Qualitative Exploration of Ultrastructural Effects of Perfluorooctanoic Acid on Carp Gills: Mitochondria-Rich Cells as Candidate Biomarkers of Cytotoxicity
Abstract
1. Introduction
2. Materials and Methods
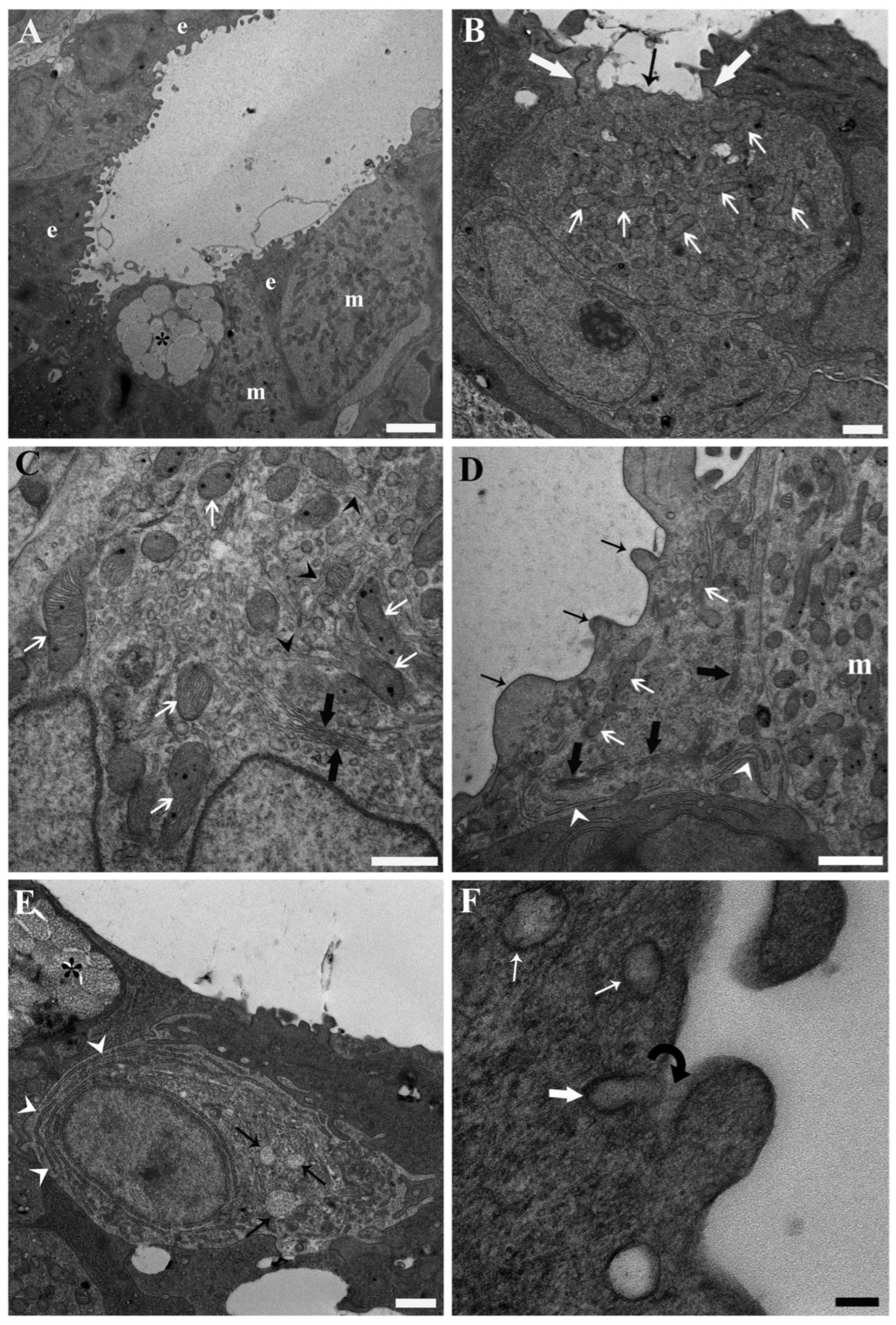
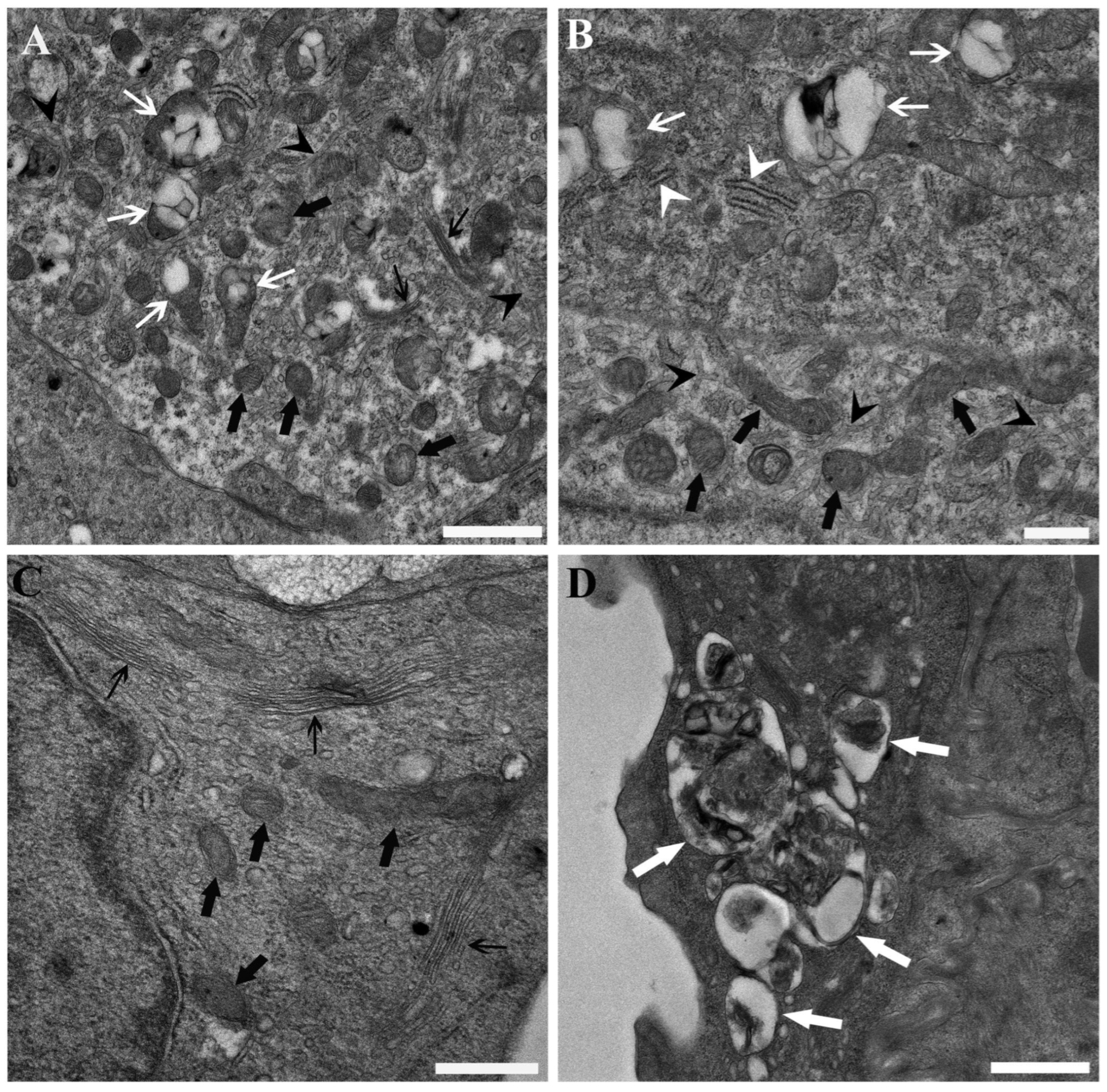
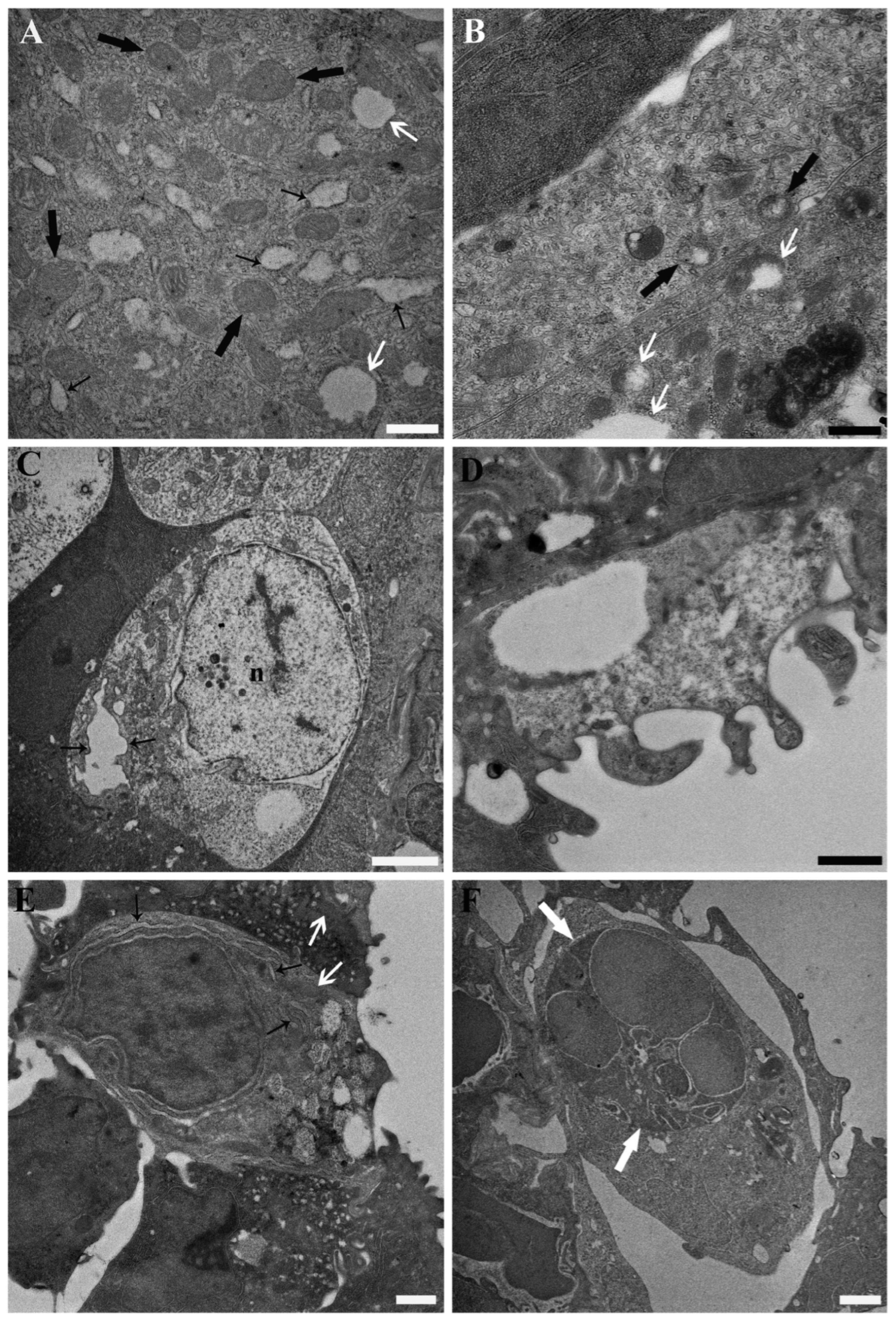
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leung, S.C.E.; Wanninayake, D.; Chen, D.; Nguyen, N.-T.; Li, Q. Physicochemical Properties and Interactions of Perfluoroalkyl Substances (PFAS)—Challenges and Opportunities in Sensing and Remediation. Sci. Total Environ. 2023, 905, 166764. [Google Scholar] [CrossRef]
- Ding, G.; Peijnenburg, W.J.G.M. Physicochemical Properties and Aquatic Toxicity of Poly- and Perfluorinated Compounds. Crit. Rev. Environ. Sci. Technol. 2013, 43, 598–678. [Google Scholar] [CrossRef]
- Cousins, I.T.; Dewitt, J.C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Ng, C.A.; Scheringer, M.; Vierke, L.; et al. Strategies for Grouping Per-and Polyfluoroalkyl Substances (PFAS) to Protect Human and Environmental Health. Environ. Sci. Process. Impacts 2020, 22, 1444–1460. [Google Scholar] [CrossRef]
- Sinclair, G.M.; Long, S.M.; Jones, O.A.H. What Are the Effects of PFAS Exposure at Environmentally Relevant Concentrations? Chemosphere 2020, 258, 127340. [Google Scholar] [CrossRef]
- Evich, M.G.; Davis, M.J.B.; McCord, J.P.; Acrey, B.; Awkerman, J.A.; Knappe, D.R.U.; Lindstrom, A.B.; Speth, T.F.; Tebes-Stevens, C.; Strynar, M.J.; et al. Per- and Polyfluoroalkyl Substances in the Environment. Science 2022, 375, 512. [Google Scholar] [CrossRef]
- Yu, R.-S.; Yu, H.-C.; Yang, Y.-F.; Singh, S. A Global Overview of Per- and Polyfluoroalkyl Substance Regulatory Strategies and Their Environmental Impact. Toxics 2025, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Vierke, L.; Staude, C.; Biegel-Engler, A.; Drost, W.; Schulte, C. Perfluorooctanoic Acid (PFOA)—Main Concerns and Regulatory Developments in Europe from an Environmental Point of View. Environ. Sci. Eur. 2012, 24, 16. [Google Scholar] [CrossRef]
- Pivato, A.; Beggio, G.; Maggi, S.; Marrone, F.; Bonato, T.; Peres, F.; Peng, W.; Lavagnolo, M.C. The Presence of PFAS in Wastes and Related Implications on the Current and Proposed European Regulatory Framework: A Systemic Critical Review. Detritus 2024, 26, 89–105. [Google Scholar] [CrossRef]
- Wasel, O.; Thompson, K.M.; Freeman, J.L. Assessment of Unique Behavioral, Morphological, and Molecular Alterations in the Comparative Developmental Toxicity Profiles of PFOA, PFHxA, and PFBA Using the Zebrafish Model System. Environ. Int. 2022, 170, 107642. [Google Scholar] [CrossRef] [PubMed]
- Hayman, N.T.; Rosen, G.; Colvin, M.A.; Conder, J.; Arblaster, J.A. Aquatic Toxicity Evaluations of PFOS and PFOA for Five Standard Marine Endpoints. Chemosphere 2021, 273, 129699. [Google Scholar] [CrossRef] [PubMed]
- Teaf, C.M.; Garber, M.M.; Covert, D.J.; Tuovila, B.J. Perfluorooctanoic Acid (PFOA): Environmental Sources, Chemistry, Toxicology, and Potential Risks. Soil Sediment Contam. Int. J. 2019, 28, 258–273. [Google Scholar] [CrossRef]
