New Methodologies and Techniques for Biomonitoring Pesticide Exposure in Agricultural Workers: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Selection Process
2.4. Data Collection Process and Data Items
2.5. Risk of Bias Assessment
3. Results
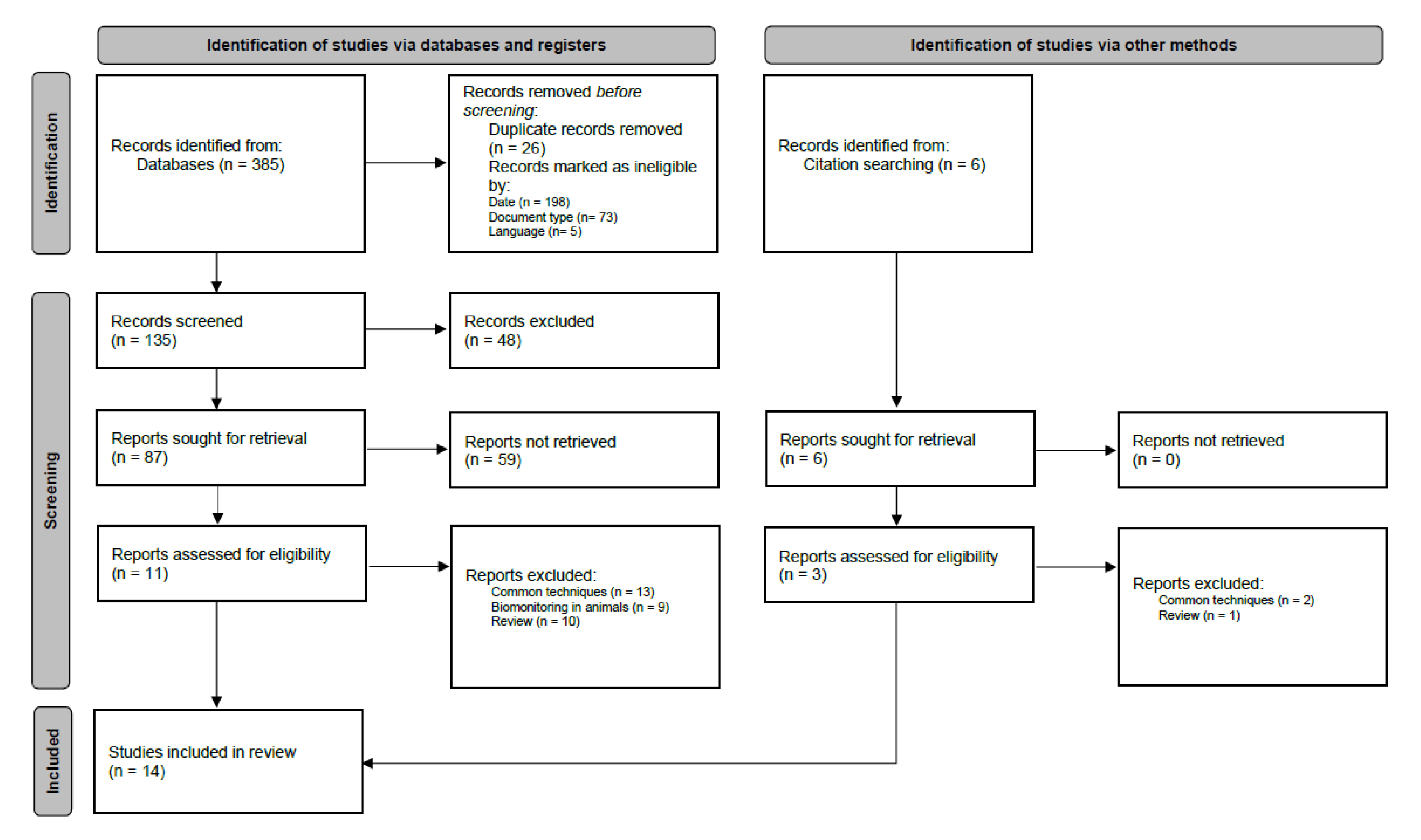
3.1. Study Selection
3.2. Included Studies’ Characteristics
3.3. New Methodologies and Techniques for Biomonitoring Pesticide Exposure
3.4. Advantages and Limitations of New Methodologies and Techniques for Biomonitoring Pesticide Exposure
3.5. Risk of Bias in Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-analyses |
| ROBINS | The Cochrane Risk of Bias in Non-Randomized Studies of Interventions |
| DNA | Deoxyribonucleic acid |
| IgG | Linear dichroism |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
| DAPs | Dialkyl phosphates |
| CBMN | Cytokinesis-block micronucleus assay |
| hOGG1 | Human 8-oxoguanine DNA glycosylase |
| ccf-DNA | Circulating cell-free DNA |
| LLE | Liquid extraction |
| ddPCR | Droplet digital PCR |
| FRET | Förster resonance energy transfer |
| ETU | Ethylene-thiourea |
| GC-MS | Gas chromatography–mass spectrometry |
| VA-SI-LLME | Vortex-assisted salt-induced liquid–liquid microextraction |
| UHPLC/MS/MS | Ultra-high-performance liquid chromatography |
| ADD | Absorbed daily dose |
| QueChERS | Quick, Easy, Cheap, Effective, Rugged, and Safe |
| ICTS | Immunochromatographic test strip |
| BChE | Butyrylcholinesterase |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| ROC | Receiver operating characteristic |
| PIXE | Particle-induced X-ray emission |
| BMCyt | Buccal micronucleus cytome assay |
| WHO-PEAM | Predicted Exposure Assessment Model by World Health Organization |
References
- Fernández, S.F.; Pastor, A.; Yusà, V.; Montesinos, L.; Pardo, O. Development of a novel methodology for determination of dialkyl phosphates in human urine using liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2019, 1130–1131, 121810. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.C.; Martins, I. Risk assessment of occupational pesticide exposure: Use of endpoints and surrogates. In Regulatory Toxicology and Pharmacology; Academic Press Inc.: New York, NY, USA, 2018; Volume 98, pp. 276–283. [Google Scholar]
- Moreira, A.; da Silva, M.V. Pesticide Application as a Risk Factor/Behaviour for Workers’ Health: A Systematic Review. Environments 2023, 10, 160. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Shin, Y.; Park, E.; Lee, J.; Keum, Y.S.; Kim, J.H. Occupational exposure and risk assessment for agricultural workers of thiamethoxam in vineyards. Ecotoxicol. Environ. Saf. 2022, 243, 113988. [Google Scholar] [CrossRef] [PubMed]
- Rattanawitoon, T.; Siriwong, W.; Shendell, D.; Fiedler, N.; Robson, M.G. An Evaluation of a Pesticide Training Program to Reduce Pesticide Exposure and Enhance Safety among Female Farmworkers in Nan, Thailand. Int. J. Environ. Res. Public Health 2023, 20, 6635. [Google Scholar] [CrossRef] [PubMed]
- Shammi, M.; Sultana, A.; Hasan, N.; Rahman, M.; Islam, S.; Doza, B.; Uddin, K. Pesticide exposures towards health and environmental hazard in Bangladesh: A case study on farmers’ perception. J. Saudi Soc. Agric. Sci. 2020, 19, 161–173. [Google Scholar] [CrossRef]
- Wong, H.L.; Garthwaite, D.G.; Ramwell, C.T.; Brown, C.D. Assessment of exposure of professional agricultural operators to pesticides. Sci. Total Environ. 2018, 619–620, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Cattelan, M.D.; Maurer, P.; Garcia, F.; Berro, L.F.; Machado, M.M.; Manfredini, V.; Piccoli, J.D. Occupational exposure to pesticides in family agriculture and the oxidative, biochemical and hematological profile in this agricultural model. Life Sci. 2018, 203, 177–183. [Google Scholar] [CrossRef]
