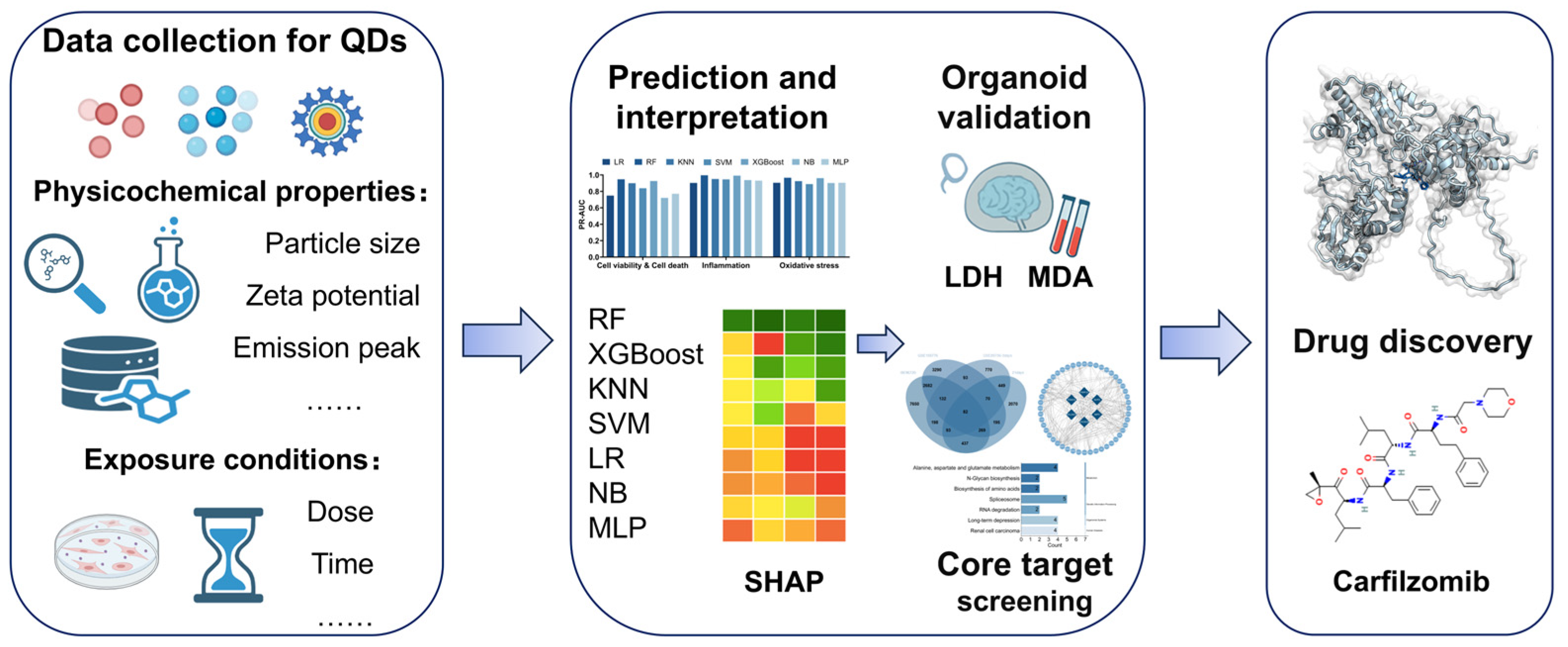
Machine Learning Framework for Multi-Endpoint Quantum Dot Toxicity Prediction with Organoid Validation and Drug Target Discovery
Abstract
1. Introduction
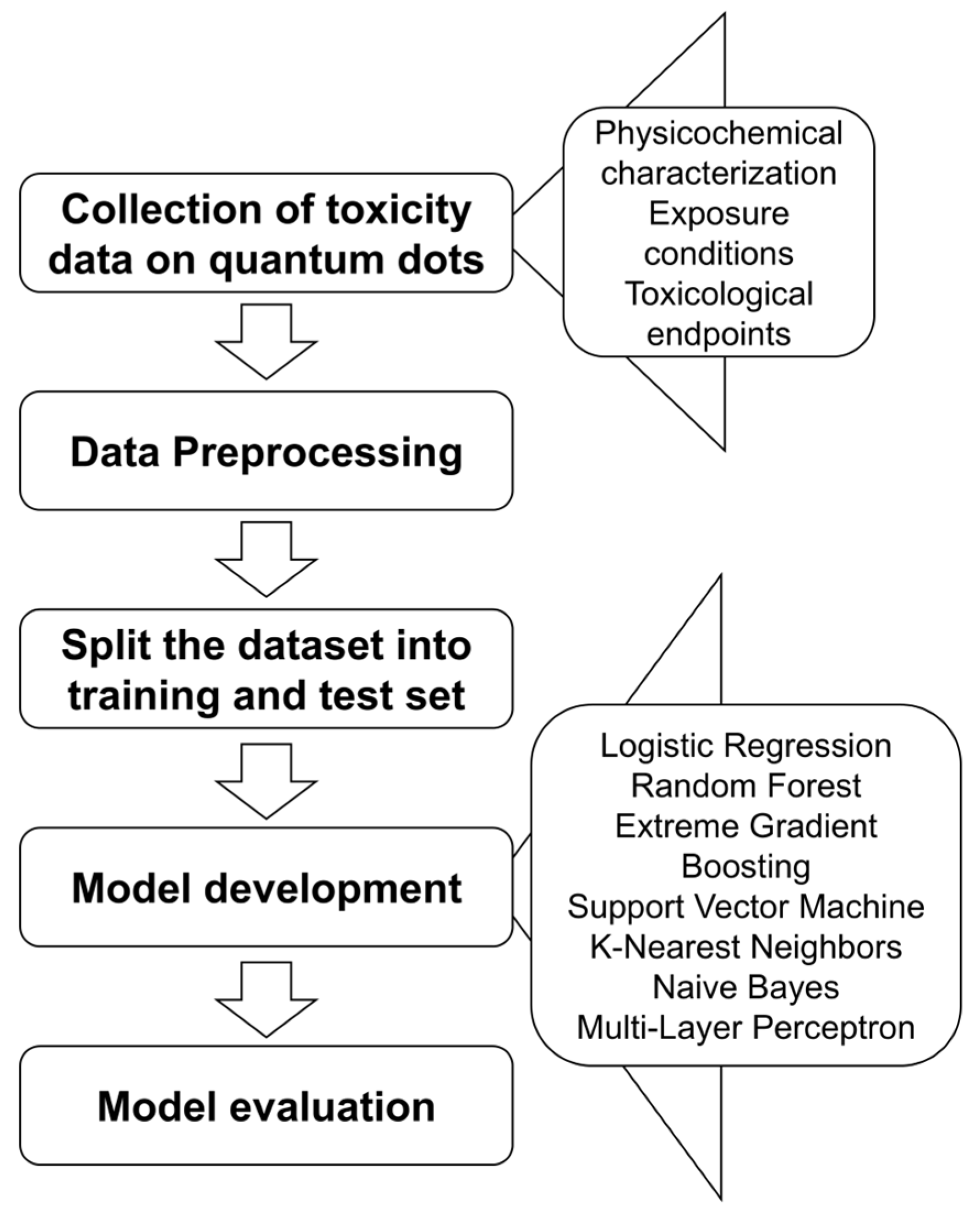
2. Materials and Methods
2.1. Data Collection, Imputation, and Multi-Model Training
2.2. Hyperparameter Tuning of Machine Learning Models
2.2.1. K-Nearest Neighbors
2.2.2. Logistic Regression
2.2.3. Naive Bayes
2.2.4. Random Forest
2.2.5. Support Vector Machine
2.2.6. Extreme Gradient Boosting
2.2.7. Multi-Layer Perceptron
2.3. Machine Learning Model Evaluation
2.4. SHAP Feature Importance Analysis
2.5. The Physicochemical Characterizations of QDs
2.6. Generation and Quantum Dot Exposure of Human Brain Organoids
2.7. Identification and Functional Analysis of Potential Target Genes in Response to QD Exposure
2.8. Screening of Potential Therapeutics for QD-Induced Toxicity
2.9. Data Analysis and Statistics
3. Results
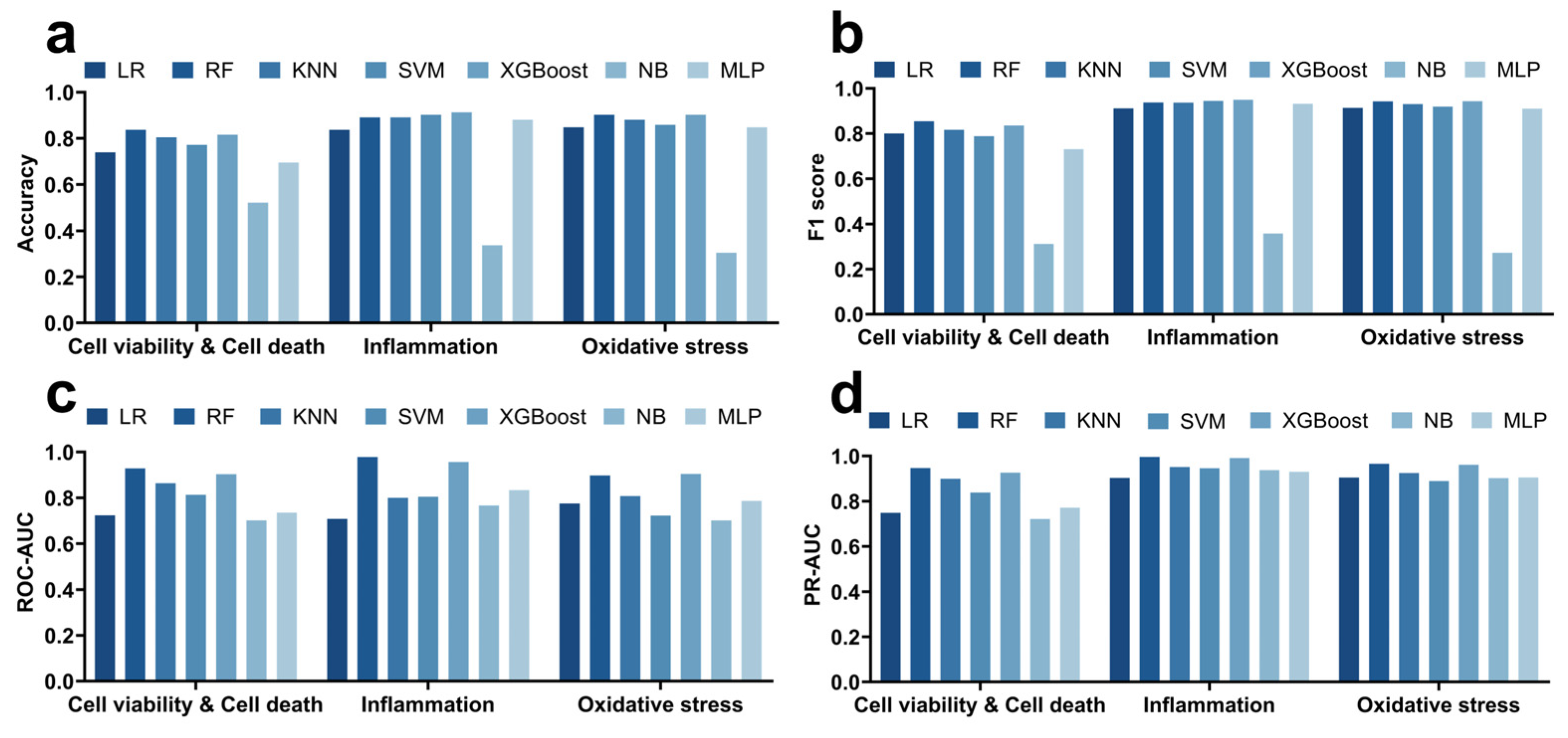
3.1. Performance Comparison of Seven Machine Learning Models in Predicting Quantum Dot-Induced Cell Viability, Inflammation, and Oxidative Stress
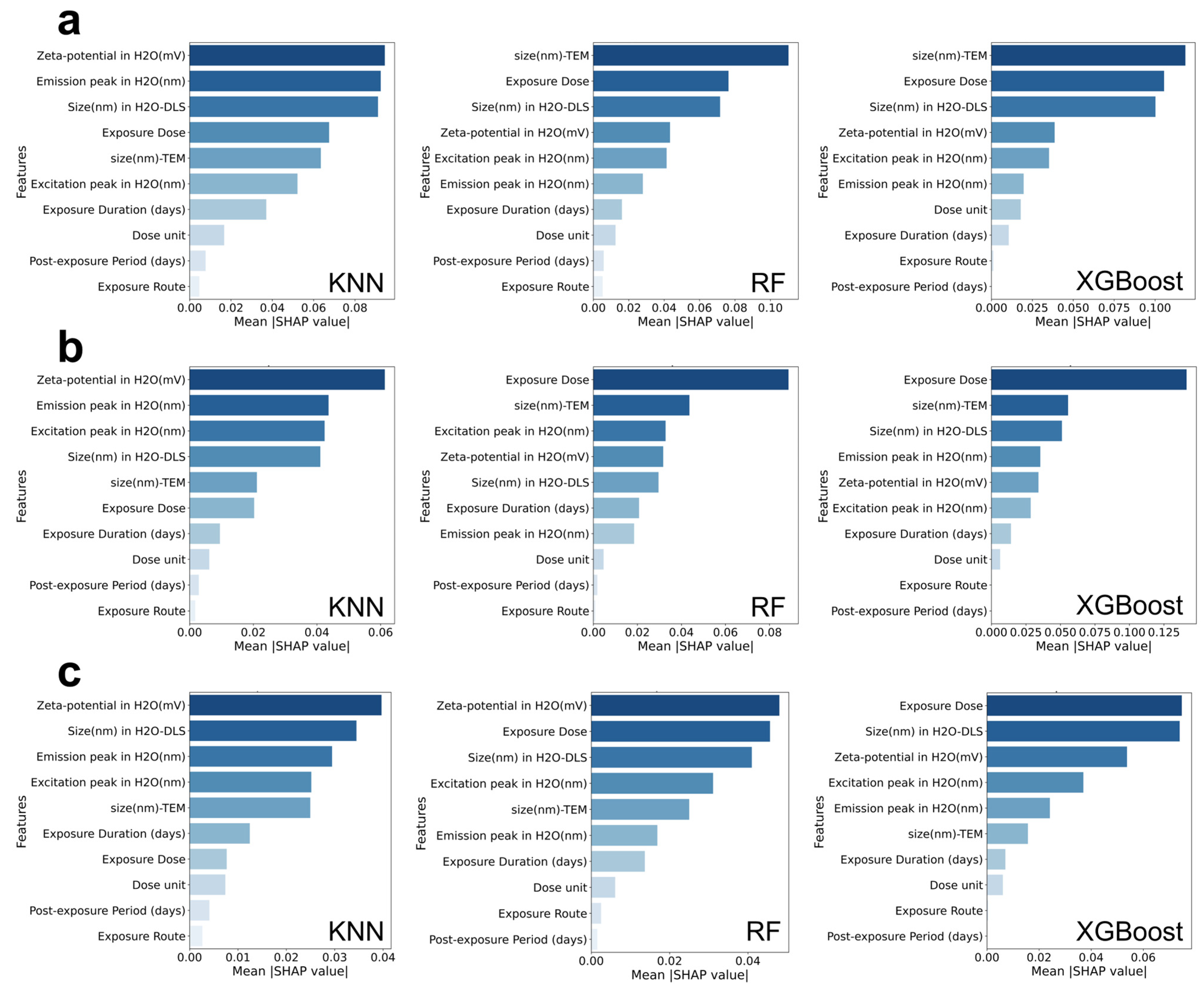
3.2. SHAP Analysis of the Physicochemical Properties and Exposure Conditions of Quantum Dots Under Different Toxicological Outcomes
3.3. Toxic Effects of Different Types of Quantum Dots in Brain Organoids and Machine Learning Validation
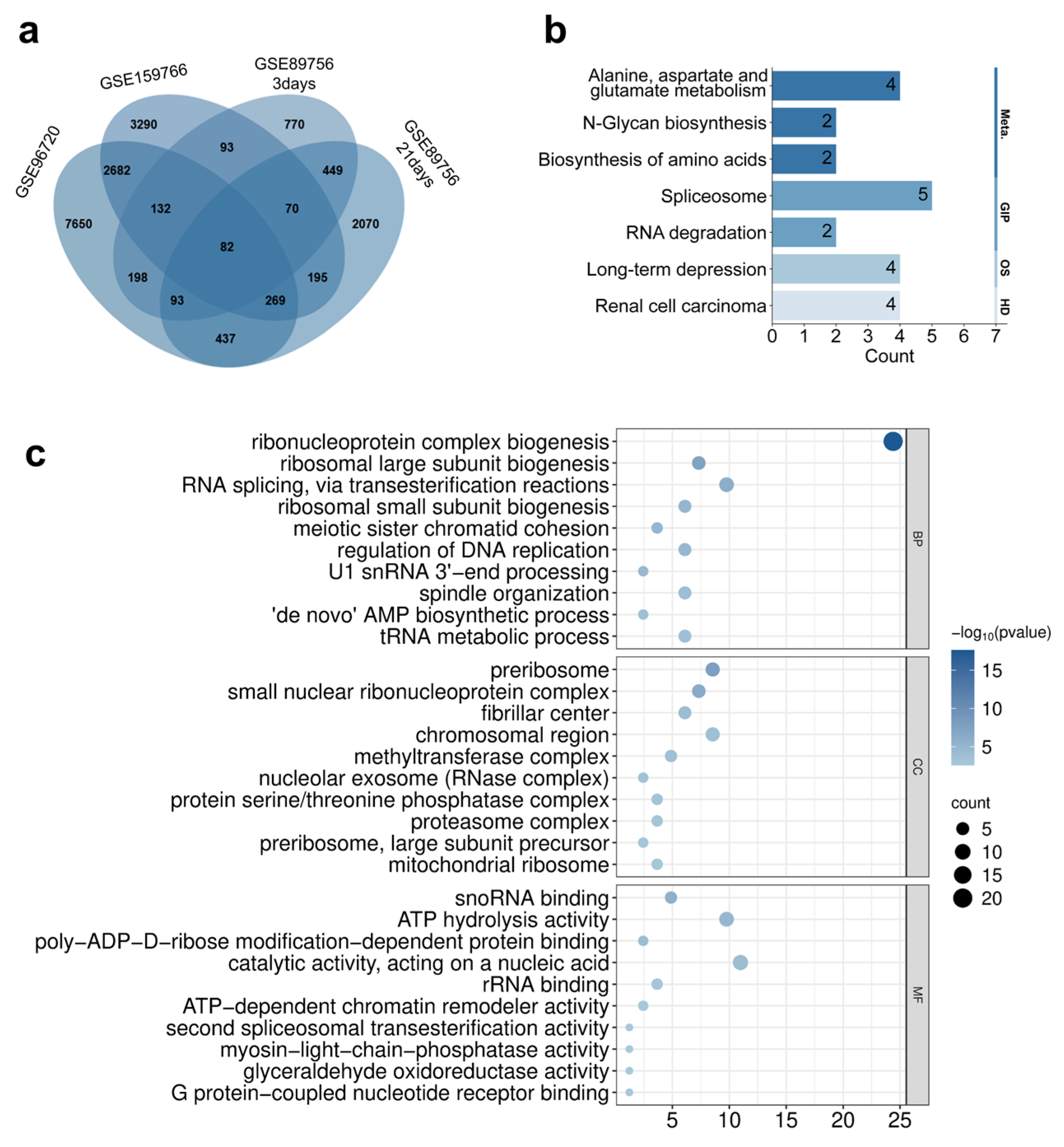
3.4. Differential Gene Expression Screening and Functional Enrichment Analysis Induced by Quantum Dots
3.5. Drug Prediction and Molecular Docking Validation of Core Target Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| QDs | Quantum Dots |
| SHAP | Shapley Additive exPlanations |
| RF | Random Forest |
| XGBoost | Extreme Gradient Boosting |
| KNN | K-Nearest Neighbors |
| SVM | Support Vector Machine |
| NB | Naive Bayes |
| LR | Logistic Regression |
| MLP | Multi-Layer Perceptron |
| EBs | Embryoid bodies |
| NIM | Neural induction medium |
| LDH | Lactate dehydrogenase |
| MDA | Malondialdehyde |
| DEGs | Differentially expressed genes |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PPI | Protein–protein interaction |
| PLIP | Protein–Ligand Interaction Profiler |
References
- Ekimov, A.I.; Onushchenko, A.A. Quantum Size Effect in the Optical-Spectra of Semiconductor Micro-Crystals. Soviet physics. Semiconductors 1982, 16, 775–778. [Google Scholar]
- Chen, B.; Li, D.; Wang, F. InP Quantum Dots: Synthesis and Lighting Applications. Small 2020, 16, 2002454. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, W.A.; Zhu, X.Y. Artificial atoms on semiconductor surfaces. Proceedings of the National Academy of Sciences 2010, 108, 965–970. [Google Scholar] [CrossRef]
- Oosterkamp, T.H.; Fujisawa, T.; van der Wiel, W.G.; Ishibashi, K.; Hijman, R.V.; Tarucha, S.; Kouwenhoven, L.P. Microwave spectroscopy of a quantum-dot molecule. Nature 1998, 395, 873–876. [Google Scholar] [CrossRef]
