Acute Intoxication After Baclofen Administration: A Review of the Literature and Methodological Proposals
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Data Sources and Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Study Selection and Data Collection Process
2.5. Case Report
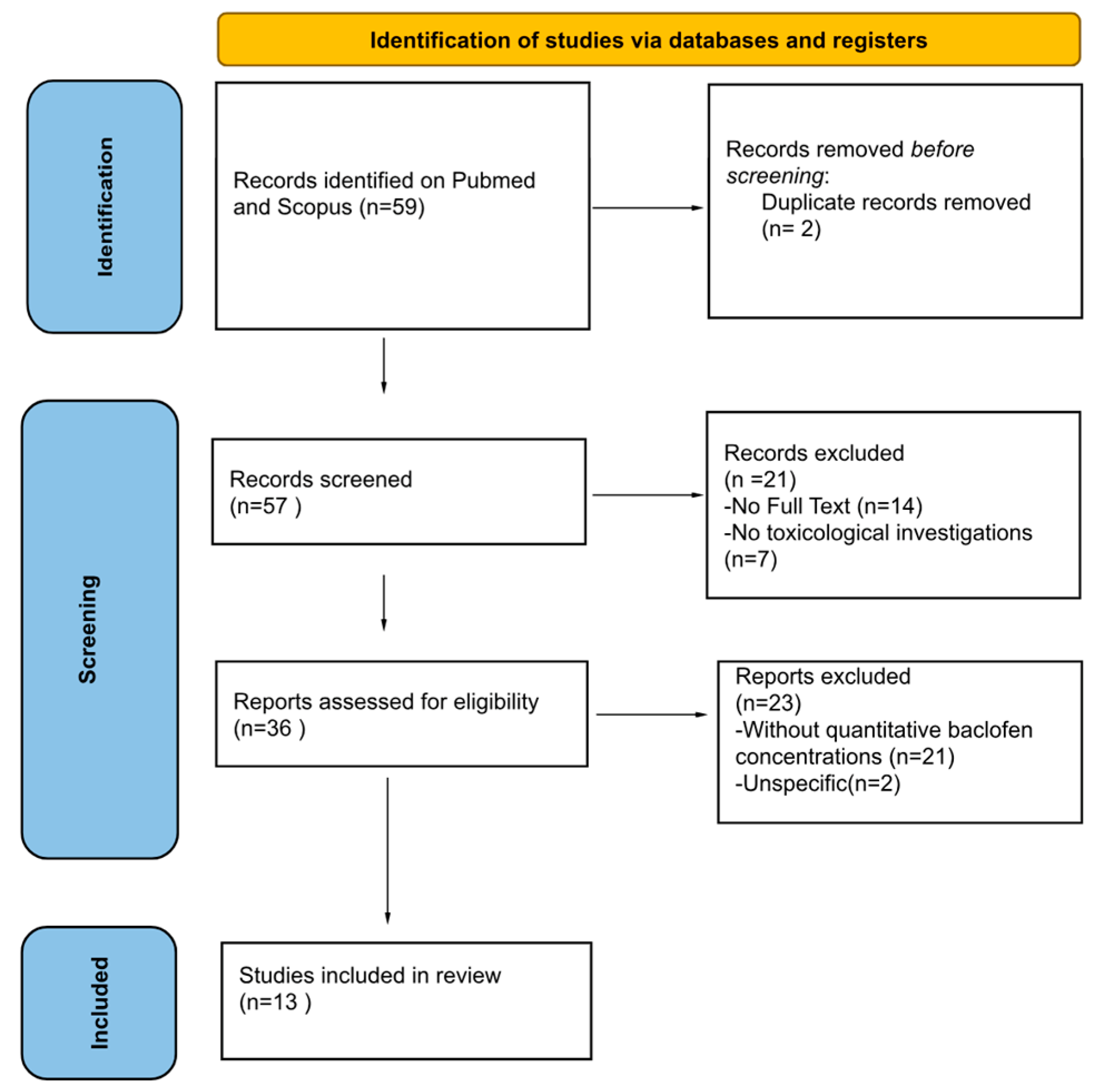
3. Results
3.1. Characteristics of Articles Included in the Systematic Review
3.2. Sociodemographic and Circumstantial Data
3.3. Analytical Toxicology Findings
4. Discussion
4.1. Scarcity of Systematic Data
4.2. Absence of Reliable Toxicological Cut-Offs
4.3. Lack of Standardized Monitoring Protocols
4.4. Differentiation of Intoxication Contexts
4.5. Postmortem Investigations
4.6. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hudgson, P.; Weightman, D. Baclofen in the treatment of spasticity. Br. Med. J. 1971, 4, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Bowery, N.G. GABAB receptor pharmacology. Annu. Rev. Pharmacol. Toxicol. 1993, 33, 109–147. [Google Scholar] [CrossRef] [PubMed]
- Slonimski, M.; Abram, S.E.; Zuniga, R.E. Intrathecal baclofen in pain management. Reg. Anesth. Pain Med. 2004, 29, 269–276. [Google Scholar] [CrossRef]
- Romito, J.W.; Turner, E.R.; Rosener, J.A.; Coldiron, L.; Udipi, A.; Nohrn, L.; Tausiani, J.; Romito, B.T. Baclofen therapeutics, toxicity, and withdrawal: A narrative review. SAGE Open Med. 2021, 9, 20503121211022197. [Google Scholar] [CrossRef]
- Ameisen, O. Complete and prolonged suppression of symptoms and consequences of alcohol-dependence using high-dose baclofen: A self-case report of a physician. Alcohol Alcohol. 2005, 40, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Franchitto, N.; Rolland, B.; Pelissier, F.; Simon, N. How to manage self-poisoning with baclofen in alcohol use disorder? Current updates. Front. Psychiatry 2018, 9, 417. [Google Scholar] [CrossRef]
- de Beaurepaire, R.; Sinclair, J.M.A.; Heydtmann, M.; Addolorato, G.; Aubin, H.-J.; Beraha, E.M.; Caputo, F.; Chick, J.D.; de La Selle, P.; Franchitto, N.; et al. The use of baclofen as a treatment for alcohol use disorder: A clinical practice perspective. Front. Psychiatry 2019, 9, 708. [Google Scholar] [CrossRef]
- Schulz, M.; Iwersen-Bergmann, S.; Andresen, H.; Schmoldt, A. Therapeutic and toxic blood concentrations of nearly 1000 drugs and other xenobiotics. Crit. Care 2012, 16, R136. [Google Scholar] [CrossRef]
- Müller, H.; Zierski, J.; Dralle, D.; Krauss, D.; Mutschler, E. Pharmacokinetics of intrathecal baclofen. In Local-Spinal Therapy of Spasticity; Springer: Berlin/Heidelberg, Germany, 1988; pp. 223–226. [Google Scholar]
- Chapple, D.; Johnson, D.; Connors, R. Baclofen overdose in two siblings. Pediatr. Emerg. Care 2001, 17, 110–112. [Google Scholar] [CrossRef]
- Drevin, G.; Briet, M.; Ghamrawi, S.; Beloncle, F.; Abbara, C. Baclofen overdose following recreational use in adolescents and young adults: A case report and review of the literature. Forensic Sci. Int. 2020, 316, 110541. [Google Scholar] [CrossRef]