- Fuhrimann, S.; Winkler, M.S.; Staudacher, P.; Weiss, F.T.; Stamm, C.; Eggen, R.I.; Lindh, C.H.; Menezes-Filho, J.A.; Baker, J.M.; Ramírez-Muñoz, F.; et al. Exposure to pesticides and health effects on farm owners and workers from conventional and organic agricultural farms in Costa Rica: Protocol for a cross-sectional study. JMIR Res. Protoc. 2019, 8, e10914. [Google Scholar] [CrossRef]
- Fargnoli, M.; Lombardi, M.; Puri, D.; Casorri, L.; Masciarelli, E.; Mandić-Rajčević, S.; Colosio, C. The safe use of pesticides: A risk assessment procedure for the enhancement of occupational health and safety (OHS) management. Int. J. Environ. Res. Public Health 2019, 16, 310. [Google Scholar] [CrossRef]
- López-Gálvez, N.; Wagoner, R.; Beamer, P.; de Zapien, J.; Rosales, C. Migrant farmworkers’ exposure to pesticides in Sonora, Mexico. Int. J. Environ. Res. Public Health 2018, 15, 2651. [Google Scholar] [CrossRef] [PubMed]
- Bossou, Y.M.; Côté, J.; Morin, É.; Dumais, É.; Bianchi, C.; Bouchard, M. Assessing the impact of coexposure on the measurement of biomarkers of exposure to the pyrethroid lambda-cyhalothrin in agricultural workers. Int. J. Hyg. Environ. Health 2023, 251, 114194. [Google Scholar] [CrossRef]
- Mueller, W.; Jones, K.; Fuhrimann, S.; Ahmad, Z.N.B.S.; Sams, C.; Harding, A.-H.; Povey, A.; Atuhaire, A.; Basinas, I.; van Tongeren, M.; et al. Factors influencing occupational exposure to pyrethroids and glyphosate: An analysis of urinary biomarkers in Malaysia, Uganda and the United Kingdom. Environ. Res. 2024, 242, 117651. [Google Scholar] [CrossRef] [PubMed]
- Kaymak, G.; Koldemir-Gündüz, M. The usages of biomarkers in occupational exposure of workers to pesticides. Interdiscip. Perspect. Occup. Saf. Health 2023, 119–131. [Google Scholar]
- Mercadante, R.; Polledri, E.; Rubino, F.M.; Mandic-Rajcevic, S.; Vaiani, A.; Colosio, C.; Moretto, A.; Fustinoni, S. Assessment of penconazole exposure in winegrowers using urinary biomarkers. Environ. Res. 2019, 168, 54–61. [Google Scholar] [CrossRef]
- HBM4EU—Science and Policy for a Healthy Future. Biomarcadores de Efeito: O Que Precisa de Saber. 2018. Available online: https://www.hbm4eu.eu/wp-content/uploads/2018/12/20166_brief_n1_biomarkers_PT_v02_HL_JG.pdf (accessed on 1 December 2024).
- Cao, L.; Zhang, H.; Li, F.; Zhou, Z.; Wang, W.; Ma, D.; Yang, L.; Zhou, P.; Huang, Q. Potential dermal and inhalation exposure to imidacloprid and risk assessment among applicators during treatment in cotton field in China. Sci. Total Environ. 2018, 624, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, M.; Wang, Y.; Xia, H.; Zhu, L.; Li, G.; Rong, L.; Dong, H.; Chen, R.; Tang, S.; et al. Cholinesterase homozygous genotype as susceptible biomarker of hypertriglyceridaemia for pesticide-exposed agricultural workers. Biomarkers 2021, 26, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Paniagua, D.; Parrón, T.; Alarcón, R.; Requena, M.; Lacasaña, M.; Hernández, A.F. Renal tubular dysfunction in greenhouse farmers exposed to pesticides unveiled by a panel of molecular biomarkers of kidney injury. Environ. Res. 2023, 238, 117200. [Google Scholar] [CrossRef] [PubMed]
