Radioprotective and Radiomitigative Effects of Resveratrol in Radiation-Induced Reproductive Toxicity in Male Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Exposure
2.2. Reagents
2.3. Sperm Count and Quality
2.4. Comet Assay
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources and Effects of Ionizing Radiation; United Nations Publication: New York, NY, USA, 2008. [Google Scholar]
- Moding, E.J.; Kastan, M.B.; Kirsch, D.G. Strategies for optimizing the response of cancer and normal tissues to radiation. Nat. Rev. Drug Discov. 2013, 12, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.; Schmid, T.E.; Baumgartner, A. Male-mediated developmental toxicity. Asian J. Androl. 2014, 16, 81–88. [Google Scholar] [CrossRef]
- Anderson, D. Overview of male-mediated developmental toxicity. Adv. Exp. Med. Biol. 2003, 518, 11–24. [Google Scholar]
- National Research Council (US) Committee on the Biological Effects of Ionizing Radiation (BEIR V). Health Effects of Exposure to Low Levels of Ionizing Radiation; National Academy Press: Washington, DC, USA, 1990. [Google Scholar]
- Fukunaga, H.; Yokoya, A.; Prise, K.M. A Brief Overview of Radiation-Induced Effects on Spermatogenesis and Oncofertility. Cancers 2022, 14, 805. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Radiological Protection (ICRP). The 2007 Recommendations of the International Commission on Radiological Protection; ICRP Publication 103; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–332. [Google Scholar]
- Liu, G.; Gong, P.; Zhao, H.; Wang, Z.; Gong, S.; Car, L. Effect of low-level radiation on the death of male germ cells. Radiat. Res. 2006, 165, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Salian, S.R.; Kalthur, G.; Uppangala, S.; Kumari, S.; Challapalli, S.; Chandraguthi, S.G.; Krishnamurthy, H.; Jain, N.; Kumar, P.; et al. Semen abnormalities, sperm DNA and global hypermethylation in health workers occupationally exposed to ionizing radiation. PLoS ONE 2013, 8, e69927. [Google Scholar] [CrossRef]
- Zhou, D.D.; Hao, J.L.; Guo, K.M.; Lu, C.W.; Liu, X.D. Sperm quality and DNA damage in men from Jilin Province, China, who are occupationally exposed to ionizing radiation. Genet. Mol. Res. 2016, 15, gmr8078. [Google Scholar] [CrossRef]
- Fukunaga, H.; Butterworth, K.T.; Yokoya, A.; Ogawa, T.; Prise, K.M. Low-dose radiation-induced risk in spermatogenesis. Int. J. Radiat. Biol. 2017, 93, 1291–1298. [Google Scholar] [CrossRef]
- Kimura, Y.; Okuda, H.; Kubo, M. Effects of stilbenes isolated from medicinal plants on arachidonate metabolism and degranulation in human polymorphonuclear leukocytes. J. Ethnopharm. 1995, 45, 131–139. [Google Scholar] [CrossRef]
- Cos, P.; de Bruyne, T.; Apers, S.; Vanden Berghe, D.; Pieters, L.; Vlietinck, A.J. Phytoetrogens: Recent developments. Plant Med. 2003, 69, 589–599. [Google Scholar]
- Zamora-Ros, R.; Rothwell, J.A.; Achaintre, D.; Ferrari, P.; Boutron-Ruault, M.C.; Mancini, F.R.; Affret, A.; Kühn, T.; Katzke, V.; Boeing, H.; et al. Evaluation of urinary resveratrol as a biomarker of dietary resveratrol intake in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Nutr. 2017, 117, 1596–1602. [Google Scholar] [CrossRef]
- Shubert, R.; Fischer, R.; Hain, R.; Schrerier, P.H.; Bahnweg, G.; Ernst, D.; Sandermann, H. An ozone-responsive region of the grapevine resveratrol synthase promoter differs from the basal pathogen-responsive sequence. Plant Mol. Biol. 1997, 34, 417–426. [Google Scholar] [CrossRef]
- Bavaresco, L.; Fregoni, C.; Cantu, E.; Trevisan, M. Stilbene compounds: From the grapevine to wine. Drugs Exp. Clin. Res. 1999, 25, 57–63. [Google Scholar]
