Transcriptomics-Based Toxicological Study of Nickel on Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Raw Materials
2.2. Cultivation and Synchronization of C. elegans
2.3. Developmental Assay for C. elegans
2.4. Reproductive Capacity Assay
2.5. Lifespan Assay
2.6. Lipofuscin Accumulation Assay
2.7. Food Preference Assay
2.8. Transcriptomic Analysis
2.9. Statistical Analysis
3. Results
3.1. Ni Exposure Impaired the Development, Reproductive Capacity, and Food Preference of Caenorhabditis elegans
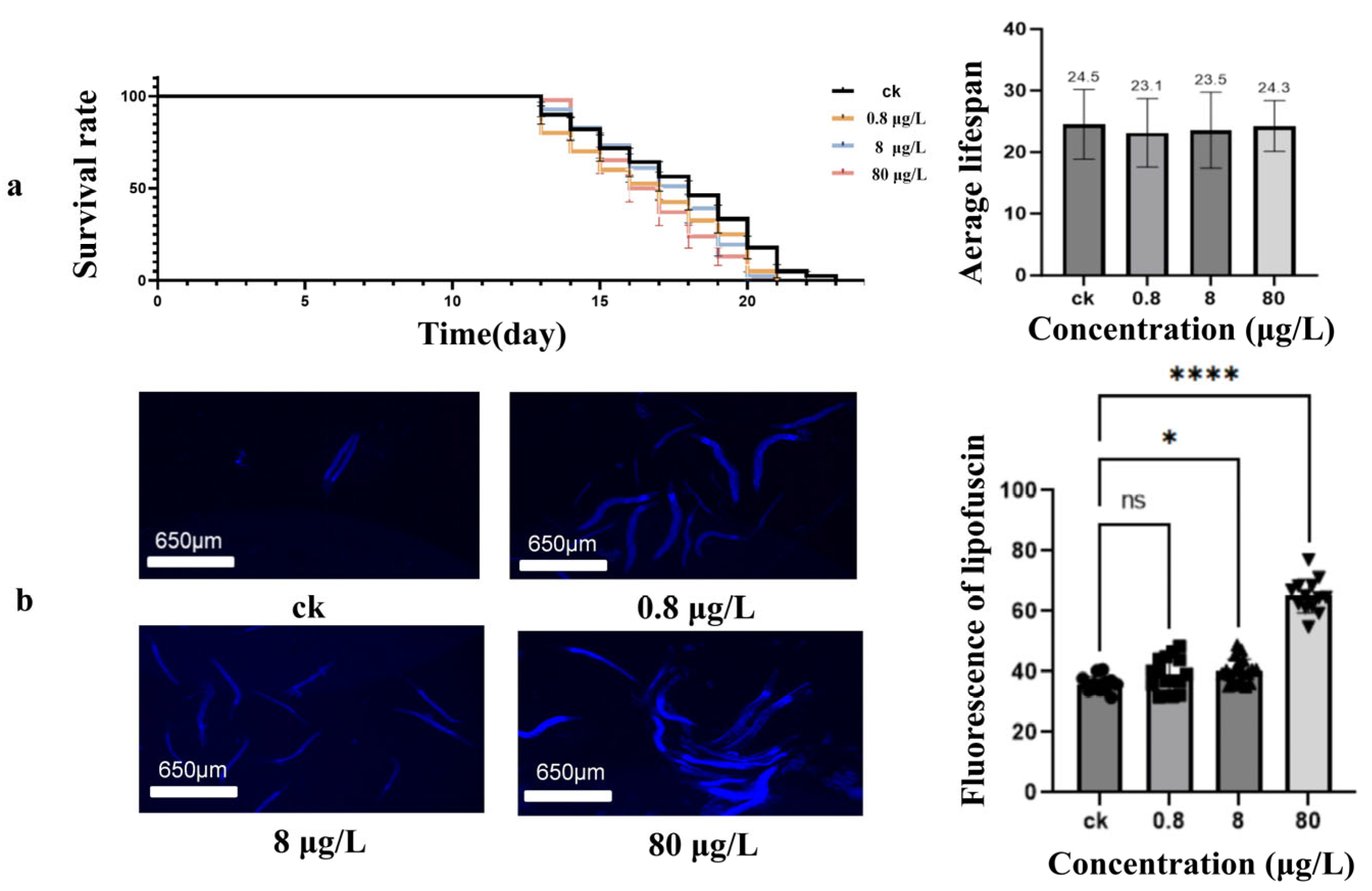
3.2. Ni Exposure Effects on Lifespan and Aging Processes of Caenorhabditis elegans
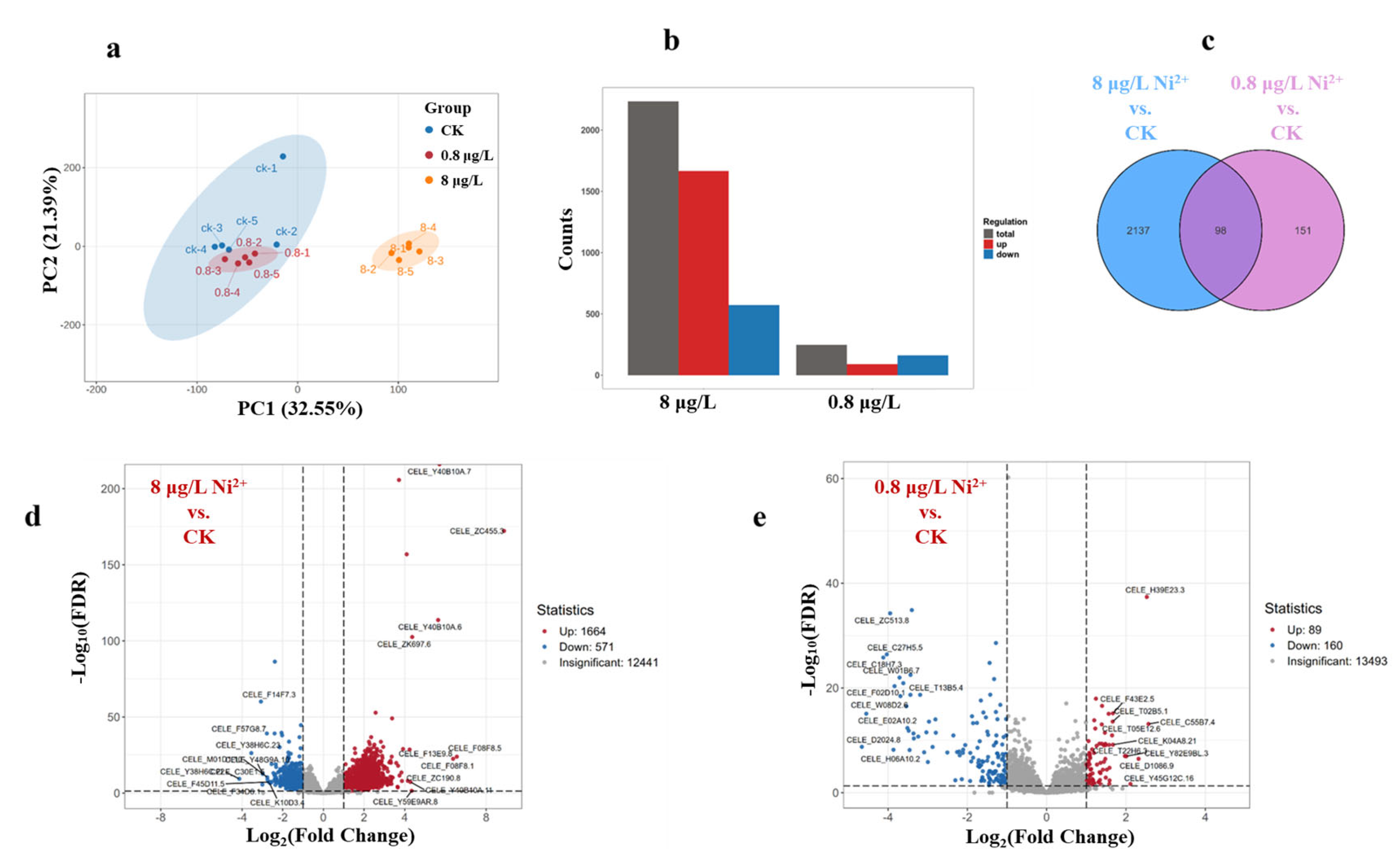
3.3. Transcriptome Analysis After Ni Exposure
3.4. Gene Ontology (GO) Enrichment Reveals Ni2+ Treatment Impacts C. elegans Stress Response, Development, and Metabolism
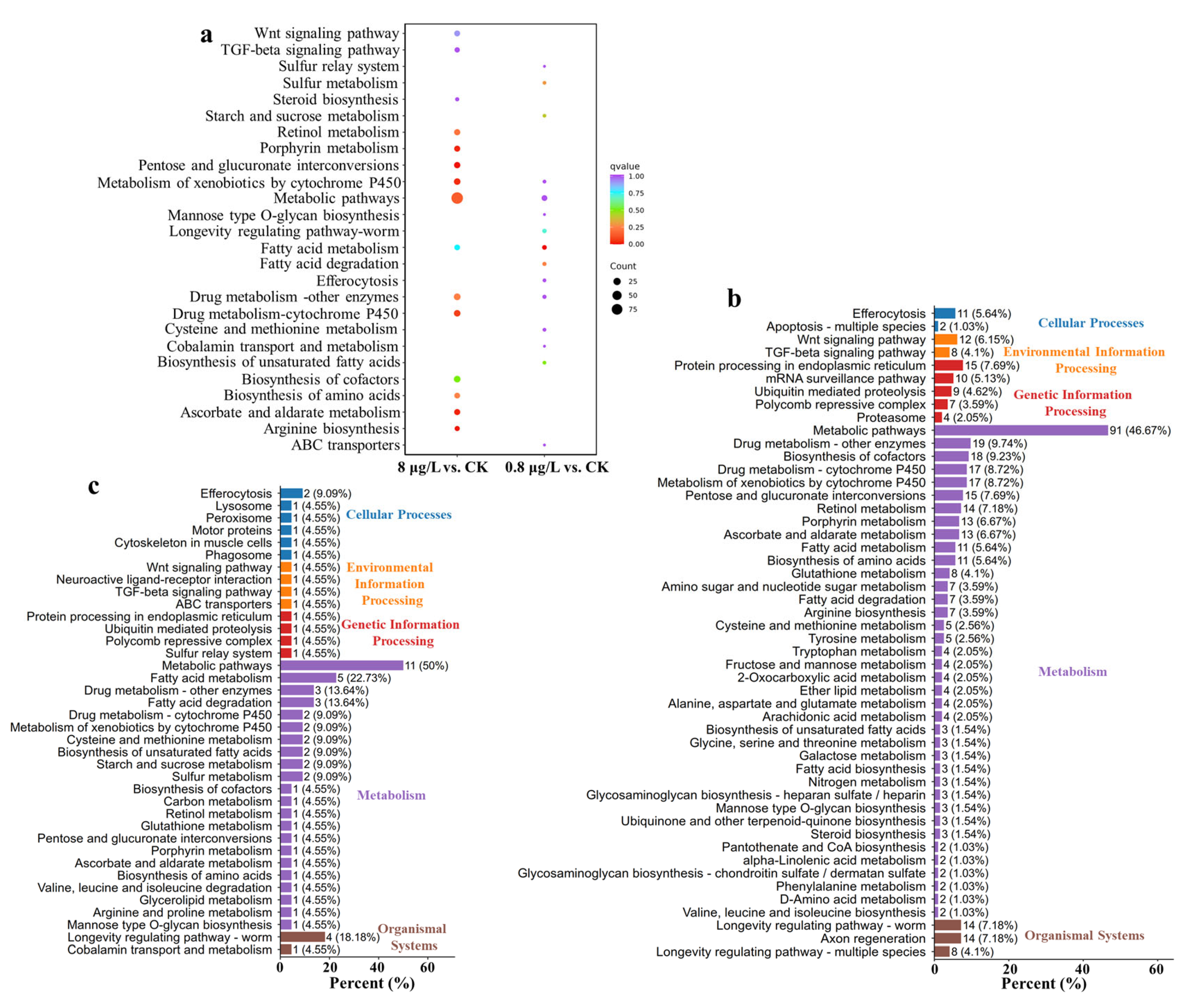
3.5. Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Unveils Ni2+ Treatment Effects on C. elegans Metabolism, Signaling, and Aging Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dilshara, P.; Abeysinghe, B.; Premasiri, R.; Dushyantha, N.; Ratnayake, N.; Senarath, S.; Sandaruwan Ratnayake, A.; Batapola, N. The role of nickel (Ni) as a critical metal in clean energy transition: Applications, global distribution and occurrences, production-demand and phytomining. J. Asian Earth Sci. 2024, 259, 105912. [Google Scholar] [CrossRef]
- Yufeng, S. Properties and Applications of Nickel. Chlor-Alkali Ind. 1995, 11, 31–35. [Google Scholar]
- Sun, X.; Jiao, Y.; Hao, H.; Liu, Z.; Zhao, F. Physical and monetary characterization of global nickel flow network. Resour. Policy 2024, 94, 105130. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2023; U.S. Geological Survey: Reston, VA, USA, 2023.
- Zhang, H.; Liu, G.; Li, J.; Qiao, D.; Zhang, S.; Li, T.; Guo, X.; Liu, M. Modeling the impact of nickel recycling from batteries on nickel demand during vehicle electrification in China from 2010 to 2050. Sci. Total Environ. 2023, 859, 159964. [Google Scholar] [CrossRef]
