Cellular and Molecular Mechanisms of Micro- and Nanoplastics Driving Adverse Human Health Effects
Abstract
1. Introduction
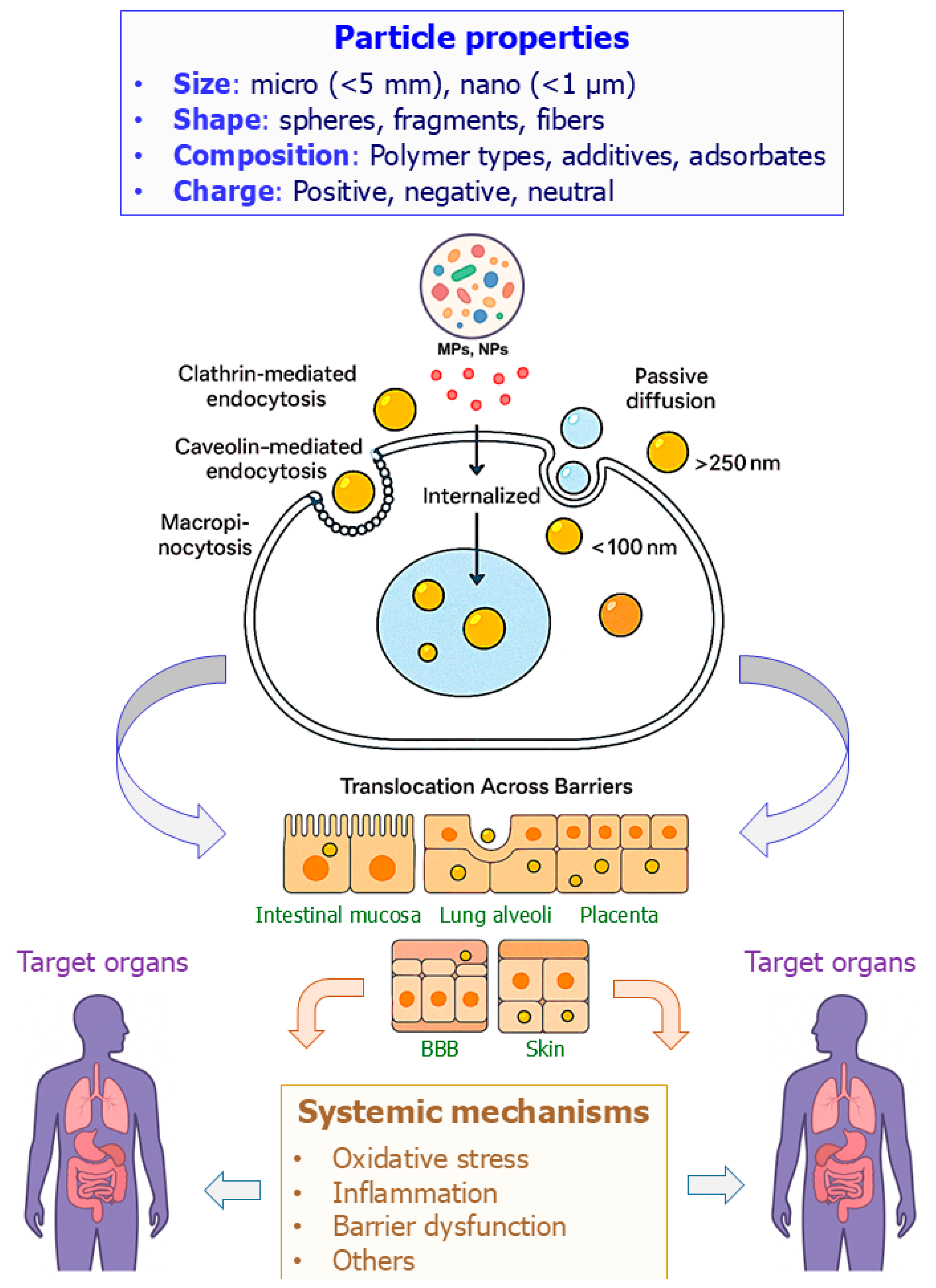
2. Physicochemical Properties of MNPs and Relevance to Toxicity
3. Cellular Uptake and Translocation Across Biological Barriers
4. Systemic (Non-Organ-Specific) Effects
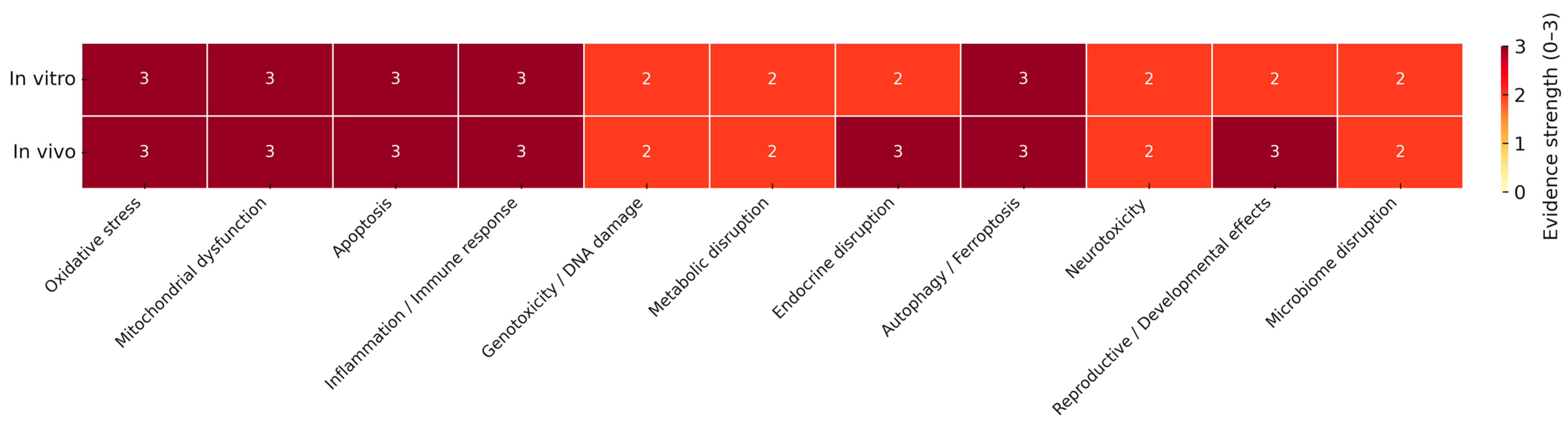
4.1. Oxidative Stress and Mitochondrial Dysfunction
4.2. Inflammatory Responses and Immune Activation
4.2.1. Inflammatory Responses
4.2.2. Immune Activation and Regulation
4.3. Genotoxicity
4.4. Endocrine Disruption
4.5. Autophagy, Apoptosis, and Other Regulated Cell Death Pathways
4.5.1. Apoptosis
4.5.2. Autophagy Dysregulation
4.5.3. Ferroptosis via Iron Dysregulation and Lipid Peroxidation Disruption
4.6. Microbiome Disruption
4.7. Omics Insights into MNPs Toxicity
5. Organ-Specific Effects
5.1. Gastrointestinal System
5.2. Respiratory System
5.3. Cardiovascular System
5.4. Nervous System
5.5. Reproductive System
6. Challenges and Methodological Limitations
6.1. Standardization and Reporting Challenges
6.2. Exposure Assessment and Detection Limitations
6.3. Mechanistic Understanding and Research Priorities
6.4. Relevance of Experimental Doses to Human Exposure
6.5. Criteria to Distinguish Polymer-Intrinsic vs. Additive/Adsorbate Effects
6.6. Methodological Overview of Analytical Detection Methods
7. Emerging Approaches and Future Directions
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; Dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, J.; Nishimura, N.; Shimada, Y. Toxicological interactions of microplastics/nanoplastics and environmental contaminants: Current knowledge and future perspectives. J. Hazard. Mater. 2021, 405, 123913. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, Y.; Xu, H. Trojan horse in the intestine: A review on the biotoxicity of microplastics combined environmental contaminants. J. Hazard. Mater. 2022, 439, 129652. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Hoh, E.; Hentschel, B.T.; Kaye, S. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: Implications for plastic marine debris. Environ. Sci. Technol. 2013, 47, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Walczak, A.P.; Kramer, E.; Hendriksen, P.J.; Tromp, P.; Helsper, J.P.; van der Zande, M.; Rietjens, I.M.; Bouwmeester, H. Translocation of differently sized and charged polystyrene nanoparticles in in vitro intestinal cell models of increasing complexity. Nanotoxicology 2015, 9, 453–461. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Microplastics in Drinking-Water; World Health Organization: Geneva, Switzerland, 2019; Available online: https://www.who.int/publications/i/item/9789241516198 (accessed on 14 October 2025).
