Evaluating the Single and Combined Effects of BMDM and PS Microplastics on Chlorella sp.: Physiological and Transcriptomic Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. Chlorella sp. Culture and Reagents
2.2. Exposure Experiment
2.3. Cell Growth and Photosynthetic Pigment Assays
2.4. Assessment of PS and BMDM Interactive Effects on Chlorella sp.
2.5. Cell Morphology Observation and Physical Property Measurement
2.6. Measurement of Oxidative Stress and Energy Metabolism Levels
2.7. Transcriptome Sequencing Analysis and qPCR Validation
2.8. Data Statistical Analysis
3. Results
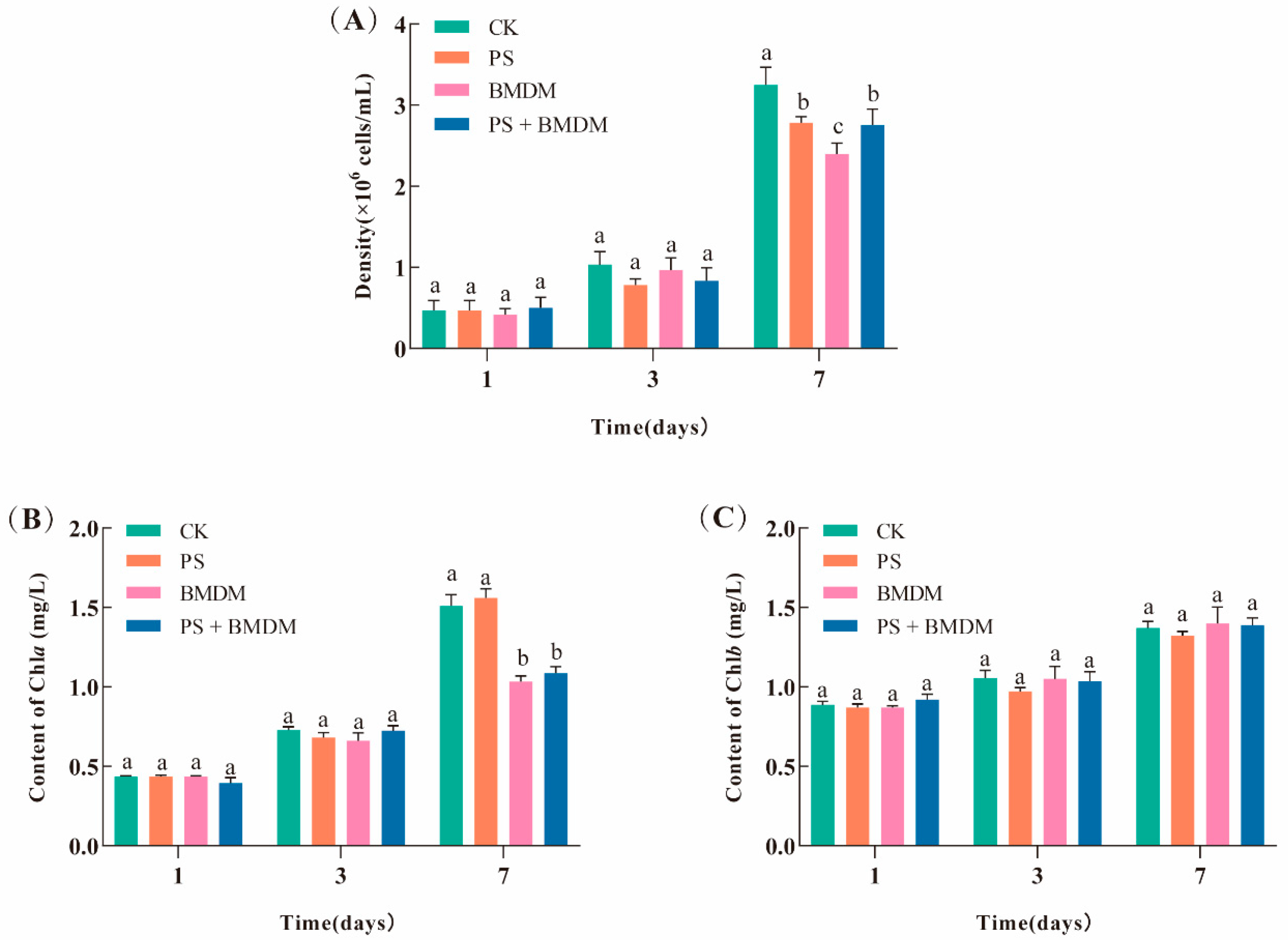
3.1. The Single and Combined Exposure Effects of MPs and BMDM on Chlorella sp. Growth
3.2. Chlorella sp. Cell Surface Morphology and Mechanical Properties
3.3. The Oxidative Stress and Energy Metabolism Levels of Chlorella sp.
3.4. RNA-Seq Analysis and Differential Expression Genes (DEGs)
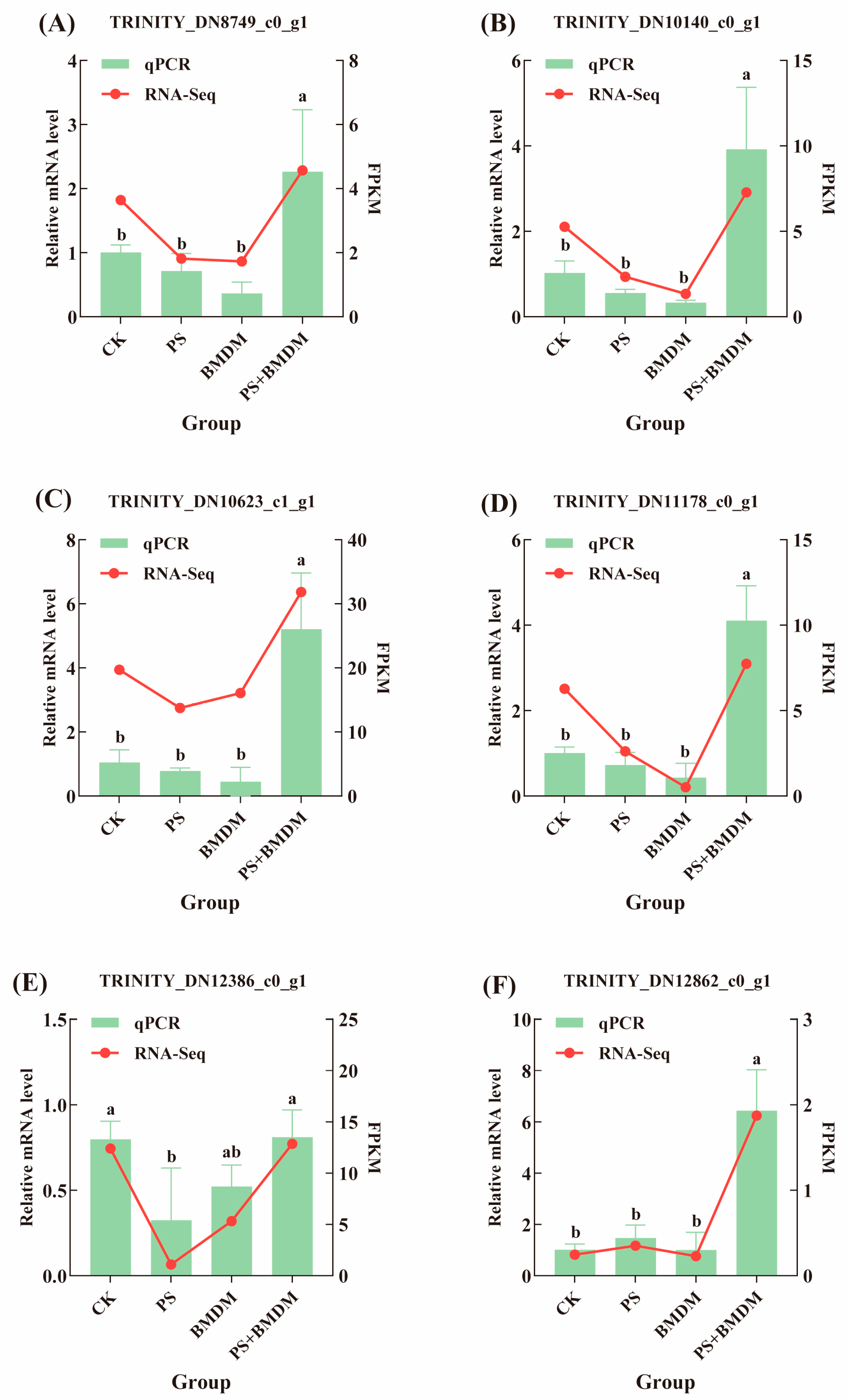
3.5. qPCR Validation
4. Discussion
4.1. Single and Combined Exposure of BMDM and PS Disrupted the Growth of Chlorella sp.
4.2. PS Reduced the Impacts of BMDM on the Morphological Structure of Chlorella sp.
4.3. PS Mitigated the Inhibitory Effect of BMDM on the Photosynthesis of Chlorella sp.
4.4. PS Declined the Inhibitory Effect of BMDM on Chlorella sp. Energy Metabolism
4.5. PS Alleviated the BMDM-Induced Damage on Chlorella sp.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1
| Gene ID | Primer Sequences |
|---|---|
| TRINITY_DN10623_c1_g1 | F: GGTGCAGTCGGTAAGACATGC |
| R: TGACCTGCTGTATCCCATAAGGAG | |
| TRINITY_DN11178_c0_g1 | F: CCACTCAAGAAGTCCACCCATAAC |
| R: AGTCAGAAACACACTCTACAAGGC | |
| TRINITY_DN12386_c0_g1 | F: CCGACAGTACCATCACCAACAAC |
| R: TGCAGAGACCAGAACAAAGAAACG | |
| TRINITY_DN12862_c0_g1 | F: TTTTGGAGTTGGGGATCGTCATC |
| R: AAATGGTGGTGGAAATGGTTTTGG | |
| TRINITY_DN8749_c0_g1 | F: GCATCAGCACCACCAACACC |
| R: AGGATTCTGGAAACCTGGTAGTGG | |
| TRINITY_DN10140_c0_g1 | F: GGACCTGGAGTTGGAATTGATGG |
| R: ACATGGTTGAGAATCAGCACAATC | |
| 18s rRNA | F: GAGTATGGTCGCAAGGCTGAA |
| R: AACCTGACAAGGCAACCCAC |
Appendix A.2
| Group | Roughness (nm) | Adhesion (nN) | Modulus (MPa) |
|---|---|---|---|
| CK | 8.715 ± 2.060 a | 44.917 ± 3.496 a | 11209.833 ± 1914.712 a |
| PS | 9.333 ± 2.092 a | 48.567 ± 8.859 a | 10724.500 ± 1227.719 a |
| BMDM | 11.422 ± 2.621 a | 44.900 ± 7.186 a | 10175.333 ± 617.196 a |
| BMDM + PS | 11.052 ± 1.939 a | 36.35 ± 5.440 b | 7759.000 ± 2202.216 b |
References
