Investigating the Effects of Long-Term Fine Particulate Matter Exposure on Autism Spectrum Disorder Severity: Evidence from Multiple Analytical Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Global Burden of Disease Analysis on Pediatric Autism Spectrum Disorder
2.2. Pilot Cohort Description and Study Design
2.3. Community-Level Participants Air Pollution Mixture Levels Calculation
2.4. Multiple Co-Exposure Statistical Models Approaches
2.5. Environmental PM2.5 Sample Collection
2.6. Experimental Environmental PM2.5 Level Calculation
- Daily Respiratory Frequency: A mouse’s respiratory rate is approximately 120 breaths per minute. Therefore, the total number of breaths per day is: 120 breaths/min × 60 min/hour × 24 h/day = 172,800 breaths/day.
- Daily Respiratory Volume: The tidal volume of a single mouse breath is 0.15 mL. Therefore, the total daily respiratory volume is: 172,800 breaths/day × 0.15 mL/breath = 25,920 mL/day = 25.92 L/day
- Conversion of Daily Respiratory Volume to Cubic Meters: 25.92 L/day ÷ 1000 L/m3 = 0.02592 m3/day.
- Daily Inhaled Mass of PM2.5: The mass of PM2.5 inhaled per day by a mouse is: 50 μg/m3 × 0.02592 m3/day ≈ 1.3 μg/day.
- Calculation of Dosage for Administration: Assuming a standard body weight of 20 g (0.02 kg) and intratracheal instillation every three days to reduce animal distress, the dosage is calculated as: (1.3 μg/day ÷ 0.02 kg) × 3 days ≈ 200 μg/kg per 3-day period.
- Final Dose Adjustment: According to principles of environmental toxicology and previous studies [18,25], a 10-fold safety factor is applied to account for intra-species individual variability and potential cumulative effects. The final administered dose is therefore: (200 μg/kg × 10) ÷ 1000 μg/mg = 2 mg/kg, administered every 3 days as the low environmental PM2.5 exposure level.
2.7. Determination of Sample Size
2.8. Animal Experimental Design
2.9. Statistical Analysis
3. Results
3.1. Air Pollution May Be Associated with Increased ASD Severity Risk
3.2. PM2.5 Maybe the Predominant Air Pollutant Affecting ASD Symptom Severity
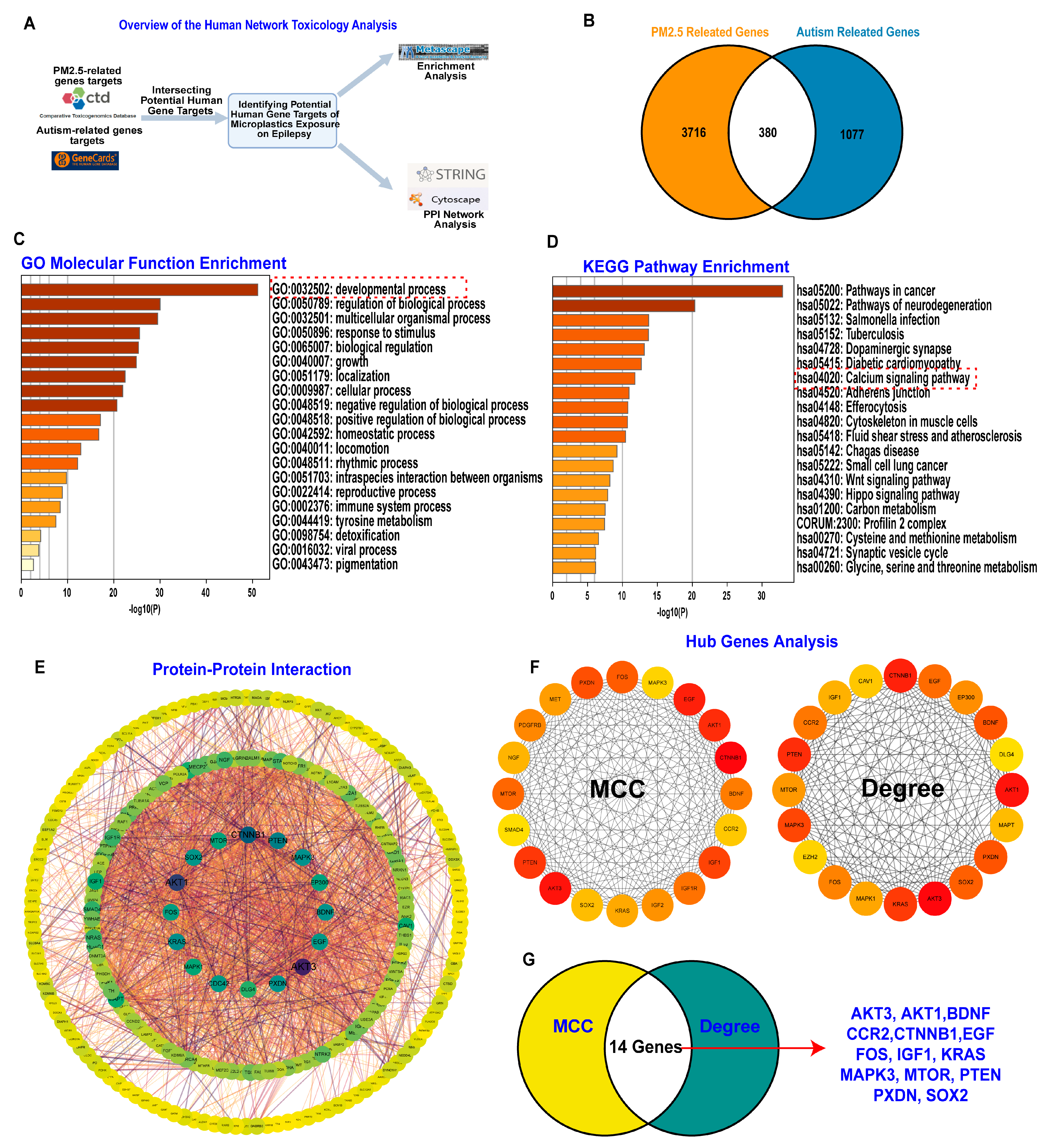
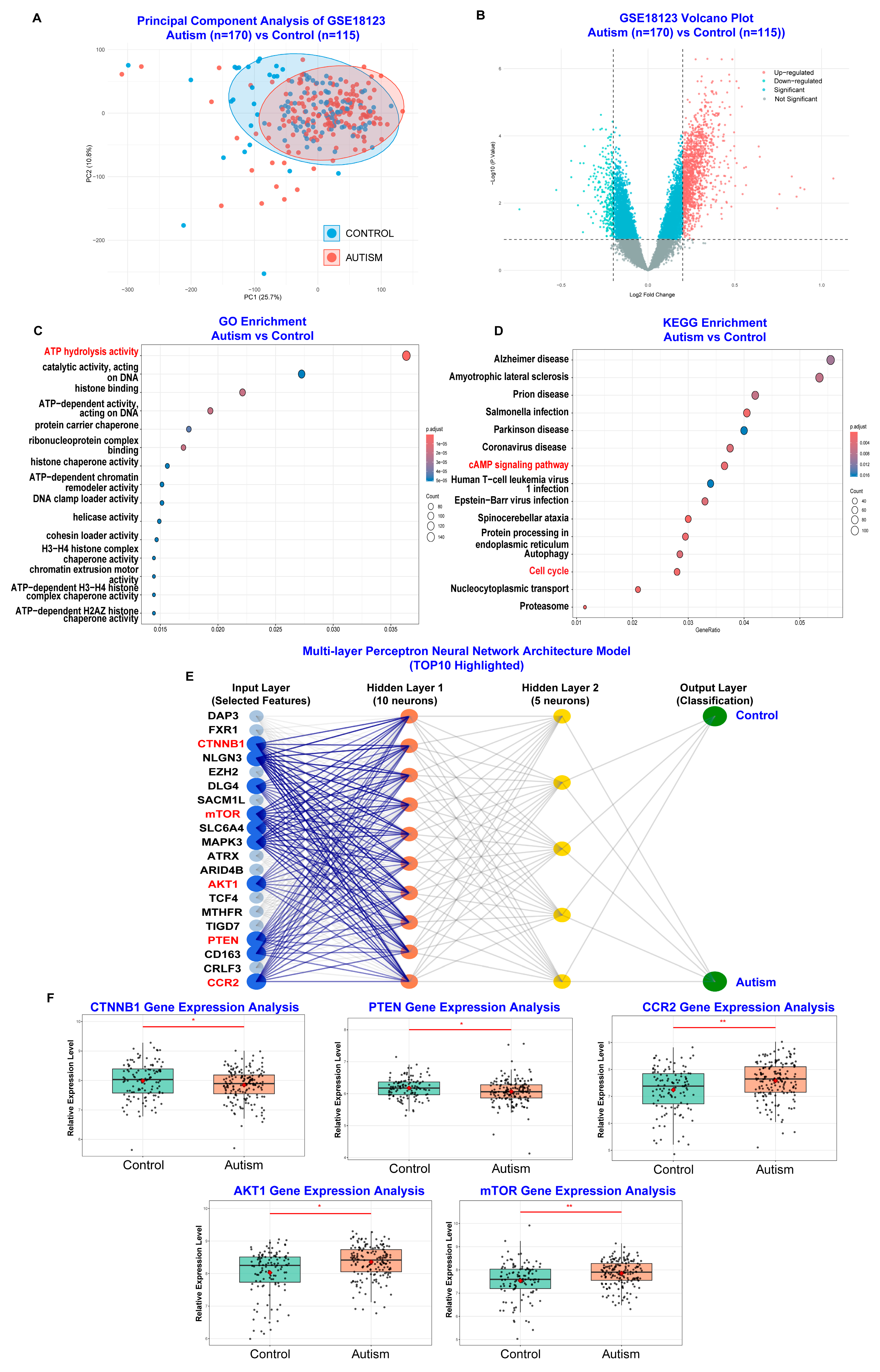
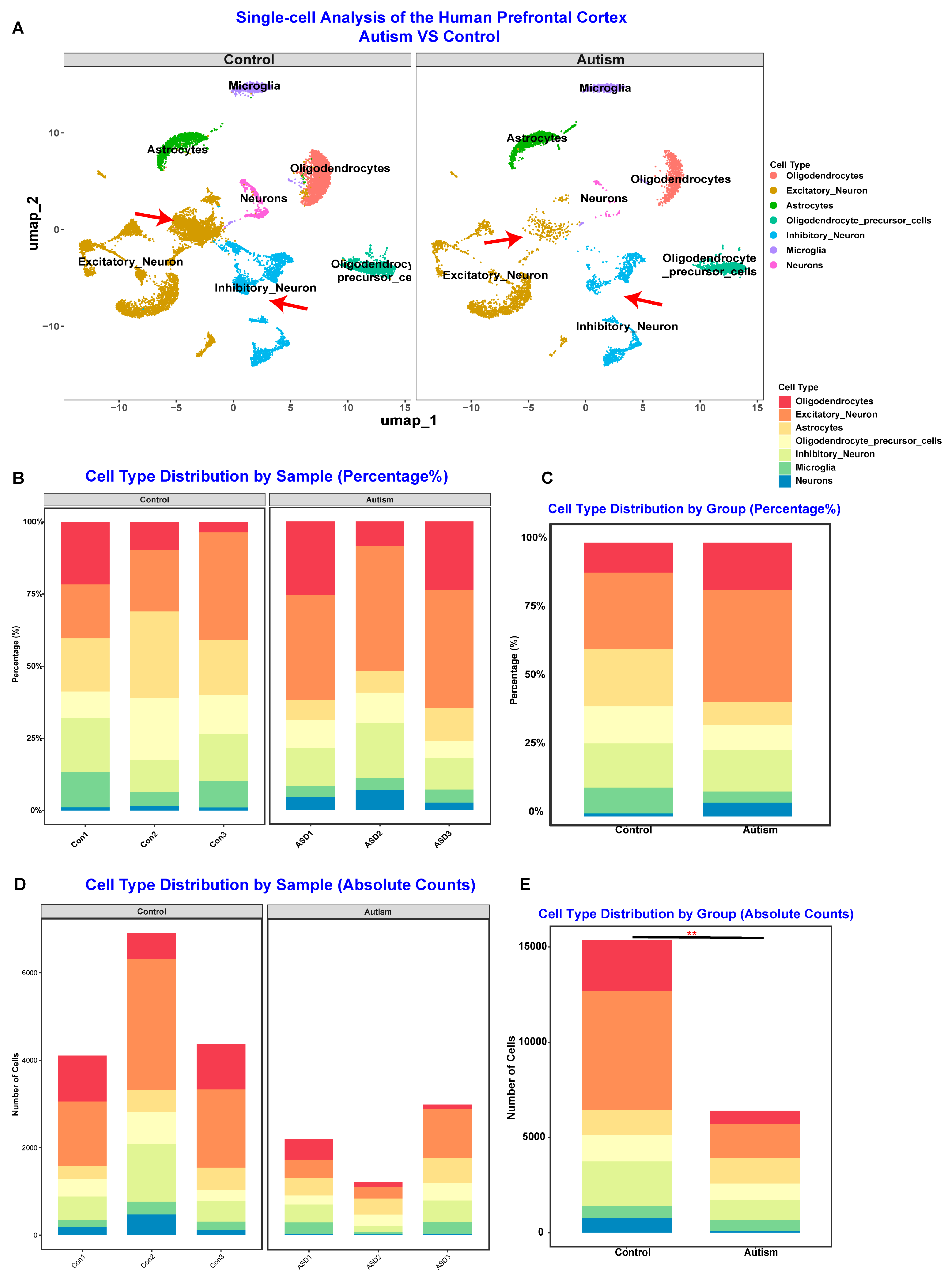
3.3. PM2.5 Exposure May Alter Gene Expression Related to Inflammation and Neuronal Development to Affect ASD Symptom Severity
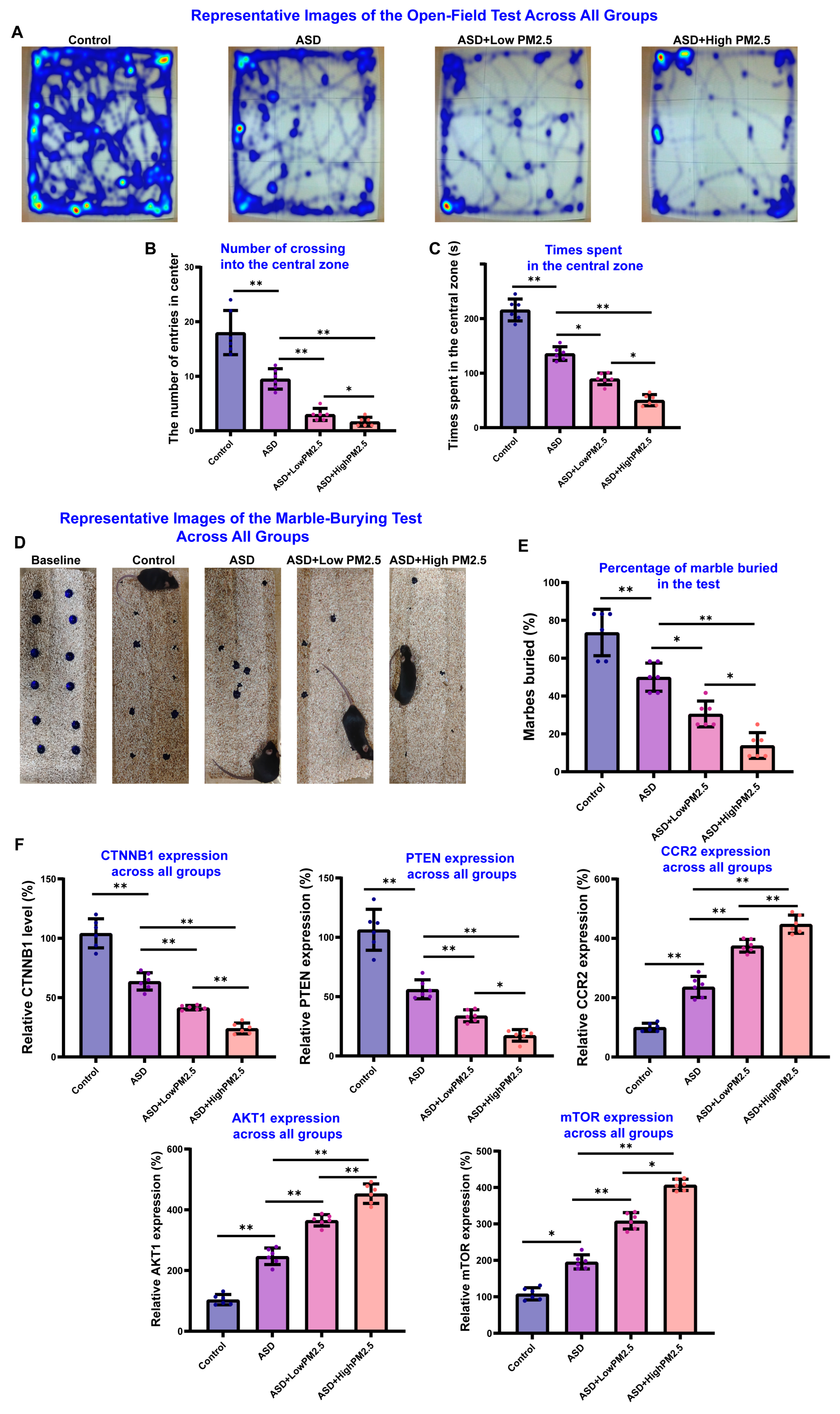
3.4. PM2.5 Exposure May Exacerbate the VPA-Induced ASD Like Behaviors in Mice
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Han, Y.; Yu, Z.; Chen, Y.; Guo, X.; Liu, Y.; Zhang, H.; Li, Z.; Chen, L. PM2.5 induces developmental neurotoxicity in cortical organoids. Environ. Pollut. 2024, 361, 124913. [Google Scholar] [CrossRef]
- Liu, F.; Liu, C.; Liu, Y.; Wang, J.; Wang, Y.; Yan, B. Neurotoxicity of the air-borne particles: From molecular events to human diseases. J. Hazard. Mater. 2023, 457, 131827. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, D.; Liang, F.; Liu, F.; Xiao, L.; An, X.; Chen, X.; Liang, X. Air pollution and hypertension in rural versus urban children: Lipidomic insights into PM2.5 impacts. Environ. Res. 2025, 278, 121715. [Google Scholar] [CrossRef]
