Sustainable Strategy Using Tung Fruit-Derived Humic Substances–Ferrihydrite for Simultaneous Pollutant Removal and Fertilizer Recovery
Abstract
1. Introduction
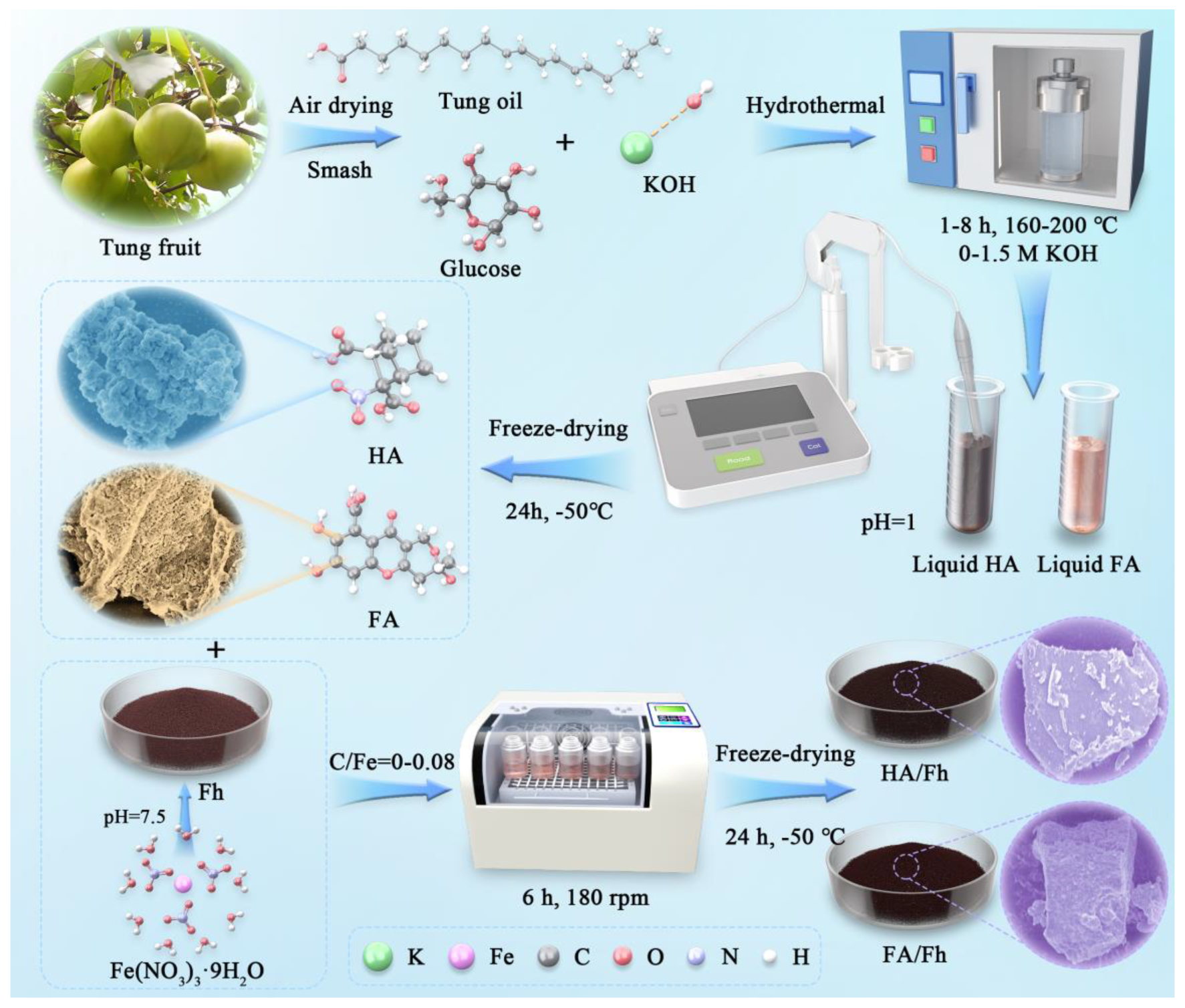
2. Materials and Methods
3. Results and Discussion
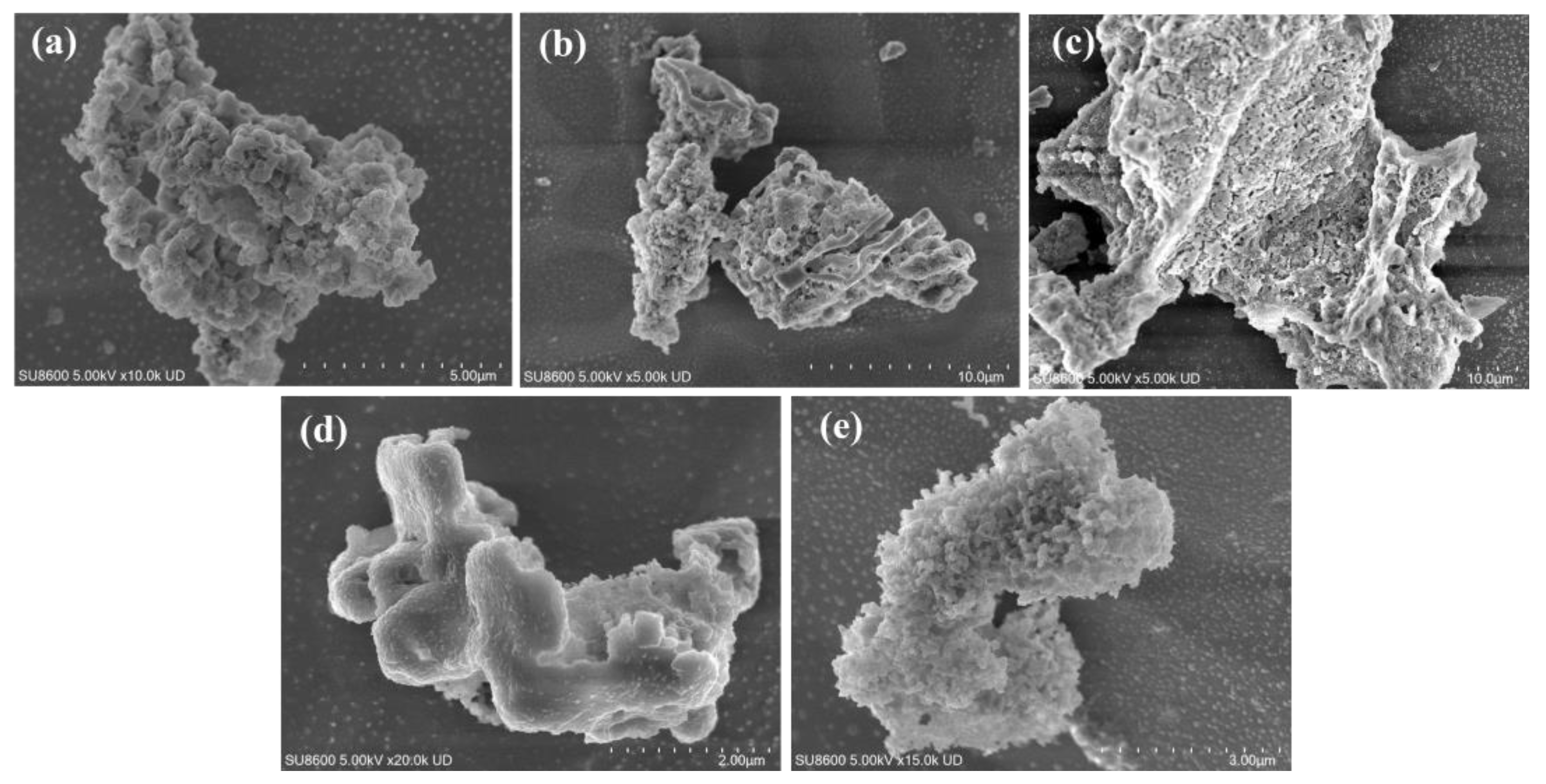
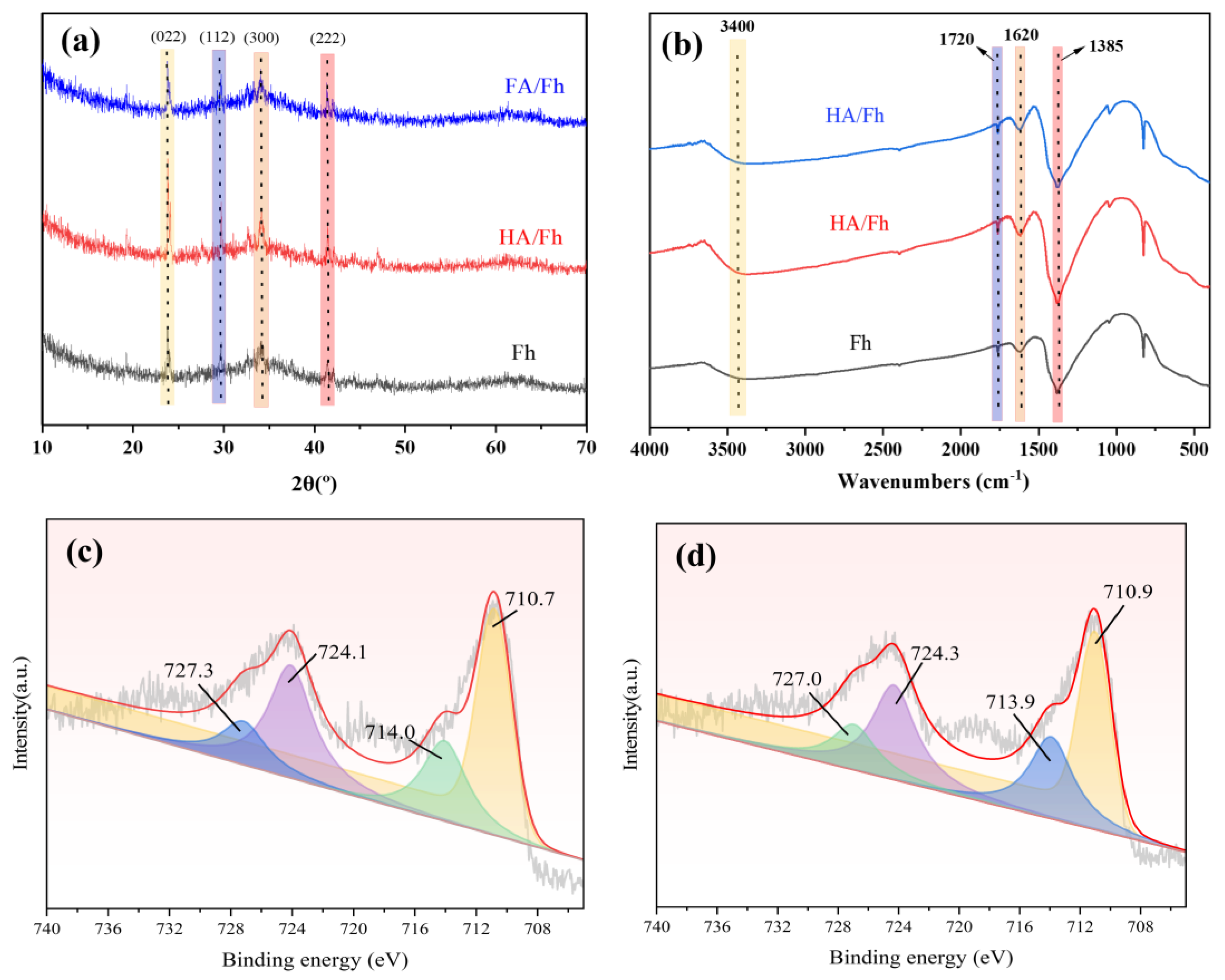
3.1. Characterization Analysis of HA/Fh and FA/Fh
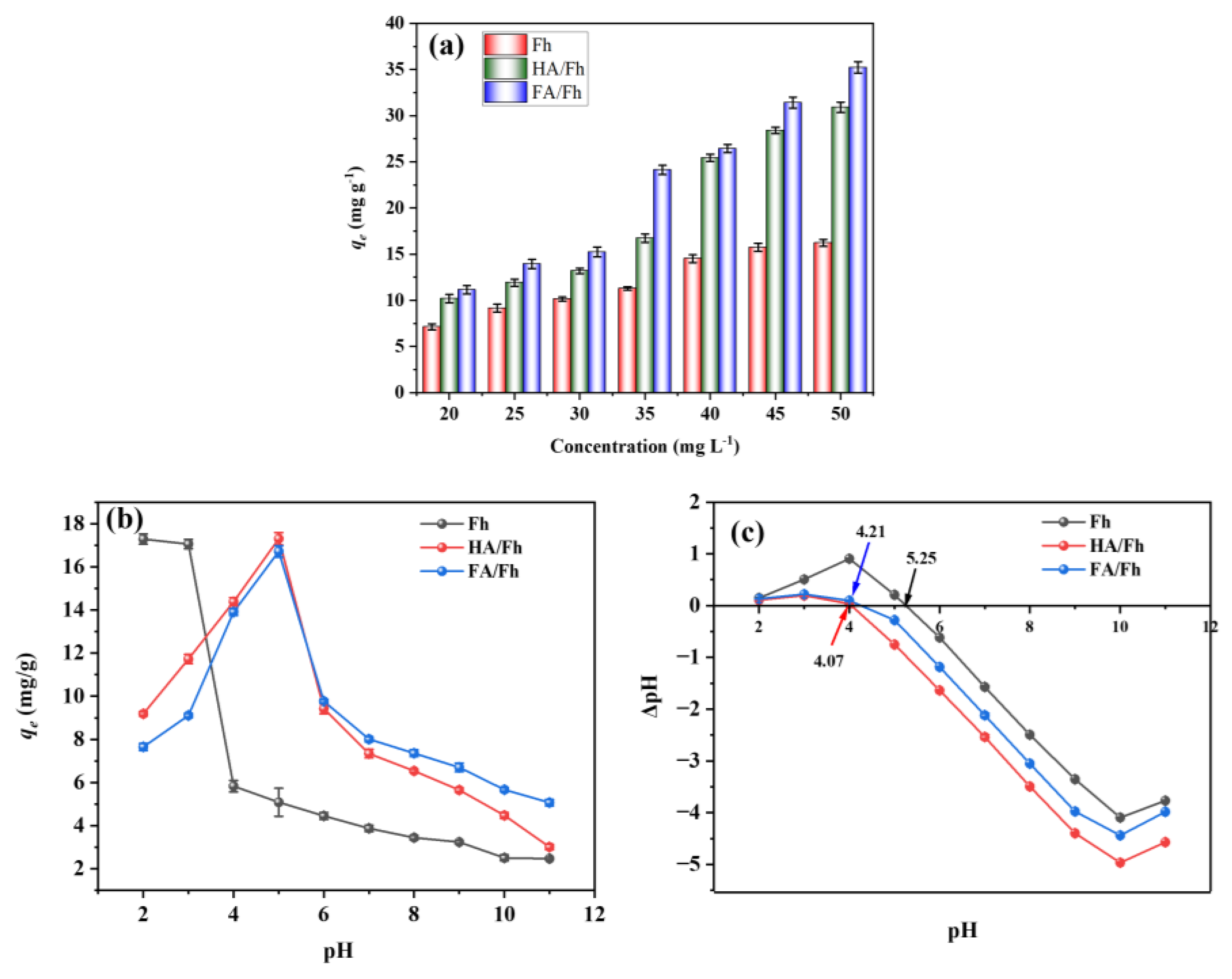
3.2. Adsorption Experiments
3.3. Adsorption Kinetics
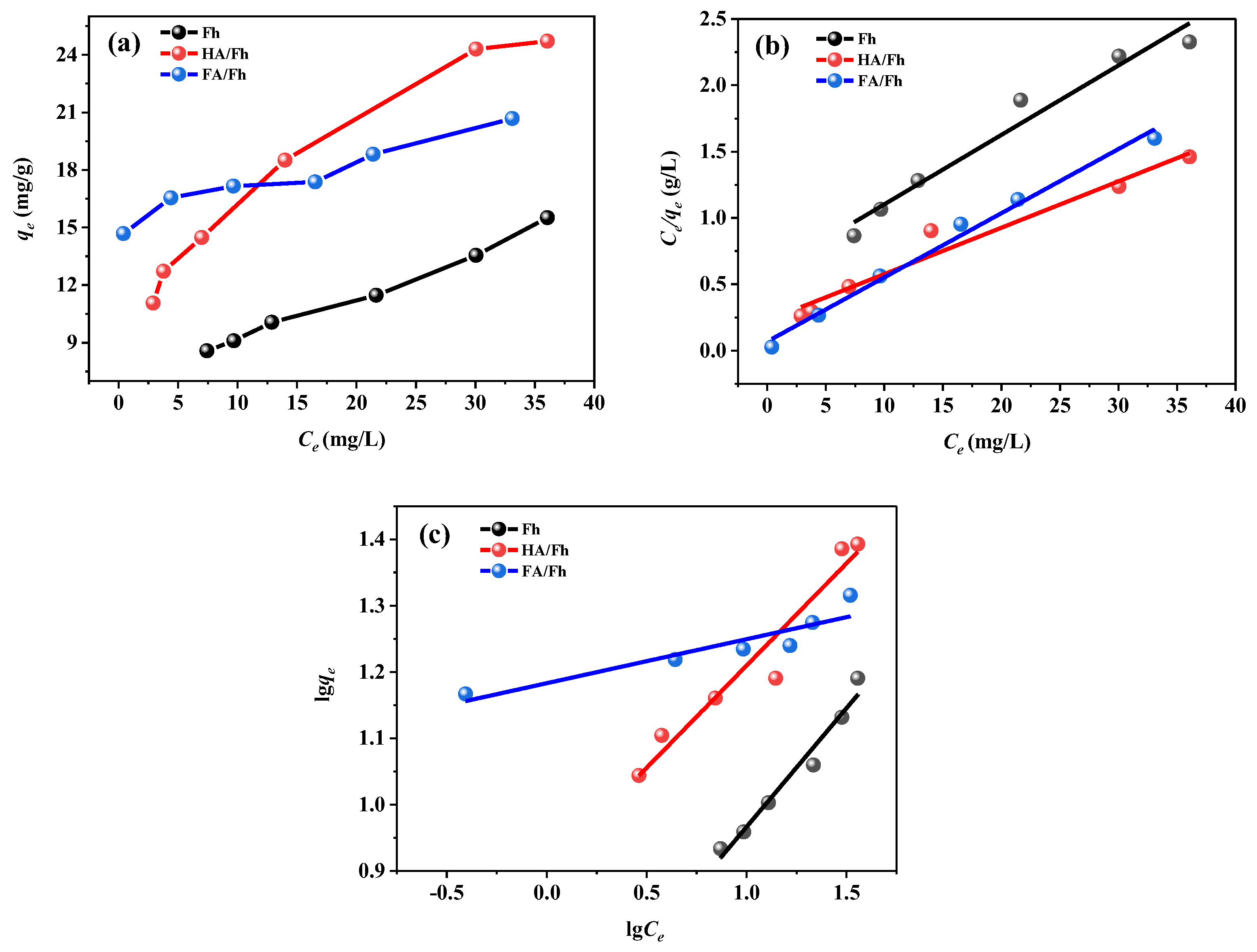
3.4. Adsorption Isotherms
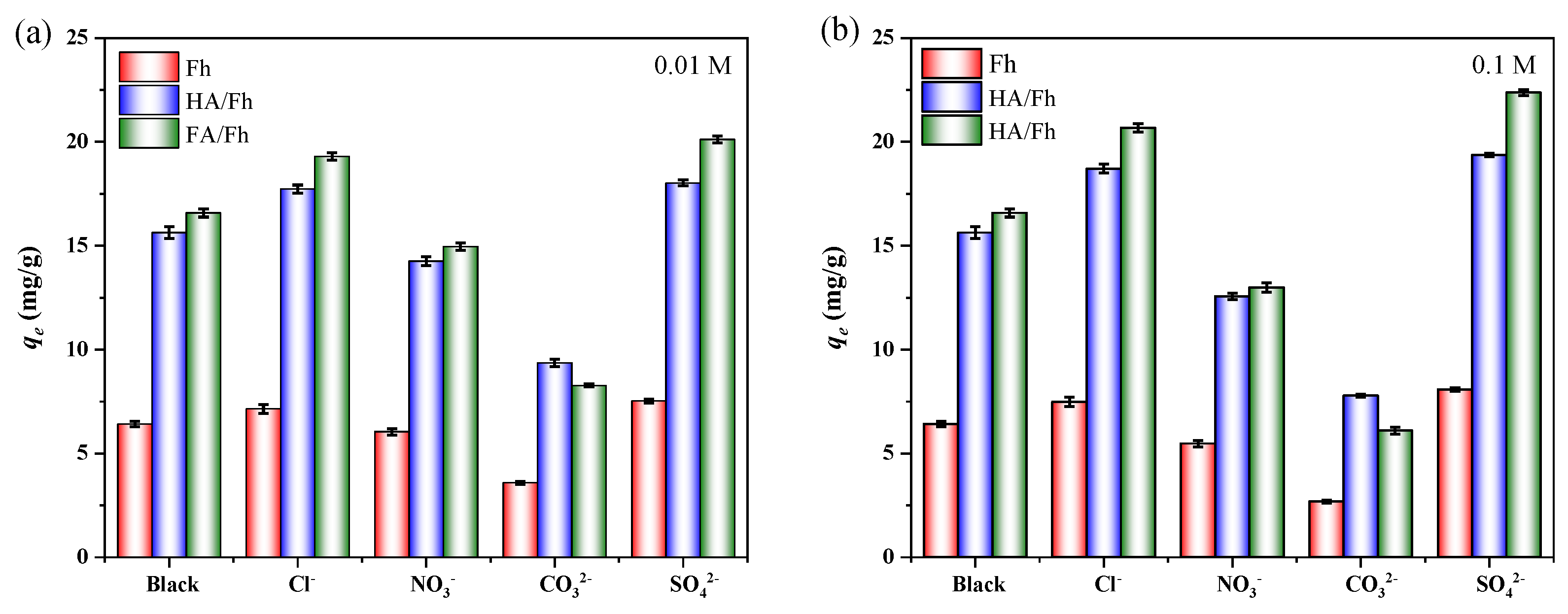
3.5. Effect of Coexisting Ions
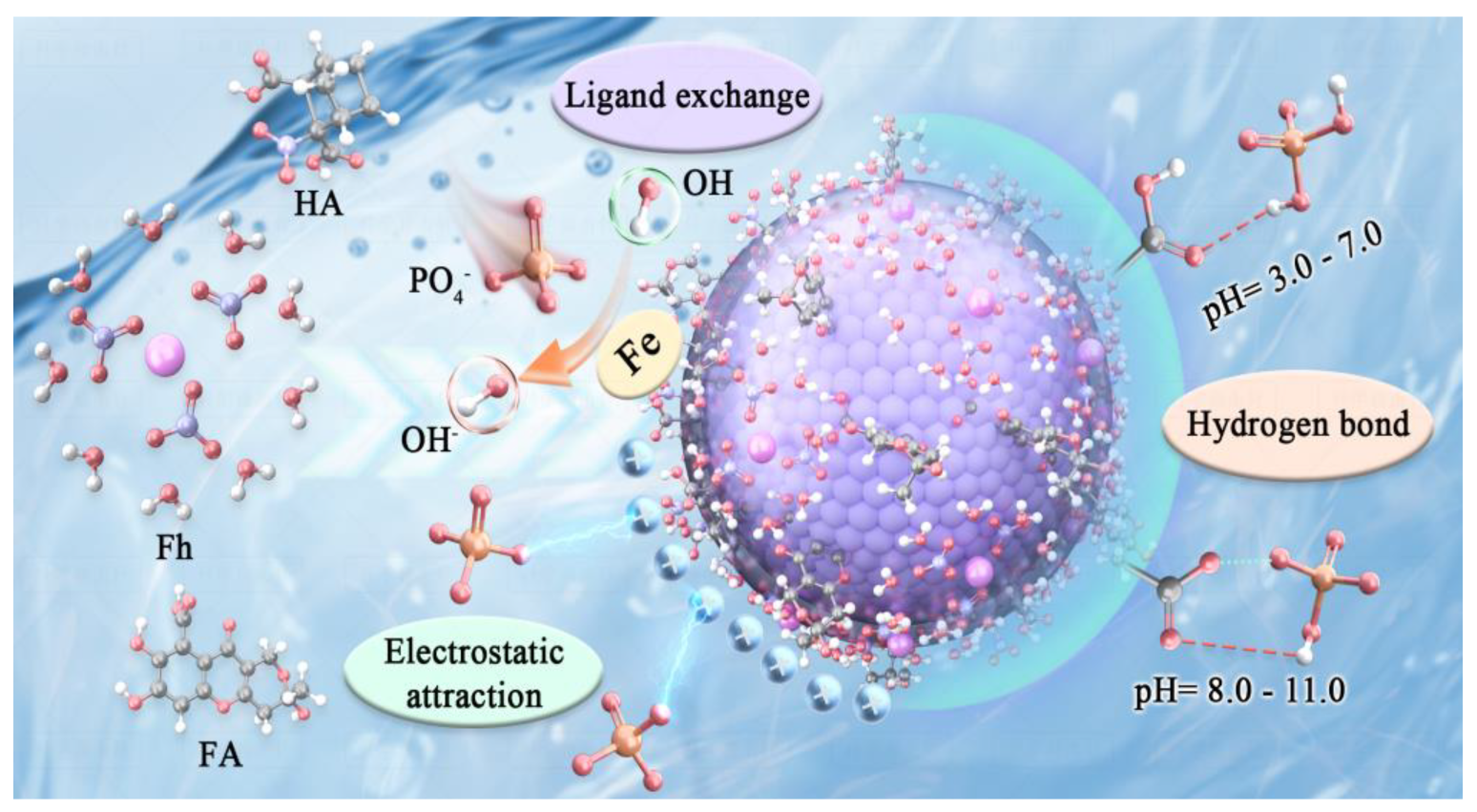
3.6. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mishra, R.K. The effect of eutrophication on drinking water. J. Multidiscip. Adv. Stud. 2023, 4, 7–20. [Google Scholar] [CrossRef]
- Zhang, P.; He, M.; Huo, S.; Li, F.; Li, K. Recent progress in metal-based composites toward adsorptive removal of phosphate: Mechanisms, behaviors, and prospects. Chem. Eng. J. 2022, 446, 137081. [Google Scholar] [CrossRef]
- Shao, Y.; Li, J.; Fang, X.; Yang, Z.; Qu, Y.; Yang, M.; Tan, W.; Li, G.; Wang, H. Chemical modification of bamboo activated carbon surface and its adsorption property of simultaneous removal of phosphate and nitrate. Chemosphere 2022, 287, 132118. [Google Scholar] [CrossRef] [PubMed]