- May, C.R. Baclofen overdose. Ann. Emerg. Med. 1983, 12, 171–173. [Google Scholar] [CrossRef]
- Perry, H.E.; Wright, R.O.; Shannon, M.W.; Woolf, A.D. Baclofen overdose: Drug experimentation in a group of adolescents. Pediatrics 1998, 101, 1045–1048. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Hodgman, M.J.; Kao, L.; Tormoehlen, L.M. Baclofen overdose mimicking brain death. Clin. Toxicol. 2012, 50, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Farhat, S.; El Halabi, T.; Makki, A.; Atweh, S.F.; Nasreddine, W.; Beydoun, A. Coma with absent brainstem reflexes and a burst suppression on EEG secondary to baclofen toxicity. Front. Neurol. 2020, 11, 404. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, T.; Abou-Khalil, B.; Blumenkopf, B. EEG changes in intrathecal baclofen overdose: A case report and review of the literature. Electroencephalogr. Clin. Neurophysiol. 1998, 107, 339–342. [Google Scholar] [CrossRef]
- Cooper, D.J.; Bergman, J. Massive baclofen overdose. Crit. Care Resusc. 2000, 2, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Sauneuf, B.; Totouom, H.K.; Savary, B.; Varin, L.; Dupeyrat, J.; Ramakers, S.; Hanouz, J.L. Clinical and EEG features of acute intrathecal baclofen overdose. Clin. Neurol. Neurosurg. 2012, 114, 84–86. [Google Scholar] [CrossRef]
- Shirley, K.W.; Kothare, S.; Piatt, J.H., Jr.; Adirim, T.A. Intrathecal baclofen overdose and withdrawal. Pediatr. Emerg. Care 2006, 22, 258–261. [Google Scholar] [CrossRef]
- Szpot, P.; Chłopaś, A.; Buszewicz, G.; Teresiński, G. Application of high-resolution mass spectrometry to determination of baclofen in a case of fatal intoxication. Forensic Toxicol. 2016, 34, 268–276. [Google Scholar] [CrossRef]
- Meulendijks, D.; Khan, S.; Koks, C.H.; Huitema, A.D.; Schellens, J.H.; Beijnen, J.H. Baclofen overdose treated with continuous venovenous hemofiltration. Eur. J. Clin. Pharmacol. 2015, 71, 357–361. [Google Scholar] [CrossRef]
- Nahar, L.K.; Murphy, K.G.; Paterson, S. Baclofen: To screen or not to screen in postmortem blood? J. Anal. Toxicol. 2021, 45, 612–618. [Google Scholar] [CrossRef]
- Zahra, E.; Darke, S.; Lappin, J.; Duflou, J.; Farrell, M. Baclofen-related deaths in Australia 2000–2022. Forensic Sci. Int. 2024, 365, 112281. [Google Scholar] [CrossRef]
- Léger, M.; Brunet, M.; Le Roux, G.; Lerolle, N.; Boels, D. Baclofen self-poisoning in the era of changing indication: Multicentric reports to a French poison control centre. Alcohol Alcohol. 2017, 52, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Drevin, G.; Briet, M.; Férec, S.; Abbara, C. Need for systematic and comprehensive toxicological screening protocols in pediatric poisoning cases. Ther. Drug Monit. 2022, 47, 562–563. [Google Scholar] [CrossRef]
- Issa, S.Y.; Hafez, E.M.; El-Banna, A.S.; Abdel Rahman, S.M.; AlMazroua, M.K.; El-Hamd, M.A. Baclofen systemic toxicity: Experimental histopathological and biochemical study. Hum. Exp. Toxicol. 2018, 37, 431–441. [Google Scholar] [CrossRef]