- Marsal, A.; Slama, R.; Lyon-Caen, S.; Borlaza, L.J.S.; Jaffrezo, J.L.; Boudier, A.; Darfeuil, S.; Elazzouzi, R.; Gioria, Y.; Lepeule, J.; et al. Prenatal Exposure to PM2.5 Oxidative Potential and Lung Function in Infants and Preschool- Age Children: A Prospective Study. Environ. Health Perspect. 2023, 131, 17004. [Google Scholar] [CrossRef]
- Thiankhaw, K.; Chattipakorn, N.; Chattipakorn, S.C. PM2.5 exposure in association with AD-related neuropathology and cognitive outcomes. Environ. Pollut. 2022, 292 Pt A, 118320. [Google Scholar] [CrossRef]
- Kang, K.; Zhang, Y.; Geng, Y.; Wang, D.; Zheng, P. Andrographolide attenuates PM2.5-induced blood-brain barrier damage via antioxidant and PI3K/AKT/mTOR/NRF2 pathways. Int. Immunopharmacol. 2025, 157, 114764. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Wu, C.; Wang, J.; Sun, M. Autism Spectrum Disorder: Neurodevelopmental Risk Factors, Biological Mechanism, and Precision Therapy. Int. J. Mol. Sci. 2023, 24, 1819. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, S.; Zhang, X.; Gao, L. ASD and ADHD: Divergent activating patterns of prefrontal cortex in executive function tasks? J. Psychiatr. Res. 2024, 172, 187–196. [Google Scholar] [CrossRef]
- Andrade, C. Autism Spectrum Disorder, 1: Genetic and Environmental Risk Factors. J. Clin. Psychiatry 2025, 86, 25f15878. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Li, J.; Qin, Z.; Machado Bressan Wilke, M.V.; Liu, Y.; Li, Q.; Liu, H.; Liang, C.; Morales-Rosado, J.A.; Cohen, A.S.A.; et al. MARK2 variants cause autism spectrum disorder via the downregulation of WNT/β-catenin signaling pathway. Am. J. Hum. Genet. 2024, 111, 2392–2410. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Sun, R.; Bai, J.; Liu, X.; He, C.; Jiang, Q.; Wang, Q.; Qi, Y.; Ding, W.; Shen, J.; et al. Calcitriol Modulates Hippocampal Axon Guidance Through Enhanced EfnA4-Mediated PI3K/AKT Signaling in an Autism Mouse Model. CNS Neurosci. Ther. 2025, 31, e70429. [Google Scholar] [CrossRef]
- Hope, S.; Shadrin, A.A.; Lin, A.; Bahrami, S.; Rødevand, L.; Frei, O.; Hübenette, S.J.; Cheng, W.; Hindley, G.; Nag, H.; et al. Bidirectional genetic overlap between autism spectrum disorder and cognitive traits. Transl. Psychiatry 2023, 13, 295. [Google Scholar] [CrossRef]
- Wood, J.J.; Kendall, P.C.; Wood, K.S.; Kerns, C.M.; Seltzer, M.; Small, B.J.; Lewin, A.B.; Storch, E.A. Cognitive Behavioral Treatments for Anxiety in Children With Autism Spectrum Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2020, 77, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Luglio, D.G.; Kleeman, M.J.; Yu, X.; Lin, J.C.; Chow, T.; Martinez, M.P.; Chen, Z.; Chen, J.C.; Eckel, S.P.; Schwartz, J.; et al. Prenatal Exposure to Source-Specific Fine Particulate Matter and Autism Spectrum Disorder. Environ. Sci. Technol. 