Abstract
Since the oral cavity comes into contact with several xenobiotics (dental materials, oral hygiene formulations, drugs, or tobacco products), it is one major site for toxicity manifestation. Multiple parameters are assessed during toxicity testing (cell viability and proliferation, apoptosis, morphological changes, genotoxicity, oxidative stress, and inflammatory response). Due to the complexity of the oral cavity environment, researchers have made great efforts to design better in vitro models that mimic natural human anatomic and functional features. The present review describes the in vitro methods currently used to investigate the toxic potential of various agents on oral cavity tissues and their evolution from simple 2D cell culture systems to complex organ-a-chip designs.
1. Introduction
Toxicity evaluation is an essential research area, especially in the medical and pharmaceutical world. The last decades have seen a change in the approach. Ethical and economic factors caused an inclination towards developing new and accurate alternative methods (in vitro, in silico) that are in accordance with the 3R principle (reduce, refine, and replace animal use). The non-animal methods have become increasingly sophisticated over the last years, stimulated especially by the progress in the tissue engineering field [1].
Oral cavity tissues are frequently exposed directly to various xenobiotics (dental materials, oral healthcare products, drugs, tobacco products, or other agents). Therefore, the development of relevant and predictive toxicity assessment methods is essential. The purpose of this literature review is to make an extensive presentation on the main in vitro techniques currently used for oral tissue toxicity evaluation.
2. Materials and Methods
The literature search was conducted on different electronic databases (PubMed, Web of Science, Scopus, Google Scholar) using keywords like “cytotoxicity”, “cell viability assays”, “apoptosis”, “genotoxicity”, “oxidative stress”, “inflammatory markers”, “2D cell cultures”, “3D cell cultures”, and “organ-on-a-chip”. Only studies that used normal human oral cell cultures, 3D, or organ-on-a-chip models reflecting oral cavity structures were considered. Original articles, reviews, and book chapters published between 1990 and now were included.
3. Two-Dimensional Cell Culture Testing
Cell culture testing still remains one of the main techniques that can be used in toxicity assays to screen the cellular response to chemical agents. When it comes to the cytotoxicity of different xenobiotics on oral cavity tissues, several types of cells can be used, for example, human oral keratinocytes and human oral fibroblasts. Cellular toxicity testing can be performed in various ways, and the design of a study is adapted depending on the characteristics of the tested materials, the type of products, or the clinical use scenario. According to the ISO 10993-5 guideline [2], there are three types of cytotoxicity test methods: the elution test (extract dilution), the direct contact test, and the indirect contact test. The direct contact test is a more sensitive method and can detect even a weak cytotoxic potential [3]. Compared with the indirect contact method, where the tested material is placed on a cell monolayer protected by an agar coat, in the case of direct contact, the cells are more vulnerable to mechanical impairment [4]. During the elution tests, extracts of the test article are used to assess the biocompatibility and cytotoxicity of compounds leached from the tested material. Preincubated cell layers are exposed to the obtained extracts, or freshly suspended cells are seeded directly in the resulting solution. When the laboratory routine is established, there are many parameters that can influence extraction tests, and researchers must evaluate them, in order to design the best experiment for cytotoxicity evaluation (e.g., the extraction medium, the extraction ratio, the extraction temperature or pH, the extraction time, and if the extraction is performed in a static or dynamic mode) [5].
As Srivastava et al. pointed out, the type of cytotoxicity method used can greatly influence the final results. They demonstrated that the extract dilution method or the indirect contact method was not appropriate to accurately assess the safety of perfluoro-octane, a derivative used intraoperatively in vitreoretinal surgery, where it comes in direct contact with the neuroretina. The direct contact method was better for providing meaningful data regarding cytotoxicity [3]. When it comes to oral cavity tissues, the concern for selecting the best cytotoxicity method is applicable especially to dental materials that come into contact with oral structures, like root canal sealers [6,7,8].
Various biological aspects can be targeted by in vitro toxicity assays, like cell viability and proliferation, morphological changes, genotoxicity, markers of oxidative stress, or inflammatory response.
3.1. Cell Viability and Cell Proliferation Assays
The most investigated aspect that reflects cytotoxicity is assessing the influence of the tested agents on cell viability. These tests have many advantages. They are low-cost, allow the assessment of a large number of samples, and give reliable and reproducible results. However, in order to obtain a clear picture of the phenomenon, a researcher must use different categories of assay. A decrease in cell viability can be the result of a cytostatic effect (the inhibition of cell metabolism or proliferation) or a cytotoxic effect of a xenobiotic (actual cell death). Colorimetric assays alone, used to evaluate cell viability via metabolic activity, are not enough to support statements about cell death induction, and additional experimental approaches must be used to detect dying cells. Furthermore, another aspect that needs investigation is the elucidation of the molecular mechanism involved in cell death (apoptosis, necrosis, or autophagy) [9].
Several methods can be used to determine the number of viable cells (Figure 1). Cell viability assays evaluate different cell functions (the permeability of cell membranes, cellular enzyme activity, ATP (adenosine triphosphate) production, and cell adherence) [10,11].
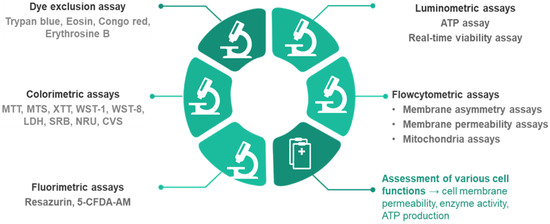
Figure 1.
Cell viability assays.
Dye exclusion assays using trypan blue, Congo red, or erythrosine B determine the integrity of the cell membranes, as only the viable cells can exclude dyes. Although these tests are fast and convenient, there are some factors that may alter the results. For example, the integrity of the membranes may not be immediately affected in lethally damaged cells. Because intracellular damage mechanisms occur before the lysis of cellular membranes, other assays can be more sensitive and accurate. Also, in some cases, the continuous proliferation of the surviving cells may underestimate cell death and cytotoxicity. Furthermore, these methods do not allow the distinction between normal, healthy cells and cells that have lost cell functions and are lethally damaged, but are still alive. Using these assays, apoptosis and necrosis processes cannot be distinguished either [12,13].
Colorimetric assays (MTT, MTS, XTT, and WST) evaluate cell viability via metabolic activity assessment. These assays are based on the conversion of a reagent (a specific dye) to a colored product in metabolically active cell populations, via mitochondrial enzymes. The tetrazolium salts are converted into colored formazan crystals, and the spectrophotometric signal becomes an indicator for cellular metabolic activity, which is related to the number of viable cells (Figure 2). WST (water-soluble tetrazolium) salts (WST-1 and WST-8) represent a new generation of reagents that are more stable and give a more efficient spectrophotometric signal. These methods are widely used, mainly because they are inexpensive, but their sensitivity is limited, the reagents are toxic to cells, and they are unsuitable for long-term studies. Furthermore, the distinction between cytotoxic and cytostatic effects is not possible [13,14].
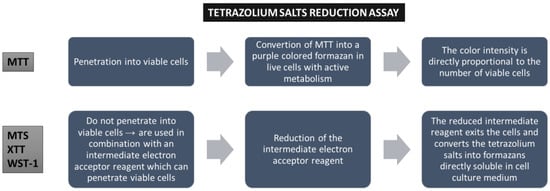
Figure 2.
Cell viability assays based on tetrazolium salt reduction.
The neutral red assay is based on the capacity of viable cells to incorporate the dye (neutral red) in lysosomes, and the reduction in uptake is associated with a lower cellular viability and cytotoxicity [15].
Sulforhodamine B assay is used to determine cell density, based on the measurement of cellular protein content. The cells are fixed with trichloroacetic acid and the sulforhodamine B dye binds to the basic amino acid residues of cellular proteins. The amount of protein-bound dye, determined spectrophotometrically, is directly proportional to the number of living cells [13,16].
Fluorometric methods (e.g., alamarBlue) were developed as an alternative to colorimetric methods, and have the advantages of a higher sensitivity. The alamarBlue assay is suitable for long-term experiments, due to the lower degree of toxicity. These assays are based on the conversion of nonfluorescent agents into fluorescent compounds by cellular enzymes (e.g., viable cells reduce resazurin to resorufin, a highly fluorescent compound). The fluorescent signal is proportional to the percentage of viable cells with an active cellular metabolism [11]. 5-CFDA-AM (5-carboxyfluorescein diacetate acetoxymethyl ester) is a cell-permeant esterase substrate that can be used for the same purpose, as it evaluates both enzymatic activity and cell-membrane integrity. Intracellular esterases activate fluorescence, while the integrity of cellular membranes is a condition for intracellular retention of the fluorescent product.
Another colorimetric assay is LDH. The method assesses cellular membrane integrity by measuring the level of lactate dehydrogenase released from the cytoplasm of affected cells into the cell culture medium after membrane disruption in necrotic or apoptotic cells. The quantitative determination of the enzyme is achieved via the conversion of tetrazolium salt into red formazan. The major limitation of this method is that it requires low-serum or serum-free conditions [12,13].
The glucose-6-phosphate dehydrogenase (G6PD) release assay is also a method based on the quantification of an enzyme released from dead cells via damaged membranes into the culture medium, but it is more sensitive than the LDH assay. It is a fluorescence-based assay, involving the reduction of resazurin to resorufin, in the presence of NADPH, generated by G6PD [13].
One of the fastest and most sensitive methods to evaluate cell viability is the ATP assay, a luminometric assay. This method is based on the transformation of luciferin to oxyluciferin, a reaction catalyzed by luciferase in the presence of Mg ions and ATP. The intensity of the luminescent signal depends on the levels of ATP synthesized by viable cells [10,12]. The major drawback of this method is the impossibility of distinguishing between cytostatic and cytotoxic effects because several factors can influence the ATP level (cell proliferation, cell death, or metabolic activities) [13]. One luminometric assay allows monitoring of cellular viability in real time (the real-time viability assay). It uses an enzyme (luciferase) and a prosubstrate. Live cells internalize the prosubstrate and transform it into an active substrate that interacts with luciferase and generates a bright signal. The main advantage is the ability to observe both dose- and time-dependent toxicity on the same set of samples, as continuous readings can be performed over extended periods [17].
Flow cytometry has become a versatile tool for qualitative and quantitative cell analysis. Flow cytometry has wide applications, including toxicity studies, because it is a rapid and reliable method to distinguish between viable and dead cell populations. This method reveals information about cells’ size and granularity, but it also allows fluorescent staining targeting various cellular parameters. One of these parameters is the composition of the outer surface of the cell membrane. In the early stages of apoptosis, this composition is altered, even while cells preserve their membrane integrity. Consecutively, phosphatidylserine can be detected at the outer surface of cells by fluorescent labeling with Annexin V. Another way to assess membrane asymmetry is using F2N12S, a molecule with dual-color fluorescence emission that is incorporated into the phospholipids of cell membranes. Apoptosis causes alterations of the cellular membrane charges, and the color of the emission is influenced by these changes, allowing the determination of the viability percentage [11,18]. Other flow cytometric assays use dyes to assess membrane integrity and permeability (inclusion dyes, exclusion dyes, and monomeric cyanine nucleic acid stains) or they determine various mitochondria parameters (mitochondria membrane potential, mitochondria mass, and mitochondria membrane permeability) [11].
The flow cytometry-based Annexin V assay is capable of distinguishing between different types of cell death (apoptosis vs. necrosis) in a single population of cells. In necrotic cells, the membrane becomes permeable to propidium iodide, a DNA-binding fluorescent dye, while apoptosis is detected using Annexin V, which binds to the phosphatidylserine [19]. For precise discernment between apoptosis and necrosis, the levels of specific biomarkers and regulators can be assessed using Western blotting [e.g., the activation of caspase-3 and PARP1 (poly (ADP-ribose) polymerase-1) for apoptosis; RIPK1 (receptor-interacting serine/threonine kinase 1) phosphorylation for necrosis]. Imaging flow cytometry can also be used to analyze the morphological differences between apoptotic and necrotic cells [9].
Cell proliferation is another parameter that characterizes cells’ health and the dynamic of the cell population. Cellular division plays a significant role in physiological and pathological processes and is usually investigated not only in studies concerning malignancies and anticancer therapy, but also in toxicity assays [13].
The clonogenic cell survival assay investigates the capacity of cells to retain their ability to proliferate and form a colony (or a clone). This method has the advantage of considering reversible damage induced by exposure to xenobiotics, and the regrowing phenomenon of resistant cells. However, the clonogenic (colony formation) assay is not suitable for agents that do not affect DNA synthesis; it is applicable only to adherent cells, it is time-consuming, and in the case of manually counting the colonies, errors may appear [13,20].
Other common assays include the detection of mitotically active cells and direct measurement of DNA synthesis via the incorporation of thymidine analogs—BrdU (5-bromo-2′-deoxyuridine) or EdU (5-ethynyl-2-deoxyuridine)—into nascent DNA during S-phase. The newly synthesized BrdU-incorporating DNA can be detected immunologically with a specific antibody against BrdU [13,21,22]. Another approach to labeling nascent DNA is using the incorporation and detection of tritium-labeled (H3) thymidine, but due to the difficulties in handling radiolabeled substances, the BrdU/EdU methods are preferred [13]. Cell proliferation can also be analyzed using the detection of proliferation markers (e.g., proliferating cell nuclear antigen (PCNA), Ki67 antigen, and phosphohistone H3 (PHH3)). PCNA confers high processivity to DNA polymerase delta and has a major role in DNA replication, but it is also involved in many other important cellular processes (cell cycle control, DNA repair, epigenetic inheritance) [23]. Ki67 is a nuclear antigen expressed in all proliferating cells (both normal and cancerous), in all phases of the interphase (G1, S, and G2) and mitosis. The Ki67 index (percentage of Ki67-positive cells) becomes an excellent marker for cell proliferation because the antigen is not only expressed in cells during the resting state (G0 phase). The quantification protocols imply the use of specific Ki67 antibodies, and the detection methods can be flow-cytometry or a colorimetric approach [13].
Cytotoxicity studies can also be performed with real-time cell analyzers that allow dynamic live-cell monitoring and electronic detection of biological processes. This approach offers continuous information about cell adhesion, growth and proliferation, morphological changes, and cell death [24,25].
Although these tests are widely used, there are several factors that can induce variability in response and affect reproducibility. These factors are biological (e.g., cells and medium characteristics) or technical (e.g., counting errors, the edge effect, treatment time, or cross-reactivity with other compounds). A suboptimal experimental design leads to significant errors, and it is very important to optimize the experimental parameters [26].
Table 1 presents a selection of studies investigating the cytotoxic potential of different materials and chemical agents on various types of normal oral cavity cell cultures using cell viability and proliferation assays.

Table 1.
Selection of studies assessing cell viability and proliferation of human oral cells during cytotoxicity testing.
Apoptosis assays
Traditionally, cell death induced by cytotoxic agents is associated with necrosis, but xenobiotics can promote toxicity by triggering apoptosis. Apoptosis appears as a defense mechanism in cells damaged by noxious agents [72]. Several methods can be used for identifying and quantifying apoptosis (Table 2). Morphological assessment is one of them, because there are typical alterations that accompany apoptosis (e.g., cell shrinkage, denser cytoplasm, tightly packed organelles, membrane blebbing, and the formation of apoptotic bodies). Apoptosis assays also evaluate other key cellular events (Figure 3) that occur during programmed cell death, such as DNA fragmentation (TUNEL assay), activation of the caspase-dependent proteolytic cascade (caspase activity assay), and the assessment of the expression levels of apoptotic proteins (Western blot analysis) [73].
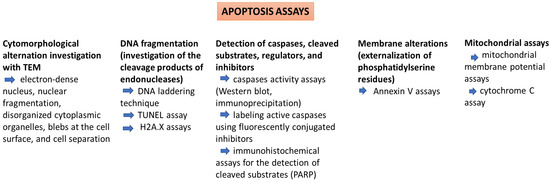
Figure 3.
Apoptosis assays.
During apoptosis, the endonucleases cause the fragmentation of nuclear DNA and nuclear destruction. The TUNEL (Terminal deoxynucleotidyl transferase-mediated dUTP Nick End Labeling) assay allows the in situ identification of DNA strand breaks in apoptotic nuclei by fluorochrome-labeling of free 3′-hydroxyl termini of DNA strand breaks. However, other deoxynucleotides (instead of dUTP) can also be used in different variants of this assay. The cells can be analyzed by flow cytometry or laser scan cytometry (for cells attached to microscope slides). False TUNEL positivity can be determined by high DNA repair rates in proliferating or necrotic cells [74].
Caspases (cysteine-aspartic proteases) are endopeptidases that are synthesized in an inactive form and are later activated via proteolytic cleavage. These enzymes are activated as a response to cytotoxic agents, which eventually leads to the disruption of cellular processes and cell death. Caspases can be considered potential markers of apoptosis caused by cell exposure to toxins [75]. Caspases can be assessed using different methods. Colorimetric assays are based on the spectrophotometric detection of chromophores (e.g., p-nitroanilide) cleaved by the enzyme from the labeled substrates. On the other hand, fluorometric assays use fluorochrome substrates (e.g., 7-amino-4-trifluoromethyl coumarin) and the fluorescent signal is quantified [76]. Other techniques used to detect the activation of caspases are the Western blot assay which uses an antibody specific to active caspase, immunocytochemical localization of an activated caspase with specific antibodies, and the FLICA assay (using Fluorochrome-Labeled Inhibitors of Caspases) [77].

