A Nitrifying Bacteria-Based Oxygen Consumption Assay for Multifaceted Soil Toxicity Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Ammonia-Based Nitrifying Bacteria Master-Culture Reactor (NBMCR)
2.2. Soil Sampling and Characterization
2.3. Optimization and Toxicity Testing Using Nitrifying Soil Toxicity Kit
2.4. NB Kit Protocol for Soil Toxicity Testing
2.5. Stoichiometric O2 Demand in Test Kits
2.6. Quantitative Analysis and Biostatistics
3. Result and Discussion
3.1. NBMCR Operation
3.2. Comparative Physicochemical Analysis of Reference and Contaminated Soils
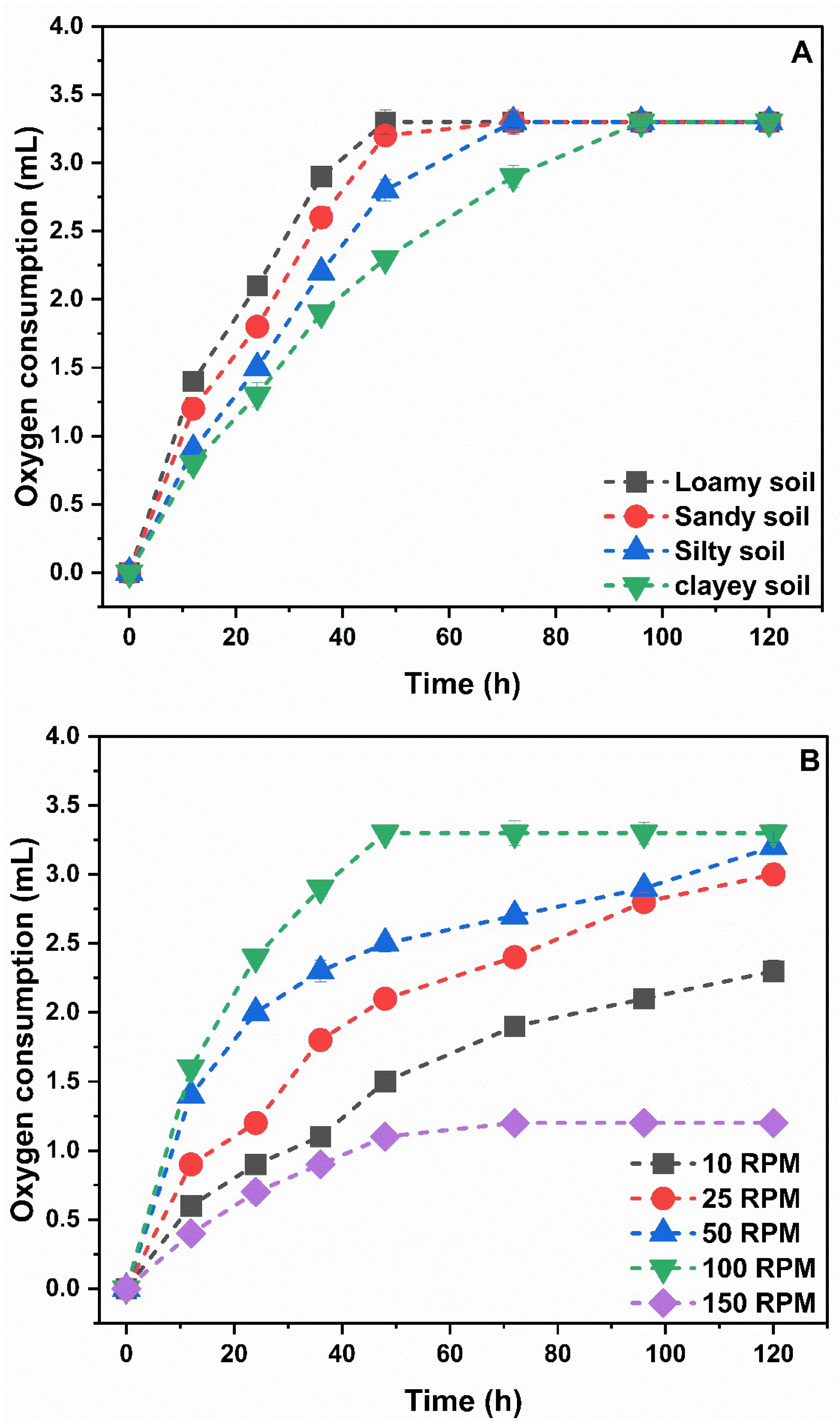
3.3. Optimization of Test Parameters
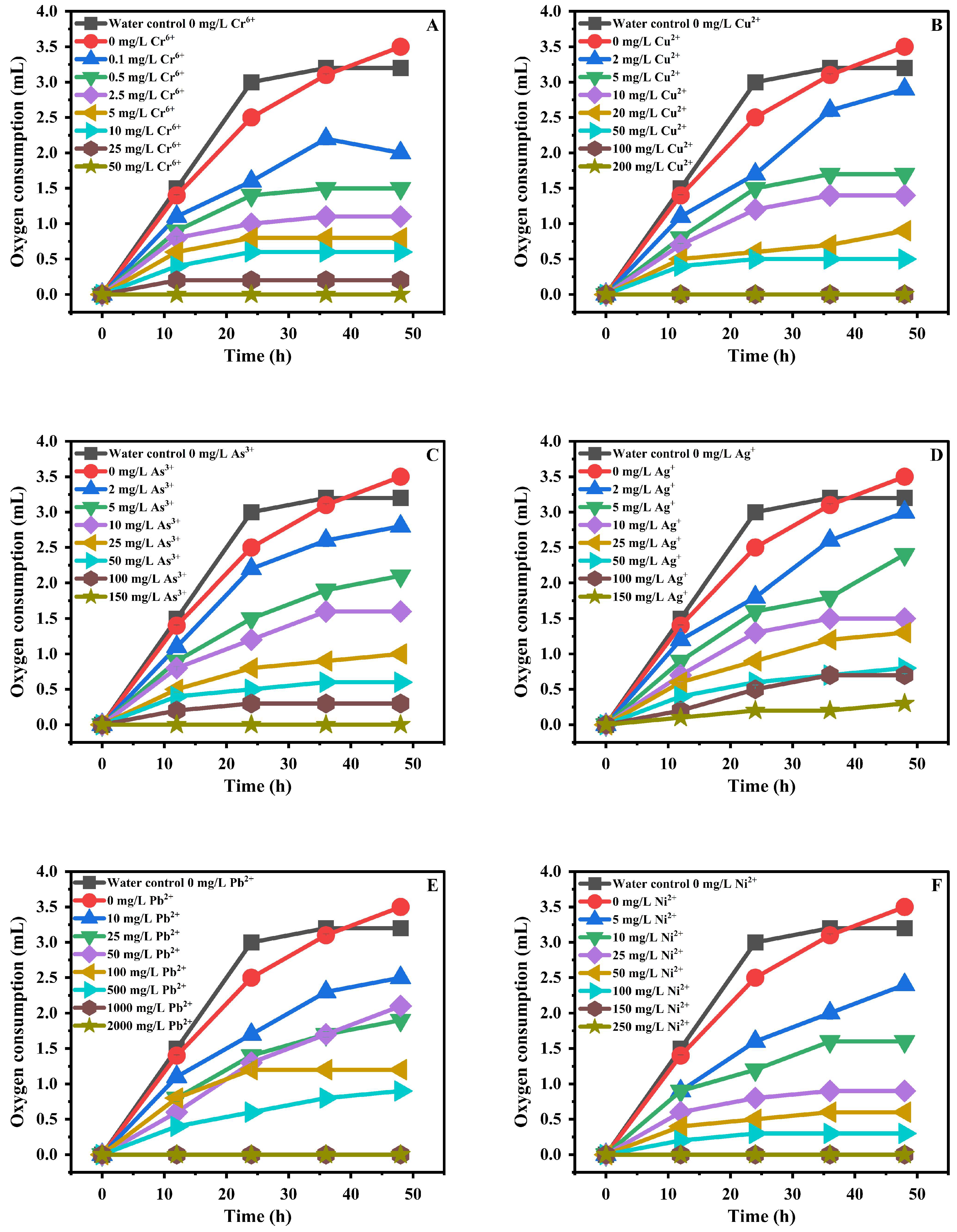
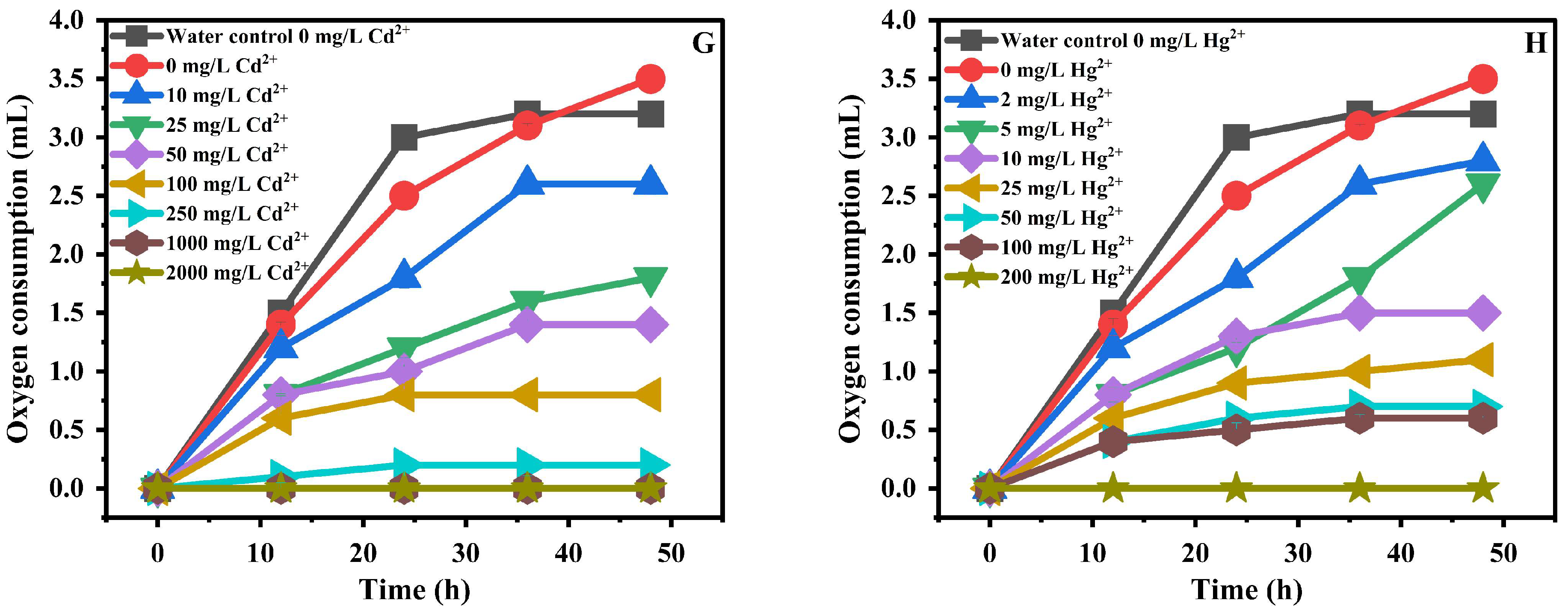
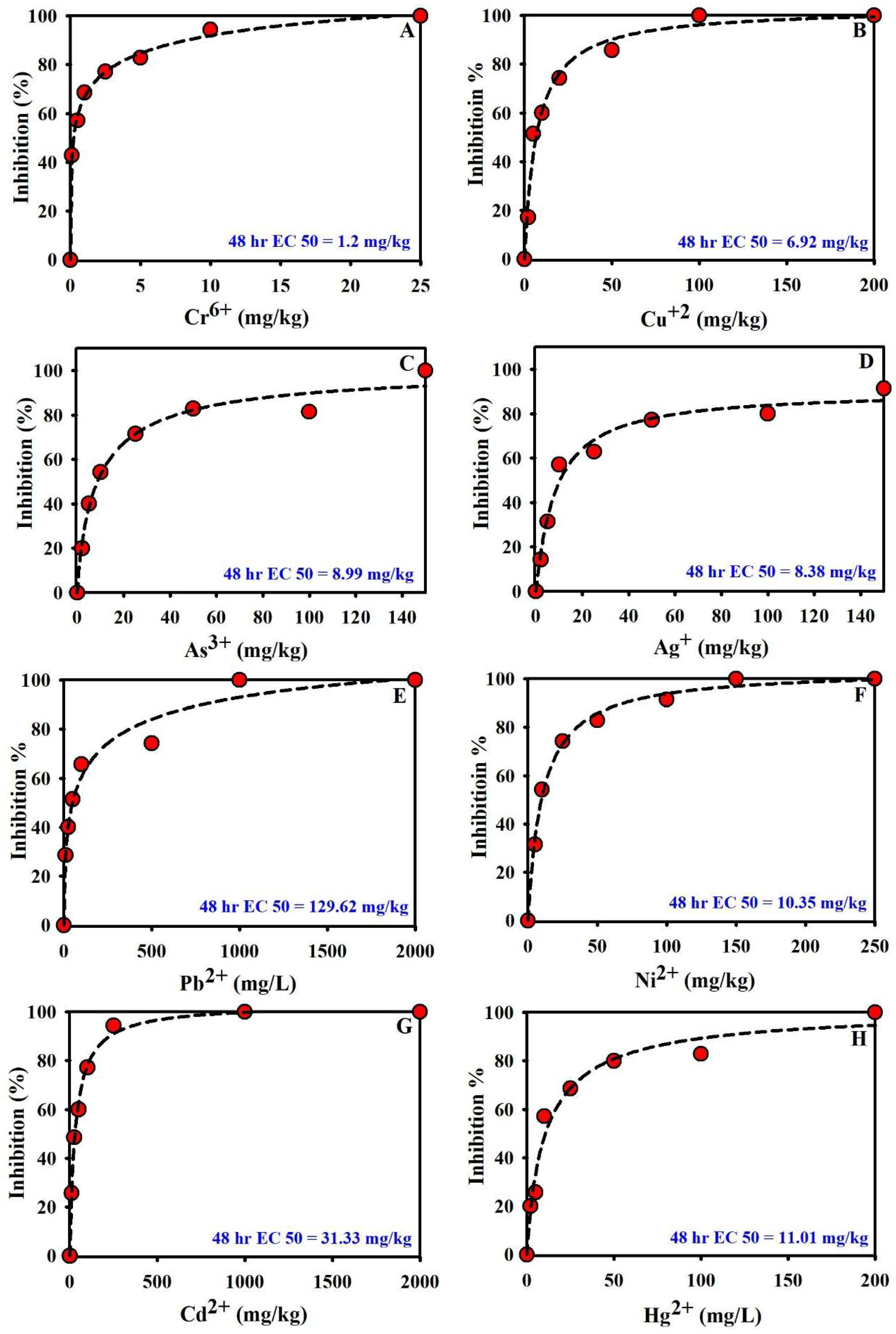
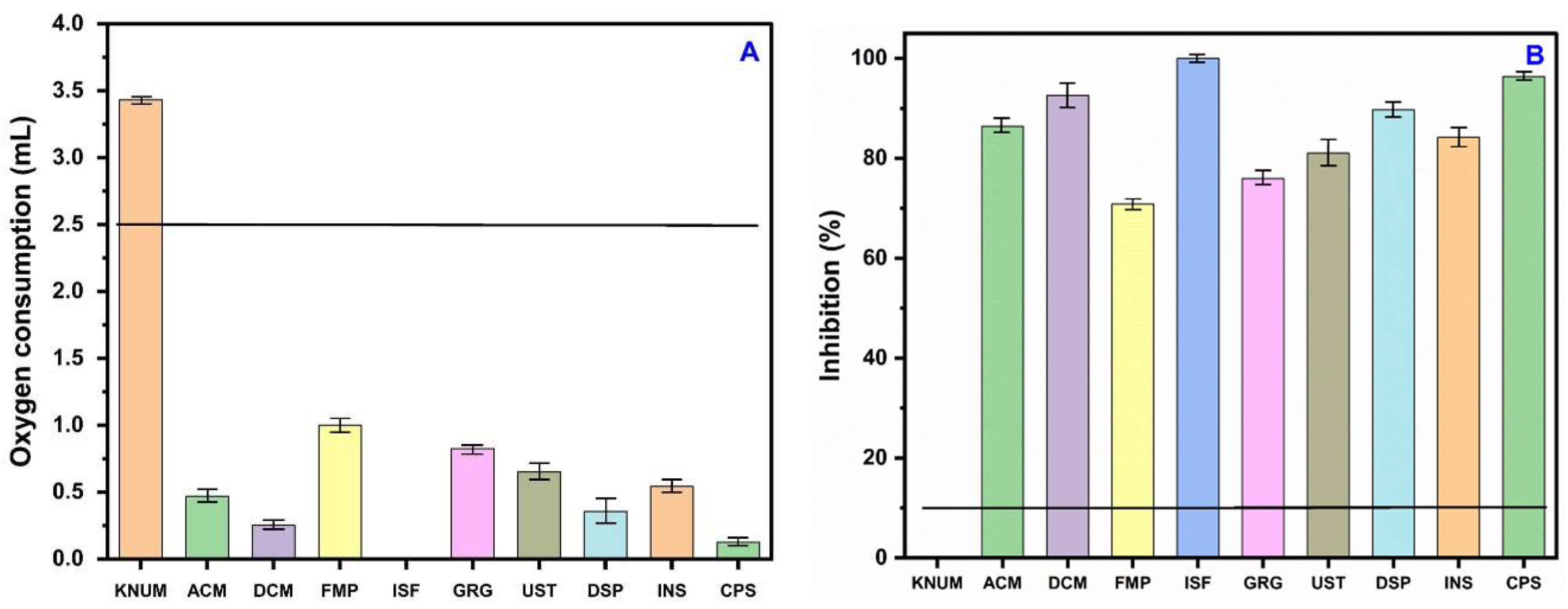
3.4. Evaluation of Heavy Metal Toxicity via Nitrifying-Bacteria Oxygen Demand
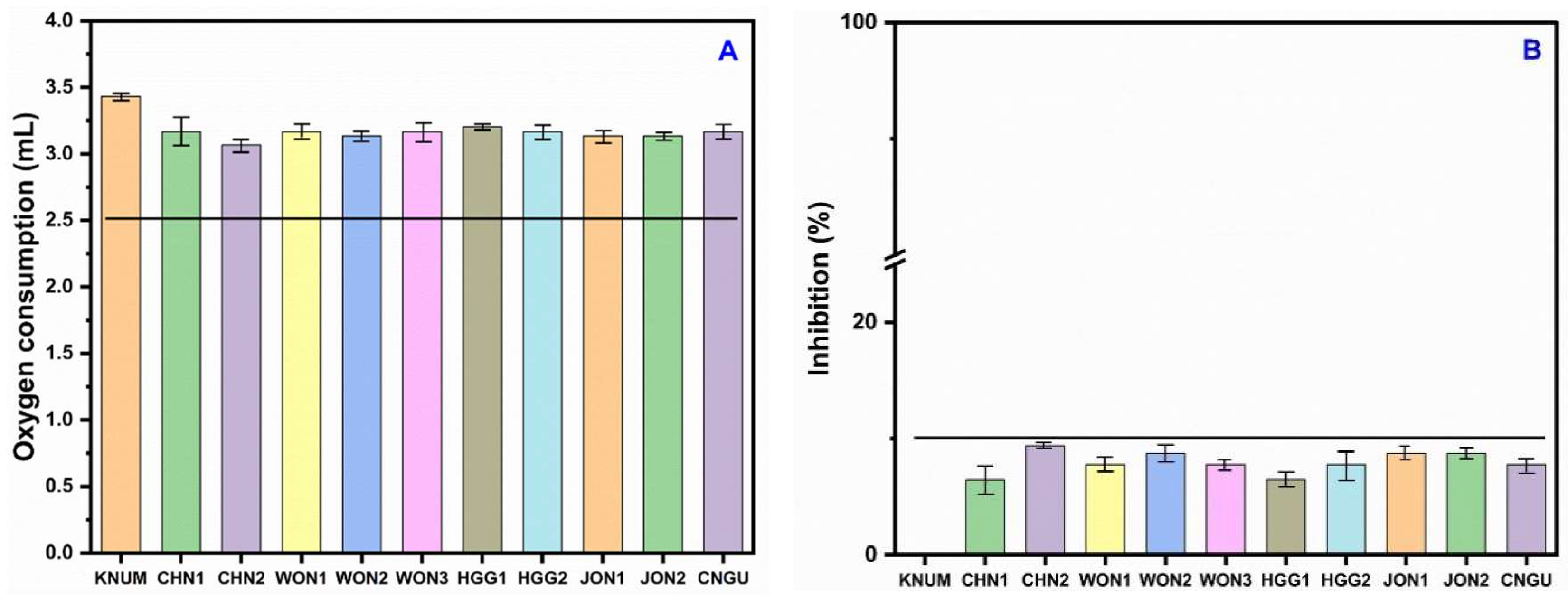
3.5. Response of Nitrifying Bacteria to Reference Soil and Contaminated Soil
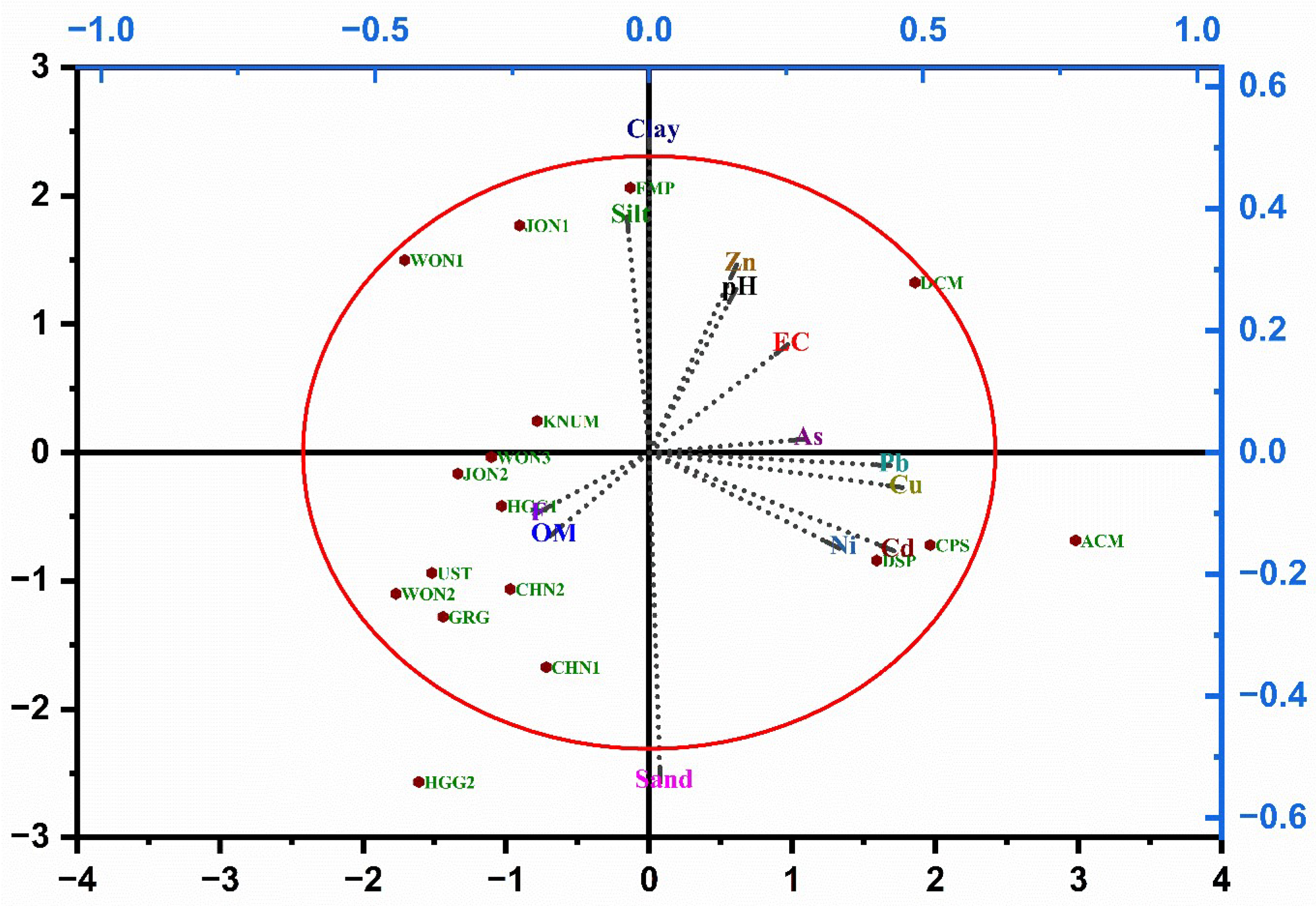
3.6. Evidence of Test Validity, Sources of Variability, and Toxicity Thresholds in NB Assays
4. Implications, Challenges, and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elshkaki, A.; Graedel, T.E.; Ciacci, L.; Reck, B.K. Resource Demand Scenarios for the Major Metals. Environ. Sci. Technol. 2018, 52, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.R.; Caetano, A.L.; Haller, A.; Gonçalves, F.; Pereira, R.; Römbke, J. Toxicity Screening of Soils from Different Mine Areas—A Contribution to Track the Sensitivity and Variability of Arthrobacter Globiformis Assay. J. Hazard. Mater. 2014, 274, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Eom, H.; Ashun, E.; Toor, U.A.; Oh, S.-E. A Solid-Phase Direct Contact Bioassay Using Sulfur-Oxidizing Bacteria (SOB) to Evaluate Toxicity of Soil Contaminated with Heavy Metals. Sens. Actuators B Chem. 2020, 305, 127510. [Google Scholar] [CrossRef]
- Ashun, E.; Toor, U.A.; Kim, H.S.; Kim, K.-R.; Park, S.J.; Hong, S.; Oh, S.-E. A Direct Contact Bioassay Using Sulfur-Oxidizing Bacteria (SOB) for Toxicity Assessment of Contaminated Field Soils. Chemosphere 2022, 286, 131599. [Google Scholar] [CrossRef]