- Huang, H.; Lyu, X.; Xiao, F.; Fu, J.; Xu, H.; Wu, J.; Sun, Y. Three-Year Field Study on the Temporal Response of Soil Microbial Communities and Functions to PFOA Exposure. J. Hazard. Mater. 2024, 476, 135008. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.L.; Small, J.; Sibley, P.K.; Boudreau, T.M.; Brain, R.A.; Mabury, S.A.; Solomon, K.R. Microcosm Evaluation of the Fate, Toxicity, and Risk to Aquatic Macrophytes from Perfluorooctanoic Acid (PFOA). Arch. Environ. Contam. Toxicol. 2005, 49, 307–316. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Pan, Y.; Dai, J.; Tang, J. Trophic Behaviors of PFOA and Its Alternatives Perfluoroalkyl Ether Carboxylic Acids (PFECAs) in a Coastal Food Web. J. Hazard. Mater. 2023, 452, 131353. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, G.; Sonne, C.; Rigét, F.F.; Dietz, R.; Letcher, R.J. Bioaccumulation and Biomagnification of Perfluoroalkyl Acids and Precursors in East Greenland Polar Bears and Their Ringed Seal Prey. Environ. Pollut. 2019, 252, 1335–1343. [Google Scholar] [CrossRef]
- Simonnet-Laprade, C.; Budzinski, H.; Maciejewski, K.; Menach, K.L.; Santos, R.; Alliot, F.; Goutte, A.; Labadie, P. Biomagnification of Perfluoroalkyl Acids (PFAAs) in the Food Web of an Urban River: Assessment of the Trophic Transfer of Targeted and Unknown Precursors and Implications. Environ. Sci. Process. Impacts 2019, 21, 1864–1874. [Google Scholar] [CrossRef]
- Cheng, H.; Lv, C.; Li, J.; Wu, D.; Zhan, X.; Song, Y.; Zhao, N.; Jin, H. Bioaccumulation and Biomagnification of Emerging Poly- and Perfluoroalkyl Substances in Marine Organisms. Sci. Total Environ. 2022, 851, 158117. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Yang, X.; Geng, Z.; Zhang, L.; Huang, Z.; Shi, C.; Xie, Q.; Zhang, F. Health Effects of PFASs on Five Major Human Cancers: A Network Toxicology Perspective on Molecular Pathogenesis. J. Environ. Sci. 2025; in press. [Google Scholar] [CrossRef]
- Li, K.; Gao, P.; Xiang, P.; Zhang, X.; Cui, X.; Ma, L.Q. Molecular Mechanisms of PFOA-Induced Toxicity in Animals and Humans: Implications for Health Risks. Environ. Int. 2017, 99, 43–54. [Google Scholar] [CrossRef]
- Zhou, J.-X.; Qin, X.-D.; Liu, X.; He, W.-T.; Zeeshan, M.; Dharmage, S.C.; Perret, J.; Bui, D.; Zhang, Y.-T.; Sun, M.-K.; et al. Exposure-Effect of PFOS and PFOA on Lung Function: An Integrated Approach with Epidemiological, Cellular, and Animal Studies. Environ. Res. 2025, 272, 121175. [Google Scholar] [CrossRef]
- Manera, M.; Giari, L. Segmentation of Renal Thyroid Follicle Colloid in Common Carp: Insights into Perfluorooctanoic Acid-Induced Morphometric Alterations. Toxics 2024, 12, 369. [Google Scholar] [CrossRef]
- Manera, M.; Castaldelli, G.; Giari, L. Perfluorooctanoic Acid Affects Thyroid Follicles in Common Carp (Cyprinus carpio). Int. J. Environ. Res. Public Health 2022, 19, 9049. [Google Scholar] [CrossRef] [PubMed]
- Manera, M.; Castaldelli, G.; Guerranti, C.; Giari, L. Effect of Waterborne Exposure to Perfluorooctanoic Acid on Nephron and Renal Hemopoietic Tissue of Common Carp Cyprinus carpio. Ecotoxicol. Environ. Saf. 2022, 234, 113407. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Giari, L.; Guerranti, C.; Tognon, M.; Castaldelli, G.; Fano, E.A.; Martini, F. Environmental Doses of Perfluorooctanoic Acid Change the Expression of Genes in Target Tissues of Common Carp. Environ. Toxicol. Chem. 2018, 37, 942–948. [Google Scholar] [CrossRef]