- Huang, W.; Zhao, T.; Zhu, X.; Ni, Z.; Guo, X.; Tan, L.; Wang, J. The effects and mechanisms of polystyrene and polymethyl methacrylate with different sizes and concentrations on Gymnodinium aeruginosum. Environ. Pollut. 2021, 287, 117626. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef]
- Rezania, S.; Park, J.; Md Din, M.F.; Mat Taib, S.; Talaiekhozani, A.; Kumar Yadav, K.; Kamyab, H. Microplastics pollution in different aquatic environments and biota: A review of recent studies. Mar. Pollut. Bull. 2018, 133, 191–208. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.; Zhou, X.; Kong, X.; Tao, S.; Xing, B. Sorption of four hydrophobic organic compounds by three chemically distinct polymers: Role of chemical and physical composition. Environ. Sci. Technol. 2012, 46, 7252–7259. [Google Scholar] [CrossRef]
- Su, Y.; Qi, H.; Hou, Y.; Gao, M.; Li, J.; Cai, M.; Zhu, X.; Chen, M.; Ge, C.; Fu, D.; et al. Combined Effects of Microplastics and Benzo[a]pyrene on the Marine Diatom Chaetoceros muelleri. Front. Mar. Sci. 2022, 8, 779321. [Google Scholar] [CrossRef]
- Jin, Z.; Du, L.; Cheng, Q.; Jiang, Y.; Hui, C.; Xu, L.; Zhao, Y.; Jiang, H. Physiological and transcriptional responses of Dictyosphaerium sp. under co-exposure of a typical microplastic and nonylphenol. Environ. Res. 2022, 204, 112287. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Homem, V.; Alves, A.; Santos, L. Advances in analytical methods and occurrence of organic UV-filters in the environment—A review. Sci. Total. Environ. 2015, 526, 278–311. [Google Scholar] [CrossRef]
- Huang, Y.; Law, J.C.; Lam, T.K.; Leung, K.S. Risks of organic UV filters: A review of environmental and human health concern studies. Sci. Total. Environ. 2021, 755, 142486. [Google Scholar] [CrossRef]
- Duis, K.; Junker, T.; Coors, A. Review of the environmental fate and effects of two UV filter substances used in cosmetic products. Sci. Total. Environ. 2022, 808, 151931. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Fan, Y.; Jin, J.; Xiong, S.; Liu, J.; Tang, C. Bioaccumulation and biomagnification of ultraviolet absorbents in marine wildlife of the Pearl River Estuarine, South China Sea. Environ. Pollut. 2017, 225, 55–65. [Google Scholar] [CrossRef]
- Paula, V.C.S.; Gomes, M.F.; Martins, L.R.R.; Yamamoto, F.Y.; Freitas, A.M. Acute toxicity characterization of organic UV-filters and chronic exposure revealing multigenerational effects in Daphnia magna. Ecotoxicology 2022, 31, 1413–1425. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.; An, Y.J. Microplastics enhance the toxicity and phototoxicity of UVfilter avobenzone on Daphnia magna. J. Hazard. Mater. 2023, 445, 130627. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Ling, X.; Yan, Z.; Wu, D.; Liu, J.; Lu, G. Effects of Nanoplastics and Butyl Methoxydibenzoylmethane on Early Zebrafish Embryos Identified by Single-Cell RNA Sequencing. Environ. Sci. Technol. 2021, 55, 1885–1896. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Moon, J.Y.; Lee, Y.C. Microalgal ecotoxicity of nanoparticles: An updated review. Ecotoxicol. Environ. Saf. 2020, 201, 110781. [Google Scholar] [CrossRef]
- Lin, J.; Zheng, J.Y.; Zhan, Z.G.; Zhao, Y.M.; Zhou, Q.Z.; Peng, J.; Li, Y.; Xiao, X.; Wang, J.H. Abundant small microplastics hidden in water columns of the Yellow Sea and East China Sea: Distribution, transportation and potential risk. J. Hazard. Mater. 2024, 478, 135531. [Google Scholar] [CrossRef] [PubMed]
- Sang, Z.; Leung, K.S. Environmental occurrence and ecological risk assessment of organic UV filters in marine organisms from Hong Kong coastal waters. Sci. Total. Environ. 2016, 566–567, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Bratkovics, S.; Sapozhnikova, Y. Determination of seven commonly used organic UV filters in fresh and saline waters by liquid chromatography-tandem mass spectrometry. Anal. Methods 2011, 3, 2943. [Google Scholar] [CrossRef]
- Ouédraogo, D.Y.; Mell, H.; Perceval, O.; Burga, K.; Domart-Coulon, I.; Hédouin, L.; Delaunay, M.; Guillaume, M.M.; Castelin, M.; Calvayrac, C.; et al. What are the toxicity thresholds of chemical pollutants for tropical reef-building corals? A systematic review. Environ. Evid. 2023, 12, 4. [Google Scholar] [CrossRef]
- Mwamba, T.M.; Ali, S.; Ali, B.; Lwalaba, J.L.; Liu, H.; Farooq, M.A. Interactive effects of cadmium and copper on metal accumulation, oxidative stress, and mineral composition in Brassica napus. Int. J. Environ. Sci. Technol. 2016, 13, 2163–2174. [Google Scholar] [CrossRef]
- Li, Z.; Yi, X.; Zhou, H.; Chi, T.; Li, W.; Yang, K. Combined effect of polystyrene microplastics and dibutyl phthalate on the microalgae Chlorella pyrenoidosa. Environ. Pollut. 2020, 257, 113604. [Google Scholar] [CrossRef]
- Sang, Z.; Jiang, Y.; Tsoi, Y.K.; Leung, K.S. Evaluating the environmental impact of artificial sweeteners: A study of their distributions, photodegradation and toxicities. Water Res. 2014, 52, 260–274. [Google Scholar] [CrossRef]
- Kumar, V. rDNA gene structure, transcription, and its coregulation. In Emerging Concepts in Ribosome Structure, Biogenesis, and Function; Academic Press: New York, NY, USA, 2021; pp. 33–45. [Google Scholar] [CrossRef]