- Giesy, J.P.; Rosiu, C.J.; Graney, R.L.; Newsted, J.L.; Benda, A.; Kreis, R.G.; Horvath, F.J. Toxicity of Detroit River Sediment Interstitial Water to the Bacterium Photobacterium Phosphoreum. J. Gt. Lakes Res. 1988, 14, 502–513. [Google Scholar] [CrossRef]
- Thomulka, K.W.; Lange, J.H. Use of the Bioluminescent Bacterium Vibrio Harveyi to Detect Biohazardous Chemicals in Soil and Water Extractions with and without Acid. Ecotoxicol. Environ. Saf. 1995, 32, 201–204. [Google Scholar] [CrossRef]
- Martín, F.; Escoto, M.; Fernández, J.; Fernández, E.; Arco, E.; Sierra, M.; Dorronsoro, C. Toxicity Assessment of Sediments with Natural Anomalous Concentrations in Heavy Metals by the Use of Bioassay. Int. J. Chem. Eng. 2010, 2010, 101390. [Google Scholar] [CrossRef]
- Vasseur, P.; Bonnard, M.; Palais, F.; Eom, I.C.; Morel, J.L. Bioavailability of Chemical Pollutants in Contaminated Soils and Pitfalls of Chemical Analyses in Hazard Assessment. Environ. Toxicol. 2008, 23, 652–656. [Google Scholar] [CrossRef]
- Ronnpagel, K.; Liss, W.; Ahlf, W. Microbial Bioassays to Assess the Toxicity of Solid-Associated Contaminants. Ecotoxicol. Environ. Saf. 1995, 31, 99–103. [Google Scholar] [CrossRef]
- van Gestel, C.A.M.; van der Waarde, J.J.; Derksen, J.G.M.A.; van der Hoek, E.E.; Veul, M.F.X.W.; Bouwens, S.; Rusch, B.; Kronenburg, R.; Stokman, G.N.M. The Use of Acute and Chronic Bioassays to Determine the Ecological Risk and Bioremediation Efficiency of Oil-polluted Soils. Environ. Toxicol. Chem. 2001, 20, 1438–1449. [Google Scholar] [CrossRef]
- Prokop, Z.; Holoubek, I. The Use of a Microbial Contact Toxicity Test for Evaluating Cadmium Bioavailability in Soil. J. Soils Sediments 2001, 1, 21–24. [Google Scholar] [CrossRef]
- Ramadass, K.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Ecotoxicity of Measured Concentrations of Soil-Applied Diesel: Effects on Earthworm Survival, Dehydrogenase, Urease and Nitrification Activities. Appl. Soil Ecol. 2017, 119, 1–7. [Google Scholar] [CrossRef]