- Cempel, M.; Nikel, G. Nickel: A review of its sources and environmental toxicology. Pol. J. Environ. Stud. 2006, 15, 375–382. [Google Scholar]
- Shaltout, A.A.; Harfouche, M.; Abd-Elkader, O.H.; Eichert, D. The occurrence and sources of Ni in ambient air particulates using synchrotron radiation based X-ray fluorescence and X-ray absorption near edge structure. J. Anal. At. Spectrom. 2025, 40, 1297–1308. [Google Scholar] [CrossRef]
- Rizwan, M.; Usman, K.; Alsafran, M. Ecological impacts and potential hazards of nickel on soil microbes, plants, and human health. Chemosphere 2024, 357, 142028. [Google Scholar] [CrossRef]
- El-Naggar, A.; Ahmed, N.; Mosa, A.; Niazi, N.K.; Yousaf, B.; Sharma, A.; Sarkar, B.; Cai, Y.; Chang, S.X. Nickel in soil and water: Sources, biogeochemistry, and remediation using biochar. J. Hazard. Mater. 2021, 419, 126421. [Google Scholar] [CrossRef]
- Chen, C.; Huang, D.; Liu, J. Functions and toxicity of nickel in plants: Recent advances and future prospects. CLEAN-Soil Air Water 2009, 37, 304–313. [Google Scholar] [CrossRef]
- Salehi, F.; Esmaeilbeigi, M.; Kazemi, A.; Sharafi, S.; Sahebi, Z.; Asl, A.G. Spatial health risk assessments of nickel in the groundwater sources of a mining-impacted area. Sci. Rep. 2024, 14, 11017. [Google Scholar] [CrossRef]
- Hernández, E.; Obrist-Farner, J.; Brenner, M.; Kenney, W.F.; Curtis, J.H.; Duarte, E. Natural and anthropogenic sources of lead, zinc, and nickel in sediments of Lake Izabal, Guatemala. J. Environ. Sci. 2020, 96, 117–126. [Google Scholar] [CrossRef]
- Zhao, J.S.; Shi, X.L.; Castranova, V.; Ding, M. Occupational toxicology of nickel and nickel compounds. J. Environ. Pathol. Toxicol. Oncol. 2009, 28, 177–208. [Google Scholar] [CrossRef]
- Hu, H.W.; Wang, J.T.; Li, J.; Shi, X.Z.; Ma, Y.B.; Chen, D.; He, J.Z. Long-term nickel contamination increases the occurrence of antibiotic resistance genes in agricultural soils. Environ. Sci. Technol. 2017, 51, 790–800. [Google Scholar] [CrossRef] [PubMed]
