Preliminary Insights into the Cyto/Genoprotective Properties of Propolis and Its Constituent Galangin In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Instrumentation
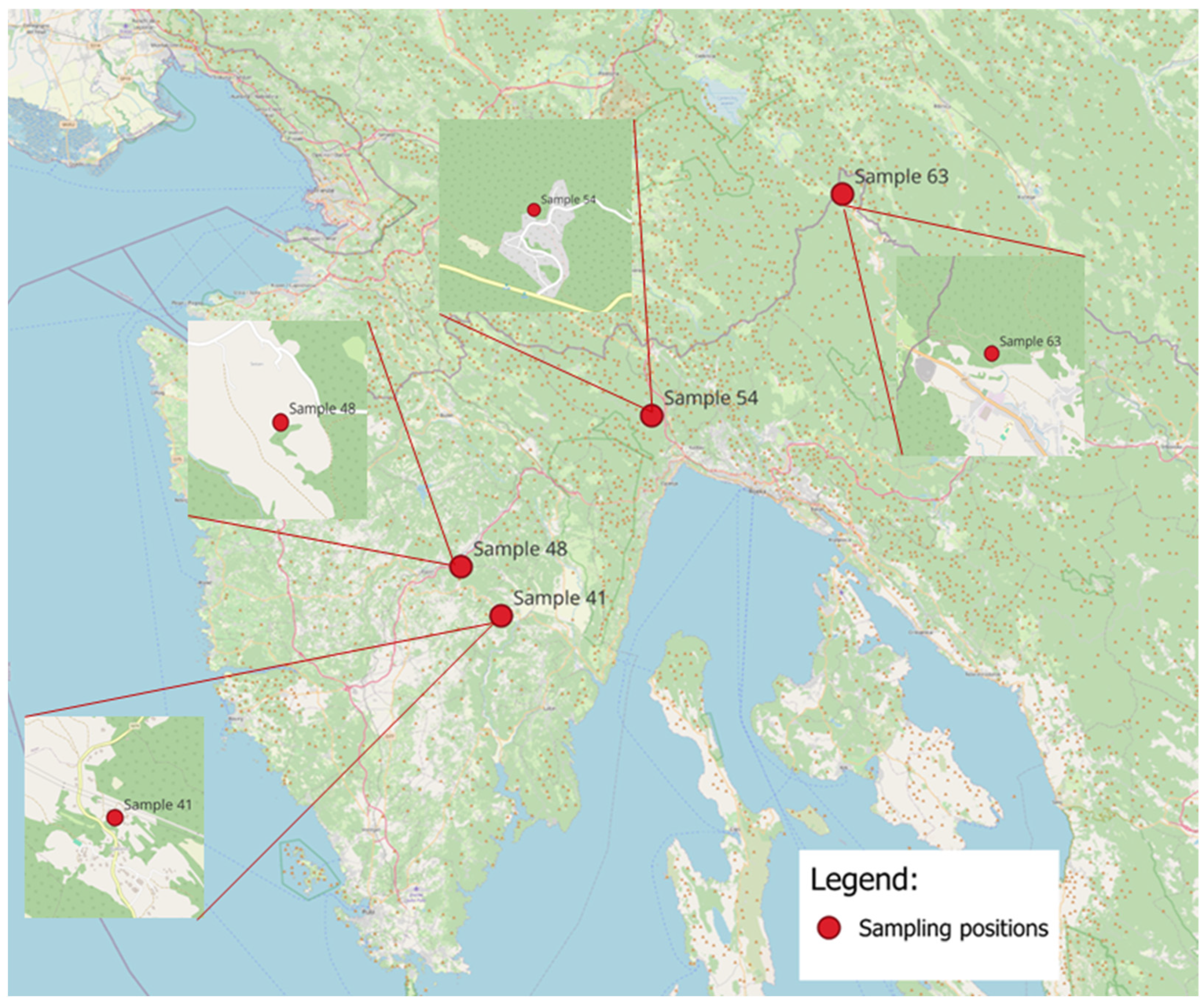
2.3. Propolis Sampling
2.4. Determination of Total Phenolic Content
2.5. Determination of Total Flavonoid Content
2.5.1. Flavones/Flavonols
2.5.2. Flavanones/Dihydroflavonols
2.6. Determination of Antioxidant Activity
2.7. Quantification of Galangin Using Ultra-High-Performance Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry (UHPLC Q-ToF MS)
2.8. Cytokinesis-Block Micronucleus (CBMN) Cytome Assay on Peripheral Blood Lymphocytes
2.8.1. Blood Sampling
2.8.2. Lymphocyte Treatment
2.8.3. Cell Harvest and Slide Preparation
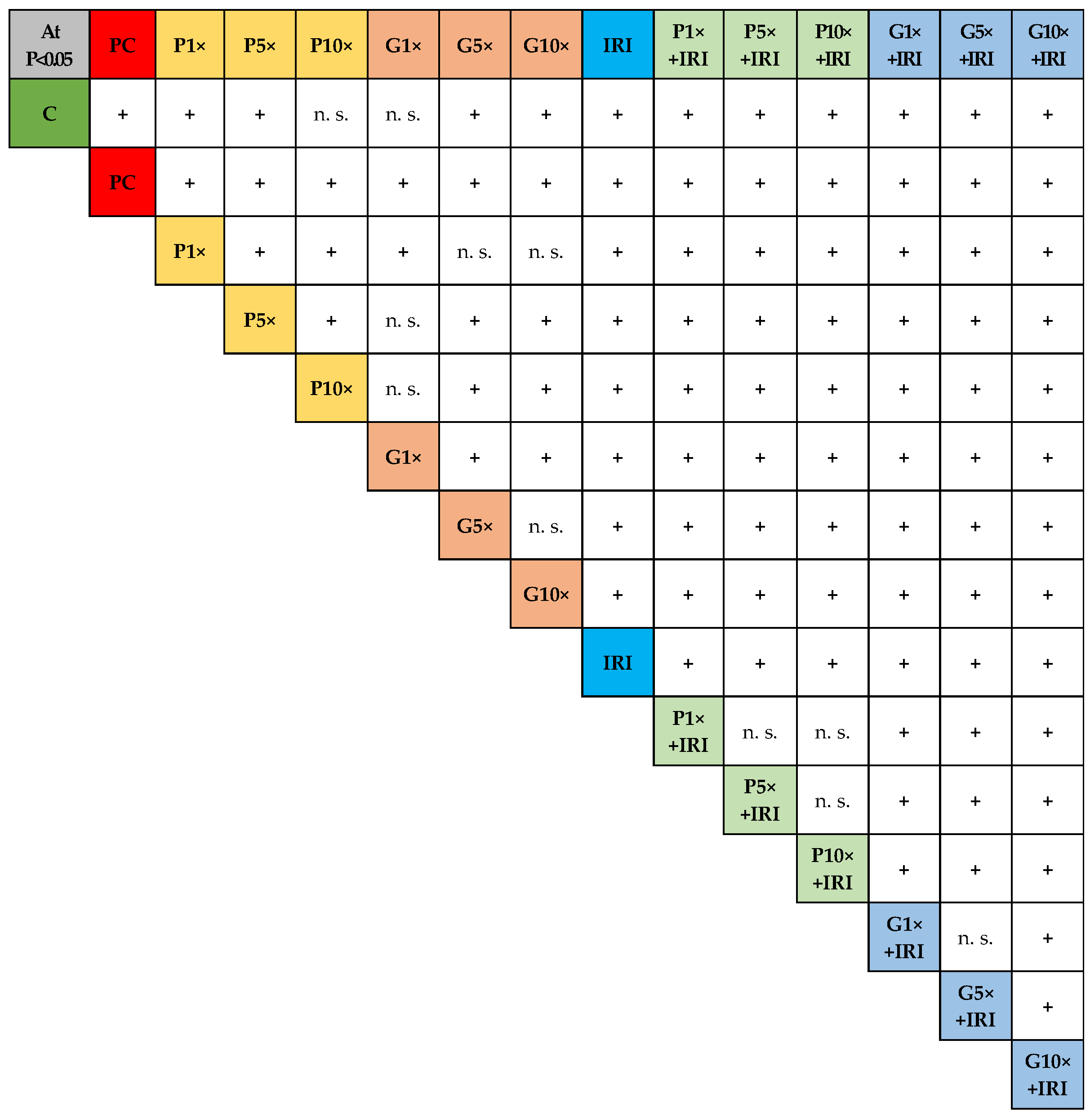
2.9. Statistics
3. Results and Discussion
3.1. Propolis Sample Selection for Research on Biological Effects