- Fremont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Juan, M.E.; Vinardell, M.P.; Planas, J.M. The daily oral administration of high doses of trans-resveratrol to rats for 28 days is not harmful. J. Nutr. 2002, 132, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Roy, M.; Bhattacharya, R.K. Prevention and repair of DNA damage by selected phytochemicals as measured by single cell gel electrophoresis. J. Environ. Pathol. Toxicol. Oncol. 2004, 23, 215–226. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Bisht, K.; Wagner, K.H.; Bulmer, A. Curcumin, resveratrol and flavonoids as anti-inflammatory, cyto- and DNA-protective dietary compounds. Toxicology 2010, 278, 88–100. [Google Scholar] [CrossRef]
- Bishayee, A. Cancer prevention and treatment with resveratrol: From rodent studies to clinical trials. Cancer Prev. Res. 2009, 2, 409–418. [Google Scholar] [CrossRef]
- Dobrzyńska, M.M. Resveratrol as promising natural radioprotector. A review. Rocz. Państw. Zakł. Hig. 2013, 64, 255–262. [Google Scholar]
- Constant, J. Alcohol, ischemic heart disease, and the French paradox. Coron. Arter. Dis. 1997, 8, 645–649. [Google Scholar] [CrossRef]
- Bhat, K.P.L.; Kosmeder, J.W.; Pezzuto, J. Biological effects of resveratrol. Antioxid. Redox Signal. 2001, 3, 1041–1064. [Google Scholar] [CrossRef]
- Carsten, R.E.; Bachand, A.M.; Bailey, S.M.; Ullrich, R.L. Resveratrol reduces radiation-induced chromosome aberration frequencies in mouse bone marrow cells. Radiat. Res. 2008, 169, 633–638. [Google Scholar] [CrossRef]
- Traversi, G.; Flore, M.; Leone, S.; Basso, E.; Di Muzzio, E.; Polticelli, E.; Degrassi, F.; Cozzi, R. Resveratrol and its methoxy-derivatives as modulators of DNA damage induced by ionizing radiation. Mutagenesis 2016, 31, 433–441. [Google Scholar] [CrossRef]
- Dobrzyńska, M.M.; Gajowik, A.; Radzikowska, J. The effect of in vivo resveratrol supplementation in irradiated mice on the induction of micronuclei in peripheral blood and bone marrow reticulocytes. Mutagenesis 2016, 31, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Ocolotobiche, E.E.; Banegas, Y.C.; Güerci, A.M. Modulation of ionizing radiation-induced damage in human blood lymphocytes by in vivo treatment with resveratrol. Int. J. Radiat. Biol. 2019, 95, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Pesta, F.; Cornetta, T.; Ricordy, R.; Cozzi, R. Resveratrol affects X-rays induced apoptosis and cell cycle delay in human cells in vitro. Int. J. Mol. Med. 2005, 15, 1005–1012. [Google Scholar] [CrossRef]
- Fang, Y.; Bradley, M.J.; Cook, K.M.; Herrick, E.J.; Nicholl, M.B. A potential role for resveratrol as a radiation sensitizer for melanoma treatment. J. Surg. Res. 2013, 183, 645–653. [Google Scholar] [CrossRef]
- Komorowska, D.; Radzik, T.; Kalenik, S.; Rodacka, A. Natural radiosensitizers in radiotherapy: Cancer treatment by combining ionizing radiation with resveratrol. Int. J. Mol. Sci. 2022, 23, 10627. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Cook, R.R. Hormesis: How it could affect the risk assessment process. Hum. Exp. Toxicol. 2005, 24, 265–270. [Google Scholar] [CrossRef]
- Searle, A.G.; Beechey, C.V. Sperm count, egg-fertilization and dominant lethality after X-irradiation of mice. Mutat. Res. 1974, 22, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.; Moore, P.C. Reduction in sperm count and increase in abnormal sperm in the mouse following X-radiation or injection of 22Na. Health Phys. 1980, 39, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Working, P.K.; Bus, J.S.; Hamm, T.E., Jr.; Reproductive effects of inhaled methyl chloride in the male Fisher 344 rat. II. Spermatogonial toxicity and sperm quality. Toxicol. Appl. Pharmacol. 1985, 77, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Wyrobek, A.J.; Bruce, W.R. Chemical induction of sperm abnormalities in mice. Proc. Natl. Acad. Sci. USA 1975, 72, 4425–4429. [Google Scholar] [CrossRef]
- Dobrzyńska, M.M. The effects in mice of combined treatments to X-rays and antineoplastic drugs in the Comet assay. Toxicology 2005, 207, 331–338. [Google Scholar] [CrossRef]
- Końca, K.; Lankoff, A.; Banasik, A.; Lisowska, H.; Kuszewski, T.; Góźdź, S.; Koza, Z.; Wójcik, A. A cross-platform public domain PC image-analysis program for comet assay. Mutat. Res. 2003, 534, 15–20. [Google Scholar] [CrossRef]