- More, S.L.; Kovochich, M.; Lyons-Darden, T.; Taylor, M.; Schulte, A.M.; Madl, A.K. Review and evaluation of the potential health effects of oxidic nickel nanoparticles. Nanomaterials 2021, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.C.; Zhang, Y.J.; He, X.F.; Li, X.F.; Zhang, X.X. Analysis of the Report on the national general survey of soil contamination. J. Agro-Environ. Sci. 2017, 36, 1689–1692. [Google Scholar]
- World Health Organization. Nickel in Drinking-Water; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Kinuthia, G.K.; Ngure, V.; Beti, D.; Lugalia, R.; Wangila, A.; Kamau, L. Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: Community health implication. Sci. Rep. 2020, 10, 8434. [Google Scholar] [CrossRef]
- Wang, C.; Hao, L.; Sun, X.; Yang, Y.; Yin, Q.; Li, M. Response mechanism of psychrotolerant Bacillus cereus D2 towards Ni (II) toxicity and involvement of amino acids in Ni (II) toxicity reduction. J. Hazard. Mater. 2022, 430, 128363. [Google Scholar] [CrossRef]
- Chen, H.; Giri, N.C.; Zhang, R.; Yamane, K.; Zhang, Y.; Maroney, M.; Costa, M. Nickel ions inhibit histone demethylase JMJD1A and DNA repair enzyme ABH2 by replacing the ferrous iron in the catalytic centers. J. Biol. Chem. 2010, 285, 7374–7383. [Google Scholar] [CrossRef]
- Li, J.; Hu, H.-W.; Ma, Y.-B.; Wang, J.-T.; Liu, Y.-R.; He, J.-Z. Long-term nickel exposure altered the bacterial community composition but not diversity in two contrasting agricultural soils. Environ. Sci. Pollut. Res. 2015, 22, 10496–10505. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Rehman, A.; Cheema, S.A.; Fahad, S.; Rehman, S.; Sharma, A. Nickel whether toxic or essential for plants and environment—A review. Plant Physiol. Bioch. 2018, 132, 641–651. [Google Scholar] [CrossRef]
- Zambelli, B.; Ciurli, S. Nickel and Human Health. In Interrelations Between Essential Metal Ions and Human Diseases; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 321–357. [Google Scholar]