- Wang, X.; Niu, X.; Chen, Y.; Sun, Z.; Han, A.; Lou, X.; Ge, J.; Li, X.; Yang, Y.; Jian, J.; et al. Transcriptome sequencing of a toxic dinoflagellate, Karenia mikimotoi subjected to stress from solar ultraviolet radiation. Harmful Algae 2019, 88, 101640. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zheng, X.; Han, Z.; Li, Y.; He, H.; Lin, T.; Xu, H. Polystyrene microplastics enhanced the effect of PFOA on Chlorella sorokiniana: Perspective from the cellular and molecular levels. J. Hazard. Mater. 2024, 465, 133455. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhu, Q.L.; Zheng, J.L.; Wen, Z.Y. Cadmium induced oxidative stress, endoplasmic reticulum (ER) stress and apoptosis with compensative responses towards the up-regulation of ribosome, protein processing in the ER, and protein export pathways in the liver of zebrafish. Aquat. Toxicol. 2022, 242, 106023. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; McFarlane, H.E. Regulation of cellulose synthesis via exocytosis and endocytosis. Curr. Opin. Plant. Biol. 2022, 69, 102273. [Google Scholar] [CrossRef]
- Huang, B.; Wei, Z.B.; Yang, L.Y.; Pan, K.; Miao, A.J. Combined Toxicity of Silver Nanoparticles with Hematite or Plastic Nanoparticles toward Two Freshwater Algae. Environ. Sci. Technol. 2019, 53, 3871–3879. [Google Scholar] [CrossRef]
- Yang, W.; Gao, P.; Ma, G.; Huang, J.; Wu, Y.; Wan, L.; Ding, H.; Zhang, W. Transcriptome analysis of the toxic mechanism of nanoplastics on growth, photosynthesis and oxidative stress of microalga Chlorella pyrenoidosa during chronic exposure. Environ. Pollut. 2021, 284, 117413. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, L.; Xu, Z.; Yang, M.; Shao, X.; Yang, S.; Zhang, H.; Wu, F.; Han, Z. Effect of polystyrene microplastics on the volatile fatty acids production from waste activated sludge fermentation. Sci. Total. Environ. 2021, 799, 149394. [Google Scholar] [CrossRef]
- Long, M.; Paul-Pont, I.; Hegaret, H.; Moriceau, B.; Lambert, C.; Huvet, A.; Soudant, P. Interactions between polystyrene microplastics and marine phytoplankton lead to species-specific hetero-aggregation. Environ. Pollut. 2017, 228, 454–463. [Google Scholar] [CrossRef]
- Su, Y.; Cheng, Z.; Hou, Y.; Lin, S.; Gao, L.; Wang, Z.; Bao, R.; Peng, L. Biodegradable and conventional microplastics posed similar toxicity to marine algae Chlorella vulgaris. Aquat. Toxicol. 2022, 244, 106097. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, Z.; Yan, Z.; Zhou, G.; Zhang, W.; Wang, Y.; Li, X. Defense pathways of Chlamydomonas reinhardtii under silver nanoparticle stress: Extracellular biosorption, internalization and antioxidant genes. Chemosphere 2022, 291, 132764. [Google Scholar] [CrossRef]