- Tindaon, F.; Benckiser, G. Evaluation of the Side Effects of Nitrification-Inhibiting Agrochemicals in Soils. In Plant Growth Promoting Rhizobacteria (PGPR): Prospects for Sustainable Agriculture; Sayyed, R.Z., Reddy, M.S., Antonius, S., Eds.; Springer: Singapore, 2019; pp. 93–107. ISBN 9789811367908. [Google Scholar]
- Griffiths, B.S.; Hallett, P.D.; Kuan, H.L.; Gregory, A.S.; Watts, C.W.; Whitmore, A.P. Functional Resilience of Soil Microbial Communities Depends on Both Soil Structure and Microbial Community Composition. Biol. Fertil. Soils 2008, 44, 745–754. [Google Scholar] [CrossRef]
- Ringwood, A.H.; DeLorenzo, M.E.; Ross, P.E.; Holland, A.F. Interpretation of Microtox® Solid-phase Toxicity Tests: The Effects of Sediment Composition. Environ. Toxicol. Chem. 1997, 16, 1135–1140. [Google Scholar] [CrossRef]
- Hussain, F.; Shahzad, S.; Mehdi, S.E.H.; Sharma, A.; Pandey, S.; Kang, W.; Oh, S.-E. Novel-Solid-Phase Bioassay kit with immobilized Chlorella vulgaris Spheres for Assessing Heavy Metals and Cyanide Toxicity in Soil. Chemosensors 2025, 13, 193. [Google Scholar] [CrossRef]
- Selivanovskaya, S.; Galitskaya, P.; Schnell, S.; Hung, Y.-T. A Comparison of Microbial Contact Bioassay with Conventional Elutriate Assays for Evaluation of Wastes Hazard. Int. J. Environ. Waste Manag. 2010, 6, 183–196. [Google Scholar] [CrossRef]
- Suwanmanon, N.; Lee, C.H.; Park, J.H.; Lee, S.; Kim, K.R. Distribution and Risk Assessment of Heavy Metals in Agricultural Soils near Industrial Complexes in South Korea. Korean J. Soil Sci. Fert. 2022, 55, 187–198. [Google Scholar] [CrossRef]
- Hayatsu, M.; Katsuyama, C.; Tago, K. Overview of Recent Researches on Nitrifying Microorganisms in Soil. Soil Sci. Plant Nutr. 2021, 67, 619–632. [Google Scholar] [CrossRef]
- Urakawa, H.; Rajan, S.; Feeney, M.E.; Sobecky, P.A.; Mortazavi, B. Ecological Response of Nitrification to Oil Spills and Its Impact on the Nitrogen Cycle. Environ. Microbiol. 2019, 21, 18–33. [Google Scholar] [CrossRef]