- Raikou, V.D. Serum phosphate and chronic kidney and cardiovascular disease: Phosphorus potential implications in general population. World J. Nephrol. 2021, 10, 76. [Google Scholar] [CrossRef]
- Erem, S.; Razzaque, M.S. Dietary phosphate toxicity: An emerging global health concern. Histochem. Cell Biol. 2018, 150, 711–719. [Google Scholar] [CrossRef]
- Ramasahayam, S.K.; Guzman, L.; Gunawan, G.; Viswanathan, T. A comprehensive review of phosphorus removal technologies and processes. J. Macromol. Sci. Part A 2014, 51, 538–545. [Google Scholar] [CrossRef]
- Zahed, M.A.; Salehi, S.; Tabari, Y.; Farraji, H.; Ataei-Kachooei, S.; Zinatizadeh, A.A.; Kamali, N.; Mahjouri, M. Phosphorus removal and recovery: State of the science and challenges. Environ. Sci. Pollut. Res. 2022, 29, 58561–58589. [Google Scholar] [CrossRef]
- Velusamy, K.; Periyasamy, S.; Kumar, P.S.; Vo, D.-V.N.; Sindhu, J.; Sneka, D.; Subhashini, B. Advanced techniques to remove phosphates and nitrates from waters: A review. Environ. Chem. Lett. 2021, 19, 3165–3180. [Google Scholar] [CrossRef]
