ROS-Mediated Unfolded Protein Response Activation Drives Hepatocyte Apoptosis in Mesaconitine-Induced Liver Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Husbandry
2.2. Chemical Exposure
2.3. Toxicity Analysis of MA
2.4. Oxidative Stress Detection
2.5. Quantitative Real-Time PCR (qRT-PCR)
2.6. Transcriptome Sequencing
2.7. Cell Apoptosis Detection
2.8. Statistical Analysis
3. Results
3.1. Effects of MA on Zebrafish Embryos Development
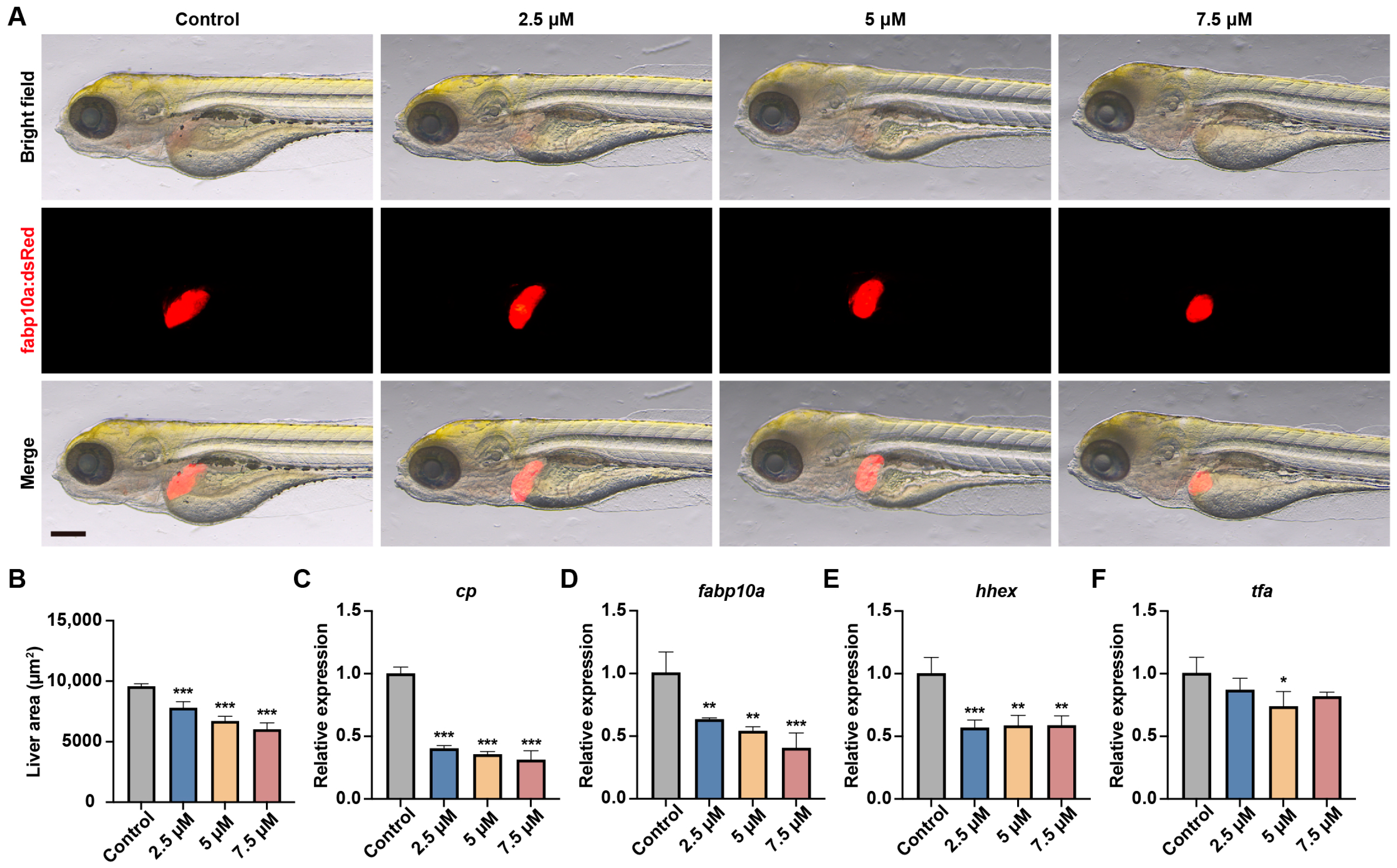
3.2. MA Impaired Liver Development in Zebrafish Embryos
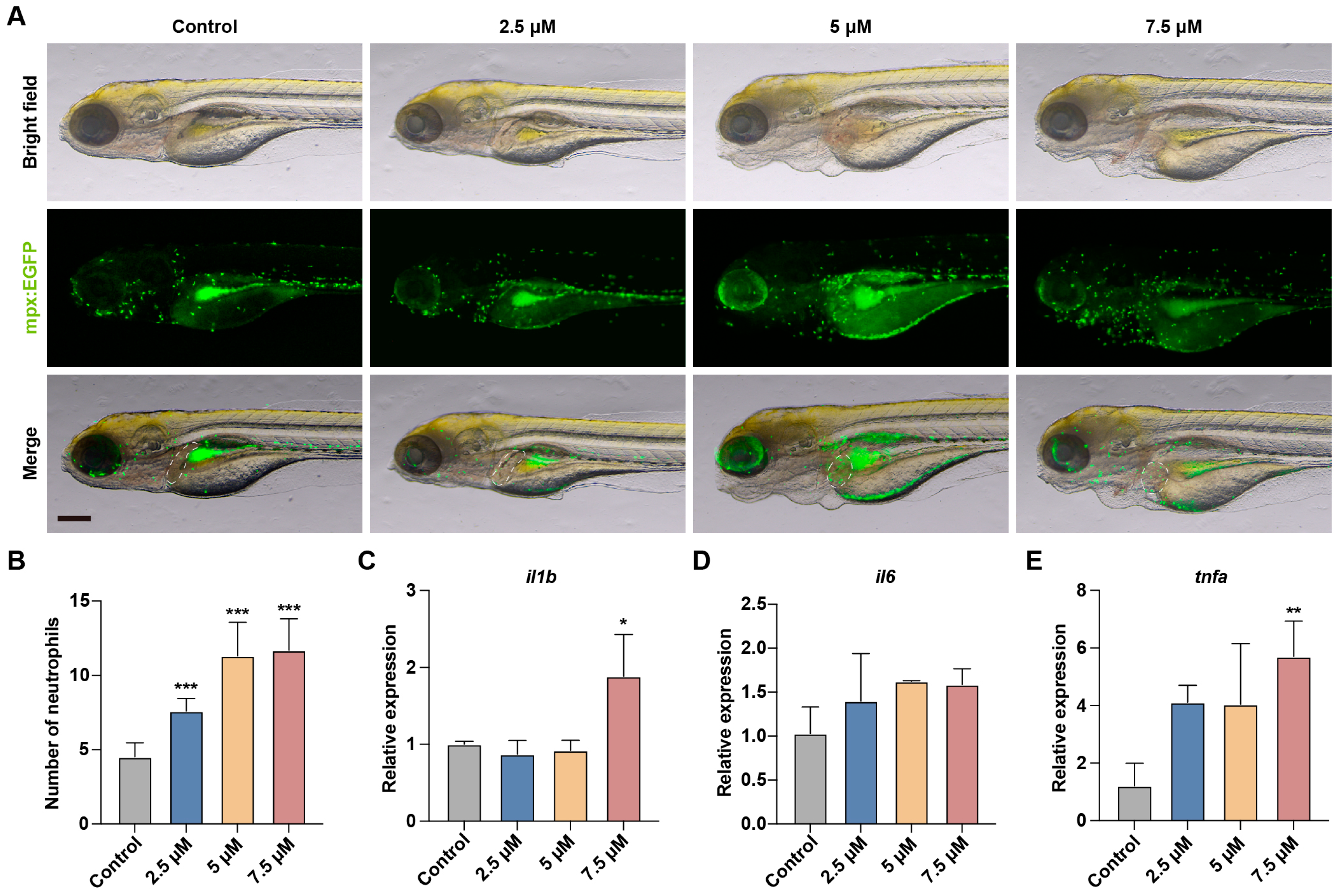
3.3. MA Induced Liver Inflammation in Zebrafish Embryos
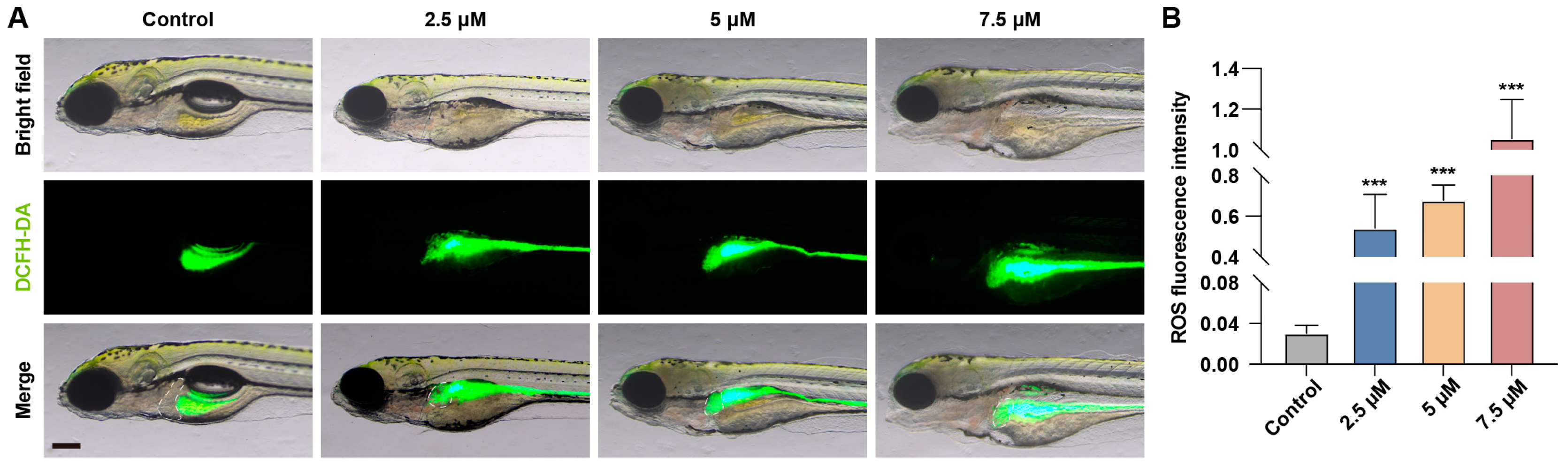
3.4. MA Induced Oxidative Stress Response in Zebrafish Embryos
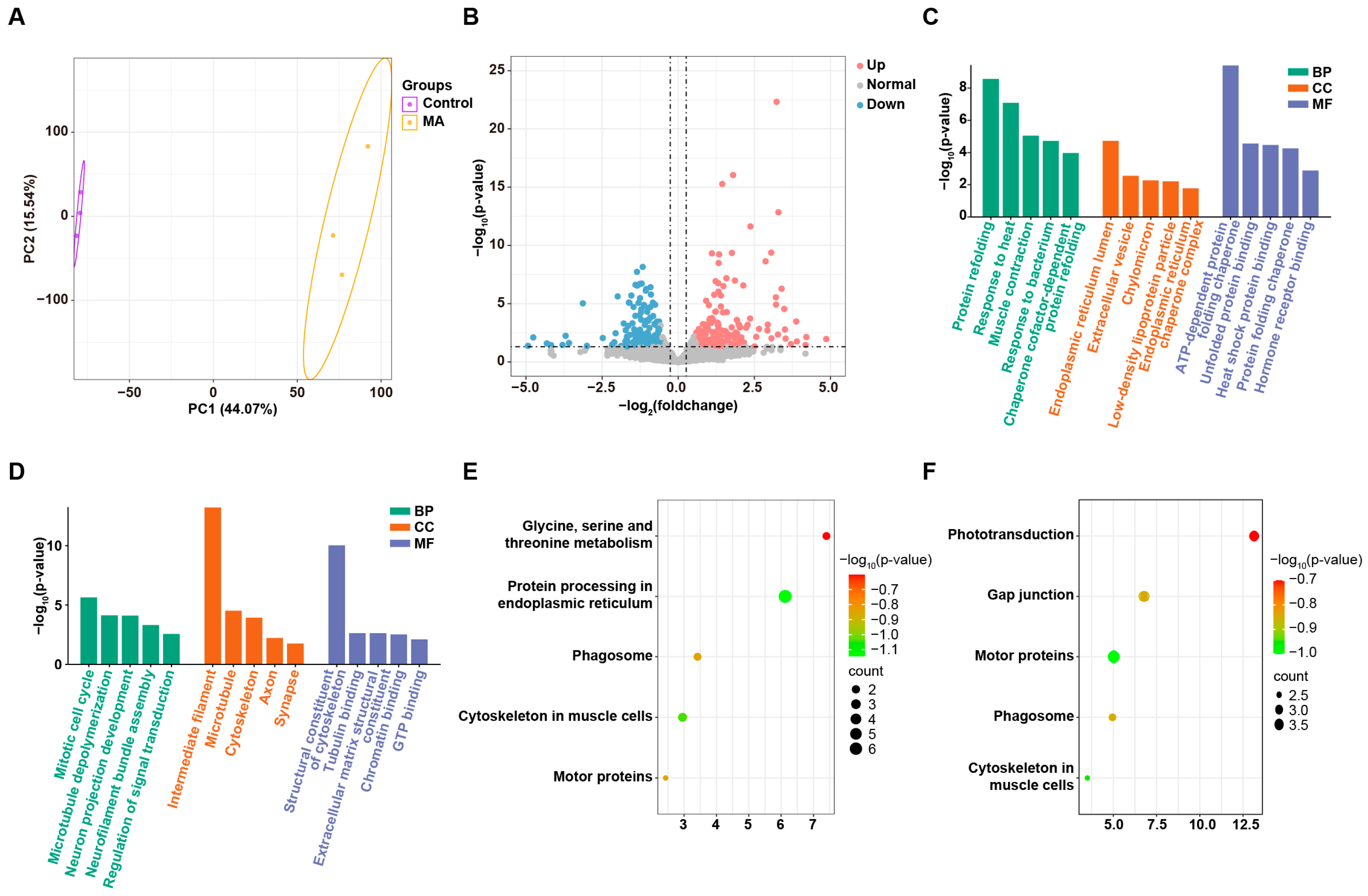
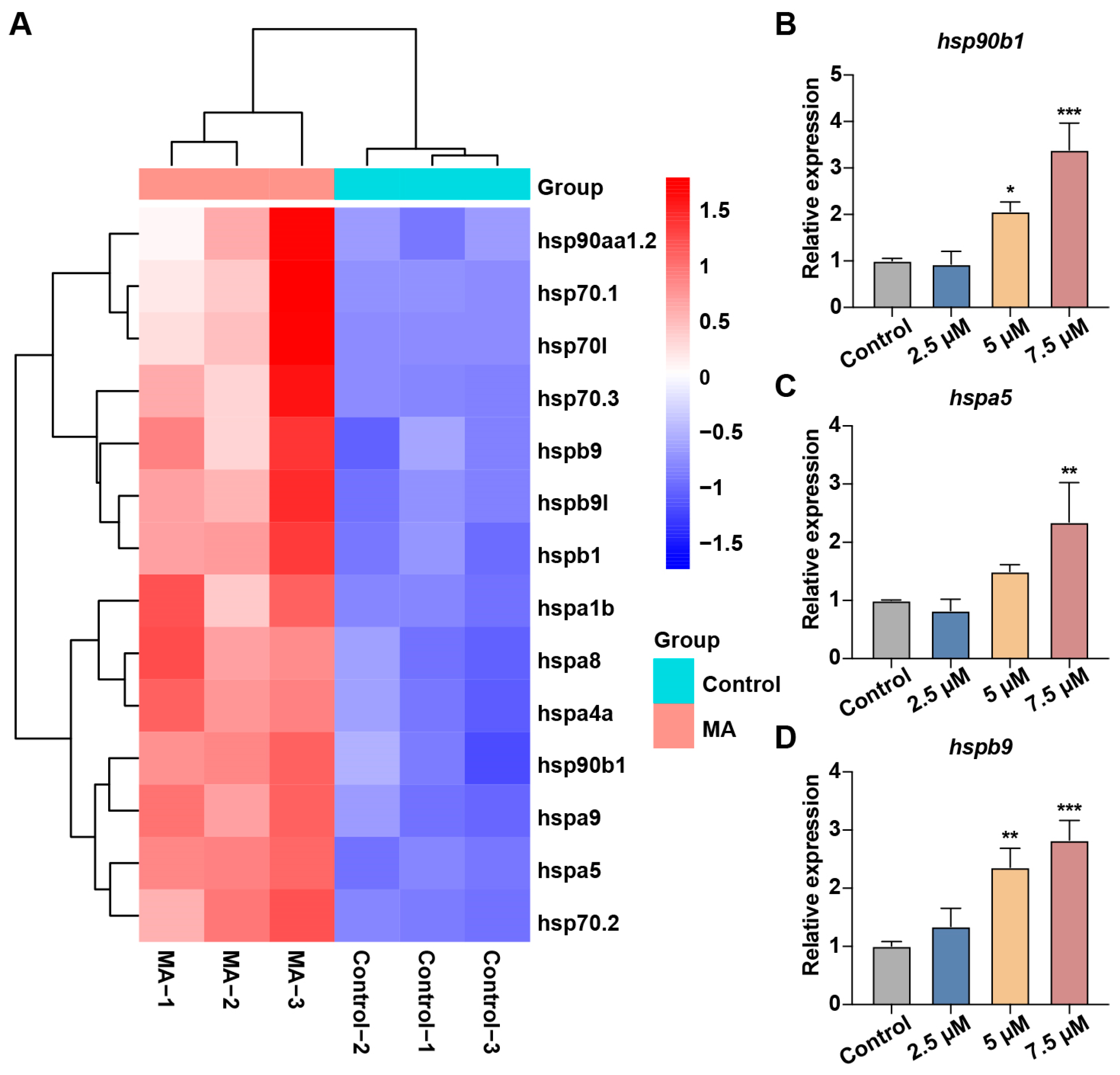
3.5. MA Induced UPR in Zebrafish Embryos
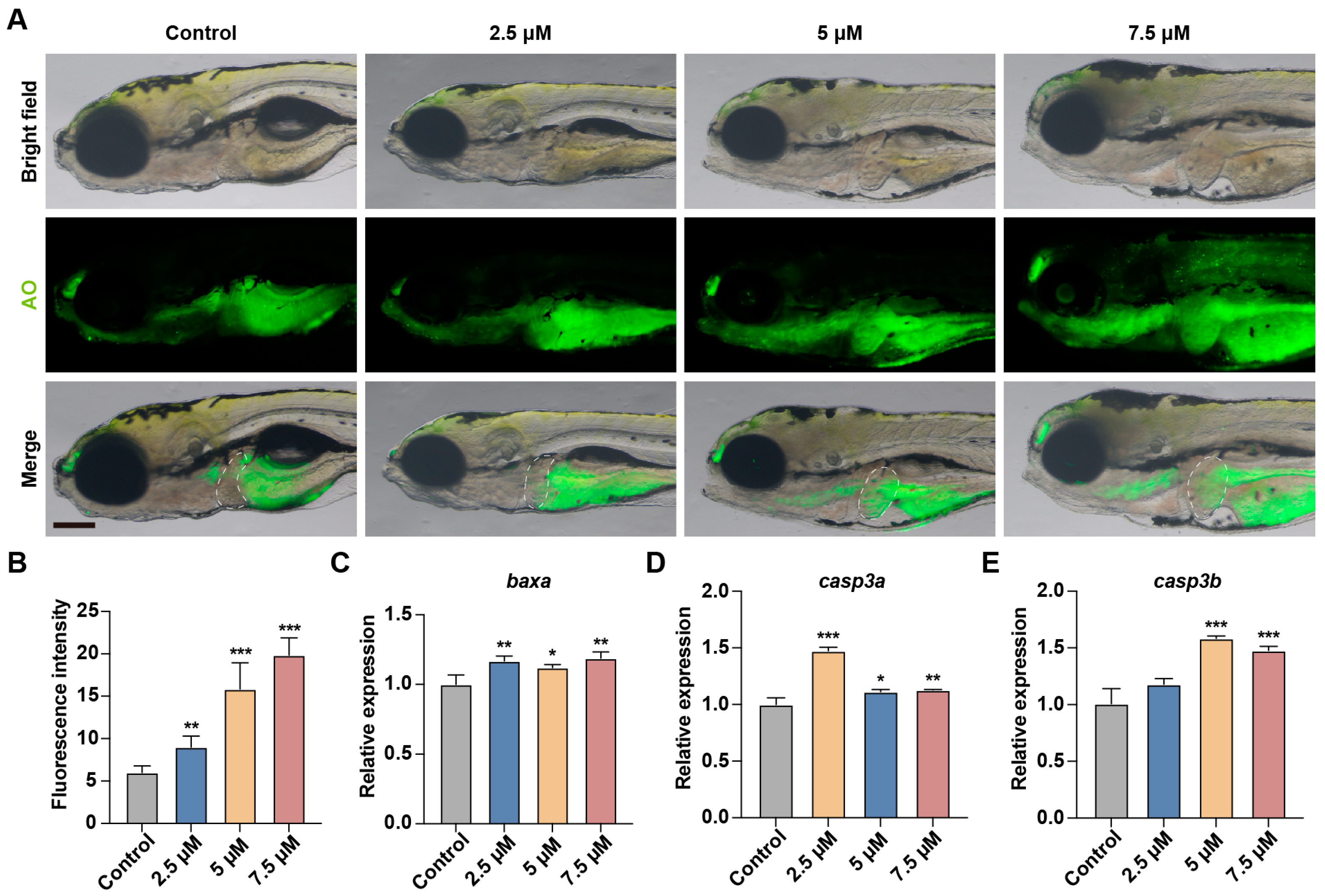
3.6. MA Induces Hepatocytes Apoptosis in Zebrafish Embryos
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MA | Mesaconitine |
| PUFAs | Polyunsaturated fatty acids |
| PPARα | Peroxisome proliferator-activated receptor α |
| ROS | Reactive oxygen species |
| UPR | Unfolded protein response |
| CZRC | China Zebrafish Resource Center |