- Dowling, J.P.; Gea-Banacloche, J. Atomic quantum dots. In Proceedings of the International Quantum Electronics Conference, QThF12, Anaheim, CA, USA, 8–13 May 1994. [Google Scholar]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, Q.; Han, S.; Zhang, S.; Ouyang, X.; Wang, X.; Duan, Z.; Wei, H.; Zhang, X.; Ma, N.; et al. Highly Efficient Photothermal Conversion of Ti3C2Tx/Ionic Liquid Gel Pen Ink for Smoothly Writing Ultrasensitive, Wide-Range Detecting, and Flexible Thermal Sensors. ACS Appl. Mater. Interfaces 2020, 12, 37637–37646. [Google Scholar] [CrossRef]
- Wang, C.W.; Wang, Q.J. Extending the detection limit: Innovations in infrared quantum dot photodetectors reaching up to 18 μm. Light Sci. Appl. 2024, 13, 154. [Google Scholar] [CrossRef]
- Klimov, V.I.; Mikhailovsky, A.A.; Xu, S.; Malko, A.; Hollingsworth, J.A.; Leatherdale, C.A.; Eisler, H.J.; Bawendi, M.G. Optical Gain and Stimulated Emission in Nanocrystal Quantum Dots. Science 2000, 290, 314–317. [Google Scholar] [CrossRef]
- Guo, D.; Xu, P.; Chen, D.; Wang, L.; Zhu, Y.; Zuo, Y.; Chen, B. Daunorubicin-Loaded CdTe QDs Conjugated with Anti-CD123 mAbs: A Novel Delivery System for Myelodysplastic Syndromes Treatment. Int. J. Nanomed. 2020, 15, 521–536. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Tawfeek, H.M.; Younis, M.A.; Alsharidah, M.; Al Rugaie, O. Biomedical Applications of Quantum Dots: Overview, Challenges, and Clinical Potential. Int. J. Nanomed. 2022, 17, 1951–1970. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, S.; Liu, K.; Ye, Z.; Qian, Y.; He, J.; Xia, J.; Xing, P.; Yang, J.; Wa Ng, Y.; et al. Putative Adverse Outcome Pathway for Parkinson’s Disease-like Symptoms Induced by Silicon Quantum Dots based on In Vivo/Vitro Approaches. ACS Nano 2024, 18, 25271–25289. [Google Scholar] [CrossRef]
- Wu, T.; Liu, K.; Chen, S.; Ye, Z.; Xia, J.; He, J.; Xing, P.; Yang, J.; Qian, Y.; Chen, M. Pulmonary microbiota disruption by respiratory exposure to carbon quantum dots induces neuronal damages in mice. J. Hazard. Mater. 2025, 487, 137255. [Google Scholar] [CrossRef]
- Wu, T.; Wang, X.; Cheng, J.; Liang, X.; Li, Y.; Chen, M.; Kong, L.; Tang, M. Nitrogen-doped graphene quantum dots induce ferroptosis through disrupting calcium homeostasis in microglia. Part. Fibre Toxicol. 2022, 19, 22. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, Z.; Huang, X.; Wei, T.; Liu, N.; Zou, L.; Niu, Y.; Hu, Y.; Fang, Q.; Wang, X.; et al. Adverse Outcome Pathway-Based Strategies to Mitigate Ag2Se Quantum Dot-Induced Neurotoxicity. ACS Nano 2025, 19, 11029–11048. [Google Scholar] [CrossRef]
- Wang, M.; Lan, S.; Zhang, W.; Jin, Q.; Du, H.; Sun, X.; He, L.; Meng, X.; Su, L.; Liu, G. Anti-Cancer Potency of Copper-Doped Carbon Quantum Dots Against Breast Cancer Progression. Int. J. Nanomed. 2024, 19, 1985–2004. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, X.; Cheng, J.; Zhang, X.; Wu, T. Ag2Se quantum dots damage the nervous system of nematode Caenorhabditis elegans. Bull. Environ. Contam. Toxicol. 2022, 109, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zheng, F.; Yang, P.; Chen, B.; Aguilar, Z.P.; Fu, F.; Xu, H. Effects of QDs exposure on the reproductive and embryonic developmental toxicity in mice at various pregnancy stages. Toxicol. Res. 2020, 9, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wang, S.; Zhang, X.; Chen, W.; Li, Y.; Yang, P.; Cao, Z.; Wang, Y.; Lu, W.; Ju, D. Quantum Dots Elicit Hepatotoxicity through Lysosome-Dependent Autophagy Activation and Reactive Oxygen Species Production. ACS Biomater. Sci. Eng. 2018, 4, 1418–1427. [Google Scholar] [CrossRef]