- Zhou, H.; Margenot, A.J.; Li, Y.; Si, B.; Wang, T.; Zhang, Y.; Li, S.; Bhattarai, R. Phosphorus pollution control using waste-based adsorbents: Material synthesis, modification, and sustainability. Crit. Rev. Environ. Sci. 2022, 52, 2023–2059. [Google Scholar] [CrossRef]
- Luo, D.; Wang, L.; Nan, H.; Cao, Y.; Wang, H.; Kumar, T.V.; Wang, C. Phosphorus adsorption by functionalized biochar: A review. Environ. Chem. Lett. 2023, 21, 497–524. [Google Scholar] [CrossRef]
- Mallet, M.; Barthélémy, K.; Ruby, C.; Renard, A.; Naille, S. Investigation of phosphate adsorption onto ferrihydrite by X-ray photoelectron spectroscopy. J. Colloid Interface Sci. 2013, 407, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Liu, J.; Chen, C.; Lin, X.; Wu, X.; Chen, Q.; He, H.; Zhu, R. Enhanced immobilization of phosphate by ferrihydrite during the photoreductive dissolution process. Sci. Total Environ. 2022, 838, 155835. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.-Y.; Yu, J.-X.; Li, H.-X.; Chi, R.-A. Removal of phosphate from aqueous solution by ferrihydrite/bagasse composite prepared through in situ precipitation method. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125144. [Google Scholar] [CrossRef]