2024, 58, 18566–18577. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Jiang, X.; Dou, J.; Xie, R.; Zhao, W.; Cao, Y.; Gao, J.; Yao, F.; Wu, D.; Mei, H.; et al. Investigating the potential risk of cadmium exposure on seizure severity and anxiety-like behaviors through the ferroptosis pathway in epileptic mice: An integrated multi-omics approach. J. Hazard. Mater. 2024, 480, 135814. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, W.; Zhong, Y.; Jiang, X.; Mei, H.; Chang, Y.; Wu, D.; Dou, J.; Vasquez, E.; Shi, X.; et al. Effects of chronic low-level lead (Pb) exposure on cognitive function and hippocampal neuronal ferroptosis: An integrative approach using bioinformatics analysis, machine learning, and experimental validation. Sci. Total Environ. 2024, 917, 170317. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L. Findings from the Global Burden of Disease Study 2021. Lancet 2024, 403, 2259–2262. [Google Scholar] [CrossRef]
- Mei, H.; Wu, D.; Yong, Z.; Cao, Y.; Chang, Y.; Liang, J.; Jiang, X.; Xu, H.; Yang, J.; Shi, X.; et al. PM(2.5) exposure exacerbates seizure symptoms and cognitive dysfunction by disrupting iron metabolism and the Nrf2-mediated ferroptosis pathway. Sci. Total Environ. 2024, 910, 168578. [Google Scholar] [CrossRef]
- Wang, Y.; Quan, F.; Cao, Q.; Lin, Y.; Yue, C.; Bi, R.; Cui, X.; Yang, H.; Yang, Y.; Birnbaumer, L.; et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2021, 28, 231–243. [Google Scholar] [CrossRef]
- Chen, N.; Ma, L.L.; Zhang, Y.; Yan, Y.X. Association of household solid fuel use and long-term exposure to ambient air pollution with estimated 10-year high cardiovascular disease risk among postmenopausal women. Environ. Pollut. 2024, 342, 123091. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.; Wu, Q.; Zhao, B.; Jiang, Y.; Zheng, H.; Wen, Y.; Zhang, S.; Wu, Y.; Hao, J. Integrated Benefits of Synergistically Reducing Air Pollutants and Carbon Dioxide in China. Environ. Sci. Technol. 2024, 58, 14193–14202. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Jiang, Y.; Lin, Y.; Liang, H.; Wang, W.; Huang, Y.; He, J. Exploring the potential associations between single and mixed volatile compounds and preserved ratio impaired spirometry using five different approaches. Ecotoxicol. Environ. Saf. 2025, 302, 118686. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Cao, J.J.; Shen, Z.X.; Han, Y.M.; Lee, S.C.; Huang, Y.; Zhu, C.S.; Wang, Q.Y.; Xu, H.M.; Huang, R.J. Spatial and seasonal variations of PM2.5 mass and species during 2010 in Xi’an, China. Sci. Total Environ. 2015, 508, 477–487. [Google Scholar] [CrossRef]
- Li, H.; Guo, X.; Li, P.; Gao, X.; Song, X.; Chen, X.; Liang, R.; Yang, J.; Li, Y.; Chen, H.; et al. Particulate matter induces depression-like behavior through systemic inflammation and brain-derived neurotrophic factors. Environ. Int. 2024, 194, 108883. [Google Scholar] [CrossRef]