- Salnikow, K.; Costa, M. Epigenetic mechanisms of nickel carcinogenesis. J. Environ. Pathol. Toxicol. Oncol. 2000, 19, 307–318. [Google Scholar]
- Lee, Y.W.; Klein, C.B.; Kargacin, B.; Salnikow, K.; Kitahara, J.; Dowjat, K.; Zhitkovich, A.; Christie, N.T.; Costa, M. Carcinogenic nickel silences gene expression by chromatin condensation and DNA methylation: A new model for epigenetic carcinogens. Mol. Cell. Biol. 1995, 15, 2547–2557. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.W.; Pons, C.; Tummolo, D.M.; Klein, C.B.; Rossman, T.G.; Christie, N.T. Mutagenicity of soluble and insoluble nickel compounds at the gpt locus in G12 Chinese hamster cells. Environ. Mol. Mutagen. 1993, 21, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Davidson, T.; Chen, H.; Kluz, T.; Costa, M. Alterations of histone modifications and transgene silencing by nickel chloride. Carcinogenesis 2006, 27, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zuo, Z.; Yang, Z.; Yin, H.; Yang, Y.; Fang, J.; Cui, H.; Du, Z.; Ouyang, P.; Chen, X.; et al. Mitochondria damage and ferroptosis involved in Ni-induced hepatotoxicity in mice. Toxicology 2022, 466, 153068. [Google Scholar] [CrossRef]
- Wu, B.; Cui, H.; Peng, X.; Fang, J.; Zuo, Z.; Deng, J.; Huang, J. Dietary nickel chloride induces oxidative stress, apoptosis and alters Bax/Bcl-2 and caspase-3 mRNA expression in the cecal tonsil of broilers. Food Chem. Toxicol. 2014, 63, 18–29. [Google Scholar] [CrossRef]
- Qin, R.; Wang, Y.; Wang, S.; Xia, B.; Xin, R.; Li, C.; Wu, Y. Nickel-refining dust regulates the expression of inflammatory factors in NIH/3T3 cells. Toxicol. Ind. Health 2019, 35, 239–247. [Google Scholar] [CrossRef]
- Saini, S.; Nair, N.; Saini, M. Effects of gestational administration of nickel on postnatal development in Swiss albino mice. Hum. Exp. Toxicol. 2014, 33, 1199–1208. [Google Scholar] [CrossRef]
- Moyson, S.; Town, R.M.; Vissenberg, K.; Blust, R. The effect of metal mixture composition on toxicity to C. elegans at individual and population levels. PLoS ONE 2019, 14, e0218929. [Google Scholar] [CrossRef]