- Reynoard, J.; Schmitt, C.; Torrents, R.; Simon, N. Toxicological considerations in the prescription of baclofen for the treatment of substance use disorders. Expert Opin. Drug Metab. Toxicol. 2020, 16, 309–317. [Google Scholar] [CrossRef]
- Fraser, A.D.; MacNeil, W.; Isner, A.F. Toxicological analysis of a fatal baclofen (Lioresal) ingestion. J. Forensic Sci. 1991, 36, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Dudek, C.M.; Yee, C.H.; Greenberg, M.; McCarver, S.C. Baclofen overdose with unique cardiovascular effects. BMJ Case Rep. CP 2024, 17, e260869. [Google Scholar] [CrossRef] [PubMed]
- Naveen, A.; Sahu, M.R.; Mohanty, M.K.; Swain, R.; Dey, A. Fatal multiorgan failure in baclofen overdose: An autopsy case report. Indian J. Forensic Community Med. 2022, 9, 124–127. [Google Scholar] [CrossRef]
- Reichmuth, P.; Blanc, A.L.; Tagan, D. Unintentional baclofen intoxication in the management of alcohol use disorder. BMJ Case Rep. 2015, 2015, bcr2015212187. [Google Scholar] [CrossRef]
- Hsieh, M.J.; Chen, S.C.; Weng, T.I.; Fang, C.C.; Tsai, T.J. Treating baclofen overdose by hemodialysis. Am. J. Emerg. Med. 2011, 30, 1654.e5–1654.e7. [Google Scholar] [CrossRef]
- Chaignot, C.; Zureik, M.; Rey, G.; Dray-Spira, R.; Coste, J.; Weill, A. Risk of hospitalisation and death related to baclofen for alcohol use disorders: Comparison with nalmefene, acamprosate, and naltrexone in a cohort study of 165,334 patients between 2009 and 2015 in France. Pharmacoepidemiol. Drug Saf. 2018, 27, 1239–1248. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, H.; Mao, J.; He, J.; Zhang, Y.; Yang, X. Neurological adverse events associated with baclofen: A pharmacovigilance study based on FDA adverse event reporting system. Front. Pharmacol. 2025, 16, 1569602. [Google Scholar] [CrossRef] [PubMed]
- Auffret, M.; Labreuche, J.; Duhamel, A.; Deheul, S.; Cottencin, O.; Bordet, R.; Rolland, B. Proactive regional pharmacovigilance system versus national spontaneous reporting for collecting safety data on concerning off-label prescribing practices: An example with baclofen and alcohol dependence in France. Drug Saf. 2017, 40, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Jiang, T.; Li, Y.; Guo, P.; Liu, Y.; Zhang, Y.; Liu, Y. Neurological adverse events associated with baclofen: A disproportionality analysis based on FDA Adverse Event Reporting System. SAGE Open Med. 2025, 13, 20503121251331812. [Google Scholar] [CrossRef]
- McGowan, J.D.; Betten, D.P. Burst suppression electroencephalography (EEG) pattern with coma and loss of brain stem reflexes following a baclofen overdose with subsequent full recovery. Am. J. Case Rep. 2022, 23, e936280. [Google Scholar] [CrossRef]
- Miller, J.J. Baclofen overdose mimicking anoxic encephalopathy: A case report and review of the literature. Ther. Adv. Drug Saf. 2017, 8, 165–167. [Google Scholar] [CrossRef]
- Schmitz, N.; Artz, M.; Walsh, K.; Gaudana, S.; Cloyd, J.; Schrogie, J.; Kriel, R. Estimating the national population of hospitalized chronic baclofen users: A cross-sectional analysis of a commercial claims database. Drugs—Real World Outcomes 2022, 9, 307–314. [Google Scholar] [CrossRef]
