan, E.; Roy, D. Chapter 4: Estrogens as Endogenous Genotoxic Agents—DNA Adducts and Mutations. JNCI Monogr. 2000, 2000, 75–94. [Google Scholar] [CrossRef]
- Kim, N.; Filipovic, D.; Bhattacharya, S.; Cuddapah, S. Epigenetic toxicity of heavy metals−implications for embryonic stem cells. Environ. Int. 2024, 193, 109084. [Google Scholar] [CrossRef]
- Meek, M.E.B.; Boobis, A.R.; Crofton, K.M.; Heinemeyer, G.; Van Raaij, M.; Vickers, C. Risk assessment of combined exposure to multiple chemicals: A WHO/IPCS framework. Regul. Toxicol. Pharmacol. 2011, 60, S1–S14. [Google Scholar] [CrossRef]
- Ahmad, N.; Muhammad, A.; Zafar, R.; Afzal, U.; Aslam, M. Assessment of lead, cadmium, and mercury levels in the breast milk in Pakistani women. Environ. Sci. Pollut. Res. 2023, 30, 85903–85909. [Google Scholar] [CrossRef] [PubMed]
- Skalnaya, M.G.; Tinkov, A.A.; Demidov, V.A.; Serebryansky, E.P.; Nikonorov, A.A.; Skalny, A.V. Hair Toxic Element Content in Adult Men and Women in Relation to Body Mass Index. Biol. Trace Elem. Res. 2014, 161, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, A.A.; Aschner, M.; Ke, T.; Ferrer, B.; Zhou, J.-C.; Chang, J.-S.; Santamaría, A.; Chao, J.C.; Aaseth, J.; Skalny, A.V. Adipotropic effects of heavy metals and their potential role in obesity. Fac. Rev. 2021, 10, 32. [Google Scholar] [CrossRef]
- Perrais, M.; Fracasso, T.; Gilardi, F.; Thomas, A.; Hausmann, E.; Lenglet, S.; Wiskott, K. Measurement of trace elements concentrations in human adipose tissue and links with phenotypic characteristics. Toxicol. Anal. Clin. 2022, 34, S154–S155. [Google Scholar] [CrossRef]
- Hinton, J.J.; Veiga, M.M.; Beinhoff, C. Women, mercury and artisanal gold mining: Risk communication and mitigation. J. Phys. IV Proc. 2003, 107, 617–620. [Google Scholar] [CrossRef]
- Mewouo, Y.C.M.; Traore, M.; Zing, B.Z.; Aboubakar, A.; Oyono, S.O.; Nzeket, A.B.; Begoude, D.; Ngoupayou, J.R.N.; Gnankambary, Z.; Nacro, H.B. Evidence of heavy metal in soil, irrigation water and vegetable cultivated in peri-urban area of Yaoundé-Cameroon. Sci. Afr. 2024, 25, e02280. [Google Scholar]
- Espitia-Pérez, L.; Brango, H.; Peñata-Taborda, A.; Galeano-Páez, C.; Jaramillo-García, M.; Espitia-Pérez, P.; Pastor-Sierra, K.; Bru-Cordero, O.; Hoyos-Giraldo, L.S.; Reyes-Carvajal, I.; et al. Influence of genetic polymorphisms of Hg metabolism and DNA repair on the frequencies of micronuclei, nucleoplasmic bridges, and nuclear buds in communities living in gold mining areas. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2024, 897, 503790. [Google Scholar] [CrossRef]
- Lin, G.F.; Du, H.; Chen, J.G.; Lu, H.C.; Kai, J.X.; Zhou, Y.S.; Guo, W.C.; Zhang, X.J.; Lu, D.R.; Golka, K.; et al. Glutathione S-transferases M1 and T1 polymorphisms and arsenic content in hair and urine in two ethnic clans exposed to indoor combustion of high arsenic coal in Southwest Guizhou, China. Arch. Toxicol. 2007, 81, 545–551. [Google Scholar] [CrossRef]
- Shaikhova, D.; Amromina, A.; Bereza, I.; Shastin, A.; Gazimova, V.; Sutunkova, M.; Gurvich, V. Effects of genetic polymorphisms of GSTM1, GSTT1, and GSTP1 genes on blood metal levels in non-ferrous metal alloy smelter operators. Health Risk Anal. 2022, 3, 176–181. [Google Scholar] [CrossRef]