- Fan, X.; Dong, T.; Yan, K.; Ci, X.; Peng, L. PM2.5 increases susceptibility to acute exacerbation of COPD via NOX4/Nrf2 redox imbalance-mediated mitophagy. Redox Biol. 2023, 59, 102587. [Google Scholar] [CrossRef]
- Xie, R.; Xiao, X.; Zhao, W.; Zhong, Y.; Wu, D.; Dou, J.; Zhao, Y.; Luo, Y.; Cao, Y.; Chang, Y.; et al. Association between long-term exposure of polystyrene microplastics and exacerbation of seizure symptoms: Evidence from multiple approaches. Ecotoxicol. Environ. Saf. 2025, 302, 118741. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Y.; Hou, W.; Bi, D.; Yin, F.; Gao, Y.; Huang, D.; Li, Y.; Cao, Z.; Yan, Y.; et al. Fecal microbiota transplantation improves VPA-induced ASD mice by modulating the serotonergic and glutamatergic synapse signaling pathways. Transl. Psychiatry 2023, 13, 17. [Google Scholar] [CrossRef]
- Jibon, M.D.K.; Islam, M.A.; Hosen, M.E.; Faruqe, M.O.; Zaman, R.; Acharjee, U.K.; Sikdar, B.; Tiruneh, Y.K.; Khalekuzzaman, M.; Jawi, M.; et al. In-silico analysis of deleterious non-synonymous SNPs in the human AVPR1a gene linked to autism. BMC Genomics 2025, 26, 492. [Google Scholar] [CrossRef]
- Lebas, M.; Chinigò, G.; Courmont, E.; Bettaieb, L.; Machmouchi, A.; Goveia, J.; Beatovic, A.; Van Kerckhove, J.; Robil, C.; Angulo, F.S.; et al. Integrated single-cell RNA-seq analysis reveals mitochondrial calcium signaling as a modulator of endothelial-to-mesenchymal transition. Sci. Adv. 2024, 10, eadp6182. [Google Scholar] [CrossRef]
- Copenhaver, A.E.; LeGates, T.A. Sex-Specific Mechanisms Underlie Long-Term Potentiation at Hippocampus→Medium Spiny Neuron Synapses in the Medial Shell of the Nucleus Accumbens. J. Neurosci. 2024, 44, e0100242024. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Z.; Guo, Y.; Li, S.; Wang, Y.A.; Gasevic, D. Air pollution increases the risk of frailty: China Health and Retirement Longitudinal Study (CHARLS). J. Hazard. Mater. 2025, 492, 138105. [Google Scholar] [CrossRef]
- Zhu, J.; Meng, H.; Zhang, L.; Li, Y. Exploring the molecular mechanism of comorbidity of autism spectrum disorder and inflammatory bowel disease by combining multiple data sets. J. Transl. Med. 2023, 21, 372. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Zhang, Z.; Sultana, N.; Ericsson, M.; Martens, Y.A.; Sun, M.; Kanekiyo, T.; Ikezu, S.; Shaffer, S.A.; Ikezu, T. ATP1A3 as a target for isolating neuron-specific extracellular vesicles from human brain and biofluids. Sci. Adv. 2023, 9, eadi3647. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Kwiatkowski, M.; Chen, H.; Hoermayer, L.; Sinclair, S.; Zou, M.; Del Genio, C.I.; Kubeš, M.F.; Napier, R.; Jaworski, K.; et al. Adenylate cyclase activity of TIR1/AFB auxin receptors in plants. Nature 2022, 611, 133–138. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.; Ang, K.S.; Ling, J.; Sethi, R.; Lee, N.Y.S.; Ginhoux, F.; Chen, J. DISCO: A database of Deeply Integrated human Single-Cell Omics data. Nucleic Acids Res. 2022, 50, D596–D602. [Google Scholar] [CrossRef]