- He, C.; Ruan, F.; Jiang, S.; Zeng, J.; Yin, H.; Liu, R.; Zhang, Y.; Huang, L.; Wang, C.; Ma, S.; et al. Black Phosphorus Quantum Dots Cause Nephrotoxicity in Organoids, Mice, and Human Cells. Small 2020, 16, 2001371. [Google Scholar] [CrossRef]
- Wu, T.; Tang, M. Toxicity of quantum dots on respiratory system. Inhal. Toxicol. 2014, 26, 128–139. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, T.; Tang, M. The DNA damage potential of quantum dots: Toxicity, mechanism and challenge. Environ. Pollut. 2023, 317, 120676. [Google Scholar] [CrossRef]
- Hardman, R. A Toxicologic Review of Quantum Dots: Toxicity Depends on Physicochemical and Environmental Factors. Environ. Health Perspect. 2006, 114, 165–172. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, F.; Wei, H.; Yang, B. The effects of composition and surface chemistry on the toxicity of quantum dots. J. Mater. Chem. B 2013, 1, 6485. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, B.; Sahu, V.; Shukla, S.S.; Pandey, R.; Joshi, V.; Jain, V.K.; Vyas, A. Quantum dots: Prospectives, toxicity, advances and applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102308. [Google Scholar] [CrossRef]
- Hu, L.; Zhong, H.; He, Z. Toxicity evaluation of cadmium-containing quantum dots: A review of optimizing physicochemical properties to diminish toxicity. Colloids Surf. B Biointerfaces 2021, 200, 111609. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Liu, R.; Nel, A.; Gemill, K.B.; Bilal, M.; Cohen, Y.; Medintz, I.L. Meta-analysis of cellular toxicity for cadmium-containing quantum dots. Nat. Nanotechnol. 2016, 11, 479–486. [Google Scholar] [CrossRef]
- Wang, X.; He, K.; Hu, Y.; Tang, M. A review of pulmonary toxicity of different types of quantum dots in environmental and biological systems. Chem.-Biol. Interact. 2022, 368, 110247. [Google Scholar] [CrossRef]
- Cui, L.W.; Fan, L.Y.; Shen, Z.Y. Application Research Progress of Nanomaterial Graphene and its Derivative Complexes in Tumor Diagnosis and Therapy. Curr. Med. Chem. 2024, 31, 6436–6459. [Google Scholar] [CrossRef]
- Gupta, J.; Vaid, P.K.; Priyadarshini, E.; Rajamani, P. Nano-bio convergence unveiled: Systematic review on quantum dots-protein interaction, their implications, and applications. Biophys. Chem. 2024, 310, 107238. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Gerasimovich, E.; Baryshnikova, M.; Sokolova, Z.; Samokhvalov, P.; Guhrenz, C.; Gaponik, N.; Karaulov, A.; Nabiev, I. Dependence of Quantum Dot Toxicity In Vitro on Their Size, Chemical Composition, and Surface Charge. Nanomaterials 2022, 12, 2734. [Google Scholar] [CrossRef]
- Hoshino, A.; Fujioka, K.; Oku, T.; Suga, M.; Sasaki, Y.F.; Ohta, T.; Yasuhara, M.; Suzuki, K.; Yamamoto, K. Physicochemical Properties and Cellular Toxicity of Nanocrystal Quantum Dots Depend on Their Surface Modification. Nano Lett. 2004, 4, 2163–2169. [Google Scholar] [CrossRef]