- Health and Ecological Criteria Division—Office of Science and Technology—Office of Water. Interim Drinking Water Health Advisory: Perfluorooctanoic Acid (PFOA) CASRN 335-67-1; Office of Water (4304T) Office of Science and Technology: Washington, DC, USA, 2022; pp. 1–27. [Google Scholar]
- Rehman, A.U.; Crimi, M.; Andreescu, S. Current and Emerging Analytical Techniques for the Determination of PFAS in Environmental Samples. Trends Environ. Anal. Chem. 2023, 37, e00198. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, K.; Park, K.; Sung, C.; Yu, S.D.; Kim, P.; Seong, C.; Yu, S.D.; Kim, P. Adverse Effects of Perfluoroalkyl Acids on Fish and Other Aquatic Organisms: A Review. Sci. Total Environ. 2020, 707, 135334. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Lu, Y.; Zhou, Y.; Li, Q.; Zhang, M.; Han, G.; Cui, H.; Jeppesen, E. Bioaccumulation, Trophic Transfer and Biomagnification of Perfluoroalkyl Acids (PFAAs) in the Marine Food Web of the South China Sea. J. Hazard. Mater. 2021, 405, 124681. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Wu, P.; Wang, L.; Li, Q.; Li, X.; Luo, Y. Toxicity of Per- and Polyfluoroalkyl Substances to Aquatic Vertebrates. Front. Environ. Sci. 2023, 11, 1101100. [Google Scholar] [CrossRef]
- Friese, C.; Nuyts, N. Posthumanist Critique and Human Health: How Nonhumans (Could) Figure in Public Health Research. Crit. Public Health 2017, 27, 303–313. [Google Scholar] [CrossRef]
- Haschek, W.M.; Rousseaux, C.G.; Wallig, M.A. Toxicologic Pathology: An Introduction. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology, Third Edition: Volume 1–3; Academic Press: Cambridge, MA, USA, 2013; Volume 1, pp. 1–9. ISBN 978-0-12-415759-0. [Google Scholar]
- Manera, M. Rodlet Cell Morpho–Numerical Alterations as Key Biomarkers of Fish Responses to Toxicants and Environmental Stressors. Toxics 2024, 12, 832. [Google Scholar] [CrossRef]
- Choi, T.-Y.; Choi, T.-I.; Lee, Y.-R.; Choe, S.-K.; Kim, C.-H. Zebrafish as an Animal Model for Biomedical Research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef]
- Manera, M. Texture Analysis as a Discriminating Tool: Unmasking Rodlet Cell Degranulation in Response to a Contaminant of Emerging Concern. Front. Biosci.-Landmark 2024, 29, 79. [Google Scholar] [CrossRef]
- Kim, W.-K.; Lee, S.-K.; Jung, J. Integrated Assessment of Biomarker Responses in Common Carp (Cyprinus carpio) Exposed to Perfluorinated Organic Compounds. J. Hazard. Mater. 2010, 180, 395–400. [Google Scholar] [CrossRef]
- Petre, V.A.; Chiriac, F.L.; Lucaciu, I.E.; Paun, I.; Pirvu, F.; Iancu, V.I.; Novac, L.; Gheorghe, S. Tissue Bioconcentration Pattern and Biotransformation of Per-Fluorooctanoic Acid (PFOA) in Cyprinus carpio (European Carp)—An Extensive In Vivo Study. Foods 2023, 12, 1423. [Google Scholar] [CrossRef] [PubMed]
- Manera, M.; Casciano, F.; Giari, L. Ultrastructural Alterations of the Glomerular Filtration Barrier in Fish Experimentally Exposed to Perfluorooctanoic Acid. Int. J. Environ. Res. Public Health 2023, 20, 5253. [Google Scholar] [CrossRef]
- Wallig, M.A.; Janovitz, E.B. Morphologic Manifestations of Toxic Cell Injury. In Fundamentals of Toxicologic Pathology; Wallig, M.A., Haschek, W.M., Rousseaux, C.G., Bolon, B., Eds.; Academic Press: London, UK, 2018; pp. 59–81. ISBN 978-0-12-809842-4. [Google Scholar]
- Grover, A.; Sinha, R.; Jyoti, D.; Faggio, C. Imperative Role of Electron Microscopy in Toxicity Assessment: A Review. Microsc. Res. Tech. 2021, 85, 1976–1989. [Google Scholar] [CrossRef] [PubMed]