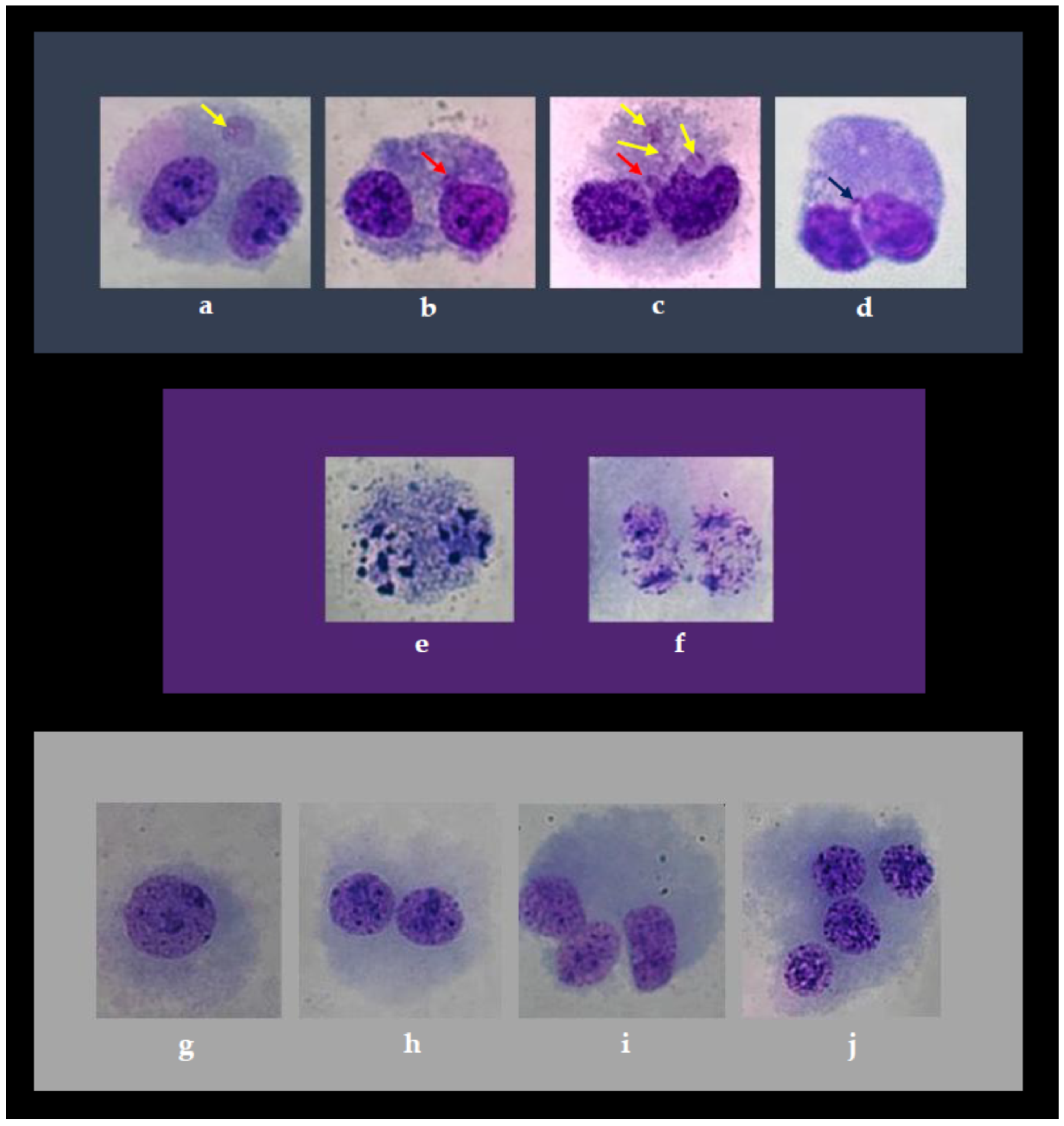
3.2. Cytokinesis-Block Micronucleus (CBMN) Cytome Assay
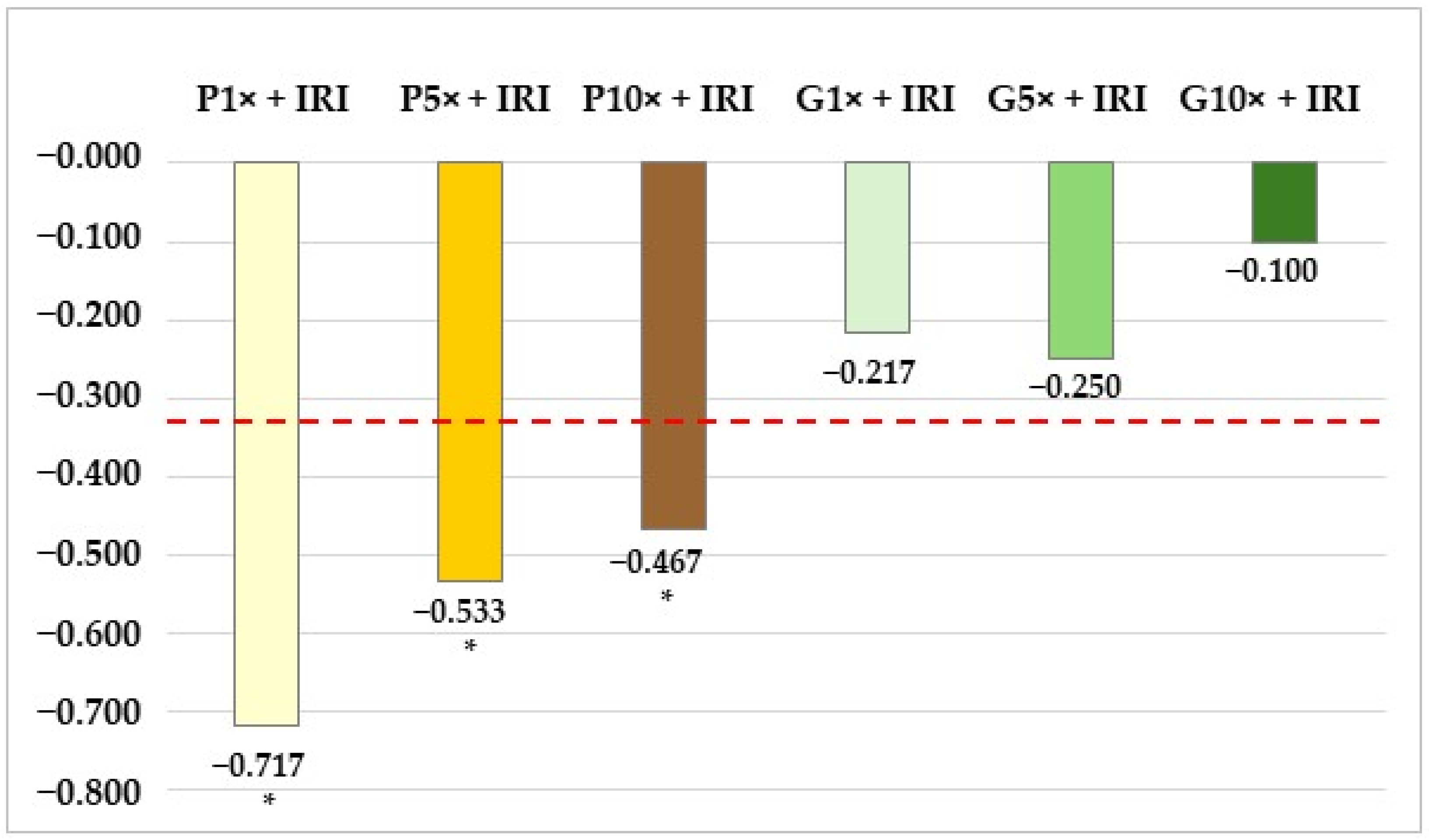
3.3. Analysis of Lymphocyte Proliferation
3.4. Limitations of This Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bogdanov, S.; Bankova, V. Propolis: Origin, Production, Composition. In The Propolis Book; Bee Product Science, 2016; pp. 2–20. Available online: https://www.bee-hexagon.net/english/bee-products/downloads-propolis-book/ (accessed on 26 January 2025).
- Santos-Buelga, C.; González-Paramás, A.M. Phenolic Composition of Propolis. In Bee Products—Chemical and Biological Properties; Alvarez-Suarez, J.M., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 99–111. ISBN 9783319596891. [Google Scholar]
- Šuran, J.; Cepanec, I.; Mašek, T.; Radić, B.; Radić, S.; Tlak Gajger, I.; Vlainić, J. Propolis Extract and Its Bioactive Compounds—From Traditional to Modern Extraction Technologies. Molecules 2021, 26, 2930. [Google Scholar] [CrossRef]
- Ristivojević, P.; Trifković, J.; Andrić, F.; Milojković-Opsenica, D. Poplar-Type Propolis: Chemical Composition, Botanical Origin and Biological Activity. Nat. Prod. Commun. 2015, 10, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; Sabzevari, O.; Wilson, J.X.; O’Brien, P.J. Prooxidant Activity and Cellular Effects of the Phenoxyl Radicals of Dietary Flavonoids and Other Polyphenolics. Toxicology 2002, 177, 91–104. [Google Scholar] [CrossRef] [PubMed]
- The Committee on Mutagenicity of Chemicals in Food, Consumer Products and the Environment. Guidance on a Strategy for Genotoxicity Testing of Chemicals–Version 4.0. 2021. Available online: https://assets.publishing.service.gov.uk/media/61c316cdd3bf7f1f71aa7ca7/strategy-for-genotoxicity-testing-of-chemicals-guidance.pdf (accessed on 26 February 2025).