- Aguera, R.G.; Freires, C.d.S.; de Oliveira, L.O.; Monteiro, L.R.; Lini, R.S.; Romoli, J.C.Z.; Freire, B.M.; Nerilo, S.B.; Junior, M.M.; Batista, B.L.; et al. Risk evaluation of occupational exposure of southern Brazilian flower farmers to pesticides potentially leading to cholinesterase inhibition and metals exposure. Environ. Toxicol. Pharmacol. 2022, 93, 103874. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Çayir, A.; Coskun, M.; Coskun, M.; Cobanoglu, H. DNA damage and circulating cell free DNA in greenhouse workers exposed to pesticides. Environ. Mol. Mutagen. 2018, 59, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Mandić-Rajčević, S.; Colosio, C. Methods for the identification of outliers and their influence on exposure assessment in agricultural pesticide applicators: A proposed approach and validation using biological monitoring. Toxics 2019, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- El Rahman, H.A.A.; Salama, M.; Gad El-Hak, S.A.; El-Harouny, M.A.; ElKafrawy, P.; Abou-Donia, M.B. A Panel of Autoantibodies Against Neural Proteins as Peripheral Biomarker for Pesticide-Induced Neurotoxicity. Neurotox. Res. 2018, 33, 316–336. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, G.; Falzone, L.; Costa, C.; Giambò, F.; Teodoro, M.; Vivarelli, S.; Libra, M.; Fenga, C. Chronic Pesticide Exposure in Farm Workers Is Associated with the Epigenetic Modulation of hsa-miR-199a-5p. Int. J. Environ. Res. Public Health 2022, 19, 7018. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, D.; Alderete, B.L.; de Souza, C.T.; Dias, J.F.; Niekraszewicz, L.; Cappetta, M.; Martínez-López, W.; Da Silva, J. DNA damage and epigenetic alteration in soybean farmers exposed to complex mixture of pesticides. Mutagenesis 2018, 33, 87–95. [Google Scholar] [CrossRef]
- Menouni, A.; Duca, R.C.; Berni, I.; Khouchoua, M.; Ghosh, M.; El Ghazi, B.; Zouine, N.; Lhilali, I.; Akroute, D.; Pauwels, S.; et al. The parental pesticide and offspring’s epigenome study: Towards an integrated use of human biomonitoring of exposure and effect biomarkers. Toxics 2021, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.F.; de Souza, M.R.; Benedetti, D.; Scotti, A.S.; Piazza, L.S.; Garcia, A.L.H.; Dias, J.F.; Niekraszewicz, L.A.B.; Duarte, A.; Bauer, D.; et al. Investigation of pesticide exposure by genotoxicological, biochemical, genetic polymorphic and in silico analysis. Ecotoxicol. Environ. Saf. 2019, 179, 135–142. [Google Scholar] [CrossRef]
- Jiang, M.; Chattopadhyay, A.N.; Geng, Y.; Rotello, V.M. An array-based nanosensor for detecting cellular responses in macrophages induced by femtomolar levels of pesticides. Chem. Commun. 2022, 58, 2890–2893. [Google Scholar] [CrossRef]
- Kumar, D.; Sinha, S.N.; Vasudev, K. Development and Validation of a New UFLC–MS/MS Method for the Detection of Organophosphate Pesticide Metabolites in Urine. Molecules 2023, 28, 5800. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, R.R.; Tsai, P.C.; Lin, P.I.D.; Wu, M.T.; Ponnusamy, V.K. Rapid and sensitive analytical procedure for biomonitoring of organophosphate pesticide metabolites in human urine samples using a vortex-assisted salt-induced liquid–liquid microextraction technique coupled with ultra-high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2020, 34 (Suppl. S1), e8565. [Google Scholar]
- Phung, D.; Miller, G.; Connell, D.; Chu, C. Is the World Health Organization predicted exposure assessment model for space spraying of insecticides applicable to agricultural farmers? Environ. Sci. Pollut. Res. 2019, 26, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Stefanelli, P.; Barbini, D.A.; Girolimetti, S. Pesticides and their metabolites in human urine: Development of multi-analyte method by LC-MS/MS and GC-MS/MS. J. Environ. Sci. Health B 2021, 56, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhao, Y.; Wang, L.; Paulsen, M.; Simpson, C.D.; Liu, F.; Du, D.; Lin, Y. Simultaneous detection of dual biomarkers from humans exposed to organophosphorus pesticides by combination of immunochromatographic test strip and ellman assay. Biosens. Bioelectron. 2018, 104, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Sine, H.; Grafel KEl Alkhammal, S.; Achbani, A.; Filali, K. Serum cholinesterase biomarker study in farmers—Souss Massa region-, Morocco: Case–control study. Biomarkers 2019, 24, 771–775. [Google Scholar] [CrossRef]