| DEGs | Differentially expressed genes |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| AO | Acridine orange |
| SD | Standard deviation |
| PCA | Principal component analysis |
| BP | Biological processes |
| CC | Cellular components |
| MF | Molecular functions |
| LDL | Low-density lipoprotein |
| ER | Endoplasmic reticulum |
| HSP | Heat shock protein |
| FABP | Fatty acid binding protein |
References
- Sun, Z.; Yang, L.; Zhao, L.; Cui, R.; Yang, W. Neuropharmacological Effects of Mesaconitine: Evidence from Molecular and Cellular Basis of Neural Circuit. Neural Plast. 2020, 2020, 8814531. [Google Scholar] [CrossRef]
- Mitamura, M.; Horie, S.; Sakaguchi, M.; Someya, A.; Tsuchiya, S.; Van de Voorde, J.; Murayama, T.; Watanabe, K. Mesaconitine-induced relaxation in rat aorta: Involvement of Ca2+ influx and nitric-oxide synthase in the endothelium. Eur. J. Pharmacol. 2002, 436, 217–225. [Google Scholar] [CrossRef]
- Nesterova, Y.V.; Povetieva, T.N.; Suslov, N.I.; Semenov, A.A.; Pushkarskiy, S.V. Antidepressant activity of diterpene alkaloids of Aconitum baicalense Turcz. Bull. Exp. Biol. Med. 2011, 151, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ji, X.; Zuo, Z. Relationships between the Toxicities of Radix Aconiti Lateralis