- Novosel, N.; Radić, T.M.; Levak Zorinc, M.; Zemla, J.; Lekka, M.; Vrana, I.; Gašparović, B.; Horvat, L.; Kasum, D.; Legović, T.; et al. Salinity-induced chemical mechanical and behavioral changes in marine microalgae. J. Appl. Phycol. 2022, 34, 1293–1309. [Google Scholar] [CrossRef]
- Radić, T.M.; Vukosav, P.; Komazec, B.; Formosa-Dague, C.; Domazet Jurašin, D.; Štefanić, P.P.; Čačković, A.; Juraić, K.; Ivošević DeNardis, N. Nanoplastic-Induced Nanostructural, Nanomechanical, and Antioxidant Response of Marine Diatom Cylindrotheca closterium. Water 2022, 14, 2163. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zheng, X.; Yang, M.; Gao, X.; Huang, J.; Zhang, L.; Fan, Z. Toxic effects and mechanisms of PFOA and its substitute GenX on the photosynthesis of Chlorella pyrenoidosa. Sci. Total. Environ. 2021, 765, 144431. [Google Scholar] [CrossRef]
- Mao, F.; He, Y.; Gin, K.Y. Antioxidant responses in cyanobacterium Microcystis aeruginosa caused by two commonly used UV filters, benzophenone-1 and benzophenone-3, at environmentally relevant concentrations. J. Hazard. Mater. 2020, 396, 122587. [Google Scholar] [CrossRef]
- Gao, L.; Xie, Y.; Su, Y.; Mehmood, T.; Bao, R.; Fan, H.; Peng, L. Elucidating the negatively influential and potentially toxic mechanism of single and combined micro-sized polyethylene and petroleum to Chlorella vulgaris at the cellular and molecular levels. Ecotoxicol. Environ. Saf. 2022, 245, 114102. [Google Scholar] [CrossRef]
- Mao, Y.; Ai, H.; Chen, Y.; Zhang, Z.; Zeng, P.; Kang, L.; Li, W.; Gu, W.; He, Q.; Li, H. Phytoplankton response to polystyrene microplastics: Perspective from an entire growth period. Chemosphere 2018, 208, 59–68. [Google Scholar] [CrossRef]
- Liao, Y.; Jiang, X.; Xiao, Y.; Li, M. Exposure of microalgae Euglena gracilis to polystyrene microbeads and cadmium: Perspective from the physiological and transcriptional responses. Aquat. Toxicol. 2020, 228, 105650. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Mukherjee, A. Ageing with algal EPS reduces the toxic effects of polystyrene nanoplastics in freshwater microalgae Scenedesmus obliquus. J. Environ. Chem. Eng. 2021, 9, 105978. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, P.; Zhang, X.; Zhang, Y.; Xie, S.; Deng, J. Effect of microplastics exposure on the photosynthesis system of freshwater algae. J. Hazard. Mater. 2019, 374, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Middepogu, A.; Hou, J.; Gao, X.; Lin, D. Effect and mechanism of TiO2 nanoparticles on the photosynthesis of Chlorella pyrenoidosa. Ecotoxicol. Environ. Saf. 2018, 161, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, J.; Chen, J.; Jin, J.; Chen, H.; Liu, H. Metabolomic analysis of Scenedesmus obliquus reveals new insights into the phytotoxicity of imidazolium nitrate ionic liquids. Sci. Total. Environ. 2022, 825, 154070. [Google Scholar] [CrossRef] [PubMed]