- Muanda, F.T.; Weir, M.A.; Bathini, L.; Blake, P.G.; Chauvin, K.; Dixon, S.N.; McArthur, E.; Sontrop, J.M.; Moist, L.; Garg, A.X. Association of baclofen with encephalopathy in patients with chronic kidney disease. JAMA 2019, 322, 1987–1995. [Google Scholar] [CrossRef] [PubMed]
- Malak, M.; Barzegar, M. Baclofen induced encephalopathy in a 6-year-old boy with advanced renal failure. Iran. J. Child Neurol. 2015, 9, 61–64. [Google Scholar] [PubMed]
- Da Silva, M.N.; da Silva, J.V.B.; da Fonsêca, N.F.; Oshiro Junior, J.A.; Dantas Medeiros, A.C. An overview of analytical methods for the identification and quantification of baclofen. Curr. Pharm. Anal. 2023, 19, 353–370. [Google Scholar] [CrossRef]
- Labat, L.; Goncalves, A.; Marques, A.R.; Duretz, B.; Granger, B.; Declèves, X. Liquid chromatography high-resolution mass spectrometry for the determination of baclofen and its metabolites in plasma: Application to therapeutic drug monitoring. Biomed. Chromatogr. 2017, 31, e3936. [Google Scholar] [CrossRef]
- He, Q.; Chhonker, Y.S.; McLaughlin, M.J.; Murry, D.J. Simultaneous quantitation of S(+)- and R(−)-baclofen and its metabolite in human plasma and cerebrospinal fluid using LC–APCI–MS/MS: An application for clinical studies. Molecules 2020, 25, 250. [Google Scholar] [CrossRef]
- Triolo, V.; Spanò, M.; Buscemi, R.; Gioè, S.; Malta, G.; Čaplinskiene, M.; Vargiano, F.; Bertol, E.; Zerbo, S.; Albano, G.D.; et al. EtG quantification in hair and different reference cut-offs in relation to various pathologies: A scoping review. Toxics 2022, 10, 682. [Google Scholar] [CrossRef]
- Treglia, M.; La Russa, R.; Napoletano, G.; Ghamlouch, A.; Del Duca, F.; Treves, B.; Frati, P.; Maiese, A. Artificial intelligence in forensic neuropathology: A systematic review. J. Forensic Leg. Med. 2025, 115, 102944. [Google Scholar] [CrossRef] [PubMed]
- Saulino, M.; Anderson, D.J.; Doble, J.; Farid, R.; Gul, F.; Konrad, P.; Boster, A.L. Best practices for intrathecal baclofen therapy: Troubleshooting. Neuromodul. Technol. NeuralInterface 2016, 19, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Mutel, N.; Lecot, J.; Lamoureux, F.; Ikhlef, D.; Bruneau, C.; Ganem, A.; Vodovar, D.; Tamion, F.; Giry, M. Delayed cerebrospinal fluid removal is ineffective in treating intrathecal baclofen overdose. Clin. Toxicol. 2025, 63, 221–222. [Google Scholar] [CrossRef] [PubMed]
- Gburek-Augustat, J.; Krause, M.; Bernhard, M.; Sorge, I.; Gräfe, D.; Siekmeyer, M.; Nestler, U.; Merkenschlager, A. Unusual mechanical failures of intrathecal baclofen pump systems: Symptoms, signs, and troubleshooting. Childs Nerv. Syst. 2021, 37, 2597–2604. [Google Scholar] [CrossRef]
- Rose, A.K.; Jones, A. Baclofen: Its effectiveness in reducing harmful drinking, craving, and negative mood. A meta-analysis. Addiction 2018, 113, 1396–1406. [Google Scholar] [CrossRef]
- Muzyk, A.J.; Rivelli, S.K.; Gagliardi, J.P. Defining the role of baclofen for the treatment of alcohol dependence: A systematic review of the evidence. CNS Drugs 2012, 26, 69–78. [Google Scholar] [CrossRef]