- Chen, S.; Wu, T. Progression and prospects of machine learning techniques in nanotoxicology: Riding the AI-driven wave. Toxicology Mechanisms and Methods 2025, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Varma, M.; Laux, P.; Choudhary, S.; Datusalia, A.K.; Gupta, N.; Luch, A.; Gandhi, A.; Kulkarni, P.; Nath, B. Artificial intelligence and machine learning disciplines with the potential to improve the nanotoxicology and nanomedicine fields: A comprehensive review. Arch. Toxicol. 2023, 97, 963–979. [Google Scholar] [CrossRef]
- Yousaf, I. AI and Machine Learning Approaches for Predicting Nanoparticles Toxicity The Critical Role of Physiochemical Properties. arXiv 2024, arXiv:2409.15322. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Bai, N.; Zheng, W.J.; Ni, S.J. MultiOmics analysis of metabolic dysregulation and immune features in breast cancer. Int. Immunopharmacol. 2025, 152, 114376. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, X.; Su, S.; Ji, F.; Shao, D.; Duan, C.; Chen, T.; Liang, C.; Zhang, D.; Lu, H. Identifying of immune-associated genes for assessing the obesity-associated risk to the offspring in maternal obesity: A bioinformatics and machine learning. CNS Neurosci. Ther. 2024, 30, e14700. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, H.; Wu, F.; Ding, Y.; Wu, J.; Lu, L.; Bajpai, A.K.; Sang, M.; Wang, X. Identification of hub genes and potential molecular mechanisms related to drug sensitivity in acute myeloid leukemia based on machine learning. Front. Pharmacol. 2024, 15, 1359832. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, X.; Zhang, X.; Cheng, J.; Zhang, J.; Chen, M.; Wu, T. A Deep Learning Analysis Reveals Nitrogen-Doped Graphene Quantum Dots Damage Neurons of Nematode Caenorhabditis elegans. Nanomaterials 2021, 11, 3314. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Yang, J.; Niu, Q.; Li, J. Exploring pesticide risk in autism via integrative machine learning and network toxicology. Ecotoxicol. Env. Saf. 2025, 297, 118233. [Google Scholar] [CrossRef]
- Guo, W.; Liu, J.; Dong, F.; Song, M.; Li, Z.; Khan, M.K.H.; Patterson, T.A.; Hong, H. Review of machine learning and deep learning models for toxicity prediction. Exp. Biol. Med. 2023, 248, 1952–1973. [Google Scholar] [CrossRef]
- Khokhlov, I.; Legashev, L.; Bolodurina, I.; Shukhman, A.; Shoshin, D.; Kolesnik, S. Prediction of Dynamic Toxicity of Nanoparticles Using Machine Learning. Toxics 2024, 12, 750. [Google Scholar] [CrossRef]
- Zang, X.; Zhou, W.; Zhang, H.; Zang, X. Using Four Machine Learning Methods to Analyze the Association Between Polycyclic Aromatic Hydrocarbons and Visual Impairment in American Adults: Evidence from NHANES. Toxics 2024, 12, 789. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Wang, S.; Ren, Y.; Chen, W.; Li, X.; Han, P.; Song, T. QuantumTox: Utilizing quantum chemistry with ensemble learning for molecular toxicity prediction. Comput. Biol. Med. 2023, 157, 106744. [Google Scholar] [CrossRef]