Preparata (Fuzi) and the Toxicokinetics of Its Main Diester-Diterpenoid Alkaloids. Toxins 2018, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, P.; Hou, Z.; Xie, J.; Wang, Y.; Yang, B.; Xu, Y.; Li, Y. Research on the Relationships between Endogenous Biomarkers and Exogenous Toxic Substances of Acute Toxicity in Radix Aconiti. Molecules 2016, 21, 1623. [Google Scholar] [CrossRef]
- Tan, Y.; Ko, J.; Liu, X.; Lu, C.; Li, J.; Xiao, C.; Li, L.; Niu, X.; Jiang, M.; He, X.; et al. Serum metabolomics reveals betaine and phosphatidylcholine as potential biomarkers for the toxic responses of processed Aconitum carmichaelii Debx. Mol. Biosyst. 2014, 10, 2305–2316. [Google Scholar] [CrossRef]
- Li, T.F.; Gong, N.; Wang, Y.X. Ester Hydrolysis Differentially Reduces Aconitine-Induced Anti-hypersensitivity and Acute Neurotoxicity: Involvement of Spinal Microglial Dynorphin Expression and Implications for Aconitum Processing. Front. Pharmacol. 2016, 7, 367. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, J.; Wu, Z.; Shi, Y.; Chen, M.; Li, M.; Hu, H.; Tian, K.; Lv, X.; Li, C.; et al. Involvement of autophagy in mesaconitine-induced neurotoxicity in HT22 cells revealed through integrated transcriptomic, proteomic, and m6A epitranscriptomic profiling. Front. Pharmacol. 2024, 15, 1393717. [Google Scholar] [CrossRef]