- Fu, H.; Yang, Y.; Zhu, R.; Liu, J.; Usman, M.; Chen, Q.; He, H. Superior adsorption of phosphate by ferrihydrite-coated and lanthanum-decorated magnetite. J. Colloid Interface Sci. 2018, 530, 704–713. [Google Scholar] [CrossRef]
- Gai, S.; Liu, B.; Lan, Y.; Han, L.; Hu, Y.; Dongye, G.; Cheng, K.; Liu, Z.; Yang, F. Artificial humic acid coated ferrihydrite strengthens the adsorption of phosphate and increases soil phosphate retention. Sci. Total Environ. 2024, 915, 169870. [Google Scholar] [CrossRef]
- Zhao, Y.; Hao, Y.; Cheng, K.; Wang, L.; Dong, W.; Liu, Z.; Yang, F. Artificial humic acid mediated migration of phosphorus in soil: Experiment and modelling. Catena 2024, 238, 107896. [Google Scholar] [CrossRef]
- Yuan, Y.; Gai, S.; Tang, C.; Jin, Y.; Cheng, K.; Antonietti, M.; Yang, F. Artificial humic acid improves maize growth and soil phosphorus utilization efficiency. Appl. Soil Ecol. 2022, 179, 104587. [Google Scholar] [CrossRef]
- Avena Maia, M.; Kranse, O.P.; Eves-van den Akker, S.; Torrente-Murciano, L. Phosphate recovery from urine-equivalent solutions for fertilizer production for plant growth. ACS Sustain. Chem. Eng. 2023, 11, 16074–16086. [Google Scholar] [CrossRef]