Table 2.
Selection of studies assessing apoptosis of human oral cells during cytotoxicity testing.
Table 2.
Selection of studies assessing apoptosis of human oral cells during cytotoxicity testing.
| Method | Type of Cells | Tested Agent | Results | Reference |
|---|---|---|---|---|
| TUNEL assay | Human gingival fibroblasts | Hydrogen peroxide | Hydrogen peroxide promoted apoptosis in human gingival fibroblasts. | [78] |
| Oral mucosa epithelial cells | Mobile phone use (radiofrequency radiation) | Apoptosis levels were not significantly influenced. | [79] | |
| Human gingival fibroblasts | Nicotine | Nicotine increased the number of TUNEL-positive cells. | [80] | |
| Human gingival epithelium keratinocytes | Traditional cigarette smoke and electronic cigarette aerosols | Exposure to traditional cigarette smoke and electronic cigarette aerosols increased the number of TUNEL-positive cells. | [39] | |
| Human oral fibroblasts | Cigarette smoke and e-vapor condensates | The DNA fragmentation assay indicated an increased number of TUNEL-positive apoptotic fibroblasts with broken nuclei. | [40] | |
| Human gingival epithelial cells | E-cigarette vapor | E-cigarette vapor exposure increased the proportion of TUNEL-positive cells. | [58] | |
| Caspase activity assay | Human gingival fibroblasts | Nicotine | Nicotine induced apoptosis via the caspase-3 pathway. | [80] |
| Human gingival epithelial cells | Cigarette smoke total particulate matter (TPM), smokeless tobacco extracted with complete artificial saliva (ST/CAS), and whole-smoke conditioned media (WS-CM). | TPM and WS-CM (combusted tobacco) significantly increased caspase-3 activity, while ST/CAS (non-combusted) showed no or only minimal activation. | [55] | |
| Human gingival epithelial cells | E-cigarette vapor | A significant induction of caspase-3 proteins was observed in e-cigarette vapor-exposed cells. | [58] | |
| Human gingival fibroblasts | E-cigarette vapors and cigarette smoke | No significant differences were recorded for the caspase 3/7 activity in the tested groups. | [81] | |
| Odontoblast-like MDPC-23 cells | NaF | NaF exposure induced the activation of caspase-3. | [82] | |
| Cementoblasts | Zinc-oxide eugenol-based root-canal sealer | The root-canal sealer stimulated caspase-3, -8, and -9 activity in a dose-dependent manner. | [76] | |
| Human oral fibroblasts | Antioxidant mixtures of resveratrol (R), ferulic acid (F), phloretin (P), and tetrahydrocurcuminoids (T)—RFT, PFR, and PFT | The tested mixtures did not increase apoptosis. | [43] | |
| Human gingival fibroblasts | Epicatechin gallate | Epicatechin gallate did not induce caspase-3 activity. | [52] | |
| Image-iTTM LIVE green caspase detection | Human oral fibroblasts | Antioxidant mixtures of resveratrol (R), ferulic acid (F), phloretin (P), and tetrahydrocurcuminoids (T)—RFT, PFR, and PFT | The tested mixtures did not increase apoptosis. | [43] |
| DAPI staining and microscopy analysis | Human gingival fibroblasts | Nicotine | Apoptotic cellular bodies, nuclear condensation, and DNA fragmentation were present. | [80] |
| Human gingival epithelium keratinocytes | Traditional cigarette smoke and electronic cigarette aerosols | Apoptotic morphology was detected; there were no significant differences in cell morphology between t-cigs and e-cigs. | [39] | |
| Annexin- V-FITC | Human gingival fibroblasts | Commercial topical fluoride varnishes | Some of the tested samples exhibited high cytotoxicity, associated with certain components present in the fluoride varnishes (cetylpyridinium chloride and ethyl acetate). | [83] |
| Human normal oral epithelial cells | Cisplatin and thymoquinone (alone and in combination) | Thymoquinone (in combination with cisplatin) decreased cisplatin-induced apoptosis and necrosis in normal cells. | [49] | |
| Human gingival fibroblasts | Vitamin C | Vitamin C reduced apoptosis rates in human gingival fibroblasts exposed to Porphyromonas gingivalis. | [84] | |
| Human gingival fibroblasts | Curcumin | The proportion of apoptotic cells increased with increasing concentrations of curcumin. | [85] | |
| Human oral fibroblast | Menthol and eucalyptol | The results indicated a concentration-dependent and exposure-time-dependent cytotoxicity for eucalyptol and menthol. | [86] | |
| Human gingival fibroblasts | Methacrylate-based resins | Methacrylate-based resins produced cell death via necrosis or apoptosis, depending on the sample. | [87] | |
| Human gingival fibroblasts | N-acetylcysteine incorporated in methacrylate-based resin cement | N-acetylcysteine diminished cytotoxicity, cell apoptosis, and necrosis in cells. | [88] | |
| Human gingival fibroblast | Dental resin polymerization initiators | Dental resin polymerization initiators induced cell death via necrosis. | [89] | |
| Western blot analysis of apoptotic proteins | Human gingival fibroblasts | Nicotine | Nicotine increased BAX expression (pro-apoptotic protein) and decreased BCL-2 expression (anti-apoptotic protein). | [80] |
| Human normal oral epithelial cells | Cisplatin and thymoquinone (alone and in combination) | Cisplatin and thymoquinone (alone and in combination) showed a significant upregulation of p53 and caspase-9 (pro-apoptotic), and a decrease in the anti-apoptotic Bcl-2 protein expression. | [49] | |
| Human gingival fibroblasts | Hydrogen peroxide | H2O2 increased the BAX expression and decreased the Bcl-2 expression. | [78] | |
| Mitochondrial membrane potential assay | Human gingival fibroblasts | Curcumin | Curcumin induced early apoptotic events characterized by the loss of the mitochondrial membrane potential. | [85] |
| Human gingival fibroblasts | TEGDMA released from resin composite | TEGDMA induced mitochondrial damage and mitochondrial membrane potential collapse. | [90] |
Abbreviations: BAX (Bcl-2-associated X protein); F (ferulic acid); P (phloretin); R (resveratrol); ST/CAS (smokeless tobacco extracted with complete artificial saliva); T (tetrahydrocurcuminoids); TEGDMA (triethyleneglycol dimethacrylate); TPM (cigarette smoke total particulate matter); and WS-CM (whole-smoke conditioned media).
3.2. Morphological Changes Assessment
Cell morphology describes the shape, size, and internal structure of cells and is considered a visual indicator of the cellular homeostasis and physiological state of cells. There is substantial evidence that cellular compartments change in response to external factors, and these modifications are accompanied by alterations in cellular functions. The interactions between the extracellular environment and the cells influence highly regulated biological processes and affect the cell shape, size, and texture of cellular compartments. Therefore, cell morphological profiling has become a valuable tool for toxicity assays, offering important clues about the intracellular targets and the toxicity mechanism [91,92,93]. Furthermore, morphological identification of cell death is more accurate than other methods and can be considered the golden standard. Microscopy analysis (e.g., SEM) completes and supports the results of cell viability assays and biochemical analysis [94]. In the -omics era, a new term has emerged. Morphomics is used to emphasize the importance of cell morphology. The morphome (the totality of the cells’ morphology parameters) is determined by molecular dynamics (genome, transcriptome, proteome, and metabolome) [95].
The main limitation of cell morphological profiling consists of the difficulty of scaling up, because, conventionally, the examination of every image was performed manually. However, the development of high-content screening systems may represent an opportunity for the emergence of assays based on cell morphological profiling, suitable for cytotoxic screening. Computational tools are able to analyze vast quantities of data and project a quantitative and statistically valid relationship between cell shape and experimental conditions [93]. The current trend is to use artificial intelligence approaches to analyze cellular images [95]. The REM-I platform is a new technology that uses artificial intelligence to analyze and extract phenotypic features of cells and sort them according to their morphology from high-resolution images [96,97].
The current advances in the field of microscopy and computer image analysis have brought this research area to an impressive level. For example, the “Cell Painting” assay represents an image-based cell profiling technique based on the fluorescent labeling of major cellular organelles that allows the detection of cellular morphological features in response to different treatments. Usually, six fluorescent stains are used, over five channels [actin + Golgi + plasma membrane (AGP), nucleus (DNA), endoplasmic reticulum (ER), nucleoli + cytoplasmic RNA (RNA), mitochondria (Mito)], imaging eight internal cellular structures and organelles [98].
Over 1500 morphological features can be acquired at the single-cell level using this assay, giving information about cellular functions alteration. This phenotypic profiling allows the investigation of the biological impact of molecular or genetic perturbations in a relatively unbiased manner, unlimited by preconceived notions or existing knowledge [99,100]. This high-throughput phenotypic profiling assay is approved for bioactivity screening and chemical risk assessment by the Center for Computational Toxicology and Exposure and the United States Environmental Protection Agency, due to its broad applicability [101,102].
Cell painting allows researchers to study cell viability, cell proliferation, DNA damage, cell cycle stage, and the dynamic organization of proteins [103]. Some modified Cell painting experiments allow not only the detection of some morphological features, but also the quantitative assessment of phenomena like apoptosis, oxidative stress, or autophagy, using fixable functional markers. Small-molecule sensor dyes were developed to detect caspase activation in cell cultures or mitochondrial superoxide generation associated with cell stress using fluorescence microscopy [104].
Fluorescence imaging techniques have been used in studies involving human oral cells [105,106,107], but, to our knowledge, no research papers using the Cell Painting assay on these types of cells have been published so far.
Several features are analyzed in morphological profiling (differences in size, shape, compactness, organelle symmetry, staining intensities, textural patterns, and spatial organization of cells. The most common morphological changes are usually cell detachment and shape alteration, due to cytoplasmatic shrinkage [108].
The morphological changes that appear in the internal cellular structure can be seen at different levels (cytoplasm, cytoskeleton, nucleus, Golgi apparatus, and endoplasmic reticulum). The alterations that affect the cell cytoplasm are among the most obvious, and Lai et al. developed a cytotoxicity assay using cytoplasm-localized fluorescent probes (CLFPs). The authors use fluorescent staining to differentiate between living and dead cells. Due to the differences in the permeability of the plasma/nuclear membrane, the live cells appear with a bright cytoplasm and a dark nucleus, while the dead cells are fully fluorescent. Dead cells without intact membranes will not be visible by fluorescence microscopy. Some companies have developed assays designed to assess changes in the morphological profile of cells by evaluating the nuclear area with a high content screening (HCS) system [94].
When it comes to assessing the cytotoxicity of materials and chemical compounds on human oral cavity cells, the most frequently tested agents can be divided into three main categories—dental materials, drugs used locally or for the treatment of different systemic diseases, and other agents/xenobiotics (Table 3).

Table 3.
Selection of studies assessing morphological changes in human oral cells during cytotoxicity testing.
3.3. Genotoxicity Assessment
Another important aspect investigated is the genotoxic potential. Genotoxic substances alter the structure, information content, and segregation of DNA. Mutagenic agents permanently modify the genetic material in the cell. There are several mechanisms involved in genotoxicity (Figure 4) and batteries of broad in vitro tests (e.g., bacterial reverse mutation assay, in vitro chromosomal aberration test, micronucleus test, comet assay, and DNA damage and repair) [119,120]. In vitro genotoxicity assays are robust and simple tools, both time- and cost-effective, with a low rate of false negative results, due to their high sensitivity. One downside of these assays is their relatively low specificity, which is associated with a high rate of false positive results. Therefore, the challenge is to develop assays with adequate sensitivity and improved specificity [120].
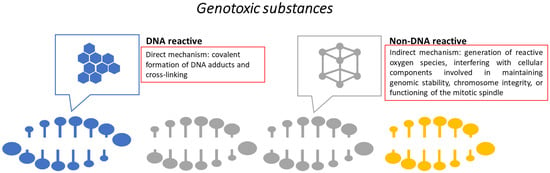
Figure 4.
Genotoxicity mechanisms.
For in vitro genotoxicity testing on human oral cells, the assays most commonly used are the micronucleus test (an assay for chromosomal abnormalities), the comet assay (a test for primary DNA damage), and the H2AX assay (Table 4).
Due to its convenience and reliability, the micronucleus assay has become a popular tool to assess genotoxicity. Micronuclei represent round-shaped extranuclear bodies, chromatin-containing, that are visible in the cytoplasm, with different origins (acentric chromosome fragments, malsegregation of chromosomes, dicentric chromosome breakage, and chromosome instability). They are considered a marker for DNA damage and genomic instability. A version of this assay is the cytokinesis-block micronucleus assay, which uses cytochalasin to stop cytokinesis (but not karyokinesis), and the micronuclei are scored only in binucleated cells. This version has the advantage of fewer false negative results [119,121].
The comet assay evaluates single or double DNA breaks, cross-linking, damage to DNA bases, and apoptotic fragments in isolated cells using gel electrophoresis. The DNA damage and relaxation of the supercoiled DNA will cause the differential migration of damaged and undamaged DNA during electrophoresis, stretching toward the anode, and forming a comet-like structure [122].
The H2AX assay reflects double-strand breaks. DNA damage and the formation of double-strand breaks (DSBs) are followed by the phosphorylation of H2AX, a component of the histone octamer in nucleosomes. A newly phosphorylated protein, γ-H2AX, is formed, which can be considered a biomarker for DSBs. γ-H2AX can be detected using specific monoclonal or polyclonal antibodies. The overall γ-H2AX levels in cell lysates can be determined by the ELISA (enzyme-linked immunosorbent assay), while the measurement of γ-H2AX levels directly in cell nuclei can be achieved by microscopy or fluorescence-activated cell sorting (FACS). The counting of γ-H2AX foci is a very sensitive marker of DNA damage, and to increase efficiency, high throughput foci counting systems were developed [119,123,124,125].

Table 4.
Selection of studies assessing genotoxicity on human oral cells.
Table 4.
Selection of studies assessing genotoxicity on human oral cells.
| Method | Type of Cells | Tested Agent | Results | Reference |
|---|---|---|---|---|
| Micronucleus assay | Oral epithelial cells | Liquids for electronic cigarettes | Positivity for micronuclei was recorded for flavored and unflavored e-liquids. | [54] |
| Human oral keratinocytes | Dental photoinitiators (BAPO and TPO) | No genotoxic effect was observed. | [30] | |
| Human gingival fibroblasts | Single-wall carbon nanotubes | The frequency of micronuclei was higher after exposure to lower doses (50–100 μg/mL), and lower for high-dose treatments (125–150 μg/mL). | [41] | |
| Human pulp fibroblasts | Aloe vera associated with endodontic medication (calcium hydroxide) and laser photo-biomodulation | The highest micronucleus rate was obtained for the association Aloe vera + laser therapy. | [126] | |
| Human gingival fibroblasts | Toothpastes | The tested toothpastes did not exhibit genotoxic effects. | [127] | |
| Human gingival fibroblasts | Static magnetic field produced by dental magnetic attachments | The micronuclei rates increased in the exposed groups, but no mitotic changes appeared. | [128] | |
| Human gingival fibroblasts | Achyrocline satureioides extract | The micronucleus count increased in a concentration-dependent manner; no genotoxic effects were recorded for concentrations up to 6.25 mg/mL. | [129] | |
| Human gingival fibroblasts | Methacrylic monomer BAPP | A dose-related increase in the number of micronuclei was recorded. | [130] | |
| Comet assay | Human gingival fibroblasts | Single-wall carbon nanotubes | The nanotubes induced a significant increase in tail moments. | [41] |
| Human gingival fibroblasts | Bracket alloys | All samples exhibited genotoxicity, but the Ni-Ti combination showed the highest DNA damage. | [131] | |
| Human gingival fibroblasts and buccal epithelial cells | Corrosion eluates from stainless-steel brackets, nanoparticle-coated brackets, and polymeric-coated brackets | The stainless-steel bracket eluates caused increased nuclear damage in cells. | [132] | |
| Human gingival fibroblasts | Metals released from three orthodontic alloys (stainless-steel, nickel-free, and titanium) | The greatest DNA damage was produced by the stainless-steel alloy. | [133] | |
| Human gingival keratinocytes | Corrosion eluates produced from orthodontic materials | No apparent DNA damage was recorded. | [134] | |
| Human oral keratinocytes | TEGDMA | The increase in tail moments after TEGDMA exposure was concentration-dependent. | [135] | |
| Human gingival fibroblasts | BAPP | The incidence of DNA strand breaks increased in a concentration-dependent manner. | [130] | |
| Human gingival fibroblasts | HEMA | HEMA induced significant fragmentation of the DNA. | [136] | |
| Human gingival fibroblasts | Non-irradiated campherquinone | The exposure to campherquinone caused significant DNA damage. | [137] | |
| Oral epithelial cells | Liquids for electronic cigarettes | No significant increase in DNA damage assessed by the comet assay was recorded. | [54] | |
| Human periodontal ligament fibroblasts | E-cigarette vapors with flavoring | An increase in fluorescent tail length was observed, indicating DNA damage. | [138] | |
| γH2AX assay | Human gingival fibroblasts | HEMA | A significant extent of DNA double-strand breaks was observed. | [136] |
| Human gingival fibroblasts | Methacrylate-based adhesives | The highest level of DNA damage induction was exhibited by the adhesive with diphenyliodonium hexafluorophosphate as a co-initiator. | [46] | |
| Human gingival fibroblasts | N-acetylcysteine incorporated in methacrylate-based resin cement | Incorporating N-acetylcysteine resulted in an anticytotoxic effect. | [88] | |
| Human gingival fibroblasts | Methacrylate-based monomers in association with antioxidants (N-acetylcysteine and ascorbic acid) | The addition of antioxidants reduced the number of DNA double-strand breaks after exposure to methacrylate-based monomers. | [125] | |
| Human gingival fibroblasts | Titanium dioxide-modified glass ionomer cements | No genotoxic effects were observed for the titanium dioxide-modified glass ionomer cements. | [139] | |
| Human gingival fibroblasts | Dental resin restoration components (TEEGDMA, Neopen, DPIC, TPSB, TPP) | The induction of DNA double-strand breaks in exposed cells was observed, with the highest incidence for diphenyliodoniumchloride and neopentylglycol dimethacrylate. | [140] | |
| DNA precipitation assay | Buccal fibroblasts | Arecoline | No DNA single-strand breaks appeared after exposure to arecoline. | [141] |
| Integrity of genomic DNA (electrophoresis) | Human gingival fibroblasts and buccal epithelial cells | Stainless-steel brackets, nanoparticle-coated brackets, and polymeric-coated brackets exposed to corrosion eluates | The number of cellular alterations was insignificant. | [132] |
| Human gingival fibroblasts | Silver tungstate microcrystals | DNA integrity was not affected. | [62] |
Abbreviations: BAPO (phenyl-bis(acyl) phosphine oxide); BAPP (2,2-bis[4-(acryloxypropoxy) phenyl] propane); DPIC (diphenyliodonium chloride); HEMA (2-hydroxylmethyl methacrylate); Neopen (neopentylglycol dimethacrylate); TEEGDMA (tetraethyleneglycol dimethacrylate); TEGDMA (triethyleneglycol dimethacrylate); TPO (diphenyl(acyl) phosphine oxide); TPP (triphenylphosphane); and TPSB (triphenyl-stibane).