- Song, L.; Mi, S.; Zhao, Y.; Liu, Z.; Wang, J.; Wang, H.; Li, W.; Wang, J.; Zu, W.; Du, H. Integrated virtual screening and in vitro studies for exploring the mechanism of triterpenoids in Chebulae Fructus alleviating mesaconitine-induced cardiotoxicity via TRPV1 channel. Front. Pharmacol. 2024, 15, 1367682. [Google Scholar] [CrossRef]
- Chen, Q.; Deng, X.; Zhang, K.; Kang, Y.; Jiao, M.; Zhang, J.; Wang, C.; Li, F. Changes to PUFA-PPAR pathway during mesaconitine induced myocardial coagulative necrosis. Food Chem. Toxicol. 2023, 177, 113831. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Han, X.; Li, N.; Liu, K.; Kang, W. Aconitum alkaloids induce cardiotoxicity and apoptosis in embryonic zebrafish by influencing the expression of cardiovascular relative genes. Toxicol. Lett. 2019, 305, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Niitsu, H.; Fujita, Y.; Fujita, S.; Kumagai, R.; Takamiya, M.; Aoki, Y.; Dewa, K. Distribution of Aconitum alkaloids in autopsy cases of aconite poisoning. Forensic Sci. Int. 2013, 227, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, K.; Jiao, M.; Jiao, J.; Chen, D.; Yin, Y.; Zhang, J.; Li, F. Study on the Mechanism of Mesaconitine-Induced Hepatotoxicity in Rats Based on Metabonomics and Toxicology Network. Toxins 2022, 14, 486. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Shi, Y.; Cui, Y.; Xing, X.; Zhang, L.; Liu, D.; Zhang, Y.; Dong, J.; Jin, L.; Pang, M.; et al. Single-cell analysis reveals an Angpt4-initiated EPDC-EC-CM cellular coordination cascade during heart regeneration. Protein Cell 2023, 14, 350–368. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Schmittgen, Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophils at the crossroads of innate and adaptive immunity. J. Leukoc. Biol. 2020, 108, 377–396. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, Y.; Hiramatsu, N.; Kasai, A.; Hayakawa, K.; Okamura, M.; Yao, J.; Kitamura, M. Induction of apoptosis by cigarette smoke via ROS-dependent endoplasmic reticulum stress and CCAAT/enhancer-binding protein-homologous protein (CHOP). Free Radic. Biol. Med. 2008, 45, 50–59. [Google Scholar] [CrossRef]
- Kopec, A.K.; Luyendyk, J.P. Coagulation in liver toxicity and disease: Role of hepatocyte tissue factor. Thromb. Res. 2014, 133 (Suppl. S1), S57–S59. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, T.; Kristinsson, J.; Torsdottir, G.; Snaedal, J. Ceruloplasmin (Cp) and iron in connection with Parkinson’s disease (PD) and Alzheimer’s disease (AD). Laeknabladid 2012, 98, 531–537. [Google Scholar]