- Ijomone, O.M.; Miah, M.R.; Akingbade, G.T.; Bucinca, H.; Aschner, M. Nickel-induced developmental neurotoxicity in C. elegans includes cholinergic, dopaminergic and GABAergic degeneration, altered behaviour, and increased SKN-1 activity. Neurotox. Res. 2020, 37, 1018–1028. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y. Nickel sulfate induces numerous defects in Caenorhabditis elegans that can also be transferred to progeny. Environ. Pollut. 2008, 151, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.W.; Williams, P.L.; Xue, K.S.; Wang, J.S.; Tang, L.L. Detoxification mechanisms of nickel sulfate in nematode Caenorhabditis elegans. Chemosphere 2020, 260, 127627. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Birdsey, J.M.; Wendolowski, M.A.; Dobbin, K.K.; Williams, P.L. Differential toxicities of nickel salts to the nematode Caenorhabditis elegans. Bull. Environ. Contam. Toxicol. 2016, 97, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Town, R.M.; van Leeuwen, H.P. Chemodynamic features of nickel(II) and its complexes: Implications for bioavailability in freshwaters. Ecotox. Environ. Saf. 2022, 241, 113840. [Google Scholar] [CrossRef]
- Huang, S.-T.; Bulaon, E.P.; Yang, K.-J.; Taw, A.; Tayo, L.L.; Hsieh, P.-H.; Tsai, J.-H.; Lu, J.-H.; Jiang, J.-J.; Wu, H.-H.; et al. The impact of boron carbide nanoparticle (B4C-NPs) toxicity on Caenorhabditis elegans models. Toxics 2025, 13, 492. [Google Scholar] [CrossRef]
- Ren, H.S.; Yin, K.; Lu, X.H.; Liu, J.J.; Li, D.D.; Liu, Z.J.; Zhou, H.L.; Xu, S.Q.; Li, H.Z. Synergy between nanoplastics and benzo[a]pyrene promotes senescence by aggravating ferroptosis and impairing mitochondria integrity in Caenorhabditis elegans. Sci. Total Environ. 2024, 946, 174418. [Google Scholar] [CrossRef]
- Tejeda, L.; Olivero-Verbel, J. Caenorhabditis elegans, a biological model for research in toxicology. Rev. Environ. Contam. Toxicol. 2015, 237, 1–35. [Google Scholar]