- Sverdrup, L.E.; Ekelund, F.; Krogh, P.H.; Nielsen, T.; Johnsen, K. Soil Microbial Toxicity of Eight Polycyclic Aromatic Compounds: Effects on Nitrification, the Genetic Diversity of Bacteria, and the Total Number of Protozoans. Environ. Toxicol. Chem. 2002, 21, 1644–1650. [Google Scholar] [CrossRef]
- Sheppard, S.C. Soil Microbial Bioassays: Quick and Relevant But Are They Useful? Hum. Ecol. Risk Assess. Int. J. 1999, 5, 697–705. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The Microbial Nitrogen-Cycling Network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, S.; Mehdi, S.E.H.; Sharma, A.; Hussain, F.; Gurung, A.; Kang, W.; Jang, M.; Oh, S.E. Characterization of Nitrifying Bacteria and Exploring a Novel Approach for Toxicity Monitoring in Water. Environ. Chem. Ecotoxicol. 2025, 7, 106–116. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer Method Improved for Making Particle Size Analyses of Soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Topper, K.; Kotuby-Amacher, J. Evaluation of a Closed Vessel Acid Digestion Method for Plant Analyses Using Inductively Coupled Plasma Spectrometry. Commun. Soil Sci. Plant Anal. 1990, 21, 1437–1455. [Google Scholar] [CrossRef]
- Villalobos, M.; Avila-Forcada, A.P.; Gutierrez-Ruiz, M.E. An Improved Gravimetric Method to Determine Total Petroleum Hydrocarbons in Contaminated Soils. Water. Air. Soil Pollut. 2008, 194, 151–161. [Google Scholar] [CrossRef]
- Brock, T.C.M.; Hammers-Wirtz, M.; Hommen, U.; Preuss, T.G.; Ratte, H.-T.; Roessink, I.; Strauss, T.; Van den Brink, P.J. The Minimum Detectable Difference (MDD) and the Interpretation of Treatment-Related Effects of Pesticides in Experimental Ecosystems. Environ. Sci. Pollut. Res. 2015, 22, 1160–1174. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal Component Analysis: A Review and Recent Developments. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Lever, J.; Krzywinski, M.; Altman, N. Points of Significance: Principal Component Analysis. Nat. Methods 2017, 14, 641–643. [Google Scholar] [CrossRef]