- Zúñiga-Venegas, L.; Pancetti, F.C. DNA damage in a Chilean population exposed to pesticides and its association with PON1 (Q192R and L55M) susceptibility biomarker. Environ. Mol. Mutagen. 2022, 63, 215–226. [Google Scholar] [CrossRef] [PubMed]
| Author(s) | Country | Study Type | Methodology | Validation |
|---|---|---|---|---|
| [27] | Brazil | Cross-sectional Farmworkers exposed (N = 137) C (N = 83) | Alkaline comet assay modified with limitation enzyme (hOGG1: human 8-oxoguanine DNA glycosylase) to identify oxidised guanine. | Comparison with buccal micronucleus cytome assay, global methylation, haematological parameters, biochemical analyses, and particle-induced X-ray emission (PIXE) method. |
| [23] | Turkey | Observational cohort Greenhouse workers (N = 72) C (N = 51) | Cytokinesis-block micronucleus assay (CBMN) + measurement of circulating cell-free DNA (ccf-DNA) in the blood of pesticide-exposed greenhouse workers using a Qubit® fluorometer to assess DNA damage in lymphocytes. | Tests with high-sensitivity kits, Quant-it™. |
| [25] | Egypt | Observational cohort Greenhouse workers (N = 72) CG (N = 51) | Western blot analysis to verify the presence of IgG class autoantibodies against ten specific neuronal and glial proteins (indicators of brain damage). | Neutralisation of each autoantibody in the serum with its target protein; unpaired t-test |
| [1] | Spain | Validation Method N = 20 | Liquid chromatography–tandem mass spectrometry (LC-MS/MS) for quantitative determination of six dialkyl phosphates (DAPs) in human urine samples + liquid extraction (LLE) method with a salting-out procedure (without pre-treatment or purification). | Linearity, accuracy, precision, quantification limits, and specificity. High recoveries and accurate results. |
| [26] | Italy | Observational case-control Farmworkers (N = 28) C (N = 9) | Analysis of levels of HSA-miR-199a-5p by droplet digital PCR (ddPCR) on liquid biopsy samples + absolute quantification of levels with QuantaSoft software + statistical analyses. | Receiver operating characteristic (ROC) curves. |
| [30] | Not specified | Experimental | Robust detection of macrophage responses to femtomolar concentrations of common pesticides (orders of magnitude lower than traditional ones) using specific Förster resonance energy transfer (FRET) sensors. | Sensitivity and specificity. Linear Discriminant Analysis (LDA). |
| [31] | India | Validation Method Individuals exposed during cultivation (N = 100) C(N = 50) | A method linking ultrafast liquid chromatography with tandem mass spectrometry (UFLC–MS/MS) for the detection of dialkyl phosphates (DAPs) in human urine samples. | Selectivity, linearity, limit of detection and quantification, recovery, precision and accuracy, matrix effect, and stability. |
| [24] | Italy | Observational Agricultural workers (N = 27) | Estimative dermal exposure with a patch methodology + 24 h post-exposure urine biomonitoring for Ethylene-thiourea (ETU) + detection of outliers via multiplication of the median, the median absolute deviation, and boxplots. | Modified Z score. |