- Manera, M.; Castaldelli, G.; Fano, E.A.; Giari, L. Perfluorooctanoic Acid-Induced Cellular and Subcellular Alterations in Fish Hepatocytes. Environ. Toxicol. Pharmacol. 2021, 81, 103548. [Google Scholar] [CrossRef]
- Wu, D.-L.; Cheng, L.; Rao, Q.-X.; Wang, X.-L.; Zhang, Q.-C.; Yao, C.-X.; Chen, S.-S.; Liu, X.; Song, W.; Zhou, J.-X.; et al. Toxic Effects and Transcriptional Responses in Zebrafish Liver Cells Following Perfluorooctanoic Acid Exposure. Aquat. Toxicol. 2022, 253, 106328. [Google Scholar] [CrossRef]
- Han, P.; Xue, Y.; Sun, Z.; Liu, X.; Miao, L.; Yuan, M.; Wang, X. The Toxicological Effects of Perfluorooctanoic Acid (PFOA) Exposure in Large Yellow Croaker (Larimichthys crocea): Exploring the Relationship between Liver Damage and Gut Microbiota Dysbiosis. Environ. Res. 2025, 278, 121683. [Google Scholar] [CrossRef]
- Lee, J.W.J.W.; Lee, J.W.J.W.; Kim, K.; Shin, Y.J.; Kim, J.; Kim, S.; Kim, H.; Kim, P.; Park, K. PFOA-Induced Metabolism Disturbance and Multi-Generational Reproductive Toxicity in Oryzias latipes. J. Hazard. Mater. 2017, 340, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Zhan, M.; Shi, K.; Zhang, X.; Fan, Q.; Xu, Q.; Liu, X.; Li, Z.; Liu, H.; Xia, Y.; Sha, Z. Histological Assessment and Transcriptome Analysis Provide Insights into the Toxic Effects of Perfluorooctanoic Acid to Juvenile Half Smooth Tongue Sole Cynoglossus semilaevis. J. Ocean Univ. China 2023, 22, 1635–1648. [Google Scholar] [CrossRef]
- Lu, W.; Long, L.; Zhao, P.; Zhang, X.; Yan, C.; Dong, S.; Huang, Q. Perfluorinated Compounds Disrupted Osmoregulation in Oryzias melastigma during Acclimation to Hypoosmotic Environment. Ecotoxicol. Environ. Saf. 2021, 223, 112613. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Wei, Y.; Zhang, H.; Liu, Y.; Dai, J. Molecular Characterization of Cytochrome P450 1A and 3A and the Effects of Perfluorooctanoic Acid on Their mRNA Levels in Rare Minnow (Gobiocypris rarus) Gills. Aquat. Toxicol. 2008, 88, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Jantzen, C.E.; Toor, F.; Annunziato, K.A.; Cooper, K.R. Effects of Chronic Perfluorooctanoic Acid (PFOA) at Low Concentration on Morphometrics, Gene Expression, and Fecundity in Zebrafish (Danio rerio). Reprod. Toxicol. 2017, 69, 34–42. [Google Scholar] [CrossRef]
- Jantzen, C.E.; Annunziato, K.M.; Cooper, K.R. Behavioral, Morphometric, and Gene Expression Effects in Adult Zebrafish (Danio rerio) Embryonically Exposed to PFOA, PFOS, and PFNA. Aquat. Toxicol. 2016, 180, 123–130. [Google Scholar] [CrossRef]
- Han, Z.; Liu, Y.; Wu, D.; Zhu, Z.; Lü, C. Immunotoxicity and Hepatotoxicity of PFOS and PFOA in Tilapia (Oreochromis niloticus). Chin. J. Geochem. 2012, 31, 424–430. [Google Scholar] [CrossRef]
- Hagenaars, A.; Vergauwen, L.; Benoot, D.; Laukens, K.; Knapen, D. Mechanistic Toxicity Study of Perfluorooctanoic Acid in Zebrafish Suggests Mitochondrial Dysfunction to Play a Key Role in PFOA Toxicity. Chemosphere 2013, 91, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.F.; Trestrail, C.; Lekamge, S.; Nugegoda, D. Effects of Perfluorooctanoic Acid (PFOA) on the Thyroid Status, Vitellogenin, and Oxidant–Antioxidant Balance in the Murray River Rainbowfish. Ecotoxicology 2020, 29, 163–174. [Google Scholar] [CrossRef]