- Li, J.; Ma, H.; Yu, H.; Feng, L.; Xia, X.; He, S.; Chen, X.; Zhao, Q.; Wei, L. Effect and Potential Mechanisms of Sludge-Derived Chromium, Nickel, and Lead on Soil Nitrification: Implications for Sustainable Land Utilization of Digested Sludge. J. Hazard. Mater. 2024, 466, 133552. [Google Scholar] [CrossRef]
- Aslan, S.; Sozudogru, O. Individual and Combined Effects of Nickel and Copper on Nitrification Organisms. Ecol. Eng. 2017, 99, 126–133. [Google Scholar] [CrossRef]
- Beduk, F. Combined Effects of Cadmium and Azithromycin on Soil Nitrification Process. Water 2023, 15, 881. [Google Scholar] [CrossRef]
- Nahar, K.; Ali, M.M.; Khanom, A.; Alam, M.K.; Azad, M.A.K.; Rahman, M.M. Levels of Heavy Metal Concentrations and Their Effect on Net Nitrification Rates and Nitrifying Archaea/Bacteria in Paddy Soils of Bangladesh. Appl. Soil Ecol. 2020, 156, 103697. [Google Scholar] [CrossRef]
- Gutwiński, P.; Cema, G.; Ziembińska-Buczyńska, A.; Wyszyńska, K.; Surmacz-Górska, J. Long-Term Effect of Heavy Metals Cr(III), Zn(II), Cd(II), Cu(II), Ni(II), Pb(II) on the Anammox Process Performance. J. Water Process Eng. 2021, 39, 101668. [Google Scholar] [CrossRef]
- Shahzad, S.; Sharma, A.; Mehdi, S.E.H.; Gurung, A.; Hussain, F.; Kang, W.; Jang, M. Assessment of Metals Toxicity Using a Nitrifying Bacteria Bioassay Kit Based on Oxygen Consumption. Arch. Environ. Contam. Toxicol. 2025, 88, 437–451. [Google Scholar] [CrossRef]
- Mwalongo, D.A.; Lisuma, J.B.; Haneklaus, N.H.; Maged, A.; Brink, H.; Carvalho, F.P.; Wacławek, S.; Mpumi, N.; Amasi, A.I.; Mwimanzi, J.M.; et al. Bacterial Diversity Dynamics in Sandy Loam Soils in Tanzania Under Varying Fertilizer-Derived Uranium Concentrations. Microorganisms 2025, 13, 1886. [Google Scholar] [CrossRef]
- Hussain, F.; Ashun, E.; Jung, S.P.; Kim, T.; Lee, S.H.; Kim, D.J.; Oh, S.E. A Direct Contact Bioassay Using Immobilized Microalgal Balls to Evaluate the Toxicity of Contaminated Field Soils. J. Environ. Manag. 2022, 321, 115930. [Google Scholar] [CrossRef]