- Farjallah, A.; Roy, A.; Guéguen, C. NMR- and HRMS-based untargeted metabolomic study of metal-stressed Euglena gracilis cells. Algal Res. 2024, 78, 103383. [Google Scholar] [CrossRef]
- He, X.; Lin, G.; Zeng, J.; Yang, Z.; Wang, L. Construction of algal-bacterial consortia using green microalgae Chlorella vulgaris and As(III)-oxidizing bacteria: As tolerance and metabolomic profiling. J. Environ. Sci. 2024, 139, 258–266. [Google Scholar] [CrossRef]
- Zhou, J.L.; Yang, Z.Y.; Vadiveloo, A.; Li, C.; Chen, Q.G.; Chen, D.Z.; Gao, F. Enhancing lipid production and sedimentation of Chlorella pyrenoidosa in saline wastewater through the addition of agricultural phytohormones. J. Environ. Manag. 2024, 354, 120445. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Chen, J.; Zhou, J.; Li, J.; Wei, Y.; Regmi, B.; Bu, D. Combined Transcriptome and Metabolome Analysis of a New Species of Microalgae from the Tibetan Plateau and Its Response to Sewage Treatment. Water 2022, 14, 3391. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, L.; Liu, Y.; Deng, S.; Wu, H.; Wang, G. Toxicological effects of nanometer titanium dioxide (nano-TiO2) on Chlamydomonas reinhardtii. Ecotoxicol. Environ. Saf. 2012, 84, 155–162. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Q.; Liu, Q.; Liu, S.; Zhu, Y.; Yao, J.; Wang, H.; Guan, W. The responses of harmful dinoflagellate Karenia mikimotoi to simulated ocean acidification at the transcriptional level. Harmful Algae 2022, 111, 102167. [Google Scholar] [CrossRef]
- Wang, L.; Kaeppler, A.; Fischer, D.; Simmchen, J. Photocatalytic TiO2 Micromotors for Removal of Microplastics and Suspended Matter. ACS Appl. Mater. Interfaces 2019, 11, 32937–32944. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, X.; Ai, S.; Wang, X.; He, J.; Gao, Z.; Meng, C.; Xi, L.; Ge, B.; Huang, F. Comprehensive transcriptomic and metabolomic insights into simultaneous CO2 sequestration and nitrate removal by the Chlorella vulgaris and Pseudomonas sp. consortium. Environ. Res. 2024, 259, 119540. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Li, W.; Keller, A.A.; Slaveykova, V.I. Metabolic alterations in alga Chlamydomonas reinhardtii exposed to nTiO2 materials. Environ. Sci. Nano 2022, 9, 2922–2938. [Google Scholar] [CrossRef]