- Manera, M.; Castaldelli, G.; Giari, L. Perfluorooctanoic Acid Promotes Recruitment and Exocytosis of Rodlet Cells in the Renal Hematopoietic Tissue of Common Carp. Toxics 2023, 11, 831. [Google Scholar] [CrossRef] [PubMed]
- Giari, L.; Vincenzi, F.; Badini, S.; Guerranti, C.; Dezfuli, B.S.; Fano, E.A.; Castaldelli, G. Common Carp Cyprinus carpio Responses to Sub-Chronic Exposure to Perfluorooctanoic Acid. Environ. Sci. Pollut. Res. 2016, 23, 15321–15330. [Google Scholar] [CrossRef]
- Consoer, D.M.; Hoffman, A.D.; Fitzsimmons, P.N.; Kosian, P.A.; Nichols, J.W. Toxicokinetics of Perfluorooctanoate (PFOA) in Rainbow Trout (Oncorhynchus mykiss). Aquat. Toxicol. 2014, 156, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Coy, C.O.; Steele, A.N.; Abdulelah, S.A.; Belanger, R.M.; Crile, K.G.; Stevenson, L.M.; Moore, P.A. Differing Behavioral Changes in Crayfish and Bluegill under Short- and Long-Chain PFAS Exposures: Field Study in Northern Michigan, USA. Ecotoxicol. Environ. Saf. 2022, 247, 114212. [Google Scholar] [CrossRef]
- Manera, M.; Sayyaf Dezfuli, B.; DePasquale, J.A.; Giari, L. Multivariate Approach to Gill Pathology in European Sea Bass after Experimental Exposure to Cadmium and Terbuthylazine. Ecotoxicol. Environ. Saf. 2016, 129, 282–290. [Google Scholar] [CrossRef]
- Manera, M.; Giari, L.; De Pasquale, J.A.; Sayyaf Dezfuli, B. Local Connected Fractal Dimension Analysis in Gill of Fish Experimentally Exposed to Toxicants. Aquat. Toxicol. 2016, 175, 12–19. [Google Scholar] [CrossRef]
- Manera, M.; Giari, L.; DePasquale, J.A.; Dezfuli, B.S.S. European Sea Bass Gill Pathology after Exposure to Cadmium and Terbuthylazine: Expert versus Fractal Analysis. J. Microsc. 2016, 261, 291–299. [Google Scholar] [CrossRef]
- Giari, L.; Manera, M.; Simoni, E.; Dezfuli, B.S.S. Changes to Chloride and Rodlet Cells in Gills, Kidney and Intestine of Dicentrarchus labrax (L.) Exposed to Reduced Salinities. J. Fish Biol. 2006, 69, 590–600. [Google Scholar] [CrossRef]
- Pawert, M.; Müller, E.; Triebskorn, R. Ultrastructural Changes in Fish Gills as Biomarker to Assess Small Stream Pollution. Tissue Cell 1998, 30, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Yancheva, V.; Velcheva, I.; Stoyanova, S.; Georgieva, E. Histological Biomarkers in Fish as a Tool in Ecological Risk Assessment and Monitoring Programs: A Review. Appl. Ecol. Environ. Res. 2016, 14, 47–75. [Google Scholar] [CrossRef]
- Costa, P.M.; Diniz, M.S.; Caeiro, S.; Lobo, J.; Martins, M.; Ferreira, A.M.; Caetano, M.; Vale, C.; DelValls, T.Á.; Costa, M.H. Histological Biomarkers in Liver and Gills of Juvenile Solea senegalensis Exposed to Contaminated Estuarine Sediments: A Weighted Indices Approach. Aquat. Toxicol. 2009, 92, 202–212. [Google Scholar] [CrossRef]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Holm, P.; Wahli, T. Histopathology in Fish: Proposal for a Protocol to Assess Aquatic Pollution. J. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef]
- Mallatt, J. Fish Gill Structural Changes Induced by Toxicants and Other Irritants: A Statistical Review. Can. J. Fish. Aquat. Sci. 1985, 42, 630–648. [Google Scholar] [CrossRef]
- Wegner, N.C.; Farrell, A.P. Plasticity in Gill Morphology and Function. In Encyclopedia of Fish Physiology (Second Edition); Alderman, S.L., Gillis, T.E., Eds.; Academic Press: Oxford, UK, 2024; pp. 762–779. ISBN 978-0-323-99761-4. [Google Scholar]