| [28] | Morocco | Cross-sectional and prospective cohort Farmworkers (N = 300) Pregnant women’s (N = 1000) | Multiple sample analysis by LC-MS and GC-MS analytical methods for biomonitoring pesticide metabolites with oxidative stress biomarkers (Glutathione, Malondialdehyde, and 8-OHdG). | Calibration curves comparable to the matrix for each analytical procedure, establishing basal concentrations of pesticides with samples from participants without exposure. |
| [29] | Brazil | Cross-sectional observational Farmworkers (N = 76) C (N = 72) | Particle-induced X-ray emission (PIXE) technique for the identification and quantification of inorganic elements in blood samples + buccal micronucleus cytome assay (BMCyt) (genotoxicity biomarker) + in silico analysis. | Standard protocols, Mann–Whitney test, Spearman’s correlation + Bonferroni correction |
| [32] | China | Validation Method Farmworkers (N = 3) C (N = 3) | Vortex-assisted salt-induced liquid–liquid microextraction (VA-SI-LLME) + liquid chromatography/tandem mass spectrometry (UHPLC/MS/MS) for the biomonitoring of organophosphate pesticide metabolites in human urine samples. | Linearity, recovery, precision, limit of detection (LOD), and limit of quantification (LOQ). |
| [33] | Vietnam | Validation Method Farmworkers (N = 18) | Application of a questionnaire to obtain parameters for predicting daily doses of chlorpyrifos exposure without biological monitoring + comparison with absorbed daily dose (ADD) measured by biomonitoring. | Wilcoxon Test. |
| [34] | Italy | Method development | QueChERS procedure + UHPLC-MS/MS + GC-MS/MS method for Fluazinam, Chlorpyrifos, Ethyl, Methyl, Buprofezin, Boscalid, Imidacloprid, Thiacloprid, Phosmet, Captan, and Metiram quantification. | Linearity, recovery, and analysis of variance (one-way ANOVA). |
| [35] | United States, Pakistan | Validation Method Farmworkers (N = 124) | Immunochromatographic test strip (ICTS) for the simultaneous detection of BChE activity and total BChE in human plasma samples exposed to organophosphorus pesticides. | Comparison of results between immunochromatographic test strip (ICTS), liquid chromatography–tandem mass spectrometry (LC-MS/MS) (standard and highly accurate analytical technique), and Pearson’s correlation. |
| New Technique | Biomarkers | Advantages | Limitations | Reference |
|---|---|---|---|---|
| Alkaline Comet Assay Modified with limitation enzyme (hOGG1: human 8-oxoguanine DNA glycosylase) | Effect biomarker | Sensitive and specific detection of oxidised guanine; Assessment of DNA damage at the molecular level; Method combined with non-invasive and precise techniques. | The test does not provide information on cell repair or the persistence of DNA damage; Failure to detect variations in damage; The study design does not allow links to be established between pesticide exposure and observed health effects. | [27] |
| The potential of ccf-DNA as a biomarker for pesticide exposure | Exposure biomarker | A less invasive technique capable of detecting cellular processes in real time; A stable and adequate biomarker for pesticide biomonitoring; Quick and easy measurement with accurate equipment. | Limitations in the interpretation of ccf-DNA as the only indicator of genotoxicity. Sample characteristics and generalization; Confounding factors are not considered. | [23] |
| The detection of serum autoantibodies against neural proteins as a biomarker for pesticide-induced neurotoxicity | Effect biomarker | A non-invasive technique; Serum autoantibodies as biomarkers; High specificity and direct association with brain lesions; The use of Western blot analysis as a standard measure. | Sample characteristics and generalization; More related studies are needed to support the obtained results. | [25] |