- Bouyanfif, A.; Jayarathne, S.; Koboziev, I.; Moustaid-Moussa, N. The nematode Caenorhabditis elegans as a model organism to study metabolic effects of ω-3 polyunsaturated fatty acids in obesity. Adv. Nutr. 2019, 10, 165–178. [Google Scholar] [CrossRef]
- Papaevgeniou, N.; Hoehn, A.; Grune, T.; Chondrogianni, N. P 090—Lipofuscin effects in Caenorhabditis elegans ageing model. Free. Radic. Bio. Med. 2017, 108, S48. [Google Scholar] [CrossRef]
- Bae, J.; Benoit, D.L.; Watson, A.K. Effect of heavy metals on seed germination and seedling growth of common ragweed and roadside ground cover legumes. Environ. Pollut. 2016, 213, 112–118. [Google Scholar] [CrossRef]
- Luo, C.; Zhang, L.; Ali, M.M.; Xu, Y.; Liu, Z. Environmental risk substances in soil on seed germination: Chemical species, inhibition performance, and mechanisms. J. Hazard. Mater. 2024, 472, 134518. [Google Scholar] [CrossRef] [PubMed]
- Su-ping, Y.H.-c.W. Effects of nickel stress on seed germination and seedling growth of wheat. Seed 2011, 30, 18–20. [Google Scholar]
- Wang, N.; Chen, H.; Tian, Y. Effects of nickel, lead, and copper stress on the growth and biochemical responses of Aegilops tauschii seedlings. Sci. Rep. 2024, 14, 24832. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Liu, L.; Wu, X.; Guo, P.; Yu, Z.; Wang, P.; Song, Y.; Zheng, S.; Liu, N. Cadmium-induced reproductive toxicity combined with a correlation to the oogenesis process and competing endogenous RNA networks based on a Caenorhabditis elegans model. Ecotox. Environ. Saf. 2023, 268, 115687. [Google Scholar] [CrossRef]
- Hoy, J.L.; Day, L.M.; Tibballs, J.; Ozanne-Smith, J. Unintentional poisoning hospitalisations among young children in Victoria. Inj. Prev. 1999, 5, 31–35. [Google Scholar] [CrossRef]
- Rankin, C.H. Nematode behavior: The taste of success, the smell of danger! Curr. Biol. 2006, 16, R89–R91. [Google Scholar] [CrossRef]
- Razmara, P.; Sharpe, J.; Pyle, G.G. Rainbow trout (Oncorhynchus mykiss) chemosensory detection and reactions to copper nanoparticles and copper ions. Environ. Pollut. 2020, 260, 113925. [Google Scholar] [CrossRef]