- Adewoyin, M.; Ibrahim, M.; Roszaman, R.; Md Isa, M.L.; Mat Alewi, N.A.; Rafa, A.A.A.; Anuar, M.N.N. Male Infertility: The Effect of Natural Antioxidants and Phytocompounds on Seminal Oxidative Stress. Diseases 2017, 5, 9. [Google Scholar] [CrossRef]
- World Health Organization. WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 1999; Volume 2. [Google Scholar]
- Sabra, S.M.; Al-Harbi, M.S. An influential relationship of seminal fluid microbial infections and infertility, Taif Region, KSA. World J. Med. Sci. 2014, 10, 32–37. [Google Scholar]
- Kumar, N.; Singh, A.K. Trends of male factor infertility, an important cause of infertility: A review of literature. J. Hum. Reprod. Sci. 2015, 8, 191–196. [Google Scholar] [CrossRef]
- Makker, K.; Agarwal, A.; Sharma, R. Oxidative stress & male infertility. Ind. J. Med. Res. 2009, 129, 357–367. [Google Scholar]
- Zini, A.; San Gabriel, M.; Libman, J. Lycopene supplementation in vitro can protect human sperm deoxyribonucleic acid from oxidative damage. Fertil. Steril. 2010, 94, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Gharagozloo, P.; Aitken, R.J. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum. Reprod. 2011, 26, 1628–1640. [Google Scholar] [CrossRef]
- Agarwal, A.; Hamada, A.; Esteves, S.C. Insight into oxidative stress in varicocele-associated male infertility: Part 1. Nat. Rev. Urol. 2012, 9, 678–690. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agarwal, A. Role of reactive oxygen species in male infertility. Urology 1996, 48, 835–850. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef]
- Durairajanayagam, D.; Agarwal, A.; Ong, C.; Prashast, P. Lycopene and male infertility. Asian J. Androl. 2014, 16, 420–425. [Google Scholar]
- Agarwal, A.; Tvrda, E.; Sharma, R. Relationship amongst teratozoospermia, seminal oxidative stress and male infertility. Reprod. Biol. 2014, 12, 45. [Google Scholar] [CrossRef]
- Rato, L.; Alves, M.G.; Socorro, S.; Duarte, A.I.; Cavaco, J.E.; Oliveira, P.F. Metabolic regulation is important for spermatogenesis. Nat. Rev. Urol. 2012, 9, 330–338. [Google Scholar] [CrossRef]
- Sabeti, P.; Pourmasumi, S.; Rahiminia, T.; Akyash, F.; Talebi, A.R. Etiologies of sperm oxidative stress. Int. J. Reprod. Biomed. 2016, 14, 231–240. [Google Scholar] [CrossRef]
- Cirin, D.; Cotrim, A.P.; Hyodo, F.; Baum, B.J.; Krishna, M.C.; Mitchell, J.B. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist 2010, 15, 360–371. [Google Scholar] [CrossRef]
- Guéguen, Y.; Bontemps, A.; Ebrahimian, T.G. Adaptive responses to low doses of radiation or chemicals: Their cellular and molecular mechanism Cellular and Molecular. Life Sci. 2019, 76, 1255–1273. [Google Scholar]
- Coleman, M.A.; Yin, E.; Peterson, L.E.; Nelson, D.; Sorensen, K.; Tucker, J.D.; Wyrobek, A.J. Low-dose irradiation alters the transcript profiles of human lymphoblastoid cells including genes associated with cytogenetic radioadaptive response. Radiat. Res. 2005, 164, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Asakawa, I.; Yuki, K.; Matsumoto, T.; Kumamoto, M.; Kondo, N.; Ohnishi, K.; Tachibana, A.; Ohnishi, T. Radiation-induced apoptosis in the scid mouse spleen after low dose rate irradiation. Int. J. Radiat. Biol. 2002, 78, 689–693. [Google Scholar] [CrossRef]
- Sasaki, M.S.; Ejima, Y.; Tachibana, A.; Yamada, T.; Ishizaki, K.; Shimizu, T.; Nomura, T. DNA damage response pathway in radioadaptive response. Mutat. Res. 2002, 504, 101–118. [Google Scholar] [CrossRef]
- Nosel, I.; Vaurijoux, A.; Barquinero, J.F.; Gruel, G. Characteri zation of gene expression profiles at low and verylow doses of ionizing radiation. DNA Repair 2013, 12, 508–517. [Google Scholar] [CrossRef]
- Shimizu, T.; Kato, T., Jr.; Tachibana, A.; Sasaki, M.S. Coordinated regulation of radioadaptive response by protein kinase C and p38 mitogen-activated protein kinase. Exp. Cell Res. 1999, 251, 424–432. [Google Scholar] [CrossRef]