| Group | Sampling Areas | Total | |
|---|---|---|---|
| Reference | Exposed | ||
| Number of individuals | 37 | 71 | |
| Individuals by area (Department) | |||
| Cotorra-Reference area (Córdoba) | 37 | - | 37 |
| Magangué (Bolivar) | - | 15 | 15 |
| Ayapel (Córdoba) | - | 16 | 16 |
| Caimito (Sucre) | - | 40 | 40 |
| Gender N (%) | |||
| Women | 20 (54.05) | 39 (54.92) | 59 (54.62) |
| Men | 17 (45.94) | 32 (45.07) | 49 (45.37) |
| Age (mean ± S.D) | 36.51 ± 9.71 | 35.49 ± 11.25 | 35.84 ± 10.71 |
| Occupation N (%) | |||
| Women | |||
| Housewives | 20 (54.05) | 39 (54.92) | 59 (54.62) |
| Men | |||
| Fishermen | - | 32 (45.07) | 32 (45.09) |
| Peasants | 17 (45.94) | - | 17 (15.74) |
| Consumption habits | |||
| Alcohol consumption N (%) | |||
| Non-alcohol consumers | 34 (91.89) | 62 (87.32) | 96 (94.74) |
| Alcohol consumers * | |||
| Low | 3 (8.10) | 7 (9.85) | 10 (9.25) |
| Moderate | - | 2 (2.81) | 2 (1.85) |
| Tobacco consumption N (%) | |||
| Smokers (%) | 2 (5.40) | 6 (8.45) | 8 (7.40) |
| Non-tobacco smokers (%) | 35 (94.59) | 65 (91.54) | 100 (92.59) |
| Fish intake days/week N (%) | |||
| 1–2 (Low) | 8 (20.51) | 11 (15.49) | 19 (17.59) |
| 3–4 (Medium) | 16 (41.02) | 30 (42.25) | 46 (42.59) |
| 5–7 (High) | 13 (38.46) | 30 (42.25) | 43 (39.81) |
| Vegetable consumption N (%) | |||
| Green vegetables | 32 (86.48) | 56 (78.87) | 98 (81.48) |
| Fruits | 5 (13.51) | 15 (21.12) | 20 (18.51) |
| Parameters | Reference Area | Exposed Areas | |||||
|---|---|---|---|---|---|---|---|
| N | Mean ± SD | Median | N | Mean ± SD a | Median | p-Value | |
| (25th–75th) | (25th–75th) | ||||||
| MNBN | |||||||
| Women | 20 | 5.19 ± 5.04 a | 3.0 (2.5–6.5) | 39 | 6.59 ± 4.63 a | 6.0 (3.0–8.0) | NS |
| Men | 17 | 2.05 ± 2.63 | 1.0 (0.0–3.0) | 32 | 4.28 ± 2.56 | 3.0 (3.0–5.0) | ≤0.01 |
| Total | 37 | 2.52 ± 2.12 | 3.0 (1.0–3.2) | 71 | 5.54 ± 3.98 | 3.0 (3.0–8.0) | ≤0.01 |
| NBUDs | |||||||
| Women | 20 | 0.80 ± 1.60 | 0.0 (0.0–1.0) | 39 | 0.46 ± 1.02 | 0.0 (0.0–0.0) | NS |
| Men | 17 | 0.35 ± 0.78 | 0.0 (0.0–0.5) | 32 | 0.68 ± 1.28 | 0.0 (0.0–1.0) | NS |
| Total | 37 | 0.29 ± 0.62 | 0.0 (0.0–1.0) | 71 | 0.56 ± 1.14 | 0.0 (0.0–1.0) | NS |
| NPB | |||||||
| Women | 20 | 0.58 ± 0.94 | 0.0 (0.0–1.0) | 39 | 2.6 ± 5.45 a | 1.0 (0.0–2.7) | ≤0.01 |
| Men | 17 | 0.56 ± 0.80 | 0.0 (0.0–1.0) | 32 | 0.58 ± 1.73 | 0.0 (0.0–0.0) | NS |
| Total | 37 | 0.54 ± 0.87 | 0.0 (0.0–1.0) | 71 | 1.59 ± 3.96 | 1.0 (0.0–1.0) | ≤0.05 |
| Total | Reference | Exposed | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CI95% | CI95% | CI95% | ||||||||||
| MNBN | PR | Lower Limit | Higher Limit | p-Value | PR | Lower Limit | Higher Limit | p-Value | PR | Lower Limit | Higher Limit | p-Value |
| Exposure | 1.26 | 1.02 | 1.57 | 0.039 | ||||||||
| Sex | 0.51 | 0.41 | 0.64 | <0.001 | 0.58 | 0.36 | 0.96 | 0.042 | 0.53 | 0.41 | 0.69 | <0.001 |
| Age | 1.01 | 1.00 | 1.02 | 0.108 | 0.97 | 0.95 | 1.00 | 0.074 | 1.01 | 1.00 | 1.02 | 0.034 |
| PC1 | 1.03 | 0.99 | 1.07 | 0.135 | 1.1 | 0.96 | 1.25 | 0.180 | 10.45 | 9.75 | 12.18 | <0.001 |
| PC2 | 0.89 | 0.81 | 0.97 | 0.009 | 0.91 | 0.73 | 1.14 | 0.420 | 5.32 | 4.83 | 5.85 | <0.001 |