- Liang, M.; Wu, Z.; Cao, H.; Dong, K.; Bai, S.; Wang, D. The phosphorus adsorption and recovery of Mg/Fe-LDHs mulberry rod biochar composite. Separations 2024, 11, 86. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Cheng, K.; Antonietti, M. A hydrothermal process to turn waste biomass into artificial fulvic and humic acids for soil remediation. Sci. Total Environ. 2019, 686, 1140–1151. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Harrington, R.; Liu, F.; Parise, J.B.; Feng, X.; Sparks, D.L. Effect of ferrihydrite crystallite size on phosphate adsorption reactivity. Environ. Sci. Technol. 2013, 47, 10322–10331. [Google Scholar] [CrossRef]
- Carta, D.; Casula, M.F.; Corrias, A.; Falqui, A.; Navarra, G.; Pinna, G. Structural and magnetic characterization of synthetic ferrihydrite nanoparticles. Mater. Chem. Phys. 2009, 113, 349–355. [Google Scholar] [CrossRef]
- Wang, N.; Deng, N.; Qiu, Y.; Su, Z.; Huang, C.; Hu, K.; Wang, J.; Ma, L.; Xiao, E.; Xiao, T. Efficient removal of antimony with natural secondary iron minerals: Effect of structural properties and sorption mechanism. Environ. Chem. 2020, 17, 332–344. [Google Scholar] [CrossRef]
- Mu, D.; Mu, L.; Geng, X.; Mohamed, T.A.; Wei, Z. Evolution from basic to advanced structure of fulvic acid and humic acid prepared by food waste. Int. J. Biol. Macromol. 2024, 256, 128413. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Song, J.; Zhao, J.; Wang, Z.; Xu, J.; Yang, W.; Hu, J. Novel MOF (Zr)–on-MOF (Ce/La) adsorbent for efficient fluoride and phosphate removal. Chem. Eng. J. 2024, 497, 154780. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, J.; Fu, Q.; Hu, H. Adsorption of phosphate on pure and humic acid-coated ferrihydrite. J. Soils Sediments 2015, 15, 1500–1509. [Google Scholar] [CrossRef]
- Hui, W.; Jun, Z.; Qing-Ling, F.; Can, H.; Hong-Qing, H. Adsorption of phosphate onto ferrihydrite and ferrihydrite-humic acid complexes. Pedosphere 2015, 25, 405–414. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Tan, W.; Li, W.; Feng, X.; Sparks, D.L. Characteristics of phosphate adsorption-desorption onto ferrihydrite: Comparison with well-crystalline Fe (hydr) oxides. Soil Sci. 2013, 178, 1–11. [Google Scholar] [CrossRef]
- Antelo, J.; Fiol, S.; Pérez, C.; Mariño, S.; Arce, F.; Gondar, D.; López, R. Analysis of phosphate adsorption onto ferrihydrite using the CD-MUSIC model. J. Colloid Interface Sci. 2010, 347, 112–119. [Google Scholar] [CrossRef]
- Civan Çavuşoğlu, F.; Özçelik, G.; Bayazit, Ş.S. Comparative Investigation of Phosphate Adsorption Efficiencies of MOF-76 (Ce) and Metal Oxides Derived from MOF-76 (Ce). Langmuir 2024, 40, 4255–4266. [Google Scholar] [CrossRef]