3.4. Oxidative Stress
Another mechanism involved in oral tissue damage is oxidative stress. The oral cavity is considered a part of the human body that is especially susceptible to oxidative damage, as there are many sources of free radicals in the oral environment (food, xenobiotics, microorganisms, dental materials and treatments, and local inflammatory processes). The saliva contains both enzymatic and nonenzymatic antioxidants (e.g., superoxide dismutase, catalase, glutathione peroxidase, salivary peroxidase, transferrin, and lactoferrin) [142]. Oxidative stress is defined as the disruption of the balance between the free radicals produced (reactive oxygen and nitrogen species) and the capability of antioxidant systems to counteract them. Oxidative damage affects all cellular components (lipids, proteins, and nucleic acids), and can lead to oral and systemic diseases [143]. Oxidative stress is a key element involved in the progression of periodontal diseases and chronic inflammation. Free radicals’ overproduction is associated not only with the loss of periodontal tissue integrity, but also with morphological alterations in the salivary glands, or with the appearance of precancerous lesions or oral cancers [142].
Animal and cell cultures have been used to study oxidative stress effects and antioxidant mechanisms. Still, experiments conducted on live animals are more difficult to interpret due to the complexity of tissues and organs’ organization and functioning. Therefore, cell culture models are widely used as models of oxidative stress [144]. One method to evaluate oxidative stress is to determine the intracellular levels of free radicals by evaluating the intracellular oxidation of 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF) to 2′,7′-dichlorofluorescein (DCF) and measuring fluorescence by flow cytometry [145]. Other assays focus on assessing the antioxidant defense system [the concentration of reduced glutathione, the activity, and the expression of antioxidant enzymes (e.g., glutathione peroxidase, catalase, and heme oxygenase)]. The Western blot assay, a method based on polyacrylamide gel electrophoresis, is frequently used to assess antioxidant enzymes [144]. In order to maintain a stable intracellular redox state, the expression of these enzymes is modified as an adaptive cell response in an attempt to maintain the homeostatic concentrations of reactive oxygen species (ROS). The expression of several antioxidant enzymes becomes a reflection of the pro-oxidant–antioxidant balance and oxidative stress and can be measured using qRT-PCR (quantitative real-time PCR) [145].
The high reactivity and the short half-life of reactive oxygen species make their quantification difficult. Under these circumstances, the evaluation is based on the quantification of biomarkers of oxidative damage to molecules (e.g., malondialdehyde for lipid peroxidation, 8-hydroxy-2′-deoxyguanosine for DNA oxidative damage, or protein carbonylation) (Figure 5) [144,146,147].
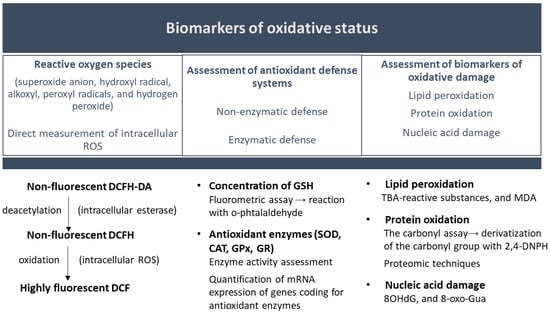
Figure 5.
Oxidative status assessment. Abbreviations: CAT (catalase); DCF (2′,7′-dichlorofluorescein); DCFH-DA (dichloro-dihydro-fluorescein diacetate); DNPH (2,4-dinitrophenylhydrazine); GPx (glutathione peroxidase family); GR (glutathione reductase); GSH (glutathione); MDA (malondialdehyde); SOD (superoxide dismutase); TBA (thiobarbituric acid); 8OHdG (8-hydroxy-2′-deoxyguanosine); and 8-oxo-Gua (8-oxoguanine).
Several techniques can be used to determine protein carbonylation. The simplest and most accessible method is the Levine spectrophotometric assay, based on the chromogenic reaction between carbonyl groups and 2,4-dinitrophenylhydrazine. 2,4-dinitrophenylhydrazine derivatization of protein carbonyls is also used in other techniques, like High Performance Liquid Chromatography (HPLC), immunoblotting (using anti-DNP antibodies), or ELISA. Although it does not give quantitative data, the immunoblot detection of carbonyl groups (Oxyblot) is used especially in cell culture studies due to its sensitivity and specificity. However, if a more detailed insight into the mechanism of protein oxidative damage is required, proteomic techniques are necessary [147,148].
Dental adhesives are involved in the induction of oxidative stress. Several research teams studied the effects on cellular redox homeostasis of photoinitiators for photo-polymerizable dental resinous materials. Conventionally, camphorquinone (CQ) is used, but there are also alternatives (e.g., phenyl-bis(acyl) phosphine oxide (BAPO) and diphenyl(acyl) phosphine oxide (TPO)). The researchers assessed the oxidative stress in oral cells by the quantification of the intracellular formation of reactive oxygen/nitrogen species (ROS/RNS) or by determining the mRNA expression levels of oxidative stress genes and discovered that the intracellular free-radical-generating activity of BAPO and TPO was significantly lower compared to CQ [30,145].
Biocompatibility and toxicity associated with dental alloys used in restorative dentistry have represented popular research topics. Several corrosion products can be released in the oral cavity, and this phenomenon can affect the health of the human oral tissues. Metal ions are frequently produced via corrosion, and one of the cellular effects is the increased formation of ROS and oxidative stress. Lee et al. studied the effects of indium ions on oral keratinocytes by detecting the intracellular ROS formation. Intracellular production of reactive oxygen species is a complex process, with several pathways being involved (the mitochondrial respiratory cycle, the NADPH oxidase, and the xanthine-oxidase pathways). Furthermore, increased production of ROS can influence the terminal differentiation of oral keratinocytes, a vital process in preserving the barrier of oral mucosa [47].
Other examples of studies investigating cellular redox homeostasis in oral cells are presented in Table 5.

Table 5.
Selection of studies assessing oxidative stress in human oral cells during cytotoxicity testing.
3.5. Inflammatory Response
Inflammation is a natural phenomenon that can be induced by various factors (infectious, physical, chemical, and autoimmune), but when it is extended or prolonged, can lead to cellular damage. Inflammatory response assessment in host cells is a key element for biocompatibility and toxicity evaluation because it can represent the starting point for toxic effects and cell damage [153].
Cytokines are soluble factors, proteins with a low molecular weight, involved in orchestrating the inflammatory response, and their quantification represents an indicator of the cellular inflammatory status. There are several categories of cytokines: tumor necrosis factors (TNFs), interleukins (ILs), lymphokines, monokines, interferons (IFNs), colony-stimulating factors (CSFs), and transforming growth factors (TGFs). Some cytokines are pro-inflammatory, while others are anti-inflammatory [154]. IL-6 is a pro-inflammatory cytokine produced by various oral cell types during the early stages of inflammation, but its dysregulated synthesis can initiate a chronic inflammatory status. Prostaglandins (e.g., PGE2) are synthesized by the cyclooxygenases COX-1 and COX-2 from arachidonic acid. COX-2 overexpression causes the cyclooxygenase pathways of inflammation [155,156]. Furthermore, activated oral cells can also secret matrix metalloproteinases (MMPs), proteolytic enzymes whose overexpression and uncontrolled release and activation can lead to inflammation and tissue destruction [157].
To assess inflammatory responses, several markers (e.g., pro-inflammatory cytokines interleukins, TNF-α, and IFN-γ) can be quantified using ELISA, but transcriptional analysis can also evaluate the expression levels of key genes associated with cellular inflammation (Table 6) [33].

Table 6.
Selection of studies assessing the inflammatory response in human oral cells during cytotoxicity testing.
4. Three-Dimensional Cell Culture Models
Although 2D cell culture models are still the most commonly used tools for in vitro studies, they exhibit several limitations. As 2D cell culture systems partially fail to replicate the architecture and physiology of living tissues, 3D models were designed to simulate better in vivo conditions (cell differentiation, gene and protein expression, and cell-to-cell communication). In monolayered cultures, the tested compounds must be dissolved in the aqueous culture medium since the cell cultures are submerged. This inconvenience disappears for 3D models cultured at the air–liquid interface; with cells placed into cell culture inserts, tissue feeding is achieved via a basal microporous membrane, and the apical tissue surface is not submerged in culture medium, which allows the direct application of xenobiotics [167,168]. Another disadvantage that appears when monolayer cell cultures are used in toxicity and exposure risk assessment studies is the direct access of xenobiotics to the proliferating cells, in contrast with in vivo systems that present an epithelial barrier [168].
Different three-dimensional oral models, histologically and biologically similar to human oral tissues, have been developed since 1997, and have been used to determine the oral mucosal irritation and cytotoxicity of oral care products and dental materials, but also to study drug delivery via the oral mucosa, oral pathology, and response to tobacco products or UV radiation (Table 7) [168].
Two types of 3D models can be developed—full- and split-thickness models. The split-thickness models comprise only the epithelium barrier (keratinocytes placed directly on permeable membranes at the liquid–air interface) and are usually used for permeation studies. The full-thickness models include a connective tissue layer beside the epithelial layer, and, usually, the connective layer represents a 3D collagen scaffold, infiltrated with fibroblast. These full-thickness models more effectively recapture the complex structural and functional characteristics of the native tissue [169].
AlFatlawi et al. published a comprehensive systematic review regarding the use of 3D gingival and mucosal models in periodontal research. They concluded that there is a lack of standardization regarding the fabrication and characterization of 3D systems and a high degree of heterogeneity among models. According to their study, most researchers used rat tail collagen as substrates for the construction of the models, but bovine collagen, acellular cadaveric dermis, and porcine collagen were also utilized. Primary human gingival keratinocytes and fibroblasts, or immortalized cell lines, were used as the cellular source, and the number of epithelial cell layers varied between 4 and 16. Most models were constructed under static cell culture conditions. The authors emphasized the importance of developing a standardized characterization protocol to confirm the validity of 3D oral models, one that includes histological confirmation and evidence of the differentiation of tissue layers using specific markers [167].
Some models are currently commercially available: the human oral mucosal epithelium models [EpiOralTM (MatTeK Corporation, Ashland, MA, USA) and SkinEthic Human Oral Epithelium (Episkin, Lyon, France)] and the human oral gingival epithelium models [EpiGingivalTM (MatTeK Corporation, Ashland, MA, USA) and SkinEthic Human Gingival Epithelium (Episkin, Lyon, France)] [170,171]. DentCytoTool is a 3D dentin/pulp tissue analog used for the cytotoxicity and biocompatibility assessment of dental restorative materials. It contains two compartments: the dentin/odontoblastic layer (the upper compartment) and the lower compartment representing a pulp analog [172].

Table 7.
Selection of studies using 3D models for toxicity assessment.
Table 7.
Selection of studies using 3D models for toxicity assessment.
| Type of 3D Model | Investigated Parameter | Tested Agent | Results | Reference |
|---|---|---|---|---|
| Dental materials and oral healthcare formulations | ||||
| Human gingival epithelial culture model EpiGingival™ | Cell viability and retention of o-cymen-5-ol and zinc | Toothpaste with o-cymen-5-ol and zinc | No cytotoxic effect appeared and the delivery of o-cymen-5-ol and zinc was successful. | [173] |
| Human oral epithelial culture model EpiOral™ | Tissue viability and cytokine release | Prototype oral care formulations | The models were useful to screen the irritation effect of oral products. | [174] |
| Three-dimensional human oral mucosal model | Histology, tissue viability, and IL-1β release | Dental composite resins | TEGDMA-based composite resin increased IL-1β release and caused mucotoxicity. | [175] |
| Human oral epithelial culture model EpiOral™ | Tissue viability, histology, and caffeine permeability | Caffeine, ethanol, and mouthrinses | Ethanol-containing mouthwashes did not exhibit a cytotoxic effect on the mucosal tissue; the permeability of caffeine was not affected. | [176] |
| Human oral epithelial culture model EpiOral™ | Viability, cytokine release, levels of protein markers for apoptosis, DNA damage, cell proliferation, cell–cell adhesion | Mucoadhesive polymer blend (pullulan, tamarindus indica polysaccharide, and sodium hyaluronate) | No cytotoxic effect was observed and the cytokine levels were not modified; there was no indication of DNA damage. | [177] |
| Reconstituted human oral epithelial tissue models (3D) (SkinEthic Laboratories) | Cell viability and morphology | Point-welded, laser-welded, and silver-soldered orthodontic wires | Viability and histological evaluation did not reveal signs of severe toxicity. | [178] |
| Oral mucosa tissue model (3D) | Transepithelial electrical resistance, cell viability, and cytokine secretion | Mouth rinsing solutions | Cell viability was not affected, but only one solution had no impact on TEER. One product increased IL 8 secretion. | [179] |
| DentCytoTool | Viability, cytotoxicity, and odontogenesis- and angiogenesis-related markers | HEMA; TEGDMA | The cytotoxic potential increased in a time- and concentration-dependent manner, and the expression of angiogenesis-related markers was reduced; the LPS/TEGDMA co-treatment aggravated the cytotoxic effects. | [172] |
| Pulp analogue (3D) | Cell viability/proliferation, morphology, and expression of angiogenic and odontogenic markers | Calcium-silicate-based cements | The materials were biocompatible, with positive angiogenic and odontogenic effects. | [180] |
| Oral mucosal models | Cytotoxicity, histological, and permeation analyses | Mucoadhesive patches with clobetasol-17-propionate | No tissue damage was recorded. | [181] |
| Nicotine, ENDS, and tobacco products | ||||
| Human oral epithelial culture model EpiOral™ | Cytotoxicity, histological analysis, cytochrome P450 activity, production of pro-inflammatory mediators, and transcriptomics | Cigarette smoke and aerosols obtained from a modified-risk tobacco product | Cytotoxicity, morphological alterations, production of inflammatory mediators, and transcriptomic changes were significantly more pronounced for cigarette smoke. | [182] |
| Human gingival epithelial culture model EpiGingival™ | Cytotoxicity, histological analysis, cytochrome P450 activity, production of pro-inflammatory mediators, transcriptomics, and metabolomics | Cigarette smoke and aerosols obtained from a modified-risk tobacco product | The aerosol from the modified-risk tobacco product caused minimal cytotoxicity and minor histopathological alterations, and had a low impact on transcriptomic and metabolomic data. | [183] |
| Human gingival epithelial culture model EpiGingival™ | Pro-inflammatory cytokine levels, inflammation and DNA damage markers (RAGE, COX-2, and γH2A.X) | E-cigarette aerosols | E-cigarette exposure augmented inflammation and DNA damage markers. | [138] |
| Human oral epithelial culture model EpiOral™ | Viability, morphology, and tissue absorption | E-cigarette aerosol | No decrease in viability and no change in morphology and tissue structure were observed. | [184] |
| Human oral epithelial culture model EpiOral™ Human gingival epithelial culture model EpiGingival™ | Cytotoxicity (adenylate kinase assay), histology, inflammatory mediator secretion, transcriptomics, and targeted proteomics | Cigarette smoke and a carbon-heated tobacco-product aerosol | The carbon-heated tobacco-product aerosol exhibited a significantly lower biological impact in comparison to cigarette smoke. | [185] |
| Human gingival epithelial culture model EpiGingival™ | Irritant potential | Extracts from nicotine pouch products | The tested products were not irritants. | [186] |
| Oral mucosa tissue model (3D) with normal fibroblasts and cancerous TR146 keratinocytes | Histological examination and viability | E-cigarette liquids | Prolonged exposure and high concentrations of e-liquids caused cytotoxic effects for normal cells but stimulated the growth of cancerous cells. | [187] |
| Other agents | ||||
| Gingival culture system (3D) | DNA analysis | Sodium butyrate | Sodium butyrate induced DNA release in a time- and dose-dependent manner, and stimulated the release of SAP130. | [188] |
| Human oral epithelial culture model EpiOral™ | Cell viability and histological analysis | Sodium lauryl sulfate | EpiOral is a suitable system for oral mucosal irritation test. | [170] |
| Human gingival epithelial culture model EpiGingival™ Human oral epithelial culture model EpiOral™ | DNA damage, CPD repair rate, and apoptotic cell numbers | UV radiation | UVB radiation presented a higher carcinogenic risk for oral tissues. | [189] |
| Human gingival epithelial culture model EpiGingival™ Human oral epithelial culture model EpiOral™ | Cytokine release, DNA damage, CPD repair rate, and apoptotic cell numbers | UVB radiation | Increased interleukin-8 (IL-8) release, low rates of CPD repair, and decreased apoptotic cell numbers. | [190] |
| Cell culture model (3D) (gingival fibroblasts in collagen matrix) | Cell viability and morphology, and gene expression of growth factors | Low-level laser therapy | Low-level laser therapy increased viability and promoted biostimulation of gingival fibroblasts. | [191] |
| Reconstituted human oral epithelial tissue models (3D) (EpiSkin Laboratories) | Cell viability and morphology | Carrageenan | No loss of viability was recorded for the carrageenan-treated sample. | [31] |
| Human gingival epithelial culture model EpiGingival™ | Cytotoxicity, viability, histology, immunohistochemistry (Ki67, VEGF-A), and TUNEL assay | Low-temperature plasma | Low-temperature plasma had low cytotoxicity and high cellular viability. | [192] |
| Human gingival epithelial culture model EpiGingival™ | Viability | Lipophilic vehicles | No signs of cytotoxicity were recorded. | [193] |
Abbreviations: COX-2 (cyclooxygenase-2); CPD (cyclobutane pyrimidine dimer); γH2A.X (phosphorylated form of the histone variant H2AX); LPS (lipopolysaccharides); RAGE (receptor for advanced glycation end); SAP130 (Sin3A-associated protein 130 kDa); TEGDMA (triethyleneglycol dimethacrylate); TEER (transepithelial electrical resistance); and VEGF-A (vascular endothelial growth factor-A).