- Höss, S.; Ahlf, W.; Fahnenstich, C.; Gilberg, D.; Hollert, H.; Melbye, K.; Meller, M.; Hammers-Wirtz, M.; Heininger, P.; Neumann-Hensel, H.; et al. Variability of Sediment-Contact Tests in Freshwater Sediments with Low-Level Anthropogenic Contamination—Determination of Toxicity Thresholds. Environ. Pollut. 2010, 158, 2999–3010. [Google Scholar] [CrossRef]
- Neumann-Hensel, H.; Melbye, K. Optimisation of the Solid-Contact Test with Arthrobacter globiformis. J. Soils Sediments 2006, 6, 201–207. [Google Scholar] [CrossRef]
- Höss, S.; Jänsch, S.; Moser, T.; Junker, T.; Römbke, J. Assessing the Toxicity of Contaminated Soils Using the Nematode Caenorhabditis elegans as Test Organism. Ecotoxicol. Environ. Saf. 2009, 72, 1811–1818. [Google Scholar] [CrossRef]

| Soil | Land Use | pH | EC (mS/cm) | OM (%) | Sand (%) | Silt (%) | Clay (%) | F (mg/kg) | As (mg/kg) | Cd (mg/kg) | Cu (mg/kg) | Ni (mg/kg) | Pb (mg/kg) | Zn (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KNUM | Mountain | 6.17 | 0.54 | 5.33 | 73.13 | 19.9 | 6.97 | 343.3 | 0 | 2.35 | * ND | * ND | * ND | * ND |
| CHN1 | Upland | 7.53 | 0.44 | 15.5 | 95.1 | 4.2 | 0.7 | 371.3 | 2.5 | 0.23 | 3.9 | 4.37 | 18.02 | 3.97 |
| CHN2 | Forest | 6.47 | 0.45 | 33.97 | 85.2 | 8.4 | 6.4 | 190.3 | 2.44 | 0.17 | 3.17 | 3.63 | 18 | 2.17 |
| WON1 | Paddy | 5.6 | 0.42 | 34.93 | 73.4 | 12.4 | 14.2 | 432.6 | 3.43 | 0.4 | 9.87 | 16.43 | 17.58 | 3.2 |
| WON2 | Urban | 7.07 | 0.23 | 40.47 | 80.6 | 15 | 4.4 | 441.3 | 4.07 | 0.37 | 7.57 | 9.5 | 14.86 | 4.37 |
| WON3 | Upland | 6.67 | 0.35 | 22.23 | 74.9 | 14.4 | 10.7 | 272.3 | 3.6 | 0.4 | 1.43 | 6.13 | 17.78 | 4.27 |
| HGG1 | Paddy | 6.73 | 0.48 | 22.17 | 79.7 | 10.2 | 10.1 | 466 | 3.1 | 0.23 | 12.17 | 11.73 | 12.18 | 4.77 |
| HGG2 | Field | 5.67 | 0.22 | 26.37 | 93 | 3.3 | 3.7 | 402.6 | 4.4 | 0.3 | 13.27 | 6.97 | 13.63 | 4.27 |
| JON1 | Grassland | 7.03 | 0.20 | 19.03 | 77 | 13.6 | 9.4 | 199 | 8.5 | 0.43 | 11 | 14.1 | 22.64 | 2.4 |
| JON2 | Upland | 6.23 | 0.29 | 25.17 | 72.93 | 12.8 | 14.27 | 386 | 5.37 | 0.23 | 10.13 | 23.87 | 15.76 | 4.33 |
| CNGU | Paddy | 6.52 | 0.40 | 22.3 | 45.6 | 28.0 | 26.4 | 287 | 4.8 | 0.3 | 7.0 | 12.5 | 15.50 | 69.0 |