- Yue, D.; Zhang, Q.; Zhang, J.; Liu, W.; Chen, L.; Wang, M.; Li, R.; Qin, S.; Song, X.; Ji, Y. Diesel exhaust PM2.5 greatly deteriorates fibrosis process in pre-existing pulmonary fibrosis via ferroptosis. Environ. Int. 2023, 171, 107706. [Google Scholar] [CrossRef] [PubMed]
- Szabó, J.; Renczés, E.; Borbélyová, V.; Ostatníková, D.; Celec, P. Assessing sociability using the Three-Chamber Social Interaction Test and the Reciprocal Interaction Test in a genetic mouse model of ASD. Behav. Brain Funct. 2024, 20, 24. [Google Scholar] [CrossRef]
- Louis, S.; Carlson, A.K.; Suresh, A.; Rim, J.; Mays, M.; Ontaneda, D.; Dhawan, A. Impacts of Climate Change and Air Pollution on Neurologic Health, Disease, and Practice: A Scoping Review. Neurology 2023, 100, 474–483. [Google Scholar] [CrossRef]
- Zundel, C.G.; Ryan, P.; Brokamp, C.; Heeter, A.; Huang, Y.; Strawn, J.R.; Marusak, H.A. Air pollution, depressive and anxiety disorders, and brain effects: A systematic review. Neurotoxicology 2022, 93, 272–300. [Google Scholar] [CrossRef]
- Lamanna, J.; Meldolesi, J. Autism Spectrum Disorder: Brain Areas Involved, Neurobiological Mechanisms, Diagnoses and Therapies. Int. J. Mol. Sci. 2024, 25, 2423. [Google Scholar] [CrossRef]
- Li, K.; Liang, X.; Liu, X.; Geng, Y.; Yan, J.; Tian, L.; Liu, H.; Lai, W.; Shi, Y.; Xi, Z.; et al. Early-life exposure to PM2.5 leads to ASD-like phenotype in male offspring rats through activation of PI3K-AKT signaling pathway. Ecotoxicol. Environ. Saf. 2024, 274, 116222. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Vant, J.W.; Zhang, A.; Sanchez, R.G.; Wu, Y.; Micou, M.L.; Luczak, V.; Whiddon, Z.; Carlson, N.M.; Yu, S.B.; et al. Organization of a functional glycolytic metabolon on mitochondria for metabolic efficiency. Nat. Metab. 2024, 6, 1712–1735. [Google Scholar] [CrossRef]
- Gholipour, P.; Ebrahimi, Z.; Mohammadkhani, R.; Ghahremani, R.; Salehi, I.; Sarihi, A.; Komaki, A.; Karimi, S.A. Effects of (S)-3,4-DCPG, an mGlu8 receptor agonist, on hippocampal long-term potentiation at perforant pathway-dentate gyrus synapses in prenatal valproic acid-induced rat model of autism. Sci. Rep. 2024, 14, 13168. [Google Scholar] [CrossRef]
- Koek, L.A.; Sanderson, T.M.; Georgiou, J.; Collingridge, G.L. The role of calcium stores in long-term potentiation and synaptic tagging and capture in mouse hippocampus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2024, 379, 20230241. [Google Scholar] [CrossRef]
- Wang, S.; Li, W.; Zhang, P.; Wang, Z.; Ma, X.; Liu, C.; Vasilev, K.; Zhang, L.; Zhou, X.; Liu, L.; et al. Mechanical overloading induces GPX4-regulated chondrocyte ferroptosis in osteoarthritis via Piezo1 channel facilitated calcium influx. J. Adv. Res. 2022, 41, 63–75. [Google Scholar] [CrossRef]
- Yan, X.; Xia, Y.; Li, B.; Ye, Z.; Li, L.; Yuan, T.; Song, B.; Yu, W.; Rao, T.; Ning, J.; et al. The SOX4/EZH2/SLC7A11 signaling axis mediates ferroptosis in calcium oxalate crystal deposition-induced kidney injury. J. Transl. Med. 2024, 22, 9. [Google Scholar] [CrossRef]