As presented in Table 7, the assessment of toxicity and biocompatibility using 3D oral models can be performed using the same parameters as in 2D cell culture studies (cell viability and proliferation, morphological changes, genotoxicity assessment, oxidative stress, and inflammatory response).
4.1. Cell Viability
Evaluating cell viability and proliferation remains a key aspect of cytotoxicity assessment for 3D cellular systems.
Most studies performed on 3D oral models have used the MTT assay to assess the metabolic activity of the cellular population and cytotoxicity [173,176,178,179,180,181,184,186,193]. The MTT assay also allows indirect measurement of the xenobiotics’ effect on the barrier function of the tissue [174]. Other researchers have used resazurin-based assays to evaluate cell viability (the alamarBlue assay [175] and PrestoBlue assay [187]), the advantages being the lack of toxicity of the dye (allowing continuous monitoring) and the greater sensitivity of detection. The quantification of lactate dehydrogenase (LDH assay) [193] and the adenylate kinase test (which detects ATP generated from ADP and the final product is determined colorimetrically or fluorometrically) [182,183,185] were also used for the evaluation of cell viability. To evaluate the safety of a mucoadhesive polymer blend, cell proliferation markers PCNA, Cyclin A, and Cyclin D1 were determined in EpiOral tissue models [177].
However, the transition of the standardized viability assays from 2D to 3D systems can be challenging due to the structural differences and the more sophisticated architecture of the 3D models, leading to inaccurate results and misleading conclusions [194].
Dominijanni et al. investigated the accuracy of cell viability assays in 3D hydrogel systems. They pointed out that indirect viability assays (the MTS assay, PrestoBlue fluorometric assay, CellTiter-Glo luminescent assay, and measuring ATP) developed for 2D monolayer cultures presented significant differences when used in 3D hydrogels. Several factors can influence performance, like the construct size, the hydrogels’ formulation, porosity, and the diffusion process, causing some reagents or metabolites to become trapped in the 3D construct, affecting the final results. The direct viability methods (BrU incorporation and FACS—fluorescence-activated cell sorting) give more reliable results. Direct evaluation via histological techniques (live/dead assay by fluorescence microscopy) offers a good alternative, the stained histological sections indicating healthy and apoptotic cells and their spatial distribution in the 3D model, but it is time-consuming. To obtain the best results, indirect viability assays can be validated via direct microscope techniques [195].
4.2. Morphological Changes
Exposure to xenobiotics can alter the tissue morphology, and for 3D models, this aspect can be analyzed using a histological examination with light microscopy after performing hematoxylin and eosin staining. Tissues (epithelia) react to harmful and irritating agents through morphological adaptations, while highly toxic compounds cause tissue damage. Various parameters can be investigated: cell morphology, the continuity and thickness of the epithelium, keratinization, the aspect of the connective tissue layer, and the presence of pyknotic nuclei [187].
Signs of morphological alteration of the human oral and gingival epithelial culture model (hyperkeratinization, keratohyalin granules, and the accumulation of nuclei in the stratum corneum) were visible after exposure to cigarette smoke [182,183,185].
4.3. Genotoxicity Assessment
The International Workshop on Genotoxicity Testing Working Group (IWGT WG), one of the leading groups in regulatory genotoxicology, has analyzed the topic of genotoxicity testing using 3D models and agreed that 3D systems are more relevant than 2D assays to evaluate human genotoxicity because the 3D models are based on human cells. Three-dimensional assays are more difficult to perform, more expensive, and time-consuming, but they also address all the endpoints of genotoxicity mentioned for 2D cell culture systems (mutagenicity, clastogenicity, and aneugenicity). So far, 3D-skin comet and micronucleus assays have gained sufficient validation to support the development of individual OECD Test Guidelines [196].
Three-dimensional oral and gingival models also proved useful for genotoxicity assessment. Sundar et al. evaluated the DNA-damaging effect of e-cigarette vapors using the comet assay and γ-H2AX levels [138]. Furthermore, to assess the genotoxicity of UV radiation for oral tissues, Agrawal et al. and Breger et al. determined DNA damage (cyclobutane pyrimidine dimers), the repair rate of cyclobutane pyrimidine dimers, and the number of apoptotic cells [189,190].
4.4. Oxidative Stress and Inflammatory Response
In vitro studies of toxicological processes using 3D oral models can also investigate the induced oxidative stress and inflammatory response.
Transcriptomics and metabolomics can reveal the changes induced by oxidative stress. The adaptative response is reflected in the alteration of the expression of genes involved in the reactive oxygen species pathway, but also in the perturbation of the metabolite profile, in an attempt to counteract the oxidative challenge. For example, cigarette smoke influenced the perturbation of metabolites in the glutathione pathway [182,183].
An increase in cytokines, chemokines, and growth factors released from oral epithelial models can be a response to exposure to dental materials, oral hygiene formulations, or other xenobiotics. To quantify the secreted inflammatory mediators [tumor necrosis factor α (TNFα), colony-stimulating factor (CSF2, CSF3), interleukin (IL-6, IL-8, IL-1A, IL-1B), chemokine (CXCL8, CXCL10), matrix metalloproteinase (MMP-1, MMP-9), and vascular endothelial growth factor alpha (VEGFA)], the ELISA technique [175,179] or Luminex xMAP technology can be used, which allows simultaneous identification and quantitation via a flow-based methodology that uses a dual-laser system and color-coded beads with unique spectral codes. The inflammatory response is also visible in the changes that appear in the expression of inflammation-related genes [182,183].
To sum up, 3D oral tissue models are valid and accurate tools for in vitro studies and present many advantages when compared to 2D monolayer cultures, and also to animal models. The structures and functions of oral human native tissues are closely reproduced, and some issues associated with animal testing (e.g., species extrapolation and ethical problems) are avoided when using tissue models.
5. Organ-on-a-Chip Systems
The continuous search for more human-relevant in vitro models is directed towards the development of models that not only can capture and recreate organ structures and tissue–tissue interfaces using multiple cell layers, but also mimic the functionality of an organ. Organ-on-a-chip devices are in vitro micro-physiological systems, and, so far, represent the best in vitro tools for toxicity prediction [197,198,199,200].
These devices use microfluidic technology to allow human cells and tissues to be cultured under dynamic fluid flow. They recreate the architecture of an organ but also mimic vascular perfusion, interstitial flow, and the complex interactions that occur in live tissues. Furthermore, these systems can replicate the immune response by circulating immune cells through the vascular endothelium-lined channel, and the complex living microbiome can be co-cultured with human cells. This is a big step forward since there is substantial scientific evidence that supports the important role that the microbiome has in both human physiological and pathological processes [197,199].
Since the beginning of the 21st century, when microfluidic technology was adapted for the development of in vitro organ models, multiple organ-on-a-chip systems were developed, covering almost all the organs in the human body (lung, liver, kidney, gut, skin, brain/blood–brain barrier, bone/bone marrow, fat, muscle, and retina) [200].
This technology was also used in dental research, although the organ-on-a-chip field is underdeveloped in this research domain compared to other areas. However, different types of microphysiological systems have been designed and used so far in dental and oral research. One-chamber design chips, with a unique culture chamber connected to channels for the transport of fluids, are usually used to simulate the impact of the oral environment (e.g., salivary flow) on biofilm formation. Parallel-chamber chips, with two or more parallel chambers interconnected, are the most used models to simulate the architecture of natural tissues and are usually used in cytotoxicity testing [201,202].
Research conducted to evaluate the oral toxicity of different agents has pointed out that in some monolayer cell culture experiments, the cytotoxic effect was overestimated due to the direct contact between the tested compounds and oral cells. The oral cavity environment is complex, influenced by processes like salivary flow and microbial colonization, with the gingival epithelium and dentin acting as barriers against chemicals and toxins. Furthermore, dental tissues have a unique characteristic, combining soft (dental pulp) and mineralized tissues (dentin, enamel), which poses extra challenges for accurate in vitro testing. Organ-on-a-chip models offer an alternative closer to in vivo conditions and actual clinical practice [203,204,205].
The first attempts to recreate the natural oral environment date back to the 1980s, when barrier models for dental pulp studies were designed. Today, the dentin barrier cytotoxicity test (ISO 7405 [206]) is used to assess the biocompatibility of dental materials. At the beginning of the 21st century, a new ex vivo model was developed, an entire human tooth culture, but the static conditions (pH and CO2/O2 exchange) were its main limitations [205].
Regarding toxicity testing on oral cavity cells, the biomaterials used for dental treatment occupy first place in biocompatibility and toxicity assessment. França et al. were the first to develop a “tooth-on-a-chip”. They designed a microfluidic chip of the pulp–dentin–biomaterials interface to test the cytotoxicity of dental materials on pulp cells. The chip has the great advantage of closely recapturing the physiological microenvironment at the pulp–dentin interface and enabling live-cell imaging to study the morphological profile of dental pulp cells exposed to dental materials [207].
Zhang et al. constructed a dentin-on-a-chip device using microfluidic chip technology by encapsulating stem cells from the apical papilla in gelatin methacrylate [208]. Muniraj et al. created a gingiva-on-a-chip model with a multi-chamber design and a vertically stacked configuration of polymethyl methacrylate sheets. Primary human gingival fibroblasts and human oral keratinocytes were used as cell cultures and upper and lower channels were created to deliver media, air, chemicals, or nutrients and to collect the metabolic waste [204]. Jin et al. designed a gingival epithelium–capillary interface-on-a-chip to study the inflammation of the periodontal soft tissue and the response to therapeutic agents [209].
There are several advantages of using organ-on-a-chip models as in vitro testing and monitoring tools. The main advantage include the ability to obtain cellular structures and dynamic experimental conditions similar to in vivo ones. Regarding oral and dental research, different scenarios of mechanical stress were recreated using these models (e.g., orthodontic forces, periodontal ligament–alveolar bone interface environment, or gradient flow in gingival crevices). Furthermore, these micro-sized culture chambers allow cost-effective parallelization for high-throughput experiments [201].
To assess morphological changes, cell viability, and proliferation of cell cultures on-chip, the technology currently used is light-phase and fluorescence imaging. The design of the organ-on-a-chip systems is made from optically transparent materials and is compatible with direct visualization in real time. Therefore, live/dead staining can be used to detect cellular viability. H–E (hematoxylin and eosin)-stained images give information regarding the integrity of various structures (e.g., the gingival epithelial layer), some of the histological parameters investigated being desquamation, cellular vacuolization, or loss of intercellular cohesion [203,207,209]. Furthermore, fluorescent dyes or antibodies can be used to evaluate the expression of certain biomarkers (e.g., immunostaining was used to observe the expression of the proteins F-actin and VE-cadherin) [209]. Makkar et al. also evaluated the tissue viability depending on the lactate dehydrogenase (LDH) release for his “gingival crevice-on-a-chip” microfluidic platform [210].
The organ-on-a-chip models allow the collection of media and the quantification of different biomarkers, evaluating the inflammatory response of the host tissue. Makkar et al. observed that microfluidic devices better mimic in vivo inflammatory events because, under traditional static experimental conditions, an over-estimation of the innate immune response can occur [210].
Examples of studies using organ-on-a-chip systems for toxicity and biocompatibility are presented in Table 8.

Table 8.
Selection of studies using organ-on-a-chip models for toxicity assessment.
6. In Vitro Models for Toxicity Assessment: Advantages, Challenges, and Future Perspectives
Studying cell populations is an essential aspect of research in many domains, from drug discovery, toxicity assessment, cancer research, or other areas of research trying to offer some insight into other pathological mechanisms. Two-dimensional cell cultures have been used for decades and are valuable, simple, and inexpensive screening tools with multiple applications and highly replicable results, suitable for large-scale studies. However, as a monolayer of cells grown on a plate, the natural environment is not recreated; cell proliferation is unnaturally fast, and differentiation is poor. Furthermore, testing drug response or toxicity can be inaccurate due to higher substance-induced apoptosis rates [214].
With 3D cell culture systems, the environment better mimics the in vivo conditions in terms of cell shape, differentiation and proliferation rates, as well as the expression levels of genes and proteins. Nevertheless, there are disadvantages associated with this technique. It is more expensive and time-consuming, the experiments are harder to replicate, and some assays established for 2D cultures must be validated for 3D models to ensure correct and accurate results [214].
As the techniques for tissue engineering have advanced, organ-on-a-chip systems have emerged, models that replicate tissue functionality and represent a bridge that can cover the gap between traditional in vitro studies and animal models. They represent predictable models for human physiological and pathological processes and recreate natural barriers, being more accurate tools for assessing bioactivity and toxicity. Another advantage is the possibility for continuous real-time measurements and visualization due to the various sensors that can be adapted to the model. The main challenges associated with this technology are the costs and the need for model standardization. The current designs are characterized by a high degree of heterogeneity, making it difficult to carry out comparative analyses [198,201,215,216].
Despite the limitations of these new microfluidic devices, future advancements will probably overcome them, bringing researchers closer to achieving the “human-on-a-chip” concept.
7. Conclusions
The oral cavity is a major site for toxicity manifestation, as it comes into contact with several xenobiotics (dental materials, oral hygiene formulations, drugs, or tobacco products). Assessing toxicity for oral cavity tissues is challenging due to the complex structure of the tissues and the particular physiology. Most studies still apply traditional 2D cell culture systems despite their limitations. However, the emergence of new technologies (3D cell cultures and organ-on-a-chip models) offers the possibility to investigate physiological and pathological processes in a dynamic and complex environment, resembling in vivo scenarios. Continued research is directed towards overcoming the difficulties associated with these innovative techniques, leading to a rapid evolution of the in vitro methods used in this research area. We expect future research to focus mainly on organ-on-a-chip systems designed to closely mimic the anatomy and physiology of oral cavity tissues.