- Thompson, A.; Owens, L.; Richardson, P.; Pirmohamed, M. Systematic review: Baclofen dosing protocols for alcohol use disorders used in observational studies. Eur. Neuropsychopharmacol. 2017, 27, 1077–1089. [Google Scholar] [CrossRef]
- Franchitto, N.; Pelissier, F.; Lauque, D.; Simon, N.; Lançon, C. Self-intoxication with baclofen in alcohol-dependent patients with co-existing psychiatric illness: An emergency department case series. Alcohol Alcohol. 2014, 49, 79–83. [Google Scholar] [CrossRef]
- Chan, W.L.; Dargan, P.I.; Haynes, C.M.; Green, J.L.; Black, J.C.; Dart, R.C.; Wood, D.M. Misuse of prescription medicines is as prevalent as the use of recreational drugs and novel psychoactive substances in Singapore: An unrecognised public health issue? Singap. Med. J. 2022, 63, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Pelerin, J.M.; Fristot, L.; Gibaja, V.; Revol, B.; Gillet, P.; Lima-Tournebize, J. Non-medical use of baclofen: A case series and review of the literature. Therapies 2023, 78, 615–637. [Google Scholar] [CrossRef] [PubMed]
- Ivanhoe, C.B.; Francisco, G.E.; McGuire, J.R.; Subramanian, T.; Grissom, S.P. Intrathecal baclofen management of poststroke spastic hypertonia: Implications for function and quality of life. Arch. Phys. Med. Rehabil. 2006, 87, 1509–1515. [Google Scholar] [CrossRef]
- Francisco, G.E.; Latorre, J.M.; Ivanhoe, C.B. Intrathecal baclofen therapy for spastic hypertonia in chronic traumatic brain injury. Brain Inj. 2007, 21, 335–338. [Google Scholar] [CrossRef]
- Weißhaar, G.F.; Hoemberg, M.; Bender, K.; Bangen, U.; Herkenrath, P.; Eifinger, F.; Rothschild, M.; Roth, B.; Oberthuer, A. Baclofen intoxication: A “fun drug” causing deep coma and nonconvulsive status epilepticus—A case report and review of the literature. Eur. J. Pediatr. 2012, 171, 1541–1547. [Google Scholar] [CrossRef]
- Gill, D.; Mann, K.; Liu, K. QT prolongation by baclofen overdose. Am. J. Ther. 2017, 24, e625–e627. [Google Scholar] [CrossRef] [PubMed]
- Zerbo, S.; Spano, M.; Albano, G.D.; Buscemi, R.; Malta, G.; Argo, A. A fatal suicidal sodium nitrite ingestion determined six days after death. J. Forensic Leg. Med. 2023, 98, 102576. [Google Scholar] [CrossRef]
- Boster, A.L.; Bennett, S.E.; Bilsky, G.S.; Gudesblatt, M.; Koelbel, S.F.; McManus, M.; Saulino, M. Best practices for intrathecal baclofen therapy: Screening test. Neuromodul. Technol. Neural Interface 2016, 19, 616–622. [Google Scholar] [CrossRef]
- Pittelkow, T.P.; Bendel, M.A.; Lueders, D.R.; Beck, L.A.; Pingree, M.J.; Hoelzer, B.C. Quantifying the change of spasticity after intrathecal baclofen administration: A descriptive retrospective analysis. Clin. Neurol. Neurosurg. 2018, 171, 163–167. [Google Scholar] [CrossRef]
- Patel, A.; Stegelmann, S.; Ordaz, J.D.; Desai, V.; Angulo-Parker, F.; Cartwright, S.; Fraitskich, G.; Flory, P.; Coon, A.; Johnson, S.K.; et al. Characterization of standard work tools for intrathecal baclofen therapy. Childs Nerv. Syst. 2021, 37, 3073–3081. [Google Scholar] [CrossRef] [PubMed]