- Jonz, M.G. Cell Proliferation and Regeneration in the Gill. J. Comp. Physiol. B 2024, 194, 583–593. [Google Scholar] [CrossRef]
- Nilsson, G.E.; Dymowska, A.; Stecyk, J.A.W. New Insights into the Plasticity of Gill Structure. Respir. Physiol. Neurobiol. 2012, 184, 214–222. [Google Scholar] [CrossRef]
- Wilson, J.M.; Laurent, P. Fish Gill Morphology: Inside Out. J. Exp. Zool. 2002, 293, 192–213. [Google Scholar] [CrossRef]
- Perry, S.F. The Chloride Cell: Structure and Function in the Gills of Freshwater Fishes. Annu. Rev. Physiol. 1997, 59, 325–347. [Google Scholar] [CrossRef]
- Pisam, M.; Boeuf, G.; Prunet, P.; Rambourg, A. Ultrastructural Features of Mitochondria-rich Cells in Stenohaline Freshwater and Seawater Fishes. Am. J. Anat. 1990, 187, 21–31. [Google Scholar] [CrossRef]
- Pisam, M.; Caroff, A.; Rambourg, A. Two Types of Chloride Cells in the Gill Epithelium of a Freshwater-adapted Euryhaline Fish: Lebistes reticulatus; Their Modifications during Adaptation to Saltwater. Am. J. Anat. 1987, 179, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.-P.; Lee, T.-H.; Lin, L.-Y. Ion Regulation in Fish Gills: Recent Progress in the Cellular and Molecular Mechanisms. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 301, R28–R47. [Google Scholar] [CrossRef] [PubMed]
- Dezfuli, B.S.; Simoni, E.; Giari, L.; Manera, M. Effects of Experimental Terbuthylazine Exposure on the Cells of Dicentrarchus labrax (L.). Chemosphere 2006, 64, 1684–1694. [Google Scholar] [CrossRef]
- Fiedler, S.; Wünnemann, H.; Hofmann, I.; Theobalt, N.; Feuchtinger, A.; Walch, A.; Schwaiger, J.; Wanke, R.; Blutke, A. A Practical Guide to Unbiased Quantitative Morphological Analyses of the Gills of Rainbow Trout (Oncorhynchus mykiss) in Ecotoxicological Studies. PLoS ONE 2020, 15, e0243462. [Google Scholar] [CrossRef] [PubMed]
- Uğurlu, P.; Satar, E.İ.; Çiçek, T. The Histopathological, Cytopathological and Ultrastructural Effects of Carbaryl on Gills of Oreochromis niloticus (Linnaeus, 1758). Environ. Toxicol. Pharmacol. 2019, 71, 103217. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.; Locoro, G.; Huber, T.; Wollgast, J.; Christoph, E.H.; de Jager, A.; Manfred Gawlik, B.; Hanke, G.; Umlauf, G.; Zaldívar, J.M. Analysis of Perfluorooctanoate (PFOA) and Other Perfluorinated Compounds (PFCs) in the River Po Watershed in N-Italy. Chemosphere 2008, 71, 306–313. [Google Scholar] [CrossRef]
- Wei, Y.; Dai, J.; Liu, M.; Wang, J.; Xu, M.; Zha, J.; Wang, Z. Estrogen-like Properties of Perfluorooctanoic Acid as Revealed by Expressing Hepatic Estrogen-Responsive Genes in Rare Minnows (Gobiocypris rarus). Environ. Toxicol. Chem./SETAC 2007, 26, 2440–2447. [Google Scholar] [CrossRef]
- Laurent, P. Gill Internal Morphology. In Fish Physiology; Hoar, W.S., Randall, D.J., Eds.; Gills; Academic Press: Cambridge, MA, USA, 1984; Volume 10, pp. 73–183. [Google Scholar]
- Olson, K.R. Branchial Anatomy. In Encyclopedia of Fish Physiology (Second Edition); Alderman, S.L., Gillis, T.E., Eds.; Academic Press: Oxford, UK, 2011; pp. 138–146. ISBN 978-0-323-99761-4. [Google Scholar]
- Monteiro, S.M.; Oliveira, E.; Fontaínhas-Fernandes, A.; Sousa, M. Fine Structure of the Branchial Epithelium in the Teleost Oreochromis niloticus. J. Morphol. 2010, 271, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Watanabe, S.; Lee, K.M. Functional Morphology of Mitochondrion-Rich Cells in Euryhaline and Stenohaline Teleosts. Aqua-BioSci. Monogr. (ABSM) 2008, 1, 1–62. [Google Scholar] [CrossRef]