Author Contributions
Conceptualization, A.J. and L.A.; methodology, M.V.; validation, L.V. and I.M.; investigation, F.-D.C. and I.-C.C.; data curation, I.-C.C.; writing—original draft preparation, A.J., M.V. and L.A.; writing—review and editing, I.M.; visualization, F.-D.C.; supervision, L.V.; and project administration, A.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 5-CFDA-AM | 5-carboxyfluorescein diacetate acetoxymethyl ester |
| 3H-TdR | tritiated thymidine |
| 8OHdG | 8-hydroxy-2′-deoxyguanosine |
| 8-oxo-Gua | 8-oxoguanine |
| ATP | adenosine triphosphate |
| BAPO | phenyl-bis(acyl) phosphine oxide |
| BAPP | 2,2-bis[4-(acryloxypropoxy) phenyl] propane |
| BAX | Bcl-2-associated X protein |
| BisGMA | bisphenol A-glycidylmethacrylate |
| BrdU | 5-bromo-2′-deoxyuridine |
| C | catechin |
| CAPE | caffeic acid phenethyl ester |
| CAT | catalase |
| CG | catechin gallate |
| CLFPs | cytoplasm-localized fluorescent probes |
| CPD | cyclobutane pyrimidine dimer |
| CQ | camphorquinone |
| CRP1.1 | a smokeless tobacco reference product—snus |
| CSFs | colony-stimulating factors |
| DCF | 2′,7′-dichlorofluorescein |
| DPIC | diphenyliodonium chloride |
| DNPH | 2,4-dinitrophenylhydrazine |
| EC | epicatechin |
| ECG | epicatechin gallate |
| EGC | epigallocatechin |
| EGCG | epigallocatechin gallate |
| ENDS | electronic nicotine delivery systems |
| EdU | 5-ethynyl-2-deoxyuridine |
| Er:YAG | erbium-doped yttrium aluminum garnet |
| FACS | fluorescence-activated cell sorting |
| G6PD | glucose-6-phosphate dehydrogenase |
| GPx | glutathione peroxidase family |
| GR | glutathione reductase |
| GSH | glutathione |
| H2DCF | 2′,7′-dichlorodihydrofluorescein diacetate |
| HEMA | 2-hydroxy-ethyl-methacrylate |
| IWGT WG | the International Workshop on Genotoxicity Testing Working Group |
| LDH | lactate dehydrogenase |
| LPS | lipopolysaccharides |
| MDA | malondialdehyde |
| MIP | macrophage inflammatory proteins |
| MMPs | matrix metalloproteinases |
| MTS | dimethylthiazolcarboxymethoxyphenylsulfophenyl-tetrazolium |
| MTT | methylthiazolyldiphenyltetrazolium bromide |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NRU | neutral red uptake |
| PARP1 | poly-(ADP-ribose) polymerase-1 |
| PCNA | proliferating cell nuclear antigen |
| PHH3 | phosphohistone H3 |
| RAGE | receptor for advanced glycation end |
| RIPK1 | receptor interacting serine/threonine kinase 1 |
| SAP130 | Sin3A associated protein 130 kDa |
| SDF-1 | stromal cell-derived factor 1 |
| SOD | superoxide dismutase |
| SRB | sulforhodamine B |
| TBA | thiobarbituric acid |
| TEGD-MA | triethyleneglycol-dimethacrylate |
| TEEGDMA | tetraethyleneglycol dimethacrylate |
| TEER | transepithelial electrical resistance |
| TGFs | transforming growth factors |
| TNFα | tumor necrosis factor α |
| TPO | diphenyl(acyl) phosphine oxide |
| TPSB | triphenyl-stibane |
| TPP | triphenylphosphane |
| UDMA | urethane dimethacrylate |
| VEGF | vascular endothelial growth factor |
| WST | water-soluble tetrazolium salt |
| XTT | methoxynitrosulfophenyl-tetrazolium carboxanilide |
References
- Poh, W.T.; Stanslas, J. The new paradigm in animal testing—“3Rs alternatives”. Regul. Toxicol. Pharmacol. 2024, 153, 105705. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Srivastava, G.K.; Alonso-Alonso, M.L.; Fernandez-Bueno, I.; Garcia-Gutierrez, M.T.; Rull, F.; Medina, J.; Coco, R.M.; Pastor, J.C. Comparison between direct contact and extract exposure methods for PFO cytotoxicity evaluation. Sci. Rep. 2018, 8, 1425. [Google Scholar] [CrossRef]
- Liu, X.; Rodeheaver, D.P.; White, J.C.; Wright, A.M.; Walker, L.M.; Zhang, F.; Shannon, S. A comparison of in vitro cytotoxicity assays in medical device regulatory studies. Regul. Toxicol. Pharmacol. 2018, 97, 24–32. [Google Scholar] [CrossRef]
- Gruber, S.; Nickel, A. Toxic or not toxic? The specifications of the standard ISO 10993-5 are not explicit enough to yield comparable results in the cytotoxicity assessment of an identical medical device. Front. Med. Technol. 2023, 5, 1195529. [Google Scholar] [CrossRef]
- Schwarze, T.; Fiedler, I.; Leyhausen, G.; Geurtsen, W. The cellular compatibility of five endodontic sealers during the setting period. J. Endod. 2002, 28, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Badole, G.P.; Warhadpande, M.M.; Meshram, G.K.; Bahadure, R.N.; Tawani, S.G.; Tawani, G.; Badole, S.G. A comparative evaluation of cytotoxicity of root canal sealers: An in vitro study. Restor. Dent. Endod. 2013, 38, 204–209. [Google Scholar] [CrossRef]
- Kumar, A.; Kour, S.; Kaul, S.; Malik, A.; Dhani, R.; Kaul, R. Cytotoxicity evaluation of Bio-C, CeraSeal, MTA—Fillapex, and AH Plus root canal sealers by microscopic and 3-(4, 5 dimethythiazol-2yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. J. Conserv. Dent. 2023, 26, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Sazonova, E.V.; Chesnokov, M.S.; Zhivotovsky, B.; Kopeina, G.S. Drug toxicity assessment: Cell proliferation versus cell death. Cell Death Discov. 2022, 8, 417. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual [Internet]; Markossian, S., Grossman, A., Arkin, M., Auld, D., Austin, C., Baell, J., Brimacombe, K., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK53196/ (accessed on 20 November 2024).
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Aslantürk, Ö.S. In Vitro Cytotoxicity and Cell Viability Assays: Principles, Advantages, and Disadvantages [Internet]. In Genotoxicity—A Predictable Risk to Our Actual World; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Adan, A.; Kiraz, Y.; Baran, Y. Cell Proliferation and Cytotoxicity Assays. Curr. Pharm. Biotechnol. 2016, 17, 1213–1221. [Google Scholar] [CrossRef]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. In Cell Viability Assays. Methods in Molecular Biology; Gilbert, D., Friedrich, O., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1601. [Google Scholar]
- Ates, G.; Vanhaecke, T.; Rogiers, V.; Rodrigues, R.M. Assaying Cellular Viability Using the Neutral Red Uptake Assay. In Cell Viability Assays. Methods in Molecular Biology; Gilbert, D., Friedrich, O., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1601. [Google Scholar]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Duellman, S.J.; Zhou, W.; Meisenheimer, P.; Vidugiris, G.; Cali, J.J.; Gautam, P.; Wennerberg, K.; Vidugiriene, J. Bioluminescent, Nonlytic, Real-Time Cell Viability Assay and Use in Inhibitor Screening. Assay. Drug Dev. Technol. 2015, 13, 456–465. [Google Scholar] [CrossRef]
- Drescher, H.; Weiskirchen, S.; Weiskirchen, R. Flow Cytometry: A Blessing and a Curse. Biomedicines 2021, 9, 1613. [Google Scholar] [CrossRef]
- Cummings, B.S.; Schnellmann, R.G. Measurement of Cell Death in Mammalian Cells. Curr. Protoc. 2021, 1, e210. [Google Scholar] [CrossRef]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Flomerfelt, F.A.; Gress, R.E. Analysis of Cell Proliferation and Homeostasis Using EdU Labeling. Methods Mol. Biol. 2016, 1323, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Salic, A.; Mitchison, T.J. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 2415–2420. [Google Scholar] [CrossRef]
- Boehm, E.M.; Gildenberg, M.S.; Washington, M.T. The Many Roles of PCNA in Eukaryotic DNA Replication. Enzymes 2016, 39, 231–254. [Google Scholar] [CrossRef]
- Stefanowicz-Hajduk, J.; Ochocka, J.R. Real-time cell analysis system in cytotoxicity applications: Usefulness and comparison with tetrazolium salt assays. Toxicol. Rep. 2020, 7, 335–344. [Google Scholar] [CrossRef]
- Urcan, E.; Haertel, U.; Styllou, M.; Hickel, R.; Scherthan, H.; Reichl, F.X. Real-time xCELLigence impedance analysis of the cytotoxicity of dental composite components on human gingival fibroblasts. Dent. Mater. 2010, 26, 51–58. [Google Scholar] [CrossRef]
- Larsson, P.; Engqvist, H.; Biermann, J.; Werner Rönnerman, E.; Forssell-Aronsson, E.; Kovács, A.; Karlsson, P.; Helou, K.; Parris, T.Z. Optimization of cell viability assays to improve replicability and reproducibility of cancer drug sensitivity screens. Sci. Rep. 2020, 10, 5798. [Google Scholar] [CrossRef] [PubMed]
- Frigaard, J.; Liaaen Jensen, J.; Kanli Galtung, H.; Hiorth, M. Stability and cytotoxicity of biopolymer-coated liposomes for use in the oral cavity. Int. J. Pharm. 2023, 645, 123407. [Google Scholar] [CrossRef] [PubMed]
- Basso, F.G.; Pansani, T.N.; de Oliveira, C.F.; Turrioni, A.P.; Soares, D.G.; Hebling, J.; Costa, C.A. Cytotoxic effects of zoledronic acid on human epithelial cells and gingival fibroblasts. Braz. Dent. J. 2013, 24, 551–558. [Google Scholar] [CrossRef]
- Modena, K.C.D.S.; Calvo, A.M.; Sipert, C.R.; Dionísio, T.J.; Navarro, M.F.L.; Atta, M.T.; Santos, C.F. Dental Pulp Fibroblasts Response after Stimulation with HEMA and Adhesive System. Braz. Dent. J. 2018, 29, 419–426. [Google Scholar] [CrossRef][Green Version]
- Popal, M.; Volk, J.; Leyhausen, G.; Geurtsen, W. Cytotoxic and genotoxic potential of the type I photoinitiators BAPO and TPO on human oral keratinocytes and V79 fibroblasts. Dent. Mater. 2018, 34, 1783–1796. [Google Scholar] [CrossRef]
- Alli, B.Y.; Upadhyay, A.; Zhang, Y.; Nicolau, B.; Tran, S.D. In Vitro Toxicity Evaluation of Carrageenan on Cells and Tissues of the Oral Cavity. Mar. Drugs 2022, 20, 502. [Google Scholar] [CrossRef] [PubMed]
- Best, M.; Phillips, G.; Fowler, C.; Rowland, J.; Elsom, J. Characterisation and cytotoxic screening of metal oxide nanoparticles putative of interest to oral healthcare formulations in non-keratinised human oral mucosa cells in vitro. Toxicol. In Vitro 2015, 30 Pt B, 402–411. [Google Scholar] [CrossRef]
- Pagano, S.; Lombardo, G.; Costanzi, E.; Balloni, S.; Bruscoli, S.; Flamini, S.; Coniglio, M.; Valenti, C.; Cianetti, S.; Marinucci, L. Morpho-functional effects of different universal dental adhesives on human gingival fibroblasts: An in vitro study. Odontology 2021, 109, 524–539. [Google Scholar] [CrossRef]
- Sampath Kumar, L.; Shantha, V.; Janardhana, K. Cell viability studies using human gingival fibroblast, human oral fibroblast cells and mechanical characterization of Calcite/Zincite nanoparticles. World J. Adv. Res. Rev. 2022, 16, 812–824. [Google Scholar] [CrossRef]
- Nirwana, I.; Munadziroh, E.; Yogiartono, R.M.; Thiyagu, C.; Ying, C.S.; Dinaryanti, A. Cytotoxicity and proliferation evaluation on fibroblast after combining calcium hydroxide and ellagic acid. J. Adv. Pharm. Technol. Res. 2021, 12, 27–31. [Google Scholar] [CrossRef]
- Strauss, F.J.; Stähli, A.; Beer, L.; Mitulović, G.; Gilmozzi, V.; Haspel, N.; Schwab, G.; Gruber, R. Acid bone lysate activates TGFβ signalling in human oral fibroblasts. Sci. Rep. 2018, 8, 16065. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.G.; Basso, F.G.; Hebling, J.; de Souza Costa, C.A. Concentrations of and application protocols for hydrogen peroxide bleaching gels: Effects on pulp cell viability and whitening efficacy. J. Dent. 2014, 42, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Boza, Y.; Yefi, R.; Rudolph, M.I.; Smith, P.C.; Oberyszyn, T.M.; Tober, K.L.; Rojas, I.G. Single Exposure of Human Oral Mucosa Fibroblasts to Ultraviolet B Radiation Reduces Proliferation and Induces COX-2 Expression and Activation. Rev. Clín. Periodoncia Implant. Rehabil. Oral 2010, 3, 123–127. [Google Scholar] [CrossRef]
- Ramenzoni, L.L.; Schneider, A.; Fox, S.C.; Meyer, M.; Meboldt, M.; Attin, T.; Schmidlin, P.R. Cytotoxic and Inflammatory Effects of Electronic and Traditional Cigarettes on Oral Gingival Cells Using a Novel Automated Smoking Instrument: An In Vitro Study. Toxics 2022, 10, 179. [Google Scholar] [CrossRef]
- Alanazi, H.; Park, H.J.; Chakir, J.; Semlali, A.; Rouabhia, M. Comparative study of the effects of cigarette smoke and electronic cigarettes on human gingival fibroblast proliferation, migration and apoptosis. Food Chem. Toxicol. 2018, 118, 390–398. [Google Scholar] [CrossRef]
- Cicchetti, R.; Divizia, M.; Valentini, F.; Argentin, G. Effects of single-wall carbon nanotubes in human cells of the oral cavity: Geno-cytotoxic risk. Toxicol. In Vitro 2011, 25, 1811–1819. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.-G.; Liang, W.-M.; Wu, T.-C.; Chen, S.-Y. Evaluation of cytotoxicity of resin bonding materials toward human oral epithelial cells using three assay systems. J. Dent. Sci. 2009, 4, 178–186. [Google Scholar] [CrossRef]
- San Miguel, S.M.; Opperman, L.A.; Allen, E.P.; Zielinski, J.; Svoboda, K.K. Bioactive antioxidant mixtures promote proliferation and migration on human oral fibroblasts. Arch. Oral Biol. 2011, 56, 812–822. [Google Scholar] [CrossRef]
- Silva, D.; Arancibia, R.; Tapia, C.; Acuña-Rougier, C.; Diaz-Dosque, M.; Cáceres, M.; Martínez, J.; Smith, P.C. Chitosan and platelet-derived growth factor synergistically stimulate cell proliferation in gingival fibroblasts. J. Periodontal Res. 2013, 48, 677–686. [Google Scholar] [CrossRef]
- Soares, A.S.L.S.; Scelza, M.Z.; Spoladore, J.; Gallito, M.A.; Oliveira, F.; Moraes, R.C.M.; Alves, G.G. Comparison of primary human gingival fibroblasts from an older and a young donor on the evaluation of cytotoxicity of denture adhesives. J. Appl. Oral Sci. 2018, 26, e20160594. [Google Scholar] [CrossRef]
- Taubmann, A.; Willershausen, I.; Walter, C.; Al-Maawi, S.; Kaina, B.; Gölz, L. Genotoxic and cytotoxic potential of methacrylate-based orthodontic adhesives. Clin. Oral Investig. 2021, 25, 2569–2581. [Google Scholar] [CrossRef]
- Lee, J.H.; Seo, S.H.; Lee, S.B.; Om, J.Y.; Kim, K.M.; Kim, K.N. Cytotoxicity and terminal differentiation of human oral keratinocyte by indium ions from a silver-palladium-gold-indium dental alloy. Dent. Mater. 2015, 31, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Aoki, A.; Iwasaki, K.; Mizutani, K.; Katagiri, S.; Suda, T.; Ichinose, S.; Ogita, M.; Pavlic, V.; Izumi, Y. Biological effects of Er:YAG laser irradiation on the proliferation of primary human gingival fibroblasts. J. Biophotonics 2018, 11, e201700157. [Google Scholar] [CrossRef]