- OECD. Test No. 471: Bacterial Reverse Mutation Test. OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2020. [Google Scholar]
- OECD. Test No. 487: In Vitro Mammalian Cell Micronucleus Test. OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2023. [Google Scholar]
- OECD. Test No. 473: In Vitro Mammalian Chromosome Aberration Test. OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2016. [Google Scholar]
- Collins, A.; Møller, P.; Gajski, G.; Vodenková, S.; Abdulwahed, A.; Anderson, D.; Bankoglu, E.E.; Bonassi, S.; Boutet-Robinet, E.; Brunborg, G.; et al. Measuring DNA Modifications with the Comet Assay: A Compendium of Protocols. Nat. Protoc. 2023, 18, 929–989. [Google Scholar] [CrossRef] [PubMed]
- Jurič, A.; Brčić Karačonji, I.; Gašić, U.; Milojković Opsenica, D.; Prđun, S.; Bubalo, D.; Lušić, D.; Vahčić, N.; Kopjar, N. Protective Effects of Arbutus unedo L. Honey in the Alleviation of Irinotecan-Induced Cytogenetic Damage in Human Lymphocytes—An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 1903. [Google Scholar] [CrossRef]
- Jurič, A.; Brčić Karačonji, I.; Kopjar, N. Homogentisic Acid, a Main Phenolic Constituent of Strawberry Tree Honey, Protects Human Peripheral Blood Lymphocytes against Irinotecan-Induced Cytogenetic Damage In Vitro. Chem. Biol. Interact. 2021, 349, 109672. [Google Scholar] [CrossRef]
- Žunec, S.; Brčić Karačonji, I.; Čatalinac, M.; Jurič, A.; Katić, A.; Kozina, G.; Micek, V.; Neuberg, M.; Lucić Vrdoljak, A. Effects of Concomitant Use of THC and Irinotecan on Tumour Growth and Biochemical Markers in a Syngeneic Mouse Model of Colon Cancer. Arh. Hig. Rada Toksikol. 2023, 74, 198–206. [Google Scholar] [CrossRef]
- Svečnjak, L.; Marijanović, Z.; Okińczyc, P.; Kuś, P.M.; Jerković, I. Mediterranean Propolis from the Adriatic Sea Islands as a Source of Natural Antioxidants: Comprehensive Chemical Biodiversity Determined by GC-MS, FTIR-ATR, UHPLC-DAD-QqTOF-MS, DPPH and FRAP Assay. Antioxidants 2020, 9, 337. [Google Scholar] [CrossRef]
- Saftić, L.; Peršurić, Ž.; Fornal, E.; Pavlešić, T.; Kraljević Pavelić, S. Targeted and Untargeted LC-MS Polyphenolic Profiling and Chemometric Analysis of Propolis from Different Regions of Croatia. J. Pharm. Biomed. Anal. 2019, 165, 162–172. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Pavlović, I.; Bojić, M.; Kosalec, I.; Srečec, S.; Vlainić, T.; Vlainić, J. The Components Responsible for the Antimicrobial Activity of Propolis from Continental and Mediterranean Regions in Croatia. Czech J. Food Sci. 2017, 35, 376–385. [Google Scholar] [CrossRef]
- Barbarić, M.; Mišković, K.; Bojić, M.; Lončar, M.B.; Smolčić-Bubalo, A.; Debeljak, Ž.; Medić-Šarić, M. Chemical Composition of the Ethanolic Propolis Extracts and Its Effect on HeLa Cells. J. Ethnopharmacol. 2011, 135, 772–778. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-Block Micronucleus Cytome Assay Evolution into a More Comprehensive Method to Measure Chromosomal Instability. Genes 2020, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Jurič, A.; Huđek Turković, A.; Brčić Karačonji, I.; Prđun, S.; Bubalo, D.; Durgo, K. Cytotoxic Activity of Strawberry Tree (Arbutus unedo L.) Honey, Its Extract, and Homogentisic Acid on CAL 27, HepG2, and Caco-2 Cell Lines. Arh. Hig. Rada Toksikol. 2022, 73, 158–168. [Google Scholar] [CrossRef]
- Trusheva, B.; Trunkova, D.; Bankova, V. Different Extraction Methods of Biologically Active Components from Propolis: A Preliminary Study. Chem. Cent. J. 2007, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Tuberoso, C.I.G.; Boban, M.; Bifulco, E.; Budimir, D.; Pirisi, F.M. Antioxidant Capacity and Vasodilatory Properties of Mediterranean Food: The Case of Cannonau Wine, Myrtle Berries Liqueur and Strawberry-tree Honey. Food Chem. 2013, 140, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Kostić, A.; Milinčić, D.D.; Špirović Trifunović, B.; Nedić, N.; Gašić, U.M.; Tešić, Ž.L.; Stanojević, S.P.; Pešić, M.B. Monofloral Corn Poppy Bee-Collected Pollen—A Detailed Insight into Its Phytochemical Composition and Antioxidant Properties. Antioxidants 2023, 12, 1424. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-Block Micronucleus Cytome Assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef]
- Takano, M.; Sugiyama, T. UGT1A1 Polymorphisms in Cancer: Impact on Irinotecan Treatment. Pharmgenom. Pers. Med. 2017, 10, 61–68. [Google Scholar] [CrossRef]
- Fenech, M.; Chang, W.P.; Kirsch-Volders, M.; Holland, N.; Bonassi, S.; Zeiger, E. HUMN Project: Detailed Description of the Scoring Criteria for the Cytokinesis-Block Micronucleus Assay Using Isolated Human Lymphocyte Cultures. Mutat. Res. 2003, 534, 65–75. [Google Scholar] [CrossRef]