- Osburn, W.O.; Kensler, T.W. Nrf2 signaling: An adaptive response pathway for protection against environmental toxic insults. Mutat. Res. 2008, 659, 31–39. [Google Scholar] [CrossRef]
- Khan, N.M.; Sandur, S.K.; Checker, R.; Sharma, D.; Poduval, T.B.; Sainis, K.B. Pro-oxidants ameliorate radiation-induced apoptosis through activation of the calcium -ERK1/2-Nrf2 pathway. Free Radic. Biol. Med. 2011, 51, 115–128. [Google Scholar] [CrossRef]
- Mathew, S.T.; Bergstrom, P.; Hammarsten, O. Repeated Nrf2 stimulation using sulforaphane protects fibroblasts from ionizing radiation. Toxicol. Appl. Pharmacol. 2014, 276, 188–194. [Google Scholar] [CrossRef]
- Komatsu, M.; Kurokawa, H.; Waguri, S.; Taguchi, K.; Kobayashi, A.; Ichimura, Y.; Sou, Y.S.; Ueno, I.; Sakamoto, A.; Tong, K.I.; et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010, 12, 213–223. [Google Scholar] [CrossRef]
- Taguchi, K.; Fujikawa, N.; Komatsu, M.; Ishii, T.; Unno, M.; Akaike, T.; Motohashi, H.; Yamamoto, M. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proc. Natl. Acad. Sci. USA 2012, 109, 13561–13566. [Google Scholar] [CrossRef]
- DeNicola, G.M.; Karreth, F.A.; Humpton, T.J.; Gopinathan, A.; Wei, C.; Frese, K.; Mangal, D.; Yu, K.H.; Yeo, C.J.; Calhoun, E.S.; et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 2011, 475, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J.; Giaccia, E.J. Radiobiology for the Radiologist; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Mongioì, L.M.; Perelli, S.; Condorelli, R.A.; Barbagallo, F.; Crafa, A.; Cannarella, R.; La Vignera, S.; Calogero, A.E. The Role of Resveratrol in Human Male Fertility. Molecules 2021, 26, 2495. [Google Scholar] [CrossRef]
- Bhaskara, V.K.; Mittal, B.; Mysorekar, V.V.; Amaresh, N.; Simal-Gandara, J. Resveratrol, cancer and cancer stem cells: A review on past to future. Curr. Res. Food Sci. 2020, 3, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.D.; Burdock, G.A.; Edwards, J.A.; Beck, M.; Bausch, J. Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem. Toxicol. 2009, 47, 2170–2182. [Google Scholar] [CrossRef]
- Cottart, C.-H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.-L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef]
- Kyselova, V.; Peknicova, J.; Buckiova, D.; Boubelik, M. Effects of p-nonylphenol and resveratrol on body and organ weight and in vivo fertility of outbread CD-1 mice. Reprod. Biol. Endocrinol. 2003, 1, 30. [Google Scholar] [CrossRef]
- Juan, M.E.; González-Pons, E.; Munuera, T.; Ballester, J.; Rodríguez-Gil, J.E.; Planas, J.M. Trans-Resverartol, a natural antioxidant from grapes, increases sperm output in healthy rats. J. Nutr. 2005, 135, 757–760. [Google Scholar] [CrossRef]
- Shin, S.; Jeon, J.H.; Park, D.; Jang, M.J.; Choi, J.H.; Choi, B.H.; Joo, S.S.; Nahm, S.S.; Kim, J.C.; Kim, Y.B. Trans-resveratrol relaxes the corpus cavernosum ex vivo and enhances testosterone levels and sperm quality. Arch. Pharm. Res. 2008, 31, 83–87. [Google Scholar] [CrossRef]
- Sharpe, R.M. The role of oestrogen in the male. Trends Endocrinol. Metabol. 1998, 9, 371–377. [Google Scholar] [CrossRef]
- Fritz, W.A.; Cotroneo, M.S.; Wang, J.; Eltoum, I.-E.; Lamartiniere, C.A. Dietary Diethylstilbestrol but not genistein adversely affects rat testicular development. J. Nutr. 2003, 133, 2287–2293. [Google Scholar] [CrossRef]
- Goyal, H.O.; Braden, T.D.; Mansour, M.; Williams, C.S.; Kamaleldin, A.; Srivastava, K.K. Diethylstilbestrol-treated adult rats with altered epididymal sperm numbers and sperm motility parameters, but without alterations in sperm production and sperm morphology. Biol. Reprod. 2001, 64, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Özatik, F.Y.; Özatik, O.; Yiğitaslan, S.; Ünel, C.C.; Erol, K. Protective role of resveratrol on testicular germ cells in mice with testicular toxicity. Turk. J. Urol. 2017, 43, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Roman, S.D. Antioxidant Systems and Oxidative Stress in the Testes. Oxidat. Med. Cell Longev. 2008, 1, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Illiano, E.; Trama, F.; Zucchi, A.; Iannitti, R.G.; Fioretti, B.; Costantini, E. Resveratrol-Based Multivitamin Supplement Increases Sperm Concentration and Motility in Idiopathic Male Infertility: A Pilot Clinical Study. J. Clin. Med. 2020, 9, 4017. [Google Scholar] [CrossRef]