- Alaufi, O.M.; Noorwali, A.; Zahran, F.; Al-Abd, A.M.; Al-Attas, S. Cytotoxicity of thymoquinone alone or in combination with cisplatin (CDDP) against oral squamous cell carcinoma in vitro. Sci. Rep. 2017, 7, 13131. [Google Scholar] [CrossRef] [PubMed]
- Babich, H.; Sinensky, M.C. Indirect cytotoxicity of dental materials: A study with Transwell inserts and the neutral red uptake assay. Altern. Lab. Anim. 2001, 29, 9–13. [Google Scholar] [CrossRef]
- Tavares, S.J.d.O.; Caldas, I.P.; Gonçalves, F.P.; Scelza, P.; Oliveira, F.; Alves, G.; Scelza, M.F.Z. Cytotoxicity in fibroblasts from young and elderly donors from two mouthwashes used to prevent the spread of SARS-CoV-2. Res. Soc. Dev. 2021, 10, e56810414587. [Google Scholar] [CrossRef]
- Babich, H.; Krupka, M.E.; Nissim, H.A.; Zuckerbraun, H.L. Differential in vitro cytotoxicity of (-)-epicatechin gallate (ECG) to cancer and normal cells from the human oral cavity. Toxicol. In Vitro 2005, 19, 231–242. [Google Scholar] [CrossRef]
- Babich, H.; Pinsky, S.M.; Muskin, E.T.; Zuckerbraun, H.L. In vitro cytotoxicity of a theaflavin mixture from black tea to malignant, immortalized, and normal cells from the human oral cavity. Toxicol. In Vitro 2006, 20, 677–688. [Google Scholar] [CrossRef]
- Tellez, C.S.; Juri, D.E.; Phillips, L.M.; Do, K.; Yingling, C.M.; Thomas, C.L.; Dye, W.W.; Wu, G.; Kishida, S.; Kiyono, T.; et al. Cytotoxicity and Genotoxicity of E-Cigarette Generated Aerosols Containing Diverse Flavoring Products and Nicotine in Oral Epithelial Cell Lines. Toxicol. Sci. 2021, 179, 220–228. [Google Scholar] [CrossRef]
- Gao, H.; Prasad, G.L.; Zacharias, W. Differential cell-specific cytotoxic responses of oral cavity cells to tobacco preparations. Toxicol. In Vitro 2013, 27, 282–291. [Google Scholar] [CrossRef]
- Szkaradkiewicz, A.K.; Karpiński, T.M.; Szkaradkiewicz, A. Effect of novobiocin on the viability of human gingival fibroblasts (HGF-1). BMC Pharmacol. Toxicol. 2014, 15, 25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, F.C.; Huang, C.M.; Yu, X.W.; Chen, Y.Y. Effect of nano zinc oxide on proliferation and toxicity of human gingival cells. Hum. Exp. Toxicol. 2022, 41, 9603271221080236. [Google Scholar] [CrossRef]
- Rouabhia, M.; Park, H.J.; Semlali, A.; Zakrzewski, A.; Chmielewski, W.; Chakir, J. E-Cigarette Vapor Induces an Apoptotic Response in Human Gingival Epithelial Cells Through the Caspase-3 Pathway. J. Cell Physiol. 2017, 232, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Aati, S.; Shrestha, B.; Fawzy, A. Cytotoxicity and antimicrobial efficiency of ZrO2 nanoparticles reinforced 3D printed resins. Dent. Mater. 2022, 38, 1432–1442. [Google Scholar] [CrossRef]
- Poulsen, C.; Mehalick, L.A.; Fischer, C.L.; Lanzel, E.A.; Bates, A.M.; Walters, K.S.; Cavanaugh, J.E.; Guthmiller, J.M.; Johnson, G.K.; Wertz, P.W.; et al. Differential cytotoxicity of long-chain bases for human oral gingival epithelial keratinocytes, oral fibroblasts, and dendritic cells. Toxicol. Lett. 2015, 237, 21–29. [Google Scholar] [CrossRef]
- Adil, N.; Ali, H.; Siddiqui, A.J.; Ali, A.; Ahmed, A.; El-Seedi, H.R.; Musharraf, S.G. Evaluation of cytotoxicity of areca nut and its commercial products on normal human gingival fibroblast and oral squamous cell carcinoma cell lines. J. Hazard. Mater. 2021, 403, 123872. [Google Scholar] [CrossRef] [PubMed]
- Haro Chávez, N.L.; de Avila, E.D.; Barbugli, P.A.; de Oliveira, R.C.; de Foggi, C.C.; Longo, E.; Vergani, C.E. Promising effects of silver tungstate microcrystals on fibroblast human cells and three dimensional collagen matrix models: A novel non-cytotoxic material to fight oral disease. Colloids Surf. B Biointerfaces 2018, 170, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Willershausen, I.; Wolf, T.; Weyer, V.; Sader, R.; Ghanaati, S.; Willershausen, B. Influence of E-smoking liquids on human periodontal ligament fibroblasts. Head. Face Med. 2014, 10, 39. [Google Scholar] [CrossRef]
- Ji, E.H.; Sun, B.; Zhao, T.; Shu, S.; Chang, C.H.; Messadi, D.; Xia, T.; Zhu, Y.; Hu, S. Characterization of Electronic Cigarette Aerosol and Its Induction of Oxidative Stress Response in Oral Keratinocytes. PLoS ONE 2016, 11, e0154447, Erratum in PLoS ONE 2016, 11, e0169380. https://doi.org/10.1371/journal.pone.0169380. [Google Scholar] [CrossRef]
- Bagchi, M.; Balmoori, J.; Bagchi, D.; Ray, S.D.; Kuszynski, C.; Stohs, S.J. Smokeless tobacco, oxidative stress, apoptosis, and antioxidants in human oral keratinocytes. Free Radic. Biol. Med. 1999, 26, 992–1000, Erratum in Free Radic. Biol. Med. 1999, 26, 1599. [Google Scholar] [CrossRef]
- Cotrim, P.; Martelli-Junior, H.; Graner, E.; Sauk, J.J.; Coletta, R.D. Cyclosporin A induces proliferation in human gingival fibroblasts via induction of transforming growth factor-beta1. J. Periodontol. 2003, 74, 1625–1633. [Google Scholar] [CrossRef]
- Thomsen, A.R.; Aldrian, C.; Luka, B.; Hornhardt, S.; Gomolka, M.; Moertl, S.; Hess, J.; Zitzelsberger, H.; Heider, T.; Schlueter, N.; et al. Biopsy-derived oral keratinocytes—A model to potentially test for oral mucosa radiation sensitivity. Clin. Transl. Radiat. Oncol. 2022, 34, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.A.; Pham, T.A.V. Effects of platelet-rich plasma on human gingival fibroblast proliferation and migration in vitro. J. Appl. Oral Sci. 2018, 26, e20180077. [Google Scholar] [CrossRef] [PubMed]
- Trivedy, C.; Meghji, S.; Warnakulasuriya, K.A.; Johnson, N.W.; Harris, M. Copper stimulates human oral fibroblasts in vitro: A role in the pathogenesis of oral submucous fibrosis. J. Oral Pathol. Med. 2001, 30, 465–470. [Google Scholar] [CrossRef]
- Tipton, D.A.; Dabbous, M.K. Effects of nicotine on proliferation and extracellular matrix production of human gingival fibroblasts in vitro. J. Periodontol. 1995, 66, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Wyganowska-Swiatkowska, M.; Kotwicka, M.; Urbaniak, P.; Nowak, A.; Skrzypczak-Jankun, E.; Jankun, J. Clinical implications of the growth-suppressive effects of chlorhexidine at low and high concentrations on human gingival fibroblasts and changes in morphology. Int. J. Mol. Med. 2016, 37, 1594–1600. [Google Scholar] [CrossRef]
- Robertson, J.D.; Orrenius, S. Molecular mechanisms of apoptosis induced by cytotoxic chemicals. Crit. Rev. Toxicol. 2000, 30, 609–627. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Galkowski, D.; Zhao, H. Analysis of apoptosis by cytometry using TUNEL assay. Methods 2008, 44, 250–254. [Google Scholar] [CrossRef]
- Payne, A.M.; Zorman, J.; Horton, M.; Dubey, S.; ter Meulen, J.; Vora, K.A. Caspase activation as a versatile assay platform for detection of cytotoxic bacterial toxins. J. Clin. Microbiol. 2013, 51, 2970–2976. [Google Scholar] [CrossRef]
- Huang, F.-M.; Kuan, Y.-H.; Lee, S.-S.; Chang, Y.-C. Caspase activation by a zinc-oxide eugenol-based root-canal sealer in cementoblasts. J. Dent. Sci. 2015, 10, 338–340. [Google Scholar] [CrossRef]
- Kaushal, V.; Herzog, C.; Haun, R.S.; Kaushal, G.P. Caspase protocols in mice. Methods Mol. Biol. 2014, 1133, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Venegas, G.; Guadarrama-Solís, A.; Muñoz-Seca, C.; Arreguín-Cano, J.A. Hydrogen peroxide-induced apoptosis in human gingival fibroblasts. Int. J. Clin. Exp. Pathol. 2015, 8, 15563–15572. [Google Scholar] [PubMed]
- Khalil, A.M.; Alemam, I.F.; Al-Qaoud, K.M. Association between Mobile Phone Using and DNA Damage of Epithelial Cells of the Oral Mucosa. J. Biotechnol. Biomed. 2020, 3, 50–66. [Google Scholar] [CrossRef]
- Kang, S.W.; Park, H.J.; Ban, J.Y.; Chung, J.H.; Chun, G.S.; Cho, J.O. Effects of nicotine on apoptosis in human gingival fibroblasts. Arch. Oral Biol. 2011, 56, 1091–1097. [Google Scholar] [CrossRef]
- Vermehren, M.F.; Wiesmann, N.; Deschner, J.; Brieger, J.; Al-Nawas, B.; Kämmerer, P.W. Comparative analysis of the impact of e-cigarette vapor and cigarette smoke on human gingival fibroblasts. Toxicol. In Vitro 2020, 69, 105005. [Google Scholar] [CrossRef]
- Karube, H.; Nishitai, G.; Inageda, K.; Kurosu, H.; Matsuoka, M. NaF activates MAPKs and induces apoptosis in odontoblast-like cells. J. Dent. Res. 2009, 88, 461–465. [Google Scholar] [CrossRef]
- López-García, S.; Pecci-Lloret, M.P.; Pecci-Lloret, M.R.; Guerrero-Gironés, J.; Rodríguez-Lozano, F.J.; García-Bernal, D. Topical fluoride varnishes promote several biological responses on human gingival cells. Ann. Anat. 2021, 237, 151723. [Google Scholar] [CrossRef]
- Staudte, H.; Güntsch, A.; Völpel, A.; Sigusch, B.W. Vitamin C attenuates the cytotoxic effects of Porphyromonas gingivalis on human gingival fibroblasts. Arch. Oral Biol. 2010, 55, 40–45. [Google Scholar] [CrossRef]
- Atsumi, T.; Tonosaki, K.; Fujisawa, S. Induction of early apoptosis and ROS-generation activity in human gingival fibroblasts (HGF) and human submandibular gland carcinoma (HSG) cells treated with curcumin. Arch. Oral Biol. 2006, 51, 913–921. [Google Scholar] [CrossRef]
- Puig-Herreros, C.; Sanz, J.L.; García-Bernal, D.; Rodríguez-Lozano, F.J.; Murcia, L.; Forner, L.; Ghilotti, J.; Oñate-Sánchez, R.E.; López-García, S. Comparative Cytotoxicity of Menthol and Eucalyptol: An In Vitro Study on Human Gingival Fibroblasts. Pharmaceutics 2024, 16, 521. [Google Scholar] [CrossRef] [PubMed]
- Sulek, J.; Luczaj-Cepowicz, E.; Marczuk-Kolada, G.; Rosłan, M.; Holownia, A. Cytotoxicity of Methacrylate Dental Resins to Human Gingival Fibroblasts. J. Funct. Biomater. 2022, 13, 56. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Huang, Z.; Ge, L.; Shi, J. N-acetylcysteine as a novel methacrylate-based resin cement component: Effect on cell apoptosis and genotoxicity in human gingival fibroblasts. BMC Oral Health 2024, 24, 222. [Google Scholar] [CrossRef]
- Masuki, K.; Nomura, Y.; Bhawal, U.K.; Sawajiri, M.; Hirata, I.; Nahara, Y.; Okazaki, M. Apoptotic and necrotic influence of dental resin polymerization initiators in human gingival fibroblast cultures. Dent. Mater. J. 2007, 26, 861–869. [Google Scholar] [CrossRef]
- Lefeuvre, M.; Amjaad, W.; Goldberg, M.; Stanislawski, L. TEGDMA induces mitochondrial damage and oxidative stress in human gingival fibroblasts. Biomaterials 2005, 26, 5130–5137. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, I.; McCall, M.N. Systematic exploration of cell morphological phenotypes associated with a transcriptomic query. Nucleic Acids Res. 2018, 46, e116. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Alizadeh, E. Cell Form and Function: Interpreting and Controlling the Shape of Adherent Cells. Trends Biotechnol. 2019, 37, 347–357. [Google Scholar] [CrossRef]
- Pincus, Z.; Theriot, J.A. Comparison of quantitative methods for cell-shape analysis. J. Microsc. 2007, 227 Pt 2, 140–156. [Google Scholar] [CrossRef]
- Lai, F.; Shen, Z.; Wen, H.; Chen, J.; Zhang, X.; Lin, P.; Yin, D.; Cui, H.; Chen, X. A Morphological identification cell cytotoxicity assay using cytoplasm-localized fluorescent probe (CLFP) to distinguish living and dead cells. Biochem. Biophys. Res. Commun. 2017, 482, 257–263. [Google Scholar] [CrossRef]
- Son, R.; Yamazawa, K.; Oguchi, A.; Suga, M.; Tamura, M.; Yanagita, M.; Murakawa, Y.; Kume, S. Morphomics via next-generation electron microscopy. J. Mol. Cell Biol. 2024, 15, mjad081. [Google Scholar] [CrossRef]
- Available online: https://www.the-scientist.com/enhancing-cell-morphology-based-analysis-71249 (accessed on 25 November 2024).
- Available online: https://www.drugdiscoverytrends.com/deepcells-rem-i-platform-marries-ai-with-morphological-analysis-for-drug-discovery (accessed on 25 November 2024).
- Tromans-Coia, C.; Jamali, N.; Abbasi, H.S.; Giuliano, K.A.; Hagimoto, M.; Jan, K.; Kaneko, E.; Letzsch, S.; Schreiner, A.; Sexton, J.Z.; et al. Assessing the performance of the Cell Painting assay across different imaging systems. Cytom. A 2023, 103, 915–926. [Google Scholar] [CrossRef]
- Bray, M.A.; Gustafsdottir, S.M.; Rohban, M.H.; Singh, S.; Ljosa, V.; Sokolnicki, K.L.; Bittker, J.A.; Bodycombe, N.E.; Dancík, V.; Hasaka, T.P.; et al. A dataset of images and morphological profiles of 30,000 small-molecule treatments using the Cell Painting assay. Gigascience 2017, 6, giw014. [Google Scholar] [CrossRef]
- Bray, M.A.; Singh, S.; Han, H.; Davis, C.T.; Borgeson, B.; Hartland, C.; Kost-Alimova, M.; Gustafsdottir, S.M.; Gibson, C.C.; Carpenter, A.E. Cell Painting, a high-content image-based assay for morphological profiling using multiplexed fluorescent dyes. Nat. Protoc. 2016, 11, 1757–1774. [Google Scholar] [CrossRef] [PubMed]
- Nyffeler, J.; Willis, C.; Harris, F.R.; Foster, M.J.; Chambers, B.; Culbreth, M.; Brockway, R.E.; Davidson-Fritz, S.; Dawson, D.; Shah, I.; et al. Application of Cell Painting for chemical hazard evaluation in support of screening-level chemical assessments. Toxicol. Appl. Pharmacol. 2023, 468, 116513. [Google Scholar] [CrossRef] [PubMed]
- Willis, C.; Nyffeler, J.; Harrill, J. Phenotypic Profiling of Reference Chemicals across Biologically Diverse Cell Types Using the Cell Painting Assay. SLAS Discov. 2020, 25, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Way, G.P.; Kost-Alimova, M.; Shibue, T.; Harrington, W.F.; Gill, S.; Piccioni, F.; Becker, T.; Shafqat-Abbasi, H.; Hahn, W.C.; Carpenter, A.E.; et al. Predicting cell health phenotypes using image-based morphology profiling. Mol. Biol. Cell 2021, 32, 995–1005. [Google Scholar] [CrossRef]
- Beacham, D.; Mandavilli, B.; Holly, R.; Chambers, K.; Oon, C.; Mohr, B.; Hu, Y.-Z.; Yan, J. New-Generation, Functional Probes for Caspase 3/7 and Mitochondrial Superoxide and Ready-Use Phenotypic Cell Painting Tools. Thermo Fisher Scientific, Eugene, Oregon, USA. Available online: https://assets.thermofisher.com/TFS-Assets/BID/posters/caspase-3-7-mitochondrial-superoxide-cell-painting-poster.pdf (accessed on 15 January 2025).