- Pisam, M. Membranous Systems in the “Chloride Cell” of Teleostean Fish Gill; Their Modifications in Response to the Salinity of the Environment. Anat. Rec. 1981, 200, 401–414. [Google Scholar] [CrossRef]
- Pisam, M.; Rambourg, A. Mitochondria-Rich Cells in the Gill Epithelium of Teleost Fishes: An Ultrastructural Approach. Int. Rev. Cytol. 1991, 130, 191–232. [Google Scholar] [CrossRef]
- Komnick, H. Chloride Cells and Salt Glands. In Biology of the Integument: 2 Vertebrates; Bereiter-Hahn, J., Matoltsy, A.G., Richards, K.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 499–516. ISBN 978-3-662-00989-5. [Google Scholar]
- Kirschner, L.B. Comparison of Vertebrate Salt-Excreting Organs. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1980, 238, R219–R223. [Google Scholar] [CrossRef]
- Ogata, T. Mammalian Tuft (Brush) Cells and Chloride Cells of Other Vertebrates Share a Similar Structure and Cytochemical Reactivities. Acta Histochem. Cytochem. 2000, 33, 439–449. [Google Scholar] [CrossRef]
- Goss, G.; Perry, S.; Laurent, P. 10 Ultrastructural and Morphometric Studies on Ion and Acid-Base Transport Processes in Freshwater Fish. In Fish Physiology; Wood, C.M., Shuttleworth, T.J., Eds.; Academic Press: Cambridge, MA, USA, 1995; Volume 14, pp. 257–284. [Google Scholar]
- Burckhardt, G.; Burckhardt, B.C. In Vitro and in Vivo Evidence of the Importance of Organic Anion Transporters (OATs) in Drug Therapy. In Drug Transporters. Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 201, pp. 29–104. [Google Scholar] [CrossRef]
- Hagenbuch, B.; Meier, P.J. Organic Anion Transporting Polypeptides of the OATP/SLC21 Family: Phylogenetic Classification as OATP/SLCO Superfamily, New Nomenclature and Molecular/Functional Properties. Pflügers Arch.-Eur. J. Physiol. 2004, 447, 653–665. [Google Scholar] [CrossRef]
- Gordon, W.E.; Espinoza, J.A.; Leerberg, D.M.; Yelon, D.; Hamdoun, A. Xenobiotic Transporter Activity in Zebrafish Embryo Ionocytes. Aquat. Toxicol. 2019, 212, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Payan, P.; Girard, J.P.; Mayer-Gostan, N. 2 Branchial Ion Movements in Teleosts: The Roles of Respiratory and Chloride Cells. In Fish Physiology; Hoar, W.S., Randall, D.J., Eds.; Gills. Ion and Water Transfer; Academic Press: Cambridge, MA, USA, 1984; Volume 10, Part B, pp. 39–63. [Google Scholar]
- Dezfuli, B.S.; Giari, L.; Simoni, E.; Palazzi, D.; Manera, M. Alteration of Rodlet Cells in Chub Caused by the Herbicide Stam® M-4 (Propanil). J. Fish Biol. 2003, 63, 232–239. [Google Scholar] [CrossRef]
- Avellán-Llaguno, R.D.; Liu, X.; Liu, L.; Dong, S.; Huang, Q. Elevated Bioaccumulation of PFAAs in Oryzias melastigma Following the Increase of Salinity Is Associated with the up-Regulated Expression of PFAA-Binding Proteins. Sci. Total Environ. 2020, 725, 138336. [Google Scholar] [CrossRef]
- Starkov, A.A.; Wallace, K.B. Structural Determinants of Fluorochemical-Induced Mitochondrial Dysfunction. Toxicol. Sci. 2002, 66, 244–252. [Google Scholar] [CrossRef]
- Wallace, K.B.; Kissling, G.E.; Melnick, R.L.; Blystone, C.R. Structure-Activity Relationships for Perfluoroalkane-Induced in Vitro Interference with Rat Liver Mitochondrial Respiration. Toxicol. Lett. 2013, 222, 257–264. [Google Scholar] [CrossRef]