| Soil | Land Use | pH | EC (mS/cm) | OM (%) | Sand (%) | Silt (%) | Clay (%) | F (mg/kg) | As (mg/kg) | Cd (mg/kg) | Cu (mg/kg) | Ni (mg/kg) | Pb (mg/kg) | Zn (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACM | Abandoned Copper | 7.37 | 0.49 | 11.7 | 85 | 9.7 | 5.3 | 394.8 | 1808.9 | 18.3 | 79.2 | 42.9 | 113.7 | 295 |
| Mine | ||||||||||||||
| DCM | Decommissioned | 8 | 0.34 | 7.17 | 71.4 | 19.2 | 9.3 | 56 | 1761.5 | 2.3 | 39 | 19.4 | 119.3 | 168 |
| Coal Mine | ||||||||||||||
| FMP | Fertilizer | 7.9 | 0.6 | 12.9 | 64.7 | 23 | 12.3 | 307.6 | 10 | 0.64 | 6.7 | 3.1 | 34.5 | 176 |
| Manufacturing | ||||||||||||||
| Plant | ||||||||||||||
| ISF | Iron Smelting | 8.1 | 0.55 | 24.2 | 60.7 | 12.9 | 26.37 | 232.4 | 143.5 | 7.05 | 59.8 | 6.47 | 275.5 | 851.9 |
| Factory | ||||||||||||||
| GRG | Garage | 6.2 | 0.17 | 28.3 | 82.6 | 12.6 | 4.8 | 215.8 | 1.89 | 2.4 | 3.1 | 2.7 | 7.9 | 57.7 |
| UST | Underground | 6.3 | 0.12 | 32.3 | 78.3 | 16.4 | 5.2 | 194.8 | 1.9 | 3.43 | 2.8 | 2.7 | 7.3 | 63.3 |
| Storage Tank | ||||||||||||||
| DSP | Decommissioned | 6.4 | 0.26 | 22 | 75.5 | 19 | 5.5 | 215.3 | 352.3 | 18.8 | 27.1 | 27.3 | 373 | 18.4 |
| Sewage Plant | ||||||||||||||
| INS | Incineration Site | 6 | 0.56 | 26.6 | 78.3 | 14.1 | 7.3 | 157.9 | 319.3 | 28.3 | 115.7 | 332.6 | 408.7 | 31.5 |
| CPS | Coking Plant Site | 7 | 0.48 | 13.8 | 80.9 | 10.8 | 8.2 | 300.7 | 124.8 | 12.8 | 97.8 | 6.47 | 200.7 | 28.4 |
| Heavy Metal | Test Concentration Range (mg/kg) |
|---|---|
| Chromium (Cr6+) | 0.1, 0.5, 1, 2.5, 5, 10, 25 |
| Silver (Ag+) | 2, 5, 10, 25, 50, 100, 150 |
| Mercury (Hg2+) | 2, 5, 10, 25, 50, 100, 200 |
| Nickel (Ni2+) | 5, 10, 25, 50, 100, 150, 250 |
| Arsenic (As3+) | 2, 5, 10, 25, 50, 100, 150 |
| Copper (Cu2+) | 2, 5, 10, 20, 50, 100, 200 |
| Cadmium (Cd2+) | 10, 25, 50, 100, 250, 1000, 2000 |
| Lead (Pb2+) | 10, 25, 50, 100, 250, 1000, 2000 |
| CVi (min, max) % | 2.12 (1.7, 4.7) |
| CVns | 9.11 (7.8, 14.6) |
| MDD (min, max) | 3.22 (2.9, 5.9) |
| MTI | 11.1 |
| Toxicity threshold | 13 |
| Number of Reference soils | 11 |
| Bioassay | Endpoint Parameter | Exposure Time (h) | Toxicity Threshold (%) | References |
|---|---|---|---|---|