- Albano, G.D.; Malta, G.; La Spina, C.; Rifiorito, A.; Provenzano, V.; Triolo, V.; Vaiano, F.; Bertol, E.; Zerbo, S.; Argo, A. Toxicological findings of self-poisoning suicidal deaths: A systematic review by countries. Toxics 2022, 10, 654. [Google Scholar] [CrossRef] [PubMed]
- Argo, A.; Zerbo, S.; Buscemi, R.; Trignano, C.; Bertol, E.; Albano, G.D.; Vaiano, F. A Forensic Diagnostic Algorithm for Drug-Related Deaths: A Case Series. Toxics 2022, 10, 152. [Google Scholar] [CrossRef] [PubMed]
| Year | Authors | Study Type | Sample (N)/Gender/Mean Age | Toxicological Method | Baclofen Analytical Data | Key Findings |
|---|---|---|---|---|---|---|
| 2006 | Shirley K.W. et al. [19] | Case Report | 1; M; 12 years old | Not specified | 7471 ng/mL (CSF) | Baclofen overdose and withdrawal are potentially life-threatening complications of pump and spinal catheter system malfunction |
| 2012 | Hsieh M.J. et al. [32] | Case Report | 1; M; 55 years old | Not specified | 1167 ng/mL (blood) | Patients with baclofen overdose could have prolonged elimination even with normal renal function |
| 2012 | Sauneuf B. et al. [18] | Case Report | 1; M; 60 years old | High-performance liquid chromatography | 2062 μg/mL (CSF) | Clinicians must be aware of the risk of intrathecal baclofen overdose and its acute management |
| 2012 | Sullivan R. et al. [14] | Brief Communication | 2; 1 F and 1 M; 40 and 51 years old | Not specified | 2.7 mcg/mL (blood; Not specified) | The need for more precise criteria to define brain death in intoxicated comatose patients |
| 2015 | Meulendijks D. et al. [21] | Case Report | 1; M; 57 years old | LC-MS/MS method | 1.81–0.05 mg/L (blood) | Continuous venovenous hemofiltration (CVVH)may be an alternative to hemodialysis in baclofen overdose |
| 2015 | Reichmuth P. et al. [31] | Case Report | 1; M; 66 years old | Not specified | 1510 mg/L (blood) | Off-label high-dose baclofen for a chronic alcohol use can precipitate severe toxicity, especially with renal dysfunction |
| 2016 | Szpot P. et al. [20] | Original Article | N = 1; F; 25 years old | High-Resolution Mass Spectrometry (HRMS) | 30.7 μg/mL (blood) | A simple and rapid method for routine baclofen testing in autopsy blood |
| 2017 | Leger M. et al. [24] | Retrospective Study | 190;~50% female, Median age 39–41 years | Not specified | Plasma baclofen concentrations were available for 30 patients with severe intoxications: median 3.24 mg/L compared to 0.32 mg/L in non-severe cases | Baclofen self-poisoning has increased with its use for alcohol use disorder |
| 2020 | Drevin G. et al. [11] | Review Article and Literature review | 1; M; 16 years old; other not specified | Liquid chromatography-tandem mass spectrometry (LC–MS/MS) system | 420 ng/mL in plasma and 64,900 ng/mL in urine; literature plasma <20–1322 ng/mL (non-fatal) | Psychotropic drug abuse is common among adolescents and young adults |