- Aitken, R.J.; De Luliis, G.N. Value of DNA integrity assays for fertility evaluation. Soc. Reprod. Fertil. 2007, 65, 81–92. [Google Scholar]
- Garcez, M.E.; dos Santos Branco, C.; Venturin Lara, L.; Pasqualotto, F.F.; Salvador, M. Effects of resveratrol supplementation on cryopreservation medium of human semens. Fertil. Steril. 2010, 94, 2118–2121. [Google Scholar] [CrossRef]
- Collodel, G.; Federico, M.G.; Geminiani, M.; Martini, S.; Bonechi, C.; Rossi, C.; Figura, N.; Moretti, E. Effect of trans-resveratrol on induced oxidative stress in human sperm and in rat germinal cells. Reprod. Toxicol. 2011, 31, 239–246. [Google Scholar] [CrossRef]
- Nashtaei, M.S.; Nekoonam Naji, S.M.; Bakhshalizadeh, S.; Amidi, F. Cryoprotective effect of resveratrol on DNA damage and crucial human sperm messenger RNAs, possibly through 50 AMP-activated protein kinase activation. Cell Tissue Bank. 2018, 19, 87–95. [Google Scholar] [CrossRef]
- Makherjee, S.; Dudley, J.I.; Das, D.K. Dose-response of resveratrol in providing health benefits. Dose Resp. 2010, 8, 478–500. [Google Scholar]
- Brown, L.; Kroon, P.A.; Das, D.K.; Das, S.; Tosaki, A.; Chan, V.; Singer, M.V.; Feick, P. The Biological Responses to Resveratrol and Other Polyphenols from Alcoholic Beverages. Alcoh. Clin. Exp. Res. 2009, 33, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Meamar, M.; Zribi, N.; Cambi, M.; Tamburrino, L.; Marchiani, S.; Filimberti, E.; Fino, M.G.; Biggeri, A.; Menezo, Y.; Forti, G.; et al. Sperm DNA fragmentation induced by cryopreservation: New insights and effect of a natural extract from Opuntia ficus-indica. Fertil. Steril. 2012, 98, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Ourique, G.M.; Finamor, I.A.; Saccol, E.M.; Riffel, A.P.; Pês, T.S.; Gutierrez, K.; Gonçalves, P.B.; Baldisserotto, B.; Pavanato, M.A.; Barreto, K.P. Resveratrol improves sperm motility, prevents lipid peroxidation and enhances antioxidant defences in the testes of hyperthyroid rats. Reprod. Toxicol. 2013, 37, 31–39. [Google Scholar] [CrossRef]
- Yun, H.; Park, S.; Kim, M.J.; Yang, W.K.; Im, D.U.; Yang, K.R.; Hong, J.; Choe, W.; Kang, I.; Kim, S.S.; et al. AMP-activated protein kinase mediates the antioxidant effects of resveratrol through regulation of the transcription factor FoxO1. FEBS J. 2014, 281, 4421–4438. [Google Scholar] [CrossRef]
- Bitterman, J.L.; Chung, J.H. Metabolic effects of resveratrol: Addressing the controversies. Cell Mol. Life Sci. 2015, 72, 1473–1488. [Google Scholar] [CrossRef]
- Nashtaei, M.; Amidi, F.; Sedighi Gilani, M.A.; Aleyasin, A.; Bakhshalizadeh, S.; Naji, M.; Nekoonam, S. Protective features of resveratrol on human spermatozoa cryopreservation may be mediated through 5′ AMP-activated protein kinase activation. Andrology 2017, 5, 313–326. [Google Scholar] [CrossRef]
- Yu, X.; Jia, Y.; Ren, F. Multidimensional biological activities od resveratrol and its prospects and challenges in the health field. Front. Nutr. 2024, 11, 1408651. [Google Scholar] [CrossRef]
- Darwish, M.A.; Abo-Youssef, A.M.; Messiha, B.A.S.; Abo-Saif, A.A.; Abdel-Bakky, M.S. Resveratrol inhibits macrophage infiltration of pancreatic islets in streptozotocin induced type 1 diabetic mice via attenuation of the CXCL16/NF-κB p65 signaling pathway. Life Sci. 2021, 272, 119250. [Google Scholar] [CrossRef]
- Jehan, Z.; Hussain, A.R.; Ahmed, M.; Ahmed, S.O.; Al-Dayel, F.; Bavi, P.; Uddin, S.; Al-Kuraya, K.S. Resveratrol suppresses constitutive activation of AKT via generation of reactive oxygen species and induces apoptosis in diffuse large B cell lymphoma cell lines. Cancer Res. 2011, 71, 4778. [Google Scholar] [CrossRef]