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. Eur. Food Saf. Auth. 2016, 14, e04501. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.M.; Mota, C.; Ramos, H.; Faria, M.A.; Carvalho, M.; Ferreira, I.M.P.L.V.O. The neurotoxic threat of micro- and nanoplastics: Evidence from In Vitro and In Vivo models. Arch. Toxicol. 2025, 99, 3505–3525. [Google Scholar] [CrossRef] [PubMed]
- Yee, M.S.; Hii, L.W.; Looi, C.K.; Lim, W.M.; Wong, S.F.; Kok, Y.Y.; Tan, B.K.; Wong, C.Y.; Leong, C.O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.A.; Poland, C.A.; Duffin, R.; Al-Jamal, K.T.; Ali-Boucetta, H.; Nunes, A.; Byrne, F.; Prina-Mello, A.; Volkov, Y.; Li, S.; et al. Length-dependent retention of carbon nanotubes in the pleural space of mice initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am. J. Pathol. 2011, 178, 2587–2600. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef] [PubMed]
- Arezki, Y.; Delalande, F.; Schaeffer-Reiss, C.; Cianférani, S.; Rapp, M.; Lebeau, L.; Pons, F.; Ronzani, C. Surface charge influences protein corona, cell uptake and biological effects of carbon dots. Nanoscale 2022, 14, 14695–14710. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yang, Q.; Jiang, J.; Dalu, T.; Kadushkin, A.; Singh, J.; Fakhrullin, R.; Wang, F.; Cai, X.; Li, R. Coronas of micro/nano plastics: A key determinant in their risk assessments. Part. Fibre Toxicol. 2022, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Joksimovic, N.; Selakovic, D.; Jovicic, N.; Jankovic, N.; Pradeepkumar, P.; Eftekhari, A.; Rosic, G. Nanoplastics as an invisible threat to humans and the environment. J. Nanomater. 2022, 2022, 6707819. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Ahmad, S.; Guo, X.; Ullah, S.; Ullah, S.; Nabi, G.; Wanghe, K. A review of the endocrine disrupting effects of micro and nano plastic and their associated chemicals in mammals. Front. Endocrinol. 2023, 13, 1084236. [Google Scholar] [CrossRef] [PubMed]
- Casella, C.; Ballaz, S.J. Genotoxic and neurotoxic potential of intracellular nanoplastics: A review. J. Appl. Toxicol. 2024, 44, 1657–1678. [Google Scholar] [CrossRef]
- Ruan, Y.; Zhong, Z.; Liu, X.; Li, Z.; Li, J.; Sun, L.; Sen, H. Correlation between cellular uptake and cytotoxicity of polystyrene micro/nanoplastics in HeLa cells: A size-dependent matter. PLoS ONE 2023, 18, e0289473. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.L.; Wang, D.; Luan, Y.L.; Huang, S.N.; Liu, L.Y.; Guo, Y. A review on micro- and nanoplastics in humans: Implication for their translocation of barriers and potential health effects. Chemosphere 2024, 361, 142424. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, D.; Chandrasekaran, N. Journey of micronanoplastics with blood components. RSC Adv. 2023, 13, 31435–31459. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Simpson, K.; Brzezinski, M.; Watt, J.; Xu, W. Cellular response of keratinocytes to the entry and accumulation of nanoplastic particles. Part. Fibre Toxicol. 2024, 21, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, Z.; Wu, Y.; Zhu, S.; Su, J. Interactions of micro- and nanoplastics with biomolecules: From public health to protein corona effect and beyond. J. Phys. Chem. B 2025, 129, 5355–5374. [Google Scholar] [CrossRef] [PubMed]