- Ozkul, Y.; Eroglu, H.E.; Ok, E. Genotoxic Potential of Turkish Propolis in Peripheral Blood Lymphocytes. Pharmazie 2006, 61, 638–640. [Google Scholar]
- Ozkul, Y.; Silici, S.; Eroǧlu, E. The Anticarcinogenic Effect of Propolis in Human Lymphocytes Culture. Phytomedicine 2005, 12, 742–747. [Google Scholar] [CrossRef]
- Benković, V.; Kopjar, N.; Horvat Kneževic, A.; Đikić, D.; Bašić, I.; Ramić, S.; Viculin, T.; Knežević, F.; Oršolić, N. Evaluation of Radioprotective Effects of Propolis and Quercetin on Human White Blood Cells In Vitro. Biol. Pharm. Bull. 2008, 31, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Benkovic, V.; Knezevic, A.H.; Orsolic, N.; Basic, I.; Ramic, S.; Viculin, T.; Knezevic, F.; Kopjar, N. Evaluation of Radioprotective Effects of Propolis and Its Flavonoid Constituents: In Vitro Study on Human White Blood Cells. Phytotherapy 2009, 23, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Montoro, A.; Barquinero, J.F.; Almonacid, M.; Montoro, A.; Sebastià, N.; Verdù, G.; Sahuquillo, V.; Serrano, J.; Saiz, M.; Villaescusa, J.I.; et al. Concentration-Dependent Protection by Ethanol Extract of Propolis against γ-Ray-Induced Chromosome Damage in Human Blood Lymphocytes. Evid.-Based Complement. Altern. Med. 2011, 2011, 174853. [Google Scholar] [CrossRef]
- Montoro, A.; Soriano, J.M.; Barquinero, J.F.; Almonacid, M.; Montoro, A.; Verdú, G.; Sahuquillo, V.; Villaescusa, J.I.; Sebastià, N. Assessment In Vitro of Cytogenetic and Genotoxic Effects of Propolis on Human Lymphocytes. Food Chem. Toxicol. 2012, 50, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Bacanlı, M.; Başaran, A.A.; Başaran, N. The Antioxidant, Cytotoxic, and Antigenotoxic Effects of Galangin, Puerarin, and Ursolic Acid in Mammalian Cells. Drug Chem. Toxicol. 2017, 40, 256–262. [Google Scholar] [CrossRef]
- Fathi-Karkan, S.; Qindeel, M.; Arshad, R.; Moafian, Z.; Ghazy, E.; Rahdar, A.; Ghotekar, S. Recent Advancements in Irinotecan-Loaded Nanomaterials as a Smart Drug Delivery System for Cancer Therapy: A State-of-Art-Review. Inorg. Chem. Commun. 2024, 161, 112028. [Google Scholar] [CrossRef]
- Elshazly, A.M.; Wright, P.A.; Xu, J.; Gewirtz, D.A. Topoisomerase I Poisons-Induced Autophagy: Cytoprotective, Cytotoxic or Non-Protective. Autophagy Rep. 2023, 2, 1–16. [Google Scholar] [CrossRef]
- Chai, Y.; Liu, J.L.; Zhang, S.; Li, N.; Xu, D.Q.; Liu, W.J.; Fu, R.J.; Tang, Y.P. The Effective Combination Therapies with Irinotecan for Colorectal Cancer. Front. Pharmacol. 2024, 15, 1356708. [Google Scholar] [CrossRef]
- Kciuk, M.; Marciniak, B.; Kontek, R. Irinotecan—Still an Important Player in Cancer Chemotherapy: A Comprehensive Overview. Int. J. Mol. Sci. 2020, 21, 4919. [Google Scholar] [CrossRef]
- Kumar, S.; Sherman, M.Y. Resistance to TOP-1 Inhibitors: Good Old Drugs Still Can Surprise Us. Int. J. Mol. Sci. 2023, 24, 7233. [Google Scholar] [CrossRef]
- Kopjar, N.; Želježić, D.; Lucić Vrdoljak, A.; Radić, B.; Ramić, S.; Milić, M.; Gamulin, M.; Pavlica, V.; Fučić, A. Irinotecan Toxicity to Human Blood Cells In Vitro: Relationship between Various Biomarkers. Basic Clin. Pharmacol. Toxicol. 2007, 100, 403–413. [Google Scholar] [CrossRef]
- Gajski, G.; Kašuba, V.; Milić, M.; Gerić, M.; Matković, K.; Delić, L.; Nikolić, M.; Pavičić, M.; Rozgaj, R.; Garaj-Vrhovac, V.; et al. Exploring Cytokinesis Block Micronucleus Assay in Croatia: A Journey through the Past, Present, and Future in Biomonitoring of the General Population. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2024, 895, 503749. [Google Scholar] [CrossRef] [PubMed]
- Kujumgiev, A.; Tsvetkova, I.; Serkedjieva, Y.; Bankova, V.; Christov, R.; Popov, S. Antibacterial, Antifungal and Antiviral Activity of Propolis of Different Geographic Origin. J. Ethnopharmacol. 1999, 64, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Scarpato, R.; Bertoli, A.; Naccarati, A.; Migliore, L.; Cocchi, L.; Barale, R.; Pistelli, L. Different Effects of Newly Isolated Saponins on the Mutagenicity and Cytotoxicity of the Anticancer Drugs Mitomycin C and Bleomycin in Human Lymphocytes. Mutat. Res.—Genet. Toxicol. Environ. Mutagen. 1998, 420, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Granchi, D.; Cenni, E.; Ciapetti, G.; Savarino, L.; Stea, S.; Gamberini, S.; Gori, A.; Pizzoferrato, A. Cell Death Induced by Metal Ions: Necrosis or Apoptosis? J. Mater. Sci. Mater. Med. 1998, 9, 31–37. [Google Scholar] [CrossRef]
- Lorge, E.; Hayashi, M.; Albertini, S.; Kirkland, D. Comparison of Different Methods for an Accurate Assessment of Cytotoxicity in the In Vitro Micronucleus Test. I. Theoretical Aspects. Mutat. Res.—Genet. Toxicol. Environ. Mutagen. 2008, 655, 1–3. [Google Scholar] [CrossRef]