- Pyle, G.G.; Mirza, R.S. Copper-impaired chemosensory function and behavior in aquatic animals. Hum. Ecol. Risk Assess. 2007, 13, 492–505. [Google Scholar] [CrossRef][Green Version]
- Amrit, F.R.G.; Ratnappan, R.; Keith, S.A.; Ghazi, A. The C. elegans lifespan assay toolkit. Methods 2014, 68, 465–475. [Google Scholar] [CrossRef]
- Krishna, M.M.; Waghmare, S.G.; Franitza, A.L.; Maccoux, E.C.; Lezi, E. Epidermal collagen reduction drives selective aspects of aging in sensory neurons. Aging Cell 2025, 24, e14459. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Wei, C.; Lin, H.; Wu, M.; Ma, B.; Jiang, J.; Li, S.; Wang, H. Negative effect of gst-35 on the health span of Caenorhabditis elegans through lysosomal dysfunction via the pmk-1 and skr Genes. Aging Cell 2025, 24, e70016. [Google Scholar] [CrossRef]
- Abraham, E.; Athapaththu, A.M.G.K.; Atanasova, K.R.; Chen, Q.-Y.; Corcoran, T.J.; Piloto, J.; Wu, C.-W.; Ratnayake, R.; Luesch, H.; Choe, K.P. Chemical genetics in C. elegans identifies anticancer mycotoxins chaetocin and chetomin as potent inducers of a nuclear metal homeostasis response. ACS Chem. Biol. 2024, 19, 1180–1193. [Google Scholar] [CrossRef]
- Earley, B.J.; Cubillas, C.; Warnhoff, K.; Ahmad, R.; Alcantar, A.; Lyon, M.D.; Schneider, D.L.; Kornfeld, K. Cadmium hijacks the high zinc response by binding and activating the HIZR-1 nuclear receptor. Proc. Natl. Acad. Sci. USA 2021, 118, e2022649118. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; Zhang, H.; Liu, R.; Wang, S.; Pu, Y.; Yin, L. Integrating transcriptomics and behavior tests reveals how the C. elegans responds to copper induced aging. Ecotox. Environ. Saf. 2021, 222, 112494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, X.; Chen, P.; Liu, L.; Xin, N.; Tian, Y.; Dillin, A. The mitochondrial unfolded protein response is mediated cell-non-autonomously by retromer-dependent Wnt signaling. Cell 2018, 174, 870–883.e17. [Google Scholar] [CrossRef] [PubMed]