- Jung, W.; Park, H.; Lee, B.-S.; Chang, Y.-S.; Kim, J.-B.; Yang, M.-J.; Lim, J.; Choi, H.; Park, E.-J. General Toxicity and Screening of Reproductive and Developmental Toxicity Following Bioaccumulation of Oral-Dosed Perfluorooctanoic Acid: Loss of the Golgi Apparatus. Food Chem. Toxicol. 2024, 191, 114867. [Google Scholar] [CrossRef]
- Sasaki, K.; Yoshida, H. Organelle Autoregulation—Stress Responses in the ER, Golgi, Mitochondria and Lysosome. J. Biochem. 2015, 157, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Manera, M.; Dezfuli, B.S.; Castaldelli, G.; DePasquale, J.A.; Fano, E.A.; Martino, C.; Giari, L. Perfluorooctanoic Acid Exposure Assessment on Common Carp Liver through Image and Ultrastructural Investigation. Int. J. Environ. Res. Public Health 2019, 16, 4923. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, H.; Wang, J.; Zheng, F.; Dai, J. Perfluorooctanoic Acid Exposure Induces Endoplasmic Reticulum Stress in the Liver and Its Effects Are Ameliorated by 4-Phenylbutyrate. Free. Radic. Biol. Med. 2015, 87, 300–311. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, W.; Zhang, B.; Gao, Z.; Zhang, Q.; Deng, H.; Han, L.; Shen, X.L. Perfluorooctanoic Acid Induces Hepatocellular Endoplasmic Reticulum Stress and Mitochondrial-Mediated Apoptosis in Vitro via Endoplasmic Reticulum-Mitochondria Communication. Chem.-Biol. Interact. 2022, 354, 109844. [Google Scholar] [CrossRef]
- Subash Peter, M.C.; Lock, R.A.C.; Wendelaar Bonga, S.E. Evidence for an Osmoregulatory Role of Thyroid Hormones in the Freshwater Mozambique Tilapia Oreochromis mossambicus. Gen. Comp. Endocrinol. 2000, 120, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.C.S. The Role of Thyroid Hormones in Stress Response of Fish. Gen. Comp. Endocrinol. 2011, 172, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cheng, W.; Jia, M.; Chen, L.; Gu, C.; Ren, H.; Wu, B. Toxicity of Perfluorooctanoic Acid on Zebrafish Early Embryonic Development Determined by Single-Cell RNA Sequencing. J. Hazard. Mater. 2022, 427, 127888. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manera, M.; Manera, C.; Castaldelli, G.; Giari, L. Qualitative Exploration of Ultrastructural Effects of Perfluorooctanoic Acid on Carp Gills: Mitochondria-Rich Cells as Candidate Biomarkers of Cytotoxicity. Toxics 2025, 13, 1020. https://doi.org/10.3390/toxics13121020
Manera M, Manera C, Castaldelli G, Giari L. Qualitative Exploration of Ultrastructural Effects of Perfluorooctanoic Acid on Carp Gills: Mitochondria-Rich Cells as Candidate Biomarkers of Cytotoxicity. Toxics. 2025; 13(12):1020. https://doi.org/10.3390/toxics13121020
Chicago/Turabian StyleManera, Maurizio, Cosma Manera, Giuseppe Castaldelli, and Luisa Giari. 2025. "Qualitative Exploration of Ultrastructural Effects of Perfluorooctanoic Acid on Carp Gills: Mitochondria-Rich Cells as Candidate Biomarkers of Cytotoxicity" Toxics 13, no. 12: 1020. https://doi.org/10.3390/toxics13121020
APA StyleManera, M., Manera, C., Castaldelli, G., & Giari, L. (2025). Qualitative Exploration of Ultrastructural Effects of Perfluorooctanoic Acid on Carp Gills: Mitochondria-Rich Cells as Candidate Biomarkers of Cytotoxicity. Toxics, 13(12), 1020. https://doi.org/10.3390/toxics13121020