- Giampieri, F.; Quiles, J.L.; Cianciosi, D.; Forbes-Hernández, T.Y.; Orantes-Bermejo, F.J.; Alvarez-Suarez, J.M.; Battino, M. Bee Products: An Emblematic Example of Underutilized Sources of Bioactive Compounds. J. Agric. Food Chem. 2022, 70, 6833–6848. [Google Scholar] [CrossRef]
- Wang, D.; Chen, J.; Pu, L.; Yu, L.; Xiong, F.; Sun, L.; Yu, Q.; Cao, X.; Chen, Y.; Peng, F.; et al. Galangin: A Food-Derived Flavonoid with Therapeutic Potential against a Wide Spectrum of Diseases. Phyther. Res. 2023, 37, 5700–5723. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Polyphenols as Antioxidant/pro-Oxidant Compounds and Donors of Reducing Species: Relationship with Human Antioxidant Metabolism. Processes 2023, 11, 2771. [Google Scholar] [CrossRef]
- Halliwell, B. Are Polyphenols Antioxidants or Pro-Oxidants? What Do We Learn from Cell Culture and In Vivo Studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef]
- Babiich, H.; Schuck, A.G.; Weisburg, J.H.; Zuckerbraun, H.L. Research Strategies in the Study of the Pro-oxidant Nature of Polyphenol Nutraceuticals. J. Toxicol. 2011, 2011, 467305. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Swierczek-Zieba, G. Structure and Antioxidant Activity of Polyphenols Derived from Propolis. Molecules 2014, 19, 78–101. [Google Scholar] [CrossRef] [PubMed]
- Dzah, C.S.; Zhang, H.; Gobe, V.; Asante-Donyinah, D.; Duan, Y. Anti- and pro-Oxidant Properties of Polyphenols and Their Role in Modulating Glutathione Synthesis, Activity and Cellular Redox Potential: Potential Synergies for Disease Management. Adv. Redox Res. 2024, 11, 100099. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed]
- Altabbal, S.; Athamnah, K.; Rahma, A.; Wali, A.F.; Eid, A.H.; Iratni, R.; Al Dhaheri, Y. Propolis: A Detailed Insight of Its Anticancer Molecular Mechanisms. Pharmaceuticals 2023, 16, 450. [Google Scholar] [CrossRef]
- Oršolić, N.; Jazvinšćak Jembrek, M. Potential Strategies for Overcoming Drug Resistance Pathways Using Propolis and Its Polyphenolic/Flavonoid Compounds in Combination with Chemotherapy and Radiotherapy. Nutrients 2024, 16, 3741. [Google Scholar] [CrossRef]
- Oršolić, N.; Jazvinšćak Jembrek, M. Molecular and Cellular Mechanisms of Propolis and Its Polyphenolic Compounds against Cancer. Int. J. Mol. Sci. 2022, 23, 10479. [Google Scholar] [CrossRef]
- Cora, M.; Üreyen Esertaş, Ü.Z.; Kara, Y.; Kolaylı, S. Antioxidant, Antimicrobial, Antiviral, and Antiproliferative Properties of Turkish Propolis Sample. Eur. Food Res. Technol. 2024, 251, 123–133. [Google Scholar] [CrossRef]
- Valivand, N.; Aravand, S.; Lotfi, H.; Esfahani, A.J.; Ahmadpour-Yazdi, H.; Gheibi, N. Propolis: A Natural Compound with Potential as an Adjuvant in Cancer Therapy—A Review of Signaling Pathways. Mol. Biol. Rep. 2024, 51, 931. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Lupia, C.; Poerio, G.; Liguori, G.; Lombardi, R.; Naturale, M.D.; Bulotta, R.M.; Biondi, V.; Passantino, A.; et al. Hive Products: Composition, Pharmacological Properties, and Therapeutic Applications. Pharmaceuticals 2024, 17, 646. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, J.; Sachan, P.; Wal, P.; Rai, A.K. In-Depth Chemistry and Pharmacological Potential of Propolis: A Critical Review of Recent Developments. Curr. Tradit. Med. 2023, 10, e180823219971. [Google Scholar] [CrossRef]
- Forma, E.; Bryś, M. Anticancer Activity of Propolis and Its Compounds. Nutrients 2021, 13, 2594. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Bustos, P.; Alday, E.; Garibay-Escobar, A.; Sforcin, J.M.; Lipovka, Y.; Hernandez, J.; Velazquez, C. Propolis: Antineoplastic Activity, Constituents, and Mechanisms of Action. Curr. Top. Med. Chem. 2023, 23, 1753–1764. [Google Scholar] [CrossRef]
- Medić-Šarić, M.; Bojić, M.; Rastija, V.; Cvek, J. Polyphenolic Profiling of Croatian Propolis and Wine. Food Technol. Biotechnol. 2013, 51, 159–170. [Google Scholar]

| Experimental Group | Description |
|---|---|
| Control | Ethanol in final concentration 0.03% in the culture medium |
| Positive Control | Bleomycin 15 mg/L |
| Treatments without cytotoxic drug | |
| Propolis 1× | 1.36 mg/L |
| Propolis 5× | 6.8 mg/L |
| Propolis 10× | 13.6 mg/L |
| Galangin 1× | 0.02 mg/L |
| Galangin 5× | 0.11 mg/L |
| Galangin 10× | 0.22 mg/L |
| Treatments with cytotoxic drug | |
| Irinotecan (IRI) | +9.0 mg/L |
| Propolis 1× + IRI | 1.36 mg/L + 9.0 mg/L |
| Propolis 5× + IRI | 6.8 mg/L + 9.0 mg/L |
| Propolis 10× + IRI | 13.6 mg/L + 9.0 mg/L |
| Galangin 1× + IRI | 0.02 mg/L + 9.0 mg/L |
| Galangin 5× + IRI | 0.11 mg/L + 9.0 mg/L |
| Galangin 10× + IRI | 0.22 mg/L + 9.0 mg/L |
| Sample | Total Phenolics (mg GAE/g) | Total Flavones/ Flavonols (mg GE/g) | Total Flavanones/ Dihydroflavonols (mg NE/g) | Antioxidant Activity (DPPH) (mmol TE/g) | Galangin Content (mg/g) |