- Zinovyeva, A.Y.; Forrester, W.C. The C. elegans Frizzled CFZ-2 is required for cell migration and interacts with multiple Wnt signaling pathways. Dev. Biol. 2005, 285, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Santiago, F.E.; Jin, H.; Lin, D.; Schedl, T.; Kipreos, E.T. The Caenorhabditis elegans skp1-related gene family: Diverse functions in cell proliferation, morphogenesis, and meiosis. Curr. Biol. 2002, 12, 277–287. [Google Scholar]
- Rousseau, A.; Bertolotti, A. Regulation of proteasome assembly and activity in health and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 697–712. [Google Scholar] [CrossRef]
- Sainio, A.; Järveläinen, H. Extracellular matrix-cell interactions: Focus on therapeutic applications. Cell. Signal. 2020, 66, 109487. [Google Scholar] [CrossRef]
- Vander Ark, A.; Cao, J.; Li, X. TGF-β receptors: In and beyond TGF-β signaling. Cell. Signal. 2018, 52, 112–120. [Google Scholar]
- Brunk, U.T.; Terman, A. Lipofuscin: Mechanisms of age-related accumulation and influence on cell function. Free Radic. Biol. Med. 2002, 33, 611–619. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Long, Y.; Wang, J.; Li, Q.; Liu, B.; Li, D.; Xu, S. Transcriptomics-Based Toxicological Study of Nickel on Caenorhabditis elegans. Toxics 2025, 13, 930. https://doi.org/10.3390/toxics13110930
He Y, Long Y, Wang J, Li Q, Liu B, Li D, Xu S. Transcriptomics-Based Toxicological Study of Nickel on Caenorhabditis elegans. Toxics. 2025; 13(11):930. https://doi.org/10.3390/toxics13110930
Chicago/Turabian StyleHe, Yutao, Yunfei Long, Jingwen Wang, Qinfen Li, Beibei Liu, Dandan Li, and Shunqing Xu. 2025. "Transcriptomics-Based Toxicological Study of Nickel on Caenorhabditis elegans" Toxics 13, no. 11: 930. https://doi.org/10.3390/toxics13110930
APA StyleHe, Y., Long, Y., Wang, J., Li, Q., Liu, B., Li, D., & Xu, S. (2025). Transcriptomics-Based Toxicological Study of Nickel on Caenorhabditis elegans. Toxics, 13(11), 930. https://doi.org/10.3390/toxics13110930