| Nitrifying bacteria | Oxygen consumption | 48 | 10 | This study |
| Chlorella vulgaris | Oxygen consumption | 72 | 15 | [38] |
| Sulfur-oxidizing bacteria | Oxygen consumption | 6 | 20 | [4] |
| Anthrobacter | Dehydrogenase | 6 | 60 | [39] |
| globitormis | activity | |||
| Caenoerhabdtis | Growth | 96 | 25 | |
| elegans | Reproduction | 96 | 50 | |
| Lumbriculus | Reproduction | 672 | 25 | |
| variegatus | ||||
| Myriophyllum | Growth | 240 | 20 | |
| aquaticum | ||||
| Danio rerio | Survival | 48 | 20 | |
| Anthrobacter globiformis | Dehydrogenase activity | 6 | 45 | [2] |
| Anthrobacter globiformis | Dehydrogenase activity | 6 | 40 | [40] |
| Caenorhabdtis | Fertility | 96 | 20 | [41] |
| elegans | Growth | 20 | ||
| Reproduction | 40 |
| Sand | Slit | Clay | OM | pH | Cd | Zn | Ni | As | F | Cu | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GC | −0.5 | 0.38 | 0.52 | −0.13 | 0.26 | −0.16 | 0.3 | −0.15 | 0.02 | −0.1 | −0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahzad, S.; Sharma, A.; Mehdi, S.E.H.; Hussain, F.; Pandey, S.; Hussain, M.; Kang, W.; Oh, S.E. A Nitrifying Bacteria-Based Oxygen Consumption Assay for Multifaceted Soil Toxicity Monitoring. Toxics 2025, 13, 937. https://doi.org/10.3390/toxics13110937
Shahzad S, Sharma A, Mehdi SEH, Hussain F, Pandey S, Hussain M, Kang W, Oh SE. A Nitrifying Bacteria-Based Oxygen Consumption Assay for Multifaceted Soil Toxicity Monitoring. Toxics. 2025; 13(11):937. https://doi.org/10.3390/toxics13110937
Chicago/Turabian StyleShahzad, Suleman, Aparna Sharma, Syed Ejaz Hussain Mehdi, Fida Hussain, Sandesh Pandey, Mudassar Hussain, Woochang Kang, and Sang Eun Oh. 2025. "A Nitrifying Bacteria-Based Oxygen Consumption Assay for Multifaceted Soil Toxicity Monitoring" Toxics 13, no. 11: 937. https://doi.org/10.3390/toxics13110937
APA StyleShahzad, S., Sharma, A., Mehdi, S. E. H., Hussain, F., Pandey, S., Hussain, M., Kang, W., & Oh, S. E. (2025). A Nitrifying Bacteria-Based Oxygen Consumption Assay for Multifaceted Soil Toxicity Monitoring. Toxics, 13(11), 937. https://doi.org/10.3390/toxics13110937