|---|---|---|---|---|---|
| P41 | 109.94 ± 3.95 | 12.48 ± 0.56 | 23.83 ± 0.89 | 176.89 ± 0.31 | 16.52 |
| P48 | 106.40 ± 7.11 | 10.06 ± 0.63 | 22.74 ± 0.63 | 174.35 ± 0.20 | 7.37 |
| P54 | 131.11 ± 8.45 | 9.23 ± 0.21 | 22.58 ± 0.57 | 176.31 ± 2.17 | ND |
| P63 | 101.44 ± 0.40 | 5.09 ± 0.23 | 17.21 ± 0.01 | 181,37 ± 1.18 | ND |
| Experimental Group | Micronuclei (MNi) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean (MNi)1000 ± SD | Total (MNi)4000 | Mean (BNMN)1000 ± SD | Total (BNMN)4000 | Distribution of BNMN Cells with | ||||
| 1 MN | 2 MN | 3 MN | 4 MN | |||||
| Control | 1.75 ± 0.50 | 7 | 1.75 ± 0.50 | 7 | 7 | 0 | 0 | 0 |
| Positive Control (Bleomycin) | 24.25 ± 6.24 * | 97 * | 19.75 ± 3.77 * | 79 * | 65 * | 11 iri, g10+iri | 2 | 1 |
| Propolis 1× | 2.00 ± 0.82 | 8 | 2.00 ± 0.82 | 8 | 8 | 0 | 0 | 0 |
| Propolis 5× | 2.00 ± 0.00 | 8 | 2.00 ± 0.00 | 8 | 8 | 0 | 0 | 0 |
| Propolis 10× | 2.00 ± 0.00 | 8 | 2.00 ± 0.00 | 8 | 8 | 0 | 0 | 0 |
| Galangin 1× | 2.00 ± 0.82 | 8 | 2.00 ± 0.82 | 8 | 8 | 0 | 0 | 0 |
| Galangin 5× | 2.25 ± 0.50 | 9 | 2.25 ± 0.50 | 9 | 9 | 0 | 0 | 0 |
| Galangin 10× | 1.75 ± 0.50 | 7 | 1.75 ± 0.50 | 7 | 7 | 0 | 0 | 0 |
| Irinotecan (IRI) | 15.00 ± 2.00 c,p1,p5,p10,g1,g5,g10,p1+iri, p5+iri, p10+iri | 60 c,p1,p5,p10,g1,g5, g10,p1+iri, p5+iri, p10+iri | 13.00 ± 0.82 c,p1,p5,p10,g1,g5, g10,p1+iri, p5+iri, p10+iri | 52 c,p1,p5,p10,g1,g5,g10, p1+iri, p5+iri, p10+iri | 45 | 6 | 1 | 0 |
| Propolis 1× + IRI | 4.25 ± 0.50 | 17 | 4.00 | 16 | 15 | 1 | 0 | 0 |
| Propolis 5× + IRI | 7.00 ± 1.41 c,p1,p5,p10,g1,g5,g10 | 28 c,p1,p5,p10,g1, g5,g10 | 6.75 ± 1.71 c,p1,p5,p10,g1,g5,g10 | 27 c,p1,p5,p10,g1,g5,g10 | 26 c,p1,p5, p10,g1,g5, g10 | 1 | 0 | 0 |
| Propolis 10× + IRI | 8.00 ± 1.41 c,p1,p5,p10,g1,g5,g10 | 32 c,p1,p5,p10,g1, g5,g10 | 7.25 ± 0.96 c,p1,p5,p10,g1,g5,g10 | 29 c,p1,p5,p10,g1,g5,g10 | 26 c,p1,p5, p10,g1,g5, g10 | 3 | 0 | 0 |
| Galangin 1× + IRI | 11.75 ± 4.11 c,p1,p5,p10,g1,g5,g10, p1+iri | 47 c,p1,p5,p10,g1,g5, g10,p1+iri | 11.50 ± 3.70 c,p1,p5,p10,g1,g5, g10,p1+iri, p5+iri, p10+iri | 46 c,p1,p5,p10,g1,g5,g10, p1+iri, p5+iri, p10+iri | 45 c,p1,p5,p10,g1,g5,g10,p1+iri, p5+iri, p10+iri | 1 | 0 | 0 |
| Galangin 5× + IRI | 11.25 ± 0.96 c,p1,p5,p10,g1,g5,g10, p1+iri | 45 c,p1,p5,p10,g1,g5, g10,p1+iri | 11.00 ± 0.82 c,p1,p5,p10,g1,g5, g10,p1+iri, p5+iri | 44 c,p1,p5,p10,g1,g5,g10, p1+iri, p5+iri | 43 c,p1,p5, p10,g1,g5, g10,p1+iri, p5+iri, p10+iri | 1 | 0 | 0 |
| Galangin 10× + IRI | 13.50 ± 1.29 c,p1,p5,p10,g1,g5,g10, p1+iri, p5+iri, p10+iri | 54 c,p1,p5,p10,g1,g5, g10,p1+iri, p5+iri, p10+iri | 12.25 ± 0.82 c,p1,p5,p10,g1,g5, g10,p1+iri, p5+iri, p10+iri | 49 c,p1,p5,p10,g1,g5,g10, p1+iri, p5+iri, p10+iri | 44 c,p1,p5, p10,g1,g5, g10,p1+iri, p5+iri, p10+iri | 5 | 0 | 0 |
| Experimental Group | Nuclear Buds (NBs) | Nucleoplasmic Bridges (NPBs) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (NBs)1000 ± SD | Total (NBs)4000 | Mean (BNNB)1000 ± SD | Total (BNNB)4000 | Distribution of BNNB Cells with | Mean (NPBs)1000 ± SD | Total (NPBs)4000 | Mean (BNNPB)1000 ± SD | Total (BNNPB)4000 | Distribution of BNNPB Cells with | |||
| 1 NB | 2 NB | 1 NPB | 2 NPB | |||||||||
| Control | 2.00 ± 0.82 | 8 | 2.00 ± 0.82 | 8 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Positive Control (Bleomycin) | 14.25 ± 0.50 * | 57 * | 13.25 ± 0.96 * | 53 * | 49 * | 4 c,p1,p5,p10,g1, g5,g10, p1+iri, p5+iri, p10+iri, g5+iri, g10+iri | 1.75 ± 1.26 c,p1,p5,p10, g1,g5,g10, p1+iri, p5+iri, p10+iri, g1+iri, g5+iri, g10+iri | 7 c,p1,p5,p10,g1,g5,g10,p1+iri, p5+iri, p10+iri, g1+iri, g5+iri, g10+iri | 1.75 ± 1.26 c,p1,p5,p10, g1,g5,g10, p1+iri, p5+iri, p10+iri, g1+iri, g5+iri, g10+iri | 7 c,p1,p5, p10,g1,g5,g10,p1+iri,p5+iri, p10+iri, g1+iri, g5+iri, g10+iri | 7 c,p1,p5,p10,g1, g5,g10, p1+iri, p5+iri, p10+iri, g1+iri, g5+iri, g10+iri | 0 |
| Propolis 1× | 2.25 ± 0.50 | 9 | 2.25 ± 0.50 | 9 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Propolis 5× | 2.75 ± 0.50 | 11 | 2.75 ± 0.50 | 11 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Propolis 10× | 3.00 ± 0.82 | 12 | 3.00 ± 0.82 | 12 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Galangin 1× | 1.75 ± 0.50 | 7 | 1.75 ± 0.50 | 7 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Galangin 5× | 2.25 ± 0.96 | 9 | 2.25 ± 0.96 | 9 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Galangin 10× | 2.25 ± 0.50 | 9 | 2.25 ± 0.50 | 9 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Irinotecan (IRI) | 11.00 ± 2.16 c,p1,p5,p10,g1,g5,g10,p1+iri, p5+iri,p10+iri, g5+iri,g10+iri | 44 c,p1,p5, p10,g1,g5, g10,p1+iri, p5+iri, p10+iri, g5+iri, g10+iri | 10.25 ± 1.71 c,p1,p5,p10,g1, g5,g10,p1+iri, p5+iri,p10+iri, g10+iri | 41 c,p1,p5,p10, g1,g5,g10, p1+iri,p5+iri,p10+iri, g10+iri | 38 c,p1,p5, p10,g1,g5, g10,p1+iri,p5+iri, p10+iri, g10+iri | 3 | 1.50 ± 1.29 | 6 | 1.25 ± 0.96 | 5 | 4 | 1 |