- Babu, D.; Leclercq, G.; Goossens, V.; Remijsen, Q.; Vandenabeele, P.; Motterlini, R.; Lefebvre, R.A. Antioxidant potential of CORM A1 and resveratrol during TNF-alpha/cycloheximide-induced oxidative stress and apoptosis in murine intestinal epithelial MODE-Kcells. Toxicol. Appl. Pharmacol. 2015, 288, 161–178. [Google Scholar] [CrossRef]
- Alesci, A.; Nicosia, N.; Cicero, N. Resveratrol and Immune Cells: A Link to Improve Human Health. Molecules 2022, 27, 424. [Google Scholar] [CrossRef]
- Dobrzyńska, M.M.; Czajka, U. Male-mediated developmental toxicity after 8 weeks’ exposure to low doses of X-rays. Int. J. Radiat. Biol. 2005, 81, 793–799. [Google Scholar] [CrossRef]
- Dobrzyńska, M.M.; Jankowska-Steifer, E.A.; Tyrkiel, E.J.; Gajowik, A.; Radzikowska, J.; Pachocki, K.A. Comparison of the effects of bisphenol A alone and in a combination with X-irradiation on sperm count and quality in male adult and pubescent mice. Environ. Toxicol. 2014, 29, 1301–1313. [Google Scholar] [CrossRef]
- Dobrzyńska, M.M.; Gajowik, A. Amelioration of sperm count and sperm quality by lycopene supplementation in irradiated mice. Reprod. Fertil. Dev. 2020, 32, 1040–1047. [Google Scholar] [CrossRef]
- Dobrzyńska, M.M. The changes in the quantity and quality of semen following subchronic exposure of mice to irradiation. In Human Monitoring for Genetic Effects; Cebulska-Wasilewska, A., Au, W.W., Sram, R.J., Eds.; IOS Press: Amsterdam, The Netherlands, 2003; pp. 309–317. [Google Scholar]
- Hawkins, M.M.; Draper, G.J.; Smith, R.A. Cancer among 1348 offspring of survivors of childhood cancer. Int. J. Cancer 1989, 43, 975–978. [Google Scholar] [CrossRef]
- Bonde, J.P.; Giwercman, A. Occupational hazards to male fecundity. Reprod. Med. Rev. 1995, 4, 59–73. [Google Scholar] [CrossRef]
- Rowley, M.J.; Leach, D.R.; Warner, G.A.; Heller, C.G. Effect of graded doses of ionizing radiation on human testis. Radiat. Res. 1974, 58, 665–678. [Google Scholar] [CrossRef]
- Pollycove, M. Nonlinearity of radiation health effects. Environ. Health Perspect. 1998, 106, 363. [Google Scholar] [CrossRef]
- Revel, A.; Raanani, H.; Younglai, E.; Xu, J.; Han, R.; Sovouret, J.F.; Casper, R.F. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects sperm from DNA damage and apoptosis caused by benzo(a)pyrene. Reprod. Toxicol. 2012, 15, 479–486. [Google Scholar] [CrossRef]
- Uguralp, S.; Usta, U.; Mizrak, B. Resveratrol may reduce apoptosis of rat testicular germ cells after experimental testicular torsion. Eur. J. Pediatr. Surg. 2005, 15, 333–336. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Alaee, S.; Namavar, M.R.; Khodabandeh, Z.; Ahmadi, N.; Rashidipour, N.; Karami-Mohajeri, S. The antioxidant properties of resveratrol on sperm parameters, testicular tissue, antioxidant capacity, and lipid peroxidation in isoflurane-induced toxicity in mice. Hum. Exp. Toxicol. 2013, 42, 09603271231215036. [Google Scholar] [CrossRef]
- Hassanin, H.M.; Kamal, A.A.; Ismail, O.I. Resveratrol ameliorates atrazine-induced caspase-dependent apoptosis and fibrosis in the testis of adult albino rats. Sci. Rep. 2024, 14, 17743. [Google Scholar] [CrossRef]
- Rohanii, A.M.; Mohammadi, T. β-Glucan and resveratrol mitigate lead induced reproductive toxicity in male mice. Sci. Rep. 2025, 15, 31277. [Google Scholar] [CrossRef]
- Tuerxun, H.; Zhao, Y.; Li, Y.; Liu, X.; Wen, S.; Zhao, Y. Resveratrol alleviates testicular toxicity induced by anti-PD-1 through regulating the NRF2-SLC7A11-GPX4 pathway. Front. Immunol. 2025, 16, 1529991. [Google Scholar] [CrossRef]
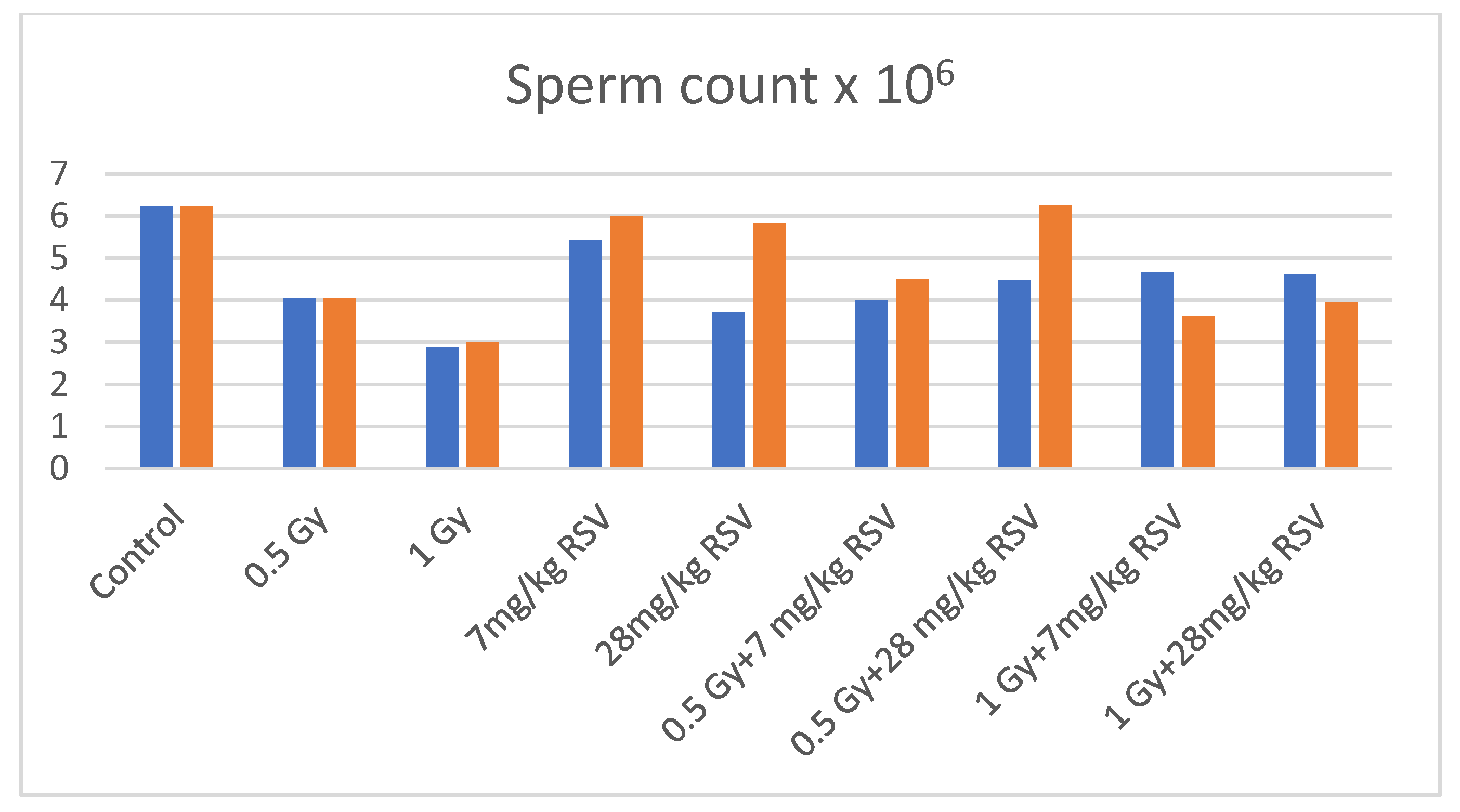
| Daily Dose | Mean Body Weight (g) ± SD | Mean Testes Weight (mg) ± SD | Mean Epididymis Weight (g) ± SD | Sperm Count × 106/mL ± SD | Percent of Motile Spermatozoa ± SD | Percent of Abnormal Spermatozoa ± SD | Comet Tail Moment ± SD | Percent of DNA in Comet Tail ± SD |
|---|---|---|---|---|---|---|---|---|