- Mariani, J.; Coppola, G.; Zhang, P.; Abyzov, A.; Provini, L.; Tomasini, L.; Amenduni, M.; Szekely, A.; Palejev, D.; Wilson, M.; et al. FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders. Cell 2015, 162, 375–390. [Google Scholar] [CrossRef]
- Zhao, H.; Mao, X.; Zhu, C.; Zou, X.; Peng, F.; Yang, W.; Li, B.; Li, G.; Ge, T.; Cui, R. GABAergic System Dysfunction in Autism Spectrum Disorders. Front. Cell Dev. Biol. 2021, 9, 781327. [Google Scholar] [CrossRef]
- Nisar, S.; Bhat, A.A.; Masoodi, T.; Hashem, S.; Akhtar, S.; Ali, T.A.; Amjad, S.; Chawla, S.; Bagga, P.; Frenneaux, M.P.; et al. Genetics of glutamate and its receptors in autism spectrum disorder. Mol. Psychiatry 2022, 27, 2380–2392. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Yang, G.; Yan, Z. Identification of a molecular network regulated by multiple ASD high risk genes. Hum. Mol. Genet. 2024, 33, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Pallarès-Sastre, M.; Amayra, I.; Pulido, R.; Nunes-Xavier, C.E.; Bañuelos, S.; Cavaliere, F.; García, M. Novel CTNNB1 Gene Variants in Spanish CTNNB1 Syndrome Patients: Clinical and Psychological Manifestations. J. Autism Dev. Disord. 2025, 1–15. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, G.; Li, Y.; Guo, Z.; Zhang, X. The molecular genetics of PI3K/PTEN/AKT/mTOR pathway in the malformations of cortical development. Genes Dis. 2024, 11, 101021. [Google Scholar] [CrossRef]
- Sharma, A.R.; Batra, G.; Saini, L.; Sharma, S.; Mishra, A.; Singla, R.; Singh, A.; Singh, R.S.; Jain, A.; Bansal, S.; et al. Valproic Acid and Propionic Acid Modulated Mechanical Pathways Associated with Autism Spectrum Disorder at Prenatal and Neonatal Exposure. CNS Neurol. Disord. Drug Targets 2022, 21, 399–408. [Google Scholar] [CrossRef]
- Huang, W.; Wu, D.; Cai, C.; Yao, H.; Tian, Z.; Yang, Y.; Pang, M.; Rong, L.; Liu, B. Inhibition of MST1 ameliorates neuronal apoptosis via GSK3β/β-TrCP/NRF2 pathway in spinal cord injury accompanied by diabetes. Redox Biol. 2024, 71, 103104. [Google Scholar] [CrossRef]
- Li, Z.Y.; Dai, Y.X.; Wu, Z.M.; Li, G.; Pu, P.M.; Hu, C.W.; Zhou, L.Y.; Zhu, K.; Shu, B.; Wang, Y.J.; et al. Network pharmacology analysis and animal experiment validation of neuroinflammation inhibition by total ginsenoside in treating CSM. Phytomedicine 2024, 126, 155073. [Google Scholar] [CrossRef]
- Moreno, R.J.; Azzam, Y.W.; Eng, S.; Rose, D.; Ashwood, P. Altered Monocyte Populations and Activation Marker Expression in Children with Autism and Co-Occurring Gastrointestinal Symptoms. Biomolecules 2025, 15, 207. [Google Scholar] [CrossRef] [PubMed]
- Alshamrani, A.A.; Alshehri, S.; Alqarni, S.S.; Ahmad, S.F.; Alghibiwi, H.; Al-Harbi, N.O.; Alqarni, S.A.; Al-Ayadhi, L.Y.; Attia, S.M.; Alfardan, A.S.; et al. DNA Hypomethylation Is Associated with Increased Inflammation in Peripheral Blood Neutrophils of Children with Autism Spectrum Disorder: Understanding the Role of Ubiquitous Pollutant Di(2-ethylhexyl) Phthalate. Metabolites 2023, 13, 458. [Google Scholar] [CrossRef] [PubMed]