| Propolis 1× + IRI | 4.50 ± 0.58 g1 | 18 g1 | 4.50 ± 0.58 g1 | 18 g1 | 18 g1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Propolis 5× + IRI | 5.75 ± 1.26 c,p1,p5,p10,g1,g5,g10 | 23 c,p1,p5, p10,g1,g5, g10 | 5.75 ± 1.26 c,p1,p5,p10, g1,g5,g10 | 23 c,p1,p5,p10, g1,g5,g10 | 23 c,p1,p5, p10,g1,g5,g10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Propolis 10× + IRI | 5.25 ± 0.96 c,p1,g1,g5,g10 | 21 c,p1,g1,g5,g10 | 5.25 ± 0.96 c,p1,p5,g1,g5,g10 | 21 c,p1,p5,g1, g5,g10 | 21 c,p1,p5, p10,g1,g5,g10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Galangin 1× + IRI | 9.25 ± 1.50 c,p1,p5,p10,g1,g5,g10, p1+iri,p5+iri, p10+iri | 37 c,p1,p5, p10,g1,g5, g10, p1+iri, p5+iri, p10+iri | 8.75 ± 0.96 c,p1,p5,p10, g1,g5,g10, p1+iri,p5+iri, p10+iri | 35 c,p1,p5,p10, g1,g5,g10, p1+iri,p5+iri,p10+iri | 33 c,p1,p5, p10,g1,g5,g10, p1+iri, p10+iri | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Galangin 5× + IRI | 8.00 ± 1.41 c,p1,p5,p10,g1,g5,g10, p1+iri,p10+iri | 32 c,p1,p5, p10,g1,g5, g10, p1+iri, p10+iri | 8.00 ± 1.41 c,p1,p5,p10, g1,g5,g10, p1+iri,p10+iri | 32 c,p1,p5,p10, g1,g5,g10, p1+iri, p10+iri | 32 c,p1,p5, p10,g1, g5,g10, p1+iri, p10+iri | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Galangin 10× + IRI | 7.50 ± 1.29 c,p1,p5,p10,g1,g5,g10,p1+iri | 30 c,p1,p5, p10,g1,g5, g10,p1+iri | 7.50 ± 1.29 c,p1,p5,p10, g1,g5,g10, p1+iri | 30 c,p1,p5,p10, g1,g5,g10, p1+iri | 30 c,p1,p5, p10,g1,g5,g10, p1+iri | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Experimental Group | Total No. of Nonviable Cells | Total No. of Cells in Apoptosis | Ratio |
|---|---|---|---|
| Control | 16 | 9 | 0.6 |
| Positive Control | 100 | 59 | 0.6 |
| Propolis 1× | 14 | 8 | 0.6 |
| Propolis 5× | 13 | 7 | 0.5 |
| Propolis 10× | 17 | 8 | 0.5 |
| Galangin 1× | 14 | 7 | 0.5 |
| Galangin 5× | 16 | 8 | 0.5 |
| Galangin 10× | 12 | 8 | 0.7 |
| Irinotecan (IRI) | 76 | 42 | 0.6 |
| Propolis 1× + IRI | 25 | 14 | 0.6 |
| Propolis 5× + IRI | 25 | 16 | 0.6 |
| Propolis 10× + IRI | 28 | 17 | 0.6 |
| Galangin 1× + IRI | 52 | 28 | 0.5 |
| Galangin 5× + IRI | 42 | 23 | 0.5 |
| Galangin 10× + IRI | 34 | 19 | 0.6 |
| Experimental Group | % of Cells with 1 to 4 Nuclei | CBPI | Replication Index (%) | |||
|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | |||
| Control | 14.5 | 72.8 | 5.2 | 7.5 | 1.983 | 100.0 |
| Positive Control | 45.5 ↑ | 46.1 ↓ | 4.2 | 4.2 ↓ | 1.629 | 64.0 |
| Propolis 1× | 12.2 ↓ | 77.5 ↑ | 3.4↓ | 6.9 | 1.982 | 99.8 |
| Propolis 5× | 11.7 ↓ | 76.2 ↑ | 4.6 | 7.5 | 2.006 | 102.3 |
| Propolis 10× | 14.9 | 73.8 | 4.6 | 6.7 | 1.963 | 98.0 |
| Galangin 1× | 13.4 | 74.7 | 4.0 | 7.9 | 1.986 | 100.3 |
| Galangin 5× | 13.3 | 76.9 ↑ | 3.3 ↓ | 6.5 | 1.967 | 98.3 |
| Galangin 10× | 10.8 ↓ | 79.0 ↑ | 4.0 | 6.2 | 1.995 | 101.2 |
| Irinotecan (IRI) | 43.1 ↑ | 52.9 ↓ | 2.0 ↓ | 2.0 ↓ | 1.609 | 62.0 |
| Propolis 1× + IRI | 16.0 L | 76.9 ↑H | 3.6 ↓H | 3.5 ↓H | 1.912 | 92.8 |
| Propolis 5× + IRI | 15.6 L | 76.6 ↑H | 3.5 ↓H | 4.3 ↓H | 1.923 | 93.8 |
| Propolis 10× + IRI | 15.3 L | 76.5 ↑H | 3.7 ↓H | 4.5 ↓H | 1.930 | 94.6 |
| Galangin 1× + IRI | 24.5 ↑L | 72.3 H | 1.4 ↓ | 1.8 ↓ | 1.788 | 80.1 |
| Galangin 5× + IRI | 22.6 ↑L | 74.2 H | 1.6 ↓ | 1.6 ↓ | 1.806 | 82.0 |
| Galangin 10× + IRI | 23.1 ↑L | 74.6 H | 1.0 ↓L | 1.3 ↓ | 1.792 | 80.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakac, M.; Brčić Karačonji, I.; Jurič, A.; Lušić, D.; Milinčić, D.; Dramićanin, A.; Pešić, M.; Landeka, N.; Kopjar, N. Preliminary Insights into the Cyto/Genoprotective Properties of Propolis and Its Constituent Galangin In Vitro. Toxics 2025, 13, 194. https://doi.org/10.3390/toxics13030194
Jakac M, Brčić Karačonji I, Jurič A, Lušić D, Milinčić D, Dramićanin A, Pešić M, Landeka N, Kopjar N. Preliminary Insights into the Cyto/Genoprotective Properties of Propolis and Its Constituent Galangin In Vitro. Toxics. 2025; 13(3):194. https://doi.org/10.3390/toxics13030194
Chicago/Turabian StyleJakac, Mateo, Irena Brčić Karačonji, Andreja Jurič, Dražen Lušić, Danijel Milinčić, Aleksandra Dramićanin, Mirjana Pešić, Nediljko Landeka, and Nevenka Kopjar. 2025. "Preliminary Insights into the Cyto/Genoprotective Properties of Propolis and Its Constituent Galangin In Vitro" Toxics 13, no. 3: 194. https://doi.org/10.3390/toxics13030194
APA StyleJakac, M., Brčić Karačonji, I., Jurič, A., Lušić, D., Milinčić, D., Dramićanin, A., Pešić, M., Landeka, N., & Kopjar, N. (2025). Preliminary Insights into the Cyto/Genoprotective Properties of Propolis and Its Constituent Galangin In Vitro. Toxics, 13(3), 194. https://doi.org/10.3390/toxics13030194