| Control | 37.79 ± 2.93 | 258.90 ± 33.58 | 244.00 ± 37.32 | 6.23 ± 0.99 | 35.93 ± 17.80 | 17.36 ± 4.49 | 3.60 ± 3.41 | 8.17 ± 5.20 |
| 0.5 Gy | 34.83 ± 4.17 | 192.70 ± 31.87 * | 159.80 ± 34.34 * | 4.05 ± 1.33 * | 14.95 ± 7.35 * | 19.63 ± 6.23 | 3.87 ± 2.75 | 8.06 ± 4.34 |
| 1 Gy | 30.59 ± 5.17 * | 208.00 ± 46.10 * | 178.30 ± 54.28 * | 2.88 ± 1.47 * | 12.08 ± 7.21 * | 22.08 ± 6.88 | 4.01 ± 2.42 | 8.47 ± 3.94 |
| 7 mg/kg RSV | 32.96 ± 4.36 * | 235.33 ± 48.29 | 198.33 ± 51.10 | 5.42 ± 4.40 | 24.82 ± 17.92 | 19.51 ± 6,96 | 4.97 ± 2.95 | 10.41 ± 4.70 |
| 28 mg/kg RSV | 34.03 ± 2.41 | 215.13 ± 59.86 | 217.50 ± 22.97 | 4.71 ± 1.61 | 16.36 ± 7.18 * | 22.54 ± 8.06 | 5.79 ± 2.66 | 11.82 ± 4.02 |
| 0.5 Gy + 7 mg/kg RSV | 28.65 ± 2.54 *a | 172.39 ± 28.15 * | 231.88 ± 75.90 a | 3.98 ± 1.79 | 9.94 ± 16.26 * | 23.69 ± 7.99 * | 7.58 ± 5.10 a | 12.32 ± 4.27 *a |
| 0.5 Gy + 28 mg/kg RSV | 29.76 ± 3.73 *a | 173.75 ± 25.09 * | 227.00 ± 60.74 a | 4.47 ± 1.75 | 16.44 ± 13.43 * | 19.69 ± 5.80 | 3.33 ± 2.85 | 6.03 ± 2.75 |
| 1 Gy + 7 mg/kg RSV | 29.01 ± 3.73 * | 170.57 ± 13.54 *b | 188.57 ± 36.13 * | 4.66 ± 2.08 b | 19.21 ± 11.18 * | 19.27 ± 3.64 | 2.07 ± 1.37 | 5.07 ± 1.50 |
| 1 Gy + 28 mg/kg RSV | 27.14 ± 3.66 * | 162.57 ± 20.31 *b | 191.57 ± 64.21 * | 4.62 ± 2.15 b | 13.71 ± 7.71 * | 19.59 ± 7.30 | 1.80 ± 1.59 b | 4.36 ± 2.81 b |
| Daily Dose | Mean Body Weight (g) ± SD | Mean Testes Weight (mg) ± SD | Mean Epididymis Weight (g) ± SD | Sperm Count × 106/mL ± SD | Percent of Motile Spermatozoa ± SD | Percent of Abnormal Spermatozoa ± SD | Comet Tail Moment ± SD | Percent of DNA in Comet Tail ± SD |
|---|---|---|---|---|---|---|---|---|
| Control | 37.79 ± 2.93 | 260.50 ± 31.52 | 248.33 ± 36.82 | 6.22 ± 1.00 | 35.93 ± 17.80 | 20.18 ± 3.29 | 3.89 ± 2.53 | 8.27 ± 3.66 |
| 0.5 Gy | 34.83 ± 4.17 | 192.70 ± 31.87 * | 159.80 ± 34.34 * | 4.05 ± 1.33 * | 14.95 ± 7.35 * | 22.40 ± 6.11 | 3.87 ± 2.75 | 8.06 ± 4.34 |
| 1 Gy | 30.59 ± 5.17 * | 204.10 ± 38.47 * | 183.40 ± 63.66 * | 3.01 ± 1.68 * | 12.08 ± 7.21 * | 21.93 ± 6.66 | 4.01 ± 2.42 | 8.47 ± 3.94 |
| 7 mg/kg RSV | 32.90 ± 3.80 | 218.60 ± 30.12 | 226.40 ± 71.90 | 5.98 ± 2.05 | 9.30 ± 7.45 * | 17.38 ± 4.03 | 5.71 ± 3.00 | 11.26 ± 4.33 |
| 28 mg/kg RSV | 34.86 ± 2.36 | 245.80 ± 34.12 | 197.20 ± 38.28 | 5.83 ± 1.56 | 8.30 ± 7.80 | 19.64 ± 5.31 | 5.72 ± 1.30 | 11.16 ± 1.36 |
| 0.5 Gy + 7 mg/kg RSV | 33.22 ± 2.32 | 213.00 ± 42.22 * | 253.33 ± 41.01 | 4.49 ± 2.07 | 10.59 ± 7.85 * | 20.80 ± 8.90 | 3.85 ± 2.08 | 7.96 ± 3.70 |
| 0.5 Gy + 28 mg/kg RSV | 33.20 ± 2.80 * | 218.33 ± 13.22 * | 191.33 ± 30.53 * | 6.25 ± 1.91 | 16.94 ± 11.32 | 16.18 ± 4.25 | 3.91 ± 1.68 | 7.91 ± 2.94 |
| 1 Gy + 7 mg/kg LYC | 27.23 ± 2.62 * | 183.86 ± 27.69 * | 255.57 ± 44.38 b | 3.63 ± 1.64 * | 8.72 ± 5.94 * | 18.08 ± 6.58 | 4.25 ± 1.66 | 8.40 ± 2.44 |
| 1 Gy + 28 mg/kg LYC | 28.60 ± 3.04 * | 186.29 ± 49.88 * | 227.71 ± 44.66 | 3.96 ± 2.30 * | 12.71 ± 12.55 * | 23.60 ± 7.45 | 6.03 ± 4.78 b | 11.26 ± 7.31 |
| Daily Dose | Mean Body Weight (g) ± SD | Mean Testes Weight (mg) ± SD | Mean Epididymis Weight (g) ± SD | Sperm Count × 106/mL ± SD | Percent of Motile Spermatozoa ± SD | Percent of Abnormal Spermatozoa ± SD | Comet Tail Moment ± SD | Percent of DNA in Comet Tail ± SD |
|---|---|---|---|---|---|---|---|---|
| Control | 36.72 ± 3.94 | 246.06 ± 91.44 | 145.60 ± 91.66 | 6.39 ± 2.31 | 18.09 ± 16.73 | 21.98 ± 4.66 | 2.60 ± 0.99 | 6.24 ± 1.91 |
| 0.5 Gy | 39.06 ± 4.17 | 158.00 ± 83.02 * | 223.11 ± 64.49 | 3.09 ± 2.07 * | 20.17 ± 15.61 | 25.66 ± 14.15 | 2.26 ± 0.60 | 5.73 ± 1.44 |
| 1 Gy | 33.97 ± 7.55 | 216.00 ± 128.92 | 245.70 ± 107.4 * | 3.14 ± 2.04 * | 34.94 ± 17.83 * | 26.44 ± 11.40 | 2.32 ± 0.66 | 5.96 ± 1.41 |
| 7 mg/kg RSV | 39.24 ± 3.47 | 236.38 ± 36.98 | 212.44 ± 123.45 | 6.04 ± 2.10 | 20.13 ± 17.52 | 20.21 ± 5,65 | 2.85 ± 1.93 | 6.59 ± 3.65 |
| 28 mg/kg RSV | 36.47 ± 2.41 | 221.89 ± 574.29 | 192.10 ± 34.38 | 6.14 ± 2.18 | 16.46 ± 7.56 | 19.20 ± 2.70 | 3.32 ± 2.06 | 7.05 ± 4.11 |