- Song, J.; Yu, Y.; Han, X.; Yang, W.; Pan, W.; Jian, S.; Duan, G.; Jiang, S.; Hu, J. Novel MOF (Zr)-on-MOF (Ce) adsorbent for elimination of excess fluoride from aqueous solution. J. Hazard. Mater. 2024, 463, 132843. [Google Scholar] [CrossRef]
- Liu, R.; Song, J.; Zhang, Z.; Ji, L.; Yang, W.; Zhao, J.; Jian, S.; Hu, J.; Ma, J. Needle-like PVP@ Ce/Zr-MOFs for the highly efficient selective of fluoride and phosphate from aqueous solution. Sep. Purif. Technol. 2025, 371, 133267. [Google Scholar] [CrossRef]
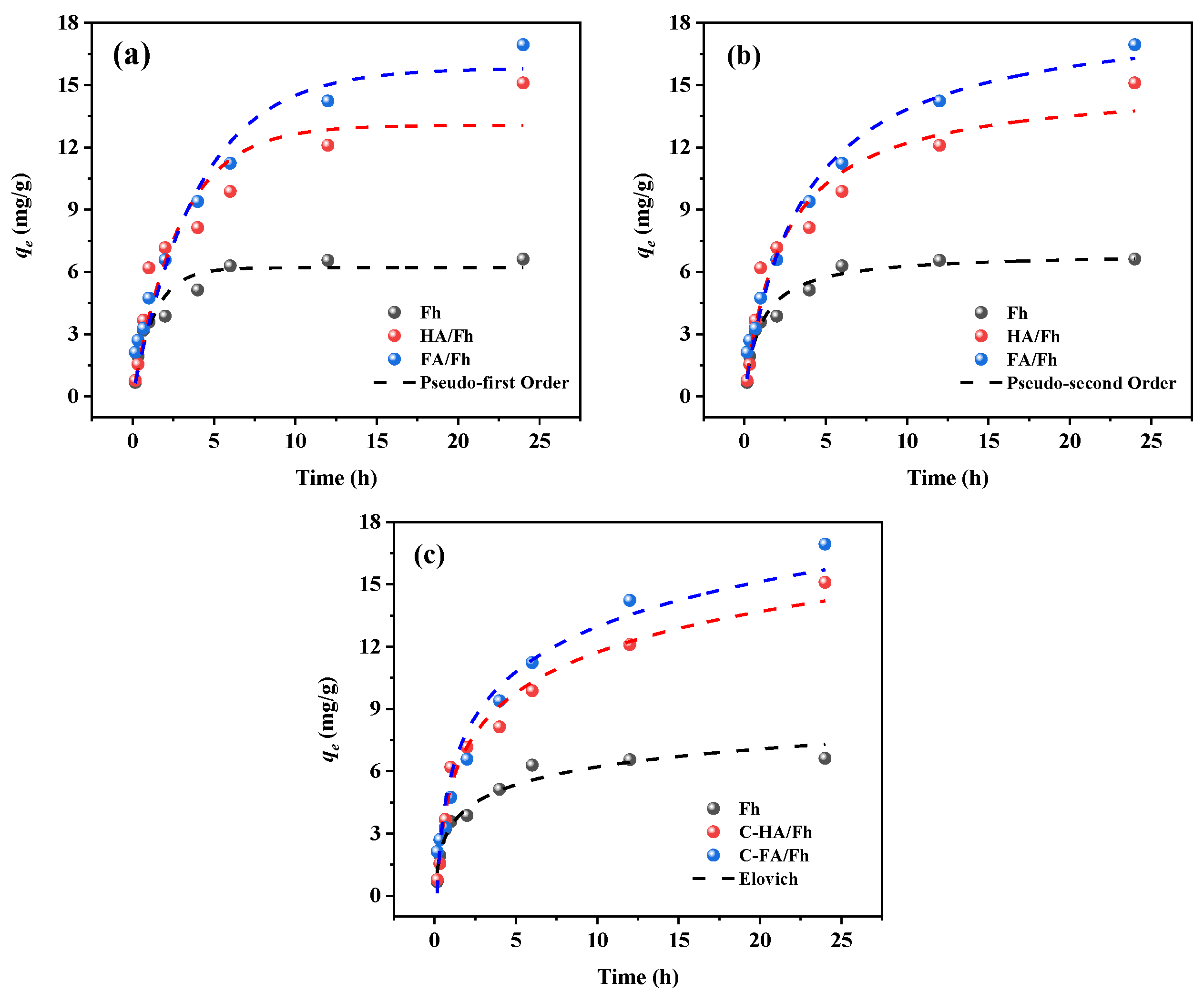
| Models | Parameter | Fh | HA/Fh | FA/Fh |
|---|---|---|---|---|
| Pseudo-first Order | R2 | 0.9219 | 0.9065 | 0.9553 |
| qe (mg g−1) | 6.203 | 13.063 | 15.818 | |
| K1 | 0.758 | 0.344 | 0.249 | |
| Pseudo-second Order | R2 | 0.9663 | 0.9552 | 0.9806 |
| qe (mg g−1) | 6.914 | 17.671 | 16.967 | |
| K2 | 0.141 | 0.028 | 0.015 | |
| Elovich | R2 | 0.9585 | 0.9789 | 0.9523 |
| A | 0.804 | 0.353 | 0.319 | |
| β | 18.3310 | 17.864 | 19.603 |
| Sample | Langmuir Equation | Freundlich Equation | ||||
|---|---|---|---|---|---|---|
| qmax (mg g−1) | KL (L mg−1) | R2 | Kf (L g−1) | n | R2 | |
| Fh | 17.634 | 0.057 | 0.9934 | 3.011 | 0.379 | 0.9832 |