- Nordin, N.; Yusof, N.; Maeda, T.; Mustapha, N.A.; Mohd Yusoff, M.Z.; Raja Khairuddin, R.F. Mechanism of carbon partitioning towards starch and triacylglycerol in Chlorella vulgaris under nitrogen stress through whole-transcriptome analysis. Biomass Bioenergy 2020, 138, 105600. [Google Scholar] [CrossRef]
- Sijil, P.V.; Sarada, R.; Chauhan, V.S. Enhanced accumulation of alpha-linolenic acid rich lipids in indigenous freshwater microalga Desmodesmus sp.: The effect of low-temperature on nutrient replete, UV treated and nutrient stressed cultures. Bioresour. Technol. 2019, 273, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Deng, R.; Lin, D. Insights into the regulation mechanisms of algal extracellular polymeric substances secretion upon the exposures to anatase and rutile TiO2 nanoparticles. Environ. Pollut. 2020, 263, 114608. [Google Scholar] [CrossRef]
- Gao, F.; Yang, L.; Chen, A.-J.; Zhou, W.-H.; Chen, D.-Z.; Chen, J.-M. Promoting effect of plant hormone gibberellin on co-metabolism of sulfamethoxazole by microalgae Chlorella pyrenoidosa. Bioresour. Technol. 2022, 351, 126900. [Google Scholar] [CrossRef]
- Takahashi, F.; Shinozaki, K. Long-distance signaling in plant stress response. Curr. Opin. Plant Biol. 2019, 47, 106–111. [Google Scholar] [CrossRef]



| Group | Pollutants | Concentration |
|---|---|---|
| CK | DMSO | 0.005% |
| PS Single toxicity | PS | 10 mg/L |
| BMDM Single toxicity | BMDM | 50 μg/L |
| Combined toxicity | BMDM + PS | 50 μg/L + 10 mg/L |
| Treatment | Tobs | Tpre | Tobs/Tpre |
|---|---|---|---|
| PS + BMDM | 0.096 ± 0.225 | 0.178 ± 0.550 | 0.476 ± 0.039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, Y.; Chen, F.; Duan, D.; Diao, X. Evaluating the Single and Combined Effects of BMDM and PS Microplastics on Chlorella sp.: Physiological and Transcriptomic Insights. Toxics 2025, 13, 946. https://doi.org/10.3390/toxics13110946
Liu J, Zhang Y, Chen F, Duan D, Diao X. Evaluating the Single and Combined Effects of BMDM and PS Microplastics on Chlorella sp.: Physiological and Transcriptomic Insights. Toxics. 2025; 13(11):946. https://doi.org/10.3390/toxics13110946
Chicago/Turabian StyleLiu, Jin, Yankun Zhang, Fengyuan Chen, Dandan Duan, and Xiaoping Diao. 2025. "Evaluating the Single and Combined Effects of BMDM and PS Microplastics on Chlorella sp.: Physiological and Transcriptomic Insights" Toxics 13, no. 11: 946. https://doi.org/10.3390/toxics13110946
APA StyleLiu, J., Zhang, Y., Chen, F., Duan, D., & Diao, X. (2025). Evaluating the Single and Combined Effects of BMDM and PS Microplastics on Chlorella sp.: Physiological and Transcriptomic Insights. Toxics, 13(11), 946. https://doi.org/10.3390/toxics13110946