- Zeng, S.; Tang, Q.; Xiao, M.; Tong, X.; Yang, T.; Yin, D.; Lei, L.; Li, S. Cell membrane-coated nanomaterials for cancer therapy. Mater. Today Bio 2023, 20, 100633. [Google Scholar] [CrossRef]
- Yang, P.; Yang, L.; Kuang, H.; Xu, H. Research advances in characteristics of biotransport and biotransformation and toxicities of quantum dots in vivo. Chin. J. Pharmacol. Toxicol. 2015, 29, 1007–1013. [Google Scholar] [CrossRef]
- Le, N.; Zhang, M.; Kim, K. Quantum Dots and Their Interaction with Biological Systems. Int. J. Mol. Sci. 2022, 23, 10763. [Google Scholar] [CrossRef]
- Liu, W.; Liao, H.; Wei, M.; Junaid, M.; Chen, G.; Wang, J. Biological uptake, distribution and toxicity of micro(nano)plastics in the aquatic biota: A special emphasis on size-dependent impacts. TrAC Trends Anal. Chem. 2024, 170, 117477. [Google Scholar] [CrossRef]
- Carnovale, C.; Bryant, G.; Shukla, R.; Bansal, V. Size, shape and surface chemistry of nano-gold dictate its cellular interactions, uptake and toxicity. Prog. Mater. Sci. 2016, 83, 152–190. [Google Scholar] [CrossRef]
- Dolai, J.; Mandal, K.; Jana, N.R. Nanoparticle Size Effects in Biomedical Applications. ACS Appl. Nano Mater. 2021, 4, 6471–6496. [Google Scholar] [CrossRef]
- Naseer, B.; Srivastava, G.; Qadri, O.S.; Faridi, S.A.; Islam, R.U.; Younis, K. Importance and health hazards of nanoparticles used in the food industry. Nanotechnol. Rev. 2018, 7, 623–641. [Google Scholar] [CrossRef]
- Cheng, D.; Zheng, D.; Jiang, M.; Jin, Y.; Liu, R.; Zhou, Y.; Shen, J.; Tang, J.; Wang, F.; Tang, J.; et al. Inhibition of iron ion accumulation alleviates polystyrene nanoplastics-induced pulmonary fibroblast proliferation and activation. Int. Immunopharmacol. 2025, 164, 115367. [Google Scholar] [CrossRef]
- He, Z.; Armaghani, D.J.; Masoumnezhad, M.; Khandelwal, M.; Zhou, J.; Murlidhar, B.R. A Combination of Expert-Based System and Advanced Decision-Tree Algorithms to Predict Air-Overpressure Resulting from Quarry Blasting. Nat. Resour. Res. 2020, 30, 1889–1903. [Google Scholar] [CrossRef]
- Ju, W.; Xing, Z. A novel technology for unraveling the spatial risk of Natech disasters based on machine learning and GIS: A case study from the city of Changzhou, China. Earth Sci. Inform. 2024, 17, 5751–5770. [Google Scholar] [CrossRef]
- Mustafa, R.; Ahmad, M.T. Internal Stability of Mechanically Stabilized Earth Wall Using Machine Learning Techniques. Transp. Infrastruct. Geotechnol. 2024, 11, 3204–3234. [Google Scholar] [CrossRef]
- Hashida, S. Dispersibility of Colloidal Particles: Basic Theory about Zeta Potential Measurement. J. Adhes. Soc. Jpn. 2019, 55, 266–270. [Google Scholar] [CrossRef]
- Inthajak, K.; Duanggate, C.; Uyyanonvara, B.; Makhanov, S.S.; Barman, S. Medical image blob detection with feature stability and KNN classification. In Proceedings of the 2011 Eighth International Joint Conference on Computer Science and Software Engineering (JCSSE), Nakhonpathom, Thailand, 11–13 May 2011; pp. 128–131. [Google Scholar] [CrossRef]
- Roberts, N.; Smith, M.; Qi, J. Data engineering for predictive machine learning of stormwater infrastructure conditions. Eng. Appl. Artif. Intell. 2024, 133, 108195. [Google Scholar] [CrossRef]