| HA/Fh | 33.669 | 0.023 | 0.9968 | 2.192 | 0.586 | 0.9917 |
| FA/Fh | 37.056 | 0.032 | 0.9962 | 3.121 | 0.513 | 0.9866 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Su, Y.; Liu, C.; Tu, J.; Liu, R.; Hu, J. Sustainable Strategy Using Tung Fruit-Derived Humic Substances–Ferrihydrite for Simultaneous Pollutant Removal and Fertilizer Recovery. Toxics 2025, 13, 974. https://doi.org/10.3390/toxics13110974
Lin H, Su Y, Liu C, Tu J, Liu R, Hu J. Sustainable Strategy Using Tung Fruit-Derived Humic Substances–Ferrihydrite for Simultaneous Pollutant Removal and Fertilizer Recovery. Toxics. 2025; 13(11):974. https://doi.org/10.3390/toxics13110974
Chicago/Turabian StyleLin, Hao, Yuhuan Su, Chengfeng Liu, Jiayi Tu, Ruilai Liu, and Jiapeng Hu. 2025. "Sustainable Strategy Using Tung Fruit-Derived Humic Substances–Ferrihydrite for Simultaneous Pollutant Removal and Fertilizer Recovery" Toxics 13, no. 11: 974. https://doi.org/10.3390/toxics13110974
APA StyleLin, H., Su, Y., Liu, C., Tu, J., Liu, R., & Hu, J. (2025). Sustainable Strategy Using Tung Fruit-Derived Humic Substances–Ferrihydrite for Simultaneous Pollutant Removal and Fertilizer Recovery. Toxics, 13(11), 974. https://doi.org/10.3390/toxics13110974