| The development of a new method to measure dialkyl phosphates in human urine using liquid chromatography–tandem mass spectrometry | Exposure biomarker | Simpler and faster analysis than gas chromatography; Optimum chromatographic determination in a quicker time; A method validated according to ISO/IEC 17025; Simplification of the extraction process; Accurate limits of quantification (LOQs); Suitable for routine urine analysis in biomonitoring. | The presence of a significant matrix effect (ME) in the determination of urinary DAP; Availability and the cost of individual isotopically labelled internal standards for each DAP; A calibration curve for synthetic urine. | [1] |
| The use of a high-sensitive droplet digital PCR assay to identify changes in the levels of the selected microRNA | Exposure biomarker | High sensitivity and accuracy in the absolute quantification of nucleic acids, ideal for the monitoring of epigenetic changes associated with exposure to pesticides; Robust against inhibitors commonly found in biological samples, allowing for reliable analysis in complex matrices such as serum and plasma. | Sample size; Possible involvement of other miRNAs or complex interactions between multiple epigenetic factors; Not approved by the ethics committee; Pilot study. | [26] |
| A FRET-based nanosensor array planned to extend responses to modifications in cell surfaces regarding pesticide exposure | Effect biomarker | High sensitivity and lower detection limits than current cell-based methods; A sensor matrix with differentiated reactions to diverse groups of pesticides. | Dependence of macrophage cell lineage models; The specificity of the sensor set for different types of pesticides; Difficulties in practical application. | [30] |
| A new UFLC–MS/MS method for the detection of organophosphate pesticide metabolites in urine | Exposure biomarker | A sensitive, accurate, and selective method; A small sample volume; Efficiency in extracting target analytes; Minimal matrix effect; Maintains analyte stability. | Not specified | [31] |
| Methods for identifying and validating outliers in biological monitoring | Exposure biomarker | Coverage of the patch methodology; Modified Z score accuracy; The use of a previously validated data collection form; A significant sample volume; A standardised methodology for application in future risk assessment studies. | Sample characteristics; A lack of “real” exposure measurement; Data variability; Limitations of the adhesive method in terms of dermal exposure. | [24] |
| The integrated use of human biomonitoring of exposure: The PaPOE study | Exposure and effect biomarkers | A comprehensive approach to assessing the impact of pesticide exposure on the epigenome; The study design allows for an analysis of the immediate and long-term epigenetic effects of exposure to pesticides; The integration of biomonitoring techniques; A contribution to policymaking. | Sample characteristics and generalization; Genetic variation is not considered; Understanding the isolated effects of pesticide mixtures can be challenging due to their complexity; A lack of couples in the sample to determine the influence of each occupational exposure on the offspring; A loss to follow-up of participants; The results are not shared individually. | [28] |