| PC3 | 0.84 | 0.76 | 0.93 | <0.001 | 0.74 | 0.59 | 0.91 | 0.009 | 5.85 | 5.26 | 6.5 | <0.001 |
| PC4 | 0.83 | 0.75 | 0.92 | <0.001 | 0.92 | 0.72 | 1.18 | 0.521 | 3.34 | 3.01 | 3.72 | <0.001 |
| NBUDs | ||||||||||||
| Exposure | 1.23 | 0.67 | 2.24 | 0.499 | ||||||||
| Sex | 1.00 | 0.56 | 1.80 | 0.988 | 0.65 | 0.16 | 2.56 | 0.539 | 1.24 | 0.61 | 2.50 | 0.551 |
| Age | 1.04 | 1.01 | 1.07 | 0.005 | 1.01 | 0.94 | 1.09 | 0.734 | 1.05 | 1.02 | 1.08 | 0.004 |
| PC1 | 0.99 | 0.87 | 1.14 | 0.912 | 1.23 | 0.87 | 1.73 | 0.255 | 1.01 | 0.86 | 1.19 | 0.920 |
| PC2 | 1.18 | 0.93 | 1.49 | 0.183 | 0.92 | 0.5 | 1.67 | 0.784 | 1.27 | 0.95 | 1.69 | 0.111 |
| PC3 | 0.76 | 0.58 | 1.00 | 0.052 | 0.63 | 0.34 | 1.15 | 0.145 | 0.79 | 0.57 | 1.08 | 0.149 |
| PC4 | 0.85 | 0.63 | 1.14 | 0.270 | 0.86 | 0.43 | 1.7 | 0.662 | 0.81 | 0.52 | 1.25 | 0.341 |
| NPB | ||||||||||||
| Exposure | 0.36 | 0.23 | 0.55 | <0.001 | ||||||||
| Sex | 0.53 | 0.32 | 0.87 | 0.014 | 1.00 | 0.26 | 3.8 | 0.999 | 1.13 | 0.55 | 2.32 | 0.737 |
| Age | 1.01 | 0.99 | 1.04 | 0.356 | 1.03 | 0.96 | 1.1 | 0.408 | 1.01 | 0.98 | 1.05 | 0.426 |
| PC1 | 1.09 | 0.98 | 1.2 | 0.112 | 1.55 | 1.14 | 2.09 | 0.009 | 1.04 | 0.85 | 1.27 | 0.735 |
| PC2 | 1.1 | 0.93 | 1.3 | 0.286 | 1.97 | 1.16 | 3.35 | 0.019 | 1.26 | 0.94 | 1.69 | 0.125 |
| PC3 | 0.92 | 0.75 | 1.12 | 0.398 | 1.16 | 0.8 | 1.67 | 0.436 | 0.71 | 0.48 | 1.05 | 0.092 |
| PC4 | 1.07 | 0.89 | 1.3 | 0.465 | 2.88 | 1.69 | 4.89 | <0.001 | 0.62 | 0.45 | 0.86 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espitia-Pérez, P.; Espitia-Pérez, L.; Peñata-Taborda, A.; Brango, H.; Pastor-Sierra, K.; Galeano-Páez, C.; Arteaga-Arroyo, G.; Humanez-Alvarez, A.; Rodríguez Díaz, R.; Salas Osorio, J.; et al. Genetic Damage and Multi-Elemental Exposure in Populations in Proximity to Artisanal and Small-Scale Gold (ASGM) Mining Areas in North Colombia. Toxics 2025, 13, 202. https://doi.org/10.3390/toxics13030202
Espitia-Pérez P, Espitia-Pérez L, Peñata-Taborda A, Brango H, Pastor-Sierra K, Galeano-Páez C, Arteaga-Arroyo G, Humanez-Alvarez A, Rodríguez Díaz R, Salas Osorio J, et al. Genetic Damage and Multi-Elemental Exposure in Populations in Proximity to Artisanal and Small-Scale Gold (ASGM) Mining Areas in North Colombia. Toxics. 2025; 13(3):202. https://doi.org/10.3390/toxics13030202
Chicago/Turabian StyleEspitia-Pérez, Pedro, Lyda Espitia-Pérez, Ana Peñata-Taborda, Hugo Brango, Karina Pastor-Sierra, Claudia Galeano-Páez, Gean Arteaga-Arroyo, Alicia Humanez-Alvarez, Ruber Rodríguez Díaz, Javier Salas Osorio, and et al. 2025. "Genetic Damage and Multi-Elemental Exposure in Populations in Proximity to Artisanal and Small-Scale Gold (ASGM) Mining Areas in North Colombia" Toxics 13, no. 3: 202. https://doi.org/10.3390/toxics13030202
APA StyleEspitia-Pérez, P., Espitia-Pérez, L., Peñata-Taborda, A., Brango, H., Pastor-Sierra, K., Galeano-Páez, C., Arteaga-Arroyo, G., Humanez-Alvarez, A., Rodríguez Díaz, R., Salas Osorio, J., Valderrama, L. A., & Saint’Pierre, T. D. (2025). Genetic Damage and Multi-Elemental Exposure in Populations in Proximity to Artisanal and Small-Scale Gold (ASGM) Mining Areas in North Colombia. Toxics, 13(3), 202. https://doi.org/10.3390/toxics13030202