- Belton, C.M.; Izutsu, K.T.; Goodwin, P.C.; Park, Y.; Lamont, R.J. Fluorescence image analysis of the association between Porphyromonas gingivalis and gingival epithelial cells. Cell Microbiol. 1999, 1, 215–223. [Google Scholar] [CrossRef]
- Sunny, S.P.; Khan, A.I.; Rangarajan, M.; Hariharan, A.; Birur N, P.; Pandya, H.J.; Shah, N.; Kuriakose, M.A.; Suresh, A. Oral epithelial cell segmentation from fluorescent multichannel cytology images using deep learning. Comput. Methods Programs Biomed. 2022, 227, 107205. [Google Scholar] [CrossRef]
- Akner, G.; Mossberg, K.; Wikström, A.C.; Sundqvist, K.G.; Gustafsson, J.A. Evidence for colocalization of glucocorticoid receptor with cytoplasmic microtubules in human gingival fibroblasts, using two different monoclonal anti-GR antibodies, confocal laser scanning microscopy and image analysis. J. Steroid Biochem. Mol. Biol. 1991, 39, 419–432. [Google Scholar] [CrossRef]
- Al-Nazhan, S.; Spangberg, L. Morphological cell changes due to chemical toxicity of a dental material: An electron microscopic study on human periodontal ligament fibroblasts and L929 cells. J. Endod. 1990, 16, 129–134. [Google Scholar] [CrossRef]
- Cecato, R.C.; Martinez, E.F.; Benfatti, C.A.M. Analysis of the Viability and Morphology of Gingival Cells on Materials Used in Novel Prosthetic Components: In Vitro Study. J. Contemp. Dent. Pract. 2022, 23, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, H.; Sen, E.; Dalcik, H.; Meseli, S.E. Evaluation of cell morphology and adhesion capacity of human gingival fibroblasts on titanium discs with different roughened surfaces: An in vitro scanning electron microscope analysis and cell culture study. Folia Morphol. 2023, 82, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Esfahanizadeh, N.; Motalebi, S.; Daneshparvar, N.; Akhoundi, N.; Bonakdar, S. Morphology, proliferation, and gene expression of gingival fibroblasts on Laser-Lok, titanium, and zirconia surfaces. Lasers Med. Sci. 2016, 31, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.P.; Jeng, J.H.; Hsieh, C.C.; Lin, C.L.; Lei, D.; Chang, M.C. Morphological alterations associated with the cytotoxic and cytostatic effects of citric acid on cultured human dental pulp cells. J. Endod. 1999, 25, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Noites, R.; Tavares, I.; Cardoso, M.; Carreira, I.M.; Bartolomeu, M.; Duarte, A.S.; Ribeiro, I.P. Human Gingival Fibroblasts Response to Different Endodontic Sealers: An In Vitro Study. Appl. Sci. 2023, 13, 10976. [Google Scholar] [CrossRef]
- Saramet, V.; Stan, M.S.; Ripszky Totan, A.; Țâncu, A.M.C.; Voicu-Balasea, B.; Enasescu, D.S.; Rus-Hrincu, F.; Imre, M. Analysis of Gingival Fibroblasts Behaviour in the Presence of 3D-Printed versus Milled Methacrylate-Based Dental Resins-Do We Have a Winner? J. Funct. Biomater. 2024, 15, 147. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.Y.; Ha, D.H.; Jeong, J.H.; Park, J.B. Effects of Valproic Acid on Morphology, Proliferation, and Differentiation of Mesenchymal Stem Cells Derived from Human Gingival Tissue. Implant. Dent. 2018, 27, 33–42. [Google Scholar] [CrossRef]
- Ha, D.H.; Yong, C.S.; Kim, J.O.; Jeong, J.H.; Park, J.B. Effects of tacrolimus on morphology, proliferation and differentiation of mesenchymal stem cells derived from gingiva tissue. Mol. Med. Rep. 2016, 14, 69–76. [Google Scholar] [CrossRef]
- Messer, R.L.; Bishop, S.; Lucas, L.C. Effects of metallic ion toxicity on human gingival fibroblasts morphology. Biomaterials 1999, 20, 1647–1657. [Google Scholar] [CrossRef]
- Jeong, S.H.; Lee, J.E.; Jin, S.H.; Ko, Y.; Park, J.B. Effects of Asiasari radix on the morphology and viability of mesenchymal stem cells derived from the gingiva. Mol. Med. Rep. 2014, 10, 3315–3319. [Google Scholar] [CrossRef]
- Dusinska, M.; Rundén-Pran, E.; Schnekenburger, J.; Kanno, J. Toxicity Tests: In Vitro and In Vivo. In Adverse Effects of Engineered Nanomaterials, 2nd ed.; Fadeel, B., Pietroiusti, A., Shvedova, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 51–82. [Google Scholar]
- Corvi, R.; Madia, F. In vitro genotoxicity testing-Can the performance be enhanced? Food Chem. Toxicol. 2017, 106 Pt B, 600–608. [Google Scholar] [CrossRef]
- Sommer, S.; Buraczewska, I.; Kruszewski, M. Micronucleus Assay: The State of Art, and Future Directions. Int. J. Mol. Sci. 2020, 21, 1534. [Google Scholar] [CrossRef] [PubMed]
- Beedanagari, S. Genetic Toxicology. In Comprehensive Medicinal Chemistry III; Chackalamannil, S., Rotella, D., Ward, S.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 195–203. [Google Scholar]
- Ivashkevich, A.; Redon, C.E.; Nakamura, A.J.; Martin, R.F.; Martin, O.A. Use of the γ-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett. 2012, 327, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.J.; Yang, L.X. Gamma-H2AX—A novel biomarker for DNA double-strand breaks. In Vivo 2008, 22, 305–309. [Google Scholar] [PubMed]
- Lottner, S.; Shehata, M.; Hickel, R.; Reichl, F.-X.; Durner, J. Effects of antioxidants on DNA-double strand breaks in human gingival fibroblasts exposed to methacrylate based monomers. Dent. Mater. 2013, 29, 991–998. [Google Scholar] [CrossRef]
- Carvalho, N.C.; Guedes, S.A.G.; Albuquerque-Júnior, R.L.C.; de Albuquerque, D.S.; de Souza Araújo, A.A.; Paranhos, L.R.; Camargo, S.E.A.; Ribeiro, M.A.G. Analysis of Aloe vera cytotoxicity and genotoxicity associated with endodontic medication and laser photobiomodulation. J. Photochem. Photobiol. B 2018, 178, 348–354. [Google Scholar] [CrossRef]
- Rode, S.M.; Sato, T.D.P.; Matos, F.S.; Correia, A.M.O.; Camargo, S.E.A. Toxicity and effect of whitening toothpastes on enamel surface. Braz. Oral Res. 2021, 35, e025. [Google Scholar] [CrossRef] [PubMed]
- Yagci, F.; Kesim, B. Cytotoxic and genotoxic effects on gingival fibroblasts from static magnetic fields produced by dental magnetic attachments. Gerodontology 2016, 33, 421–427. [Google Scholar] [CrossRef]
- Ferreira, E.C.C.; Gonçalves, T.M.; Pereira, T.C.; Hasna, A.A.; Oliveira, F.E.d.; Jorjão, A.L.; Camargo, S.E.A.; Oliveira, L.D.d.; Spalding, M. The biocompatibility of Achyrocline satureioides plant extract over human gingival fibroblasts. Res. Soc. Dev. 2021, 10, e37610111902. [Google Scholar] [CrossRef]
- Yang, M.L. The effects of cytotoxicity and genotoxicity induced by 2,2-bis[4-(acryloxypropoxy)phenyl]propane via caspases in human gingival fibroblasts. Toxicol. Ind. Health 2014, 30, 755–764. [Google Scholar] [CrossRef]
- Loyola-Rodríguez, J.P.; Lastra-Corso, I.; García-Cortés, J.O.; Loyola-Leyva, A.; Domínguez-Pérez, R.A.; Avila-Arizmendi, D.; Contreras-Palma, G.; González-Calixto, C. In Vitro Determination of Genotoxicity Induced by Brackets Alloys in Cultures of Human Gingival Fibroblasts. J. Toxicol. 2020, 2020, 1467456, Erratum in J. Toxicol. 2021, 2021, 4028481. https://doi.org/10.1155/2021/4028481. [Google Scholar] [CrossRef]
- Ahuja, D.; Jose, N.P.; Kamal, R.; Panduranga, V.; Nambiar, S.; Isloor, A.M. In vitro determination of genotoxicity and cytotoxicity induced by stainless steel brackets with and without surface coating in cultures of oral mucosal cells. BMC Oral Health 2024, 24, 1233. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.J.; Fernández, E.; Vicente, A.; Calvo, J.L.; Ortiz, C. Metallic ions released from stainless steel, nickel-free, and titanium orthodontic alloys: Toxicity and DNA damage. Am. J. Orthod. Dentofac. Orthop. 2011, 140, e115–e122, Erratum in Am. J. Orthod. Dentofacial Orthop. 2018, 153, 765. https://doi.org/10.1016/j.ajodo.2018.03.012. [Google Scholar] [CrossRef]
- Tomakidi, P.; Koke, U.; Kern, R.; Erdinger, L.; Krüger, H.; Kohl, A.; Komposch, G. Assessment of acute cyto- and genotoxicity of corrosion eluates obtained from orthodontic materials using monolayer cultures of immortalized human gingival keratinocytes. J. Orofac. Orthop. 2000, 61, 2–19, English, German. [Google Scholar] [CrossRef] [PubMed]
- Volk, J.; Leyhausen, G.; Geurtsen, W. Glutathione level and genotoxicity in human oral keratinocytes exposed to TEGDMA. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Szczepanska, J.; Poplawski, T.; Synowiec, E.; Pawlowska, E.; Chojnacki, C.J.; Chojnacki, J.; Blasiak, J. 2-hydroxylethyl methacrylate (HEMA), a tooth restoration component, exerts its genotoxic effects in human gingival fibroblasts through methacrylic acid, an immediate product of its degradation. Mol. Biol. Rep. 2012, 39, 1561–1574. [Google Scholar] [CrossRef]
- Volk, J.; Ziemann, C.; Leyhausen, G.; Geurtsen, W. Non-irradiated campherquinone induces DNA damage in human gingival fibroblasts. Dent. Mater. 2009, 25, 1556–1563. [Google Scholar] [CrossRef]
- Sundar, I.K.; Javed, F.; Romanos, G.E.; Rahman, I. E-cigarettes and flavorings induce inflammatory and pro-senescence responses in oral epithelial cells and periodontal fibroblasts. Oncotarget 2016, 7, 77196–77204. [Google Scholar] [CrossRef]
- Laiteerapong, A.; Reichl, F.X.; Yang, Y.; Hickel, R.; HÖgg, C. Induction of DNA double-strand breaks in human gingival fibroblasts by eluates from titanium dioxide modified glass ionomer cements. Dent. Mater. 2018, 34, 282–287. [Google Scholar] [CrossRef]
- Shehata, M.; Durner, J.; Eldenez, A.; Van Landuyt, K.; Styllou, P.; Rothmund, L.; Hickel, R.; Scherthan, H.; Geurtsen, W.; Kaina, B.; et al. Cytotoxicity and induction of DNA double-strand breaks by components leached from dental composites in primary human gingival fibroblasts. Dent. Mater. 2013, 29, 971–979. [Google Scholar] [CrossRef]
- Chang, Y.C.; Tai, K.W.; Cheng, M.H.; Chou, L.S.; Chou, M.Y. Cytotoxic and non-genotoxic effects of arecoline on human buccal fibroblasts in vitro. J. Oral Pathol. Med. 1998, 27, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Żukowski, P.; Maciejczyk, M.; Waszkiel, D. Sources of free radicals and oxidative stress in the oral cavity. Arch. Oral Biol. 2018, 92, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Teoh, S.L.; Das, S.; Mahakknaukrauh, P. Oxidative Stress in Oral Diseases: Understanding Its Relation with Other Systemic Diseases. Front. Physiol. 2017, 8, 693. [Google Scholar] [CrossRef] [PubMed]
- Goya, L.; Martín, M.A.; Ramos, S.; Mateos, R.; Bravo, L. A cell culture model for the assessment of the chemopreventive potential of dietary compounds. Curr. Nutr. Food Sci. 2009, 5, 56–64. [Google Scholar] [CrossRef][Green Version]
- Van Landuyt, K.L.; Krifka, S.; Hiller, K.A.; Bolay, C.; Waha, C.; Van Meerbeek, B.; Schmalz, G.; Schweikl, H. Evaluation of cell responses toward adhesives with different photoinitiating systems. Dent. Mater. 2015, 31, 916–927. [Google Scholar] [CrossRef]
- Tejchman, K.; Kotfis, K.; Sieńko, J. Biomarkers and Mechanisms of Oxidative Stress-Last 20 Years of Research with an Emphasis on Kidney Damage and Renal Transplantation. Int. J. Mol. Sci. 2021, 22, 8010. [Google Scholar] [CrossRef]
- Alomari, E.; Bruno, S.; Ronda, L.; Paredi, G.; Bettati, S.; Mozzarelli, A. Protein carbonylation detection methods: A comparison. Data Brief. 2018, 19, 2215–2220. [Google Scholar] [CrossRef]
- Weber, D.; Davies, M.J.; Grune, T. Determination of protein carbonyls in plasma, cell extracts, tissue homogenates, isolated proteins: Focus on sample preparation and derivatization conditions. Redox Biol. 2015, 5, 367–380. [Google Scholar] [CrossRef]
- Krifka, S.; Seidenader, C.; Hiller, K.A.; Schmalz, G.; Schweikl, H. Oxidative stress and cytotoxicity generated by dental composites in human pulp cells. Clin. Oral Investig. 2012, 16, 215–224. [Google Scholar] [CrossRef]
- Chen, C.-S.; Lee, S.-S.; Yu, H.-C.; Huang, F.-M.; Chang, Y.-C. Effects of nicotine on cell growth, migration, and production of inflammatory cytokines and reactive oxygen species by cementoblasts. J. Dent. Sci. 2015, 10, 154–160. [Google Scholar] [CrossRef]
- Sancilio, S.; Gallorini, M.; Cataldi, A.; di Giacomo, V. Cytotoxicity and apoptosis induction by e-cigarette fluids in human gingival fibroblasts. Clin. Oral Investig. 2016, 20, 477–483. [Google Scholar] [CrossRef]
- Lleperuma, R.P.; Kim, D.K.; Park, Y.J.; Son, H.K.; Kim, J.Y.; Kim, J.; Lee, D.Y.; Kim, K.Y.; Jung, D.W.; Tilakaratne, W.M.; et al. Areca nut exposure increases secretion of tumor-promoting cytokines in gingival fibroblasts that trigger DNA damage in oral keratinocytes. Int. J. Cancer 2015, 137, 2545–2557. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Ombredane, A.S.; Pinheiro, W.O.; Andrade, L.R.; Silva, V.R.P.; Felice, G.J.; Alves, D.S.; Albernaz, A.F.; Silveira, A.P.; Lima, M.C.F.; et al. Evaluation of Biocompatibility, Anti-Inflammatory, and Antinociceptive Activities of Pequi Oil-Based Nanoemulsions in In Vitro and In Vivo Models. Nanomaterials 2022, 12, 4260. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, e2004433. [Google Scholar] [CrossRef] [PubMed]
- Kollmuss, M.; Edelhoff, D.; Schwendicke, F.; Wuersching, S.N. In Vitro Cytotoxic and Inflammatory Response of Gingival Fibroblasts and Oral Mucosal Keratinocytes to 3D Printed Oral Devices. Polymers 2024, 16, 1336. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.R.; Scherer, D.; Muir, K.; Schildkraut, J.; Boffetta, P.; Spitz, M.R.; Le Marchand, L.; Chan, A.T.; Goode, E.L.; Ulrich, C.M.; et al. A review of the application of inflammatory biomarkers in epidemiologic cancer research. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1729–1751. [Google Scholar] [CrossRef]
- Atanasova, T.; Stankova, T.; Bivolarska, A.; Vlaykova, T. Matrix Metalloproteinases in Oral Health-Special Attention on MMP-8. Biomedicines 2023, 11, 1514. [Google Scholar] [CrossRef]
- Trubiani, O.; Toniato, E.; Di Iorio, D.; Diomede, F.; Merciaro, I.; D’ Arcangelo, C.; Caputi, S. Morphological analysis and interleukin release in human gingival fibroblasts seeded on different denture base acrylic resins. Int. J. Immunopathol. Pharmacol. 2012, 25, 637–643. [Google Scholar] [CrossRef]
- Massaro, H.; Zambelli, L.F.A.; Britto, A.A.; Vieira, R.P.; Ligeiro-de-Oliveira, A.P.; Andia, D.C.; Oliveira, M.T.; Lima, A.F. Solvent and HEMA Increase Adhesive Toxicity and Cytokine Release from Dental Pulp Cells. Materials 2019, 12, 2750. [Google Scholar] [CrossRef]
- Rinaldi, S.; Pieper, E.; Schulz, T.; Zimmermann, R.; Luch, A.; Laux, P.; Mallock-Ohnesorg, N. Oral nicotine pouches with an aftertaste? Part 2: In vitro toxicity in human gingival fibroblasts. Arch. Toxicol. 2023, 97, 2343–2356. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Kłósek, M.; Pietsz, G.; Czuba, Z.P.; Kolayli, S.; Can, Z.; Balwierz, R.; Olczyk, P. The Phenolic Profile and Anti-Inflammatory Effect of Ethanolic Extract of Polish Propolis on Activated Human Gingival Fibroblasts-1 Cell Line. Molecules 2023, 28, 7477. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.I.; Kim, S.; Baek, S.M.; Choi, S.I.; Kim, G.H.; Imm, J.Y. Ecklonia cava Extract Exerts Anti-Inflammatory Effect in Human Gingival Fibroblasts and Chronic Periodontitis Animal Model by Suppression of Pro-Inflammatory Cytokines and Chemokines. Foods 2021, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Wu, B.; Hou, Z.; Xie, Q.; Liao, T.; Wang, T.; Ma, D. Asiatic acid inhibits LPS-induced inflammatory response in human gingival fibroblasts. Int. Immunopharmacol. 2017, 50, 313–318, Erratum in Int. Immunopharmacol. 2019, 72, 523. https://doi.org/10.1016/j.intimp.2019.01.042. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Choi, C.H. Anti-inflammatory effects of bamboo salt and sodium fluoride in human gingival fibroblasts—An in vitro study. Kaohsiung J. Med. Sci. 2015, 31, 303–308. [Google Scholar] [CrossRef]
- Wang, P.-L.; Tachi, Y.; Masuno, K.; Okusa, N.; Imamura, Y. The Study of Ozone Ointment on Human Gingival Fibroblasts Cell Proliferation Ability and Anti-Inflammatory. J. Hard Tissue Biol. 2018, 27, 209–212. [Google Scholar] [CrossRef]
- Ono, M.; Kantoh, K.; Ueki, J.; Shimada, A.; Wakabayashi, H.; Matsuta, T.; Sakagami, H.; Kumada, H.; Hamada, N.; Kitajima, M.; et al. Quest for anti-inflammatory substances using IL-1β-stimulated gingival fibroblasts. Vivo 2011, 25, 763–768. [Google Scholar]
- AlFatlawi, Z.; Huang, M.; Chau, D.Y.S.; D’Aiuto, F. Three dimensional (3D) gingival models in periodontal research: A systematic review. J. Mater. Sci. Mater. Med. 2023, 34, 58. [Google Scholar] [CrossRef]
- Klausner, M.; Handa, Y.; Aizawa, S. In vitro three-dimensional organotypic culture models of the oral mucosa. Vitro Cell Dev. Biol. Anim. 2021, 57, 148–159. [Google Scholar] [CrossRef]
- Riaz, A.; Gidvall, S.; Prgomet, Z.; Hernandez, A.R.; Ruzgas, T.; Nilsson, E.J.; Davies, J.; Valetti, S. Three-Dimensional Oral Mucosal Equivalents as Models for Transmucosal Drug Permeation Studies. Pharmaceutics 2023, 15, 1513. [Google Scholar] [CrossRef]
- Aizawa, S.; Yoshida, H.; Umeshita, K.; Watanabe, S.; Takahashi, Y.; Sakane, S.; Sakaguchi, H.; Kataoka, S. Development of an oral mucosal irritation test using a three-dimensional human buccal oral mucosal model. Toxicol. Vitro 2023, 87, 105519. [Google Scholar] [CrossRef]
- Available online: www.mattek.com/mattekproduct/epioral-epigingival/ (accessed on 10 January 2025).