| Advanced molecular techniques and bioinformatics tools (particle-induced X-ray emission (PIXE)) for the detection and quantification of inorganic elements in blood samples | Effect biomarker | Comprehensive use of BmCyT; Quantitative detection of inorganic elements in the blood using PIXE as a measure of exposure to harmful substances present in pesticides; The inclusion of polymorphic genetic analyses (XRCC1 and PON1 genotypes) relevant to DNA repair and pesticide metabolism (respectively); The use of in silico analysis to model potential interactions. | Failure to analyse causal inferences between pesticide exposure and observed genotoxic effects; Sample characteristics and generalization; Limited sensitivity for a butyrylcholinesterase (BChE) biomarker in the study; Genetic variation. | [29] |
| VA-SI-LLME-UHPLC/MS/MS | Exposure biomarker | A short agitation time and fast extraction (six minutes in total); High sensitivity and accuracy; Profitable and environmentally conscious (use of only organic solvents); Efficiency in practical applications. | Not specified | [32] |
| Validation of the World Health Organization Predicted Exposure Assessment Model (WHO-PEAM) for chlorpyrifos exposure | Exposure biomarker | Exposure prediction without biomonitoring; A more accessible technique; A consistent alternative for assessing exposure to chlorpyrifos given the correlation coefficient values; A practical tool for risk management. | The influence of the sample size on statistics; The total variability is not considered; The study did not include activities that could increase exposure, such as circulation in recently treated areas; The need to improve the parameters addressed to increase accuracy. | [33] |
| A multi-analyte method using LC-MS/MS and GC-MS/MS for the quantification of pesticides and metabolites in human urine | Exposure biomarker | An accurate, consistent, easy, cheap, and fast method; Extensive applications in biomonitoring; A multi-analyte approach; Satisfactory results for linearity, recovery, and accuracy. | The PSA adsorbent is inadequate; Unacceptable results for linearity, accuracy, and precision regarding 3-OH THPI; The need for sophisticated analytical equipment and complex sample preparation; Dermal and inhalation exposure routes are not considered. | [34] |
| Innovative sandwich immunoassay-based immunochromatographic test strips (ICTSs) for measuring butyrylcholinesterase (BChE) activity and the total amount of BChE in human plasma samples | Exposure biomarker | Simultaneous measurement of BChE activity values from a small sample volume; The use of a monoclonal antibody simplifies the assay, avoiding the need to use multiple antibodies; A rapid approach; A cheap and portable method. | Not specified | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, A.; Guedes, J.; Vieira da Silva, M. New Methodologies and Techniques for Biomonitoring Pesticide Exposure in Agricultural Workers: A Systematic Review. Toxics 2025, 13, 104. https://doi.org/10.3390/toxics13020104
Moreira A, Guedes J, Vieira da Silva M. New Methodologies and Techniques for Biomonitoring Pesticide Exposure in Agricultural Workers: A Systematic Review. Toxics. 2025; 13(2):104. https://doi.org/10.3390/toxics13020104
Chicago/Turabian StyleMoreira, Andreia, Joana Guedes, and Manuela Vieira da Silva. 2025. "New Methodologies and Techniques for Biomonitoring Pesticide Exposure in Agricultural Workers: A Systematic Review" Toxics 13, no. 2: 104. https://doi.org/10.3390/toxics13020104
APA StyleMoreira, A., Guedes, J., & Vieira da Silva, M. (2025). New Methodologies and Techniques for Biomonitoring Pesticide Exposure in Agricultural Workers: A Systematic Review. Toxics, 13(2), 104. https://doi.org/10.3390/toxics13020104