| 0.5 Gy + 7 mg/kg RSV RPR | 32.50 ± 3.30 *a | 128.50 ± 40.37 *a | 147.00 ± 38.68 | 2.45 ± 1.19 * | 8.34 ± 8.34 a | 34.91 ± 19.94 * | 2.38 ± 1.18 | 5.74 ± 2.18 |
| 0.5 Gy + 28 mg/kg RSV RPR | 33.77 ± 3.70 *a | 91.80 ± 37.97 *a | 213.20 ± 57.36 | 2.58 ± 1.12 * | 14.23 ± 12.60 | 36.35 ± 15.32 a | 2.70 ± 1.76 | 5.65 ± 2.74 |
| 0.5 Gy + 7 mg/kg RSV RAR | 27.49 ± 6.57 *a | 160.96 ± 33.56 * | 184.00 ± 42.61 | 3.60 ± 1.43 | 9.71 ± 10.94 | 29.98 ± 10.85 | 2.58 ± 0.83 | 4.36 ± 1.99 |
| 0.5 Gy + 28 mg/kg RSV RAR | 29.49 ± 2.86 *a | 179.27 ± 18.41 * | 238.36 ± 46.91 * | 3.34 ± 2.72 | 17.86 ± 11.47 | 26.22 ± 16.55 | 2.83 ± 1.75 | 5.21 ± 1.18 |
| 1 Gy + 7 mg/kg RSV RAR | 28.60 ± 6.47 *b | 176.87 ± 21.55 * | 177.00 ± 66.73 | 2.42 ± 0.93 * | 15.75 ± 9.29 | 40.20 ± 35.20 * | 1.86 ± 1.60 | 4.03 ± 3.18 |
| 1 Gy + 28 mg/kg RSV RAR | 26.40 ± 4.06 * | 170.75 ± 16.48 * | 224.33 ± 49.35 | 2.83 ± 0.48 * | 14.58 ± 11.29 | 46.70 ± 15.14 *b | 3.54 ± 0.85 | 6.77 ± 1.38 |
| Daily Dose | Mean Body Weight (g) ± SD | Mean Testes Weight (mg) ± SD | Mean Epididymis Weight (g) ± SD | Sperm Count × 106/mL ± SD | Percent of Motile Spermatozoa ± SD | Percent of Abnormal Spermatozoa ± SD | Comet Tail Moment ± SD | Percent of DNA in Comet Tail ± SD |
|---|---|---|---|---|---|---|---|---|
| Control | 37.14 ± 2.72 | 263.42 ± 60.42 | 139.60 ± 35.08 | 6.19 ± 1.09 | 16.72 ± 9.80 | 18.56 ± 4.26 | 3.34 ± 1.05 | 7.48 ± 1.61 |
| 0.5 Gy | 38.72 ± 4.32 | 176.00 ± 90.95 * | 189.50- ± 41.44 | 3.35 ± 2.49 * | 24.70 ± 11.62 | 29.13 ± 9.13 | 2.11 ± 2.75 | 4.64 ± 1.87 |
| 1 Gy | 33.07 ± 4.10 | 186.50 ± 115.18 * | 185.57 ± 96.55 | 4.97 ± 2.65 | 9.48 ± 5.22 | 33.88 ± 23.57 * | 2.92 ± 2.16 | 6.60 ± 3.26 |
| 7 mg/kg RSV | 35.14 ± 2.62 | 240.63 ± 85.54 | 120.71 ± 49.42 | 5.36 ± 2.38 | 9.46 ± 8.49 | 16.63 ± 3.05 | 4.60 ± 2.09 | 9.77 ± 3.92 |
| 28 mg/kg RSV | 35.36 ± 2.72 | 234.71 ± 107.54 | 120.29 ± 58.22 | 5.96 ± 2.50 | 13.55 ± 8.19 | 18.89 ± 5.06 | 3.64 ± 1.53 | 7.61 ± 2.05 |
| 0.5 Gy + 7 mg/kg RSV RPR | 32.21 ± 4.89 * | 87.67 ± 11.81 *,a | 155.67 ± 58.07 | 3.67 ± 1.77 * | 18.11 ± 10.77 | 34.52 ± 13.15 * | 3.28 ± 1.63 | 5.94 ± 3.10 |
| 0.5 Gy + 28 mg/kg RSV RPR | 33.24 ± 2.80 * | 99.75 ± 27.26 *,a | 187.17 ± 58.07 | 2.81 ± 0.95 *,a | 19.32 ± 8.85 | 35.57 ± 8.72 * | 3.14 ± 2.30 | 6.75 ± 4.21 |
| 1 Gy + 28 mg/kg RSV RPR | 32.33 ± 3.26 * | 82.75 ± 15.78 *,b | 100.50 ± 57.24 b | 1.67 ± 1.24 *,b | 10.81 ± 3.9 | 46.95 ± 16.55 *,b | 3.50 ± 2.23 | 8.81 ± 6.38 |
| 0.5 Gy + 7 mg/kg RSV RAR | 31.60 ± 2.26 *,a | 79.33 ± 12.50 *,a | 165.33 ± 68.41 | 2.60 ± 2.66 * | 13.75 ± 13.28 | 37.82 ± 16.41 * | 1.76 ± 1.78 | 7.21 ± 3.21 |
| 0.5 Gy + 28 mg/kg RSV RAR | 33.20 ± 3.80 *,a | 218.57 ± 19.71 | 229.14 ± 52.55 * | 2.34 ± 1.33 * | 15.07 ± 11.26 | 37.3 ± 12.23 * | 2.71 ± 3.29 | 4.37 ± 2.88 |
| 1 Gy + 7 mg/kg RSV RAR | 28.87 ± 2.12 * | 212.40 ± 15.57 | 237.2 ± 39.19 * | 2.45 ± 1.57 *,b | 8.57 ± 7.87 * | 40.9 ± 6.21 * | 2.74 ± 0.83 | 2.17 ± 0.39 * |
| 1 Gy + 28 mg/kg RSV RAR | 28.20 ± 3.46 * | 203.0 ± 23.07 | 257.33 ± 38.73 * | 1.77 ± 1.55 *,b | 24.13 ± 16.40 b | 32.04 ± 13.87 * | 3.02 ± 0.96 | 2.27 ± 1.84 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrzyńska, M.M.; Gajowik, A. Radioprotective and Radiomitigative Effects of Resveratrol in Radiation-Induced Reproductive Toxicity in Male Mice. Toxics 2025, 13, 1019. https://doi.org/10.3390/toxics13121019
Dobrzyńska MM, Gajowik A. Radioprotective and Radiomitigative Effects of Resveratrol in Radiation-Induced Reproductive Toxicity in Male Mice. Toxics. 2025; 13(12):1019. https://doi.org/10.3390/toxics13121019
Chicago/Turabian StyleDobrzyńska, Małgorzata M., and Aneta Gajowik. 2025. "Radioprotective and Radiomitigative Effects of Resveratrol in Radiation-Induced Reproductive Toxicity in Male Mice" Toxics 13, no. 12: 1019. https://doi.org/10.3390/toxics13121019
APA StyleDobrzyńska, M. M., & Gajowik, A. (2025). Radioprotective and Radiomitigative Effects of Resveratrol in Radiation-Induced Reproductive Toxicity in Male Mice. Toxics, 13(12), 1019. https://doi.org/10.3390/toxics13121019