- Hadjichristou, C.; Papachristou, E.; Bonovolias, I.; Bakopoulou, A. Three-dimensional tissue engineering-based Dentin/Pulp tissue analogue as advanced biocompatibility evaluation tool of dental restorative materials. Dent. Mater. 2020, 36, 229–248. [Google Scholar] [CrossRef]
- Yang, J.; Deol, G.; Myangar, N. Retention of o-cymen-5-ol and zinc on reconstructed human gingival tissue from a toothpaste formulation. Int. Dent. J. 2011, 61 (Suppl. 3), 41–45. [Google Scholar] [CrossRef] [PubMed]
- Klausner, M.; Ayehunie, S.; Breyfogle, B.A.; Wertz, P.W.; Bacca, L.; Kubilus, J. Organotypic human oral tissue models for toxicological studies. Toxicol. Vitro 2007, 21, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Moharamzadeh, K.; Brook, I.M.; Scutt, A.M.; Thornhill, M.H.; Van Noort, R. Mucotoxicity of dental composite resins on a tissue-engineered human oral mucosal model. J. Dent. 2008, 36, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Koschier, F.; Kostrubsky, V.; Toole, C.; Gallo, M.A. In vitro effects of ethanol and mouthrinse on permeability in an oral buccal mucosal tissue construct. Food Chem. Toxicol. 2011, 49, 2524–2529, Erratum in Food Chem. Toxicol. 2012, 50, 1815. [Google Scholar] [CrossRef]
- Song, G.; Banov, D.; Bassani, A.S.; Valdez, B.C. Evaluation of the Safety, Cell Migration, and Mucoadhesive Properties of a Mucoadhesive Polymer Blend in Human Oral Mucosa. AAPS PharmSciTech 2017, 18, 1617–1623. [Google Scholar] [CrossRef]
- Vande Vannet, B.; Hanssens, J.L.; Wehrbein, H. The use of three-dimensional oral mucosa cell cultures to assess the toxicity of soldered and welded wires. Eur. J. Orthod. 2007, 29, 60–66. [Google Scholar] [CrossRef]
- Zwicker, P.; Zumpe, M.; Kramer, A.; Müller, G. A 3D Model of Human Buccal Mucosa for Compatibility Testing of Mouth Rinsing Solutions. Pharmaceutics 2023, 15, 721. [Google Scholar] [CrossRef]
- Koutrouli, A.; Machla, F.; Arapostathis, K.; Kokoti, M.; Bakopoulou, A. Biological responses of two calcium-silicate-based cements on a tissue-engineered 3D organotypic deciduous pulp analogue. Dent. Mater. 2024, 40, e14–e25. [Google Scholar] [CrossRef]
- Colley, H.E.; Said, Z.; Santocildes-Romero, M.E.; Baker, S.R.; D’Apice, K.; Hansen, J.; Madsen, L.S.; Thornhill, M.H.; Hatton, P.V.; Murdoch, C. Pre-clinical evaluation of novel mucoadhesive bilayer patches for local delivery of clobetasol-17-propionate to the oral mucosa. Biomaterials 2018, 178, 134–146. [Google Scholar] [CrossRef]
- Zanetti, F.; Sewer, A.; Mathis, C.; Iskandar, A.R.; Kostadinova, R.; Schlage, W.K.; Leroy, P.; Majeed, S.; Guedj, E.; Trivedi, K.; et al. Systems Toxicology Assessment of the Biological Impact of a Candidate Modified Risk Tobacco Product on Human Organotypic Oral Epithelial Cultures. Chem. Res. Toxicol. 2016, 29, 1252–1269. [Google Scholar] [CrossRef]
- Zanetti, F.; Titz, B.; Sewer, A.; Lo Sasso, G.; Scotti, E.; Schlage, W.K.; Mathis, C.; Leroy, P.; Majeed, S.; Torres, L.O.; et al. Comparative systems toxicology analysis of cigarette smoke and aerosol from a candidate modified risk tobacco product in organotypic human gingival epithelial cultures: A 3-day repeated exposure study. Food Chem. Toxicol. 2017, 101, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, M.; Ishikawa, S.; Kurachi, T.; Sakamoto, K.; Sakai, H. Oral Absorption across Organotypic Culture Models of the Human Buccal Epithelium after E-cigarette Aerosol Exposure. ACS Omega 2022, 7, 45574–45581. [Google Scholar] [CrossRef]
- Zanetti, F.; Sewer, A.; Scotti, E.; Titz, B.; Schlage, W.K.; Leroy, P.; Kondylis, A.; Vuillaume, G.; Iskandar, A.R.; Guedj, E.; et al. Assessment of the impact of aerosol from a potential modified risk tobacco product compared with cigarette smoke on human organotypic oral epithelial cultures under different exposure regimens. Food Chem. Toxicol. 2018, 115, 148–169. [Google Scholar] [CrossRef] [PubMed]
- Miller-Holt, J.; Baskerville-Abraham, I.; Sakimura, M.; Fukushima, T.; Puglisi, A.; Gafner, J. In vitro evaluation of mutagenic, cytotoxic, genotoxic and oral irritation potential of nicotine pouch products. Toxicol. Rep. 2022, 9, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, Z.N.; Alqahtani, A.; Almela, T.; Franklin, K.; Tayebi, L.; Moharamzadeh, K. Effects of electronic cigarette liquid on monolayer and 3D tissue-engineered models of human gingival mucosa. J. Adv. Periodontol. Implant. Dent. 2019, 11, 54–62. [Google Scholar] [CrossRef]
- Kurosawa, Y.; Yamaguchi, H.; Uemichi, K.; Shinozuka, K.; Kirihara, Y.; Tsuda, H. Butyrate-treatment induces gingival epithelial cell death in a three-dimensional gingival-connective tissue hybrid co-culture system. J. Dent. Sci. 2023, 18, 893–897. [Google Scholar] [CrossRef]
- Agrawal, A.; Shindell, E.; Jordan, F.; Baeva, L.; Pfefer, J.; Godar, D.E. UV radiation increases carcinogenic risks for oral tissues compared to skin. Photochem. Photobiol. 2013, 89, 1193–1198. [Google Scholar] [CrossRef]
- Breger, J.; Baeva, L.; Agrawal, A.; Shindell, E.; Godar, D.E. UVB-induced inflammatory cytokine release, DNA damage and apoptosis of human oral compared with skin tissue equivalents. Photochem. Photobiol. 2013, 89, 665–670. [Google Scholar] [CrossRef]
- Basso, F.G.; Soares, D.G.; de Souza Costa, C.A.; Hebling, J. Low-level laser therapy in 3D cell culture model using gingival fibroblasts. Lasers Med. Sci. 2016, 31, 973–978. [Google Scholar] [CrossRef]
- Carreiro, A.F.P.; Delben, J.A.; Guedes, S.; Silveira, E.J.D.; Janal, M.N.; Vergani, C.E.; Pushalkar, S.; Duarte, S. Low-temperature plasma on peri-implant-related biofilm and gingival tissue. J. Periodontol. 2019, 90, 507–515. [Google Scholar] [CrossRef]
- Moghaddam, B.; Yang, J.; Roohpour, N. Biologic evaluation of devices with chronic exposure using 3D human gingival model. In Proceedings of the 10th World Biomaterials Congress, Montréal, QC, Canada, 17–22 May 2016. [Google Scholar] [CrossRef]
- Avnet, S.; Di Pompo, G.; Borciani, G.; Fischetti, T.; Graziani, G.; Baldini, N. Advantages and limitations of using cell viability assays for 3Dbioprinted constructs. Biomed. Mater. 2024, 19, 025033. [Google Scholar] [CrossRef] [PubMed]
- Dominijanni, A.J.; Devarasetty, M.; Forsythe, S.D.; Votanopoulos, K.I.; Soker, S. Cell Viability Assays in Three-Dimensional Hydrogels: A Comparative Study of Accuracy. Tissue Eng. Part. C Methods 2021, 27, 401–410. [Google Scholar] [CrossRef]
- Pfuhler, S.; van Benthem, J.; Curren, R.; Doak, S.H.; Dusinska, M.; Hayashi, M.; Heflich, R.H.; Kidd, D.; Kirkland, D.; Luan, Y.; et al. Use of in vitro 3D tissue models in genotoxicity testing: Strategic fit, validation status and way forward. Report of the working group from the 7th International Workshop on Genotoxicity Testing (IWGT). Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2020, 850–851, 503135. [Google Scholar] [CrossRef] [PubMed]
- Joseph, X.; Akhil, V.; Arathi, A.; Mohanan, P.V. Comprehensive Development in Organ-On-A-Chip Technology. J. Pharm. Sci. 2022, 111, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Koyilot, M.C.; Natarajan, P.; Hunt, C.R.; Sivarajkumar, S.; Roy, R.; Joglekar, S.; Pandita, S.; Tong, C.W.; Marakkar, S.; Subramanian, L.; et al. Breakthroughs and Applications of Organ-on-a-Chip Technology. Cells 2022, 11, 1828. [Google Scholar] [CrossRef]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Deng, S.; Li, C.; Cao, J.; Cui, Z.; Du, J.; Fu, Z.; Yang, H.; Chen, P. Organ-on-a-chip meets artificial intelligence in drug evaluation. Theranostics 2023, 13, 4526–4558. [Google Scholar] [CrossRef]
- Huang, C.; Sanaei, F.; Verdurmen, W.P.R.; Yang, F.; Ji, W.; Walboomers, X.F. The Application of Organs-on-a-Chip in Dental, Oral, and Craniofacial Research. J. Dent. Res. 2023, 102, 364–375. [Google Scholar] [CrossRef]
- Pierfelice, T.V.; D’Amico, E.; Petrini, M.; Romano, M.; D’Arcangelo, C.; Sbordone, L.; Barone, A.; Plebani, R.; Iezzi, G. A Systematic Review on Organ-on-a-Chip in PDMS or Hydrogel in Dentistry: An Update of the Literature. Gels 2024, 10, 102. [Google Scholar] [CrossRef]
- Hu, S.; Muniraj, G.; Mishra, A.; Hong, K.; Lum, J.L.; Hong, C.H.L.; Rosa, V.; Sriram, G. Characterization of silver diamine fluoride cytotoxicity using microfluidic tooth-on-a-chip and gingival equivalents. Dent. Mater. 2022, 38, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Muniraj, G.; Tan, R.H.S.; Dai, Y.; Wu, R.; Alberti, M.; Sriram, G. Microphysiological Modeling of Gingival Tissues and Host-Material Interactions Using Gingiva-on-Chip. Adv. Healthc. Mater. 2023, 12, e2301472. [Google Scholar] [CrossRef]
- França, C.M.; Balbinot, G.S.; Cunha, D.; Saboia, V.P.A.; Ferracane, J.; Bertassoni, L.E. In-vitro models of biocompatibility testing for restorative dental materials: From 2D cultures to organs on-a-chip. Acta Biomater. 2022, 150, 58–66. [Google Scholar] [CrossRef]
- ISO 7405:2018; Dentistry—Evaluation of Biocompatibility of Medical Devices Used in Dentistry. International Organization for Standardization: Geneva, Switzerland, 2018.
- França, C.M.; Tahayeri, A.; Rodrigues, N.S.; Ferdosian, S.; Puppin Rontani, R.M.; Sereda, G.; Ferracane, J.L.; Bertassoni, L.E. The tooth on-a-chip: A microphysiologic model system mimicking the biologic interface of the tooth with biomaterials. Lab. Chip 2020, 20, 405–413. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Wang, S.; Sun, X.; Luo, C.; Hou, B. Construction of dentin-on-a-chip based on microfluidic technology and tissue engineering. J. Dent. 2024, 146, 105028. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Kou, N.; An, F.; Gao, Z.; Tian, T.; Hui, J.; Chen, C.; Ma, G.; Mao, H.; Liu, H. Analyzing Human Periodontal Soft Tissue Inflammation and Drug Responses In Vitro Using Epithelium-Capillary Interface On-a-Chip. Biosensors 2022, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.; Zhou, Y.; Tan, K.S.; Lim, C.T.; Sriram, G. Modeling Crevicular Fluid Flow and Host-Oral Microbiome Interactions in a Gingival Crevice-on-Chip. Adv. Healthc. Mater. 2023, 12, 2202376. [Google Scholar] [CrossRef]
- Rodrigues, N.S.; França, C.M.; Tahayeri, A.; Ren, Z.; Saboia, V.P.A.; Smith, A.J.; Ferracane, J.L.; Koo, H.; Bertassoni, L.E. Biomaterial and Biofilm Interactions with the Pulp-Dentin Complex-on-a-Chip. J. Dent. Res. 2021, 100, 1136–1143. [Google Scholar] [CrossRef]
- Ly, K.L.; Rooholghodos, S.A.; Rahimi, C.; Rahimi, B.; Bienek, D.R.; Kaufman, G.; Raub, C.B.; Luo, X. An Oral-mucosa-on-a-chip sensitively evaluates cell responses to dental monomers. Biomed. Microdevices 2021, 23, 7. [Google Scholar] [CrossRef]
- Rahimi, C.; Rahimi, B.; Padova, D.; Rooholghodos, S.A.; Bienek, D.R.; Luo, X.; Kaufman, G.; Raub, C.B. Oral mucosa-on-a-chip to assess layer-specific responses to bacteria and dental materials. Biomicrofluidics 2018, 12, 054106. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning from 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Ardila, C.M.; Jiménez-Arbeláez, G.A.; Vivares-Builes, A.M. Potential Clinical Application of Organs-on-a-Chip in Periodontal Diseases: A Systematic Review of In Vitro Studies. Dent. J. 2023, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Farhang Doost, N.; Srivastava, S.K. A Comprehensive Review of Organ-on-a-Chip Technology and Its Applications. Biosensors 2024, 14, 225. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).