- Jin, Q.; Hu, Y.; Gao, Y.; Zheng, J.; Chen, J.; Gao, C.; Peng, J. Hhex and Prox1a synergistically dictate the hepatoblast to hepatocyte differentiation in zebrafish. Biochem. Biophys. Res. Commun. 2023, 686, 149182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Peng, H.; Yu, H. Bixafen causes hepatotoxicity and pancreas toxicity in zebrafish (Danio rerio). Ecotoxicology 2023, 32, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role During Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Zininga, T.; Ramatsui, L.; Shonhai, A. Heat Shock Proteins as Immunomodulants. Molecules 2018, 23, 2846. [Google Scholar] [CrossRef] [PubMed]
- Brenner, C.; Galluzzi, L.; Kepp, O.; Kroemer, G. Decoding cell death signals in liver inflammation. J. Hepatol. 2013, 59, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Tucker, M.B.; MacKenzie, S.H.; Maciag, J.J.; Ackerman, H.D.; Swartz, P.; Yoder, J.A.; Hamilton, P.T.; Clark, A.C. Phage display and structural studies reveal plasticity in substrate specificity of caspase-3a from zebrafish. Protein Sci. 2016, 25, 2076–2088. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Ling, S.C.; Wu, K.; Luo, Z. Identification of Five Key Genes Involved in Intrinsic Apoptotic Pathway From Yellow Catfish Pelteobagrus fulvidraco and Their Transcriptional Responses to High Fat Diet (HFD). Front. Physiol. 2019, 10, 921. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells 1998, 3, 697–707. [Google Scholar] [CrossRef]

| Designed qRT-PCR Primer Sequences (5′ to 3′) | |||
|---|---|---|---|
| Gene | Forward | Reverse | |
| Reference gene | actb2 | CCCAAACCCAAGTTCAGCCA | ACCCACGATGGATGGGAAGA |
| Apoptosis-related genes | baxa | TGGCAAGTTCAACTGGG-GAA | ATAACTGCGGATTCCGTCCC |
| casp3a | CCCAGTGGAGGCAGATTTCC | AGCATTGAGAC-GATGCAGGG | |
| casp3b | ACAACACCAGAAGCAGGACTT | TTTGCATCGCTTTGTCTGGC | |
| Liver Development-related genes | cp | CGCTTCTGGAACCGTCAGTC | CTCGTTGCCTGGGCTTTCTT |
| fabp10a | CCACCATGGACGGCAAGAAG | GACTGTCAGCGTCTCCACCA | |
| hhex | AATCCTCCGTCCACCGGTAA | GGGTGAACTGATGCTCGTCC | |
| tfa | GACTGCAGCTGCTCACACAA | TCTGCCTCTCACTCTCTGGG | |
| Unfolded protein response-related genes | hspa5 | CAGATCTGGCCAAAATGCGG | ATGACGGAGTGATGCGGTTT |
| hspb9 | TCCTCAACCTTCTCCAGGCT | CCTGGGACTCAGCAGATGAC | |
| hsp90b1 | TCTGTGCACTTTTGGCGTTC | TCGATTCACTTCTGCCTGGA | |
| Inflammation-related genes | il1b | TCTGCTCAGCCTGTGTGTTT | GAGACCCGCTGATCTCCTTG |