- Virnodkar, S.S.; Pachghare, V.K.; Patil, V.C.; Jha, S.K. Remote sensing and machine learning for crop water stress determination in various crops: A critical review. Precis. Agric. 2020, 21, 1121–1155. [Google Scholar] [CrossRef]
- Amiri, A.; Peltier, N.; Goldberg, C.; Sun, Y.; Nathan, A.; Hiremath, S.; Mankodiya, K. WearSense: Detecting Autism Stereotypic Behaviors through Smartwatches. Healthcare 2017, 5, 11. [Google Scholar] [CrossRef]
- Mistry, P.; Neagu, D.; Trundle, P.R.; Vessey, J.D. Using random forest and decision tree models for a new vehicle prediction approach in computational toxicology. Soft Comput. 2015, 20, 2967–2979. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, X.; Liu, D. Model of Gradient Boosting Random Forest Prediction. In Proceedings of the 2022 IEEE International Conference on Networking, Sensing and Control (ICNSC), Shanghai, China, 15–18 December 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Mohammed, B.; Hamza, C. A Robust Estimation of Blasting-Induced Flyrock Using Machine Learning Decision Tree Algorithms: Random Forest, Gradient Boosting Machine, and XGBoost. Min. Metall. Explor. 2025, 42, 1609–1624. [Google Scholar] [CrossRef]
- Sheridan, R.P.; Wang, W.M.; Liaw, A.; Ma, J.; Gifford, E.M. Extreme Gradient Boosting as a Method for Quantitative Structure–Activity Relationships. J. Chem. Inf. Model. 2016, 56, 2353–2360. [Google Scholar] [CrossRef]
- Wang, S.; Long, W.; Wei, L.; Cheng, W.; Chen, H.; Yang, J.; Fu, H. Nano effect fluorescence visual sensor based on Au-AgNCs: A novel strategy to identify the origin and growth year of Lilium bulbs. Food Chem. 2024, 441, 138353. [Google Scholar] [CrossRef]
- Calé, A.; Elblová, P.; Andělová, H.; Lunova, M.; Lunov, O. Analyzing Molecular Determinants of Nanodrugs’ Cytotoxic Effects. Int. J. Mol. Sci. 2025, 26, 6687. [Google Scholar] [CrossRef]
- Ge, D.; Du, Q.; Ran, B.; Liu, X.; Wang, X.; Ma, X.; Cheng, F.; Sun, B. The neurotoxicity induced by engineered nanomaterials. Int. J. Nanomed. 2019, 14, 4167–4186. [Google Scholar] [CrossRef]
- Xu, S.; Pang, X.; Zhang, X.; Lv, Q.; Zhang, M.; Wang, J.; Ni, N.; Sun, X. Nanomaterials disrupting cell-cell junctions towards various diseases. Nano Res. 2023, 16, 7053–7074. [Google Scholar] [CrossRef]
- Sun, J.; Peng, S.; Yang, Q.; Yang, J.; Dai, Y.; Xing, L. Microplastics/nanoplastics and neurological health: An overview of neurological defects and mechanisms. Toxicology 2025, 511, 154030. [Google Scholar] [CrossRef] [PubMed]
- Barla, I.; Efentakis, P.; Lamprou, S.; Gavriatopoulou, M.; Dimopoulos, M.-A.; Terpos, E.; Andreadou, I.; Thomaidis, N.; Gikas, E. Metabolomics Point out the Effects of Carfilzomib on Aromatic Amino Acid Biosynthesis and Degradation. Int. J. Mol. Sci. 2023, 24, 13966. [Google Scholar] [CrossRef] [PubMed]