- Basini, G.; Grolli, S.; Bertini, S.; Bussolati, S.; Berni, M.; Berni, P.; Ramoni, R.; Scaltriti, E.; Quintavalla, F.; Grasselli, F. Nanoplastics induced oxidative stress and VEGF production in aortic endothelial cells. Environ. Toxicol. Pharmacol. 2023, 104, 104294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, H.; Wang, Y.; Duan, Z.; Cui, W.; Shi, Y.; Qin, L. Influence of functional group modification on the toxicity of nanoplastics. Front. Mar. Sci. 2022, 8, 800782. [Google Scholar] [CrossRef]
- Das, A. The emerging role of microplastics in systemic toxicity: Involvement of reactive oxygen species (ROS). Sci. Total Environ. 2023, 895, 165076. [Google Scholar] [CrossRef] [PubMed]
- Kadac-Czapska, K.; Ośko, J.; Knez, E.; Grembecka, M. Microplastics and Oxidative Stress-Current Problems and Prospects. Antioxidants 2024, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, M.; Niu, S.; Shang, M.; Chang, X.; Sun, Z.; Zhang, R.; Shen, X.; Xue, Y. ROS and DRP1 interactions accelerate the mitochondrial injury induced by polystyrene nanoplastics in human liver HepG2 cells. Chem.-Biol. Interact. 2023, 379, 110502. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Z.; Xu, T.; Luo, D.; Chi, Q.; Zhang, Y.; Li, S. Polystyrene nanoplastics deteriorate LPS-modulated duodenal permeability and inflammation in mice via ROS drived-NF-κB/NLRP3 pathway. Chemosphere 2022, 307 Pt 1, 135662. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, F.; Sarker, D.B.; Jocelyn, J.A.; Sang, Q.A. Molecular and Cellular Effects of Microplastics and Nanoplastics: Focus on Inflammation and Senescence. Cells 2024, 13, 1788. [Google Scholar] [CrossRef] [PubMed]
- Panizzolo, M.; Martins, V.H.; Ghelli, F.; Squillacioti, G.; Bellisario, V.; Garzaro, G.; Bosio, D.; Colombi, N.; Bono, R.; Bergamaschi, E. Biomarkers of oxidative stress, inflammation, and genotoxicity to assess exposure to micro- and nanoplastics. A literature review. Ecotoxicol. Environ. Saf. 2023, 267, 115645. [Google Scholar] [CrossRef] [PubMed]
- Jayavel, S.; Govindaraju, B.; Michael, J.R.; Viswanathan, B. Impacts of micro and nanoplastics on human health. Bull. Natl. Res. Cent. 2024, 48, 110. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, H.; Wang, C.; Su, X.L.; Song, Y.; Wu, P.; Yang, Z.; Wong, M.H.; Cai, Z.; Zheng, C. Metabolomics Reveal Nanoplastic-Induced Mitochondrial Damage in Human Liver and Lung Cells. Environ. Sci. Technol. 2022, 56, 12483–12493. [Google Scholar] [CrossRef]
- Wang, K.; Du, Y.; Li, P.; Guan, C.; Zhou, M.; Wu, L.; Liu, Z.; Huang, Z. Nanoplastics causes heart aging/myocardial cell senescence through the Ca2+/mtDNA/cGAS-STING signaling cascade. J. Nanobiotechnol. 2024, 22, 96. [Google Scholar] [CrossRef] [PubMed]
- Kustra, A.; Zając, M.; Bednarczyk, P.; Maliszewska-Olejniczak, K. Exposure to polystyrene nanoparticles leads to dysfunction in DNA repair mechanisms in Caco-2 cells. Biol. Res. 2025, 58, 49. [Google Scholar] [CrossRef] [PubMed]
- Ojo, A.B.; Agbeye, O.D.; Ogwa, T.O.; Adedoyin, D.; Rotimi, D.E.; Ojo, O.A. Implications of plastic-derived endocrine disruptors on human health. Toxicol. Mech