| il6 | ACTCAGAGACGAGCAGTTTGA | GCGGTCTGAAGGTTTGAGGA | |
| tnfa | TCACGCTCCATAAGACCCAG | AAATGGATGGCAGCCTTGGA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Zhuang, Y.; Liu, Y.; Zheng, Y.; Liu, X.; Lin, S.; Zheng, C.; Wu, Z. ROS-Mediated Unfolded Protein Response Activation Drives Hepatocyte Apoptosis in Mesaconitine-Induced Liver Injury. Toxics 2025, 13, 155. https://doi.org/10.3390/toxics13030155
Tian J, Zhuang Y, Liu Y, Zheng Y, Liu X, Lin S, Zheng C, Wu Z. ROS-Mediated Unfolded Protein Response Activation Drives Hepatocyte Apoptosis in Mesaconitine-Induced Liver Injury. Toxics. 2025; 13(3):155. https://doi.org/10.3390/toxics13030155
Chicago/Turabian StyleTian, Jin, Yubin Zhuang, Yinuo Liu, Yihong Zheng, Xuyang Liu, Shiyu Lin, Chenhua Zheng, and Zekai Wu. 2025. "ROS-Mediated Unfolded Protein Response Activation Drives Hepatocyte Apoptosis in Mesaconitine-Induced Liver Injury" Toxics 13, no. 3: 155. https://doi.org/10.3390/toxics13030155
APA StyleTian, J., Zhuang, Y., Liu, Y., Zheng, Y., Liu, X., Lin, S., Zheng, C., & Wu, Z. (2025). ROS-Mediated Unfolded Protein Response Activation Drives Hepatocyte Apoptosis in Mesaconitine-Induced Liver Injury. Toxics, 13(3), 155. https://doi.org/10.3390/toxics13030155