- Forghani, P.; Rashid, A.; Sun, F.; Liu, R.; Li, D.; Lee, M.R.; Hwang, H.; Maxwell, J.T.; Mandawat, A.; Wu, R.; et al. Carfilzomib Treatment Causes Molecular and Functional Alterations of Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes. J. Am. Heart Assoc. 2021, 10, e022247. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Lopez, M.; Besse, A.; Zuppinger, C.; Perez-Shibayama, C.; Gil-Cruz, C.; Florea, B.I.; De Martin, A.; Lütge, M.; Beckerova, D.; Klimovic, S.; et al. Carfilzomib-specific proteasome β5/β2 inhibition drives cardiotoxicity via remodeling of protein homeostasis and the renin-angiotensin-system. iScience 2025, 28, 113228. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, B.; Xiao, C.; Sun, Z.; Gao, Y.; Liu, C.; Dou, X.; Tong, H.; Wang, R.; Li, P.; et al. IGF2BP3 stabilizes SESN1 mRNA to mitigate oxidized low-density lipoprotein-induced oxidative stress and endothelial dysfunction in human umbilical vein endothelial cells by activating Nrf2 signaling. Prostaglandins Other Lipid Mediat. 2024, 172, 106832. [Google Scholar] [CrossRef]
- Lv, L.; Wei, Q.; Zhang, J.; Dong, Y.; Shan, Z.; Chang, N.; Zhao, Y.; Bian, P.; Yi, Q. IGF2BP3 prevent HMGB1 mRNA decay in bladder cancer and development. Cell. Mol. Biol. Lett. 2024, 29, 39. [Google Scholar] [CrossRef]
- Suvasini, R.; Shruti, B.; Thota, B.; Shinde, S.V.; Friedmann-Morvinski, D.; Nawaz, Z.; Prasanna, K.V.; Thennarasu, K.; Hegde, A.S.; Arivazhagan, A.; et al. Insulin Growth Factor-2 Binding Protein 3 (IGF2BP3) Is a Glioblastoma-specific Marker That Activates Phosphatidylinositol 3-Kinase/Mitogen-activated Protein Kinase (PI3K/MAPK) Pathways by Modulating IGF-2. J. Biol. Chem. 2011, 286, 25882–25890. [Google Scholar] [CrossRef]
- Burns, D.T.; Donkervoort, S.; Müller, J.S.; Knierim, E.; Bharucha-Goebel, D.; Faqeih, E.A.; Bell, S.K.; AlFaifi, A.Y.; Monies, D.; Millan, F.; et al. Variants in EXOSC9 Disrupt the RNA Exosome and Result in Cerebellar Atrophy with Spinal Motor Neuronopathy. Am. J. Hum. Genet. 2018, 102, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Dabaj, I.; Hassani, A.; Burglen, L.; Qebibo, L.; Guerrot, A.-M.; Marret, S.; Tebani, A.; Bekri, S. Pontocerebellar Hypoplasia Type 1D: A Case Report and Comprehensive Literature Review. J. Clin. Med. 2022, 11, 4335. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Iwama, K.; Sekiguchi, F.; Mashimo, H.; Kumada, S.; Ishigaki, K.; Okamoto, N.; Behnam, M.; Ghadami, M.; Koshimizu, E.; et al. Novel EXOSC9 variants cause pontocerebellar hypoplasia type 1D with spinal motor neuronopathy and cerebellar atrophy. J. Hum. Genet. 2020, 66, 401–407. [Google Scholar] [CrossRef]
- Georgiou, M.; Atkinson, R.; Mozaffari-Jovin, S.; Lako, M. Progressive accumulation of cytoplasmic aggregates in PRPF31 retinal pigment epithelium cells interferes with cell survival. Clin. Transl. Discov. 2022, 2, e89. [Google Scholar] [CrossRef]
- Wagle, A.S.; Vargas, M. Uncovering Pre-messenger RNA Splicing Mechanisms in Retinitis Pigmentosa. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Alnafakh, R.A.A.; Adishesh, M.; Button, L.; Saretzki, G.; Hapangama, D.K. Telomerase and Telomeres in Endometrial Cancer. Front. Oncol. 2019, 9, 344. [Google Scholar] [CrossRef] [PubMed]