- Yadollahi-Farsani, Y.; Vanani, V.R.; Lorigooini, Z.; Farahzad, A.; Amini-Khoei, H. Anethole via increase in the gene expression of PI3K/AKT/mTOR mitigates the autistic-like behaviors induced by maternal separation stress in mice. IBRO Neurosci. Rep. 2024, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Hu, C.; Cui, J.; Li, H.; Luo, X.; Hao, Y. IL-6 Enhances the Activation of PI3K-AKT/mTOR-GSK-3β by Upregulating GRPR in Hippocampal Neurons of Autistic Mice. J. Neuroimmune Pharmacol. 2024, 19, 12. [Google Scholar] [CrossRef]
- Hu, J.; Xu, J.; Li, M.; Jiang, Z.; Mao, J.; Feng, L.; Miao, K.; Li, H.; Chen, J.; Bai, Z.; et al. Identification and validation of an explainable prediction model of acute kidney injury with prognostic implications in critically ill children: A prospective multicenter cohort study. eClinicalMedicine 2024, 68, 102409. [Google Scholar] [CrossRef]
- Sanderson, E.; Glymour, M.M.; Holmes, M.V.; Kang, H.; Morrison, J.; Munafò, M.R.; Palmer, T.; Schooling, C.M.; Wallace, C.; Zhao, Q.; et al. Mendelian randomization. Nat. Rev. Methods Primers 2022, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhang, R.; Huang, H.; Zhu, Y.; Li, Y.; Dong, X.; Shen, S.; Wei, L.; Chen, X.; Christiani, D.C.; et al. Mendelian Randomization With Refined Instrumental Variables From Genetic Score Improves Accuracy and Reduces Bias. Front. Genet. 2021, 12, 618829. [Google Scholar] [CrossRef]
- Zhu, M.; Li, X.; Lin, W.; Zeng, D.; Yang, P.; Ni, W.; Chen, Z.; Lin, B.; Lai, L.; Ouyang, Z.; et al. Microplastic Particles Detected in Fetal Cord Blood, Placenta, and Meconium: A Pilot Study of Nine Mother–Infant Pairs in South China. Toxics 2024, 12, 850. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Fernandes, A.J.S.; da Costa, F.M.; Duarte, A.C.; Rocha-Santos, T. Selection of microplastics by Nile Red staining increases environmental sample throughput by micro-Raman spectroscopy. Sci. Total. Environ. 2021, 783, 146979. [Google Scholar] [CrossRef]
- Zheng, X.; Feng, Q.; Chen, J.; Yan, J.; Li, X.; Guo, L. Quantification analysis of microplastics released from disposable polystyrene tableware with fluorescent polymer staining. Sci. Total. Environ. 2022, 864, 161155. [Google Scholar] [CrossRef]
- Singh, S.; Botvinnik, A.; Shahar, O.; Wolf, G.; Yakobi, C.; Saban, M.; Salama, A.; Lotan, A.; Lerer, B.; Lifschytz, T. Effect of psilocybin on marble burying in ICR mice: Role of 5-HT1A receptors and implications for the treatment of obsessive-compulsive disorder. Transl. Psychiatry 2023, 13, 164. [Google Scholar] [CrossRef]
- Horii-Hayashi, N.; Masuda, K.; Kato, T.; Kobayashi, K.; Inutsuka, A.; Nambu, M.F.; Tanaka, K.Z.; Inoue, K.; Nishi, M. Entrance-sealing behavior in the home cage: A defensive response to potential threats linked to the serotonergic system and manifestation of repetitive/stereotypic behavior in mice. Front. Behav. Neurosci. 2024, 17, 1289520. [Google Scholar] [CrossRef]