| 2020 | Farhat S. et al. [15] | Case Report and literature review of baclofen cases with EEG burst-suppression | 1; M; 68 years old | Not specified | 4.30 ug/mL (blood) | Severe baclofen toxicity can mimic brain death; EEG can help avoid premature prognosis |
| 2020 | Khatun Nahar L. et al. [22] | Original Article | 21; 11 male (52%) and 10 female (48%); the age ranged from 31 to 69 years (median = 47 years) for male and 31 to 71 years (median = 52 years) for female | Liquid chromatography–tandem mass spectrometry (Triple Quadrupole mass spectrometer) | from 0.08 to 102.00 μg/mL (median = 0.28 μg/mL, mean = 5.90 μg/mL ± 22.2 SD) (femoral vein blood) | This study highlights that although Baclofen abuse may be occurring, deaths are rare |
| 2024 | Dudek C.M. et al. [29] | Case Report | 1; F; 70 years old | Gas chromatography-mass spectrometry and high-performance liquid chromatography (LCMS), high- resolution mass spectrometry (LC-HRMS) | 4.4 mcg/mL (blood; Not specified) | Baclofen overdose can feature cardiovascular complications (ECG changes/Takotsubo pattern) |
| 2024 | Zahra E. et al. [23] | Retrospective Study | 102; 51% being male; mean age of 45.6 years | Immuno- assay, gas chromatography, high-performance liquid chromatography (HPLC) and liquid chromatography-quadrupole time-of-flight mass spectrometry (LC-QTOF-MS | The median baclofen blood concentration for all cases was 3.10 mg/L (25th 0.70, 75th 8.10, range 0.04–110.00), unintentional toxicity 1.95 mg/L (25th 0.70, 75th 4.35, range 0.04–24.0), intentional toxicity 6.00 mg/L (25th 1.10, 75th 13.0, range 0.05–110.0) | Caution is needed in prescribing baclofen given its potential to be used in intentional and non-intentional overdose |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albano, G.D.; Midiri, M.; Gergely, P.A.; Harsányi, T.G.; Racz, K.; Nivoli, A.M.; Buscemi, R.; Zerbo, S.; Argo, A.; Trignano, C. Acute Intoxication After Baclofen Administration: A Review of the Literature and Methodological Proposals. Toxics 2025, 13, 999. https://doi.org/10.3390/toxics13110999
Albano GD, Midiri M, Gergely PA, Harsányi TG, Racz K, Nivoli AM, Buscemi R, Zerbo S, Argo A, Trignano C. Acute Intoxication After Baclofen Administration: A Review of the Literature and Methodological Proposals. Toxics. 2025; 13(11):999. https://doi.org/10.3390/toxics13110999
Chicago/Turabian StyleAlbano, Giuseppe Davide, Mauro Midiri, Péter Attila Gergely, Tamás Gergő Harsányi, Kálmán Racz, Alessandra Matilde Nivoli, Roberto Buscemi, Stefania Zerbo, Antonina Argo, and Claudia Trignano. 2025. "Acute Intoxication After Baclofen Administration: A Review of the Literature and Methodological Proposals" Toxics 13, no. 11: 999. https://doi.org/10.3390/toxics13110999
APA StyleAlbano, G. D., Midiri, M., Gergely, P. A., Harsányi, T. G., Racz, K., Nivoli, A. M., Buscemi, R., Zerbo, S., Argo, A., & Trignano, C. (2025). Acute Intoxication After Baclofen Administration: A Review of the Literature and Methodological Proposals. Toxics, 13(11), 999. https://doi.org/10.3390/toxics13110999