- Maliński, B.; Vertemara, J.; Faustini, E.; Ladenvall, C.; Norberg, A.; Zhang, Y.; von Castelmur, E.; Baliakas, P.; Tisi, R.; Cammenga, J.; et al. Novel pathological variants of NHP2 affect N-terminal domain flexibility, protein stability, H/ACA Ribonucleoprotein (RNP) complex formation and telomerase activity. Hum. Mol. Genet. 2023, 32, 2901–2912. [Google Scholar] [CrossRef]
- Rembiałkowska, N.; Sędzik, M.; Kisielewska, M.; Łuniewska, W.; Sebastianka, K.; Molik, K.; Skinderowicz, K.; Kuźnicki, J.; Tunikowska, J.; Kulbacka, J. Telomere Maintenance and DNA Repair: A Bidirectional Relationship in Cancer Biology and Therapy. Cancers 2025, 17, 2284. [Google Scholar] [CrossRef]
- Danilova, N.; Gazda, H.T. Ribosomopathies: How a common root can cause a tree of pathologies. Dis. Models Mech. 2015, 8, 1013–1026. [Google Scholar] [CrossRef]
- Kang, J.; Brajanovski, N.; Chan, K.T.; Xuan, J.; Pearson, R.B.; Sanij, E. Ribosomal proteins and human diseases: Molecular mechanisms and targeted therapy. Signal Transduct. Target. Ther. 2021, 6, 323. [Google Scholar] [CrossRef]
- Temaj, G.; Saha, S.; Dragusha, S.; Ejupi, V.; Buttari, B.; Profumo, E.; Beqa, L.; Saso, L. Ribosomopathies and cancer: Pharmacological implications. Expert. Rev. Clin. Pharmacol. 2022, 15, 729–746. [Google Scholar] [CrossRef]
- Vadivel Gnanasundram, S.; Fåhraeus, R. Translation Stress Regulates Ribosome Synthesis and Cell Proliferation. Int. J. Mol. Sci. 2018, 19, 3757. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.L. Protein degradation and protection against misfolded or damaged proteins. Nature 2003, 426, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.S.; Vierstra, R.D. Dynamic Regulation of the 26S Proteasome: From Synthesis to Degradation. Front. Mol. Biosci. 2019, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Amm, I.; Sommer, T.; Wolf, D.H. Protein quality control and elimination of protein waste: The role of the ubiquitin–proteasome system. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 182–196. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Hu, D.; Xing, P.; Zhang, Y.; Ye, Z.; Liu, K.; Xia, J.; He, J.; Qian, Y.; Wu, T. Machine Learning Framework for Multi-Endpoint Quantum Dot Toxicity Prediction with Organoid Validation and Drug Target Discovery. Toxics 2025, 13, 967. https://doi.org/10.3390/toxics13110967
Yang J, Hu D, Xing P, Zhang Y, Ye Z, Liu K, Xia J, He J, Qian Y, Wu T. Machine Learning Framework for Multi-Endpoint Quantum Dot Toxicity Prediction with Organoid Validation and Drug Target Discovery. Toxics. 2025; 13(11):967. https://doi.org/10.3390/toxics13110967
Chicago/Turabian StyleYang, Jiafu, Dayu Hu, Pengcheng Xing, Yikai Zhang, Zongjian Ye, Kehan Liu, Jieyi Xia, Jing He, Yijing Qian, and Tianshu Wu. 2025. "Machine Learning Framework for Multi-Endpoint Quantum Dot Toxicity Prediction with Organoid Validation and Drug Target Discovery" Toxics 13, no. 11: 967. https://doi.org/10.3390/toxics13110967
APA StyleYang, J., Hu, D., Xing, P., Zhang, Y., Ye, Z., Liu, K., Xia, J., He, J., Qian, Y., & Wu, T. (2025). Machine Learning Framework for Multi-Endpoint Quantum Dot Toxicity Prediction with Organoid Validation and Drug Target Discovery. Toxics, 13(11), 967. https://doi.org/10.3390/toxics13110967