- Samra, A.I.; Kamel, A.S.; Abdallah, D.M.; El Fattah, M.A.A.; Ahmed, K.A.; El-Abhar, H.S. Preclinical Evidence for the Role of the Yin/Yang Angiotensin System Components in Autism Spectrum Disorder: A Therapeutic Target of Astaxanthin. Biomedicines 2023, 11, 3156. [Google Scholar] [CrossRef]
- Dang, R.; Wang, M.; Li, X.; Wang, H.; Liu, L.; Wu, Q.; Zhao, J.; Ji, P.; Zhong, L.; Licinio, J.; et al. Edaravone ameliorates epressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J. Neuroinflammation 2022, 19, 41. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Yun, J.Y.; Gregory, A.; Hogarth, P.; Hayflick, S.J. Brain MRI Pattern Recognition in Neurodegeneration With Brain Iron Accumulation. Front. Neurol. 2020, 11, 1024. [Google Scholar] [CrossRef]
- Xie, R.; Zhao, W.; Lowe, S.; Bentley, R.; Hu, G.; Mei, H.; Jiang, X.; Sun, C.; Wu, Y.; Liu, Y. Quercetin alleviates kainic acid-induced seizure by inhibiting the Nrf2-mediated ferroptosis pathway. Free. Radic. Biol. Med. 2022, 191, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Li, T.; Qiao, X.; Mei, H.; Hu, G.; Li, L.; Sun, C.; Cheng, C.; Cui, Y.; Hong, N.; et al. The Protective Role of E-64d in Hippocampal Excitotoxic Neuronal Injury Induced by Glutamate in HT22 Hippocampal Neuronal Cells. Neural Plast. 2021, 2021, 7174287. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Hu, B. Effects of gut microbiota on prostatic cancer: A two-sample Mendelian randomization study. Front. Microbiol. 2023, 14, 1250369. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, J.; Zhang, K.; Xie, R.; Xu, H.; Pan, Q.; Xiao, X.; Luo, Y.; Xu, S.; Xiao, W.; Wu, D.; et al. Investigating the Effects of Long-Term Fine Particulate Matter Exposure on Autism Spectrum Disorder Severity: Evidence from Multiple Analytical Approaches. Toxics 2025, 13, 922. https://doi.org/10.3390/toxics13110922
Dou J, Zhang K, Xie R, Xu H, Pan Q, Xiao X, Luo Y, Xu S, Xiao W, Wu D, et al. Investigating the Effects of Long-Term Fine Particulate Matter Exposure on Autism Spectrum Disorder Severity: Evidence from Multiple Analytical Approaches. Toxics. 2025; 13(11):922. https://doi.org/10.3390/toxics13110922
Chicago/Turabian StyleDou, Jianrui, Kaiyue Zhang, Ruijin Xie, Hua Xu, Qiyang Pan, Xue Xiao, Yufan Luo, Shengjie Xu, Wei Xiao, Dongqin Wu, and et al. 2025. "Investigating the Effects of Long-Term Fine Particulate Matter Exposure on Autism Spectrum Disorder Severity: Evidence from Multiple Analytical Approaches" Toxics 13, no. 11: 922. https://doi.org/10.3390/toxics13110922
APA StyleDou, J., Zhang, K., Xie, R., Xu, H., Pan, Q., Xiao, X., Luo, Y., Xu, S., Xiao, W., Wu, D., Wang, B., Zhang, L., Sun, C., & Liu, Y. (2025). Investigating the Effects of Long-Term Fine Particulate Matter Exposure on Autism Spectrum Disorder Severity: Evidence from Multiple Analytical Approaches. Toxics, 13(11), 922. https://doi.org/10.3390/toxics13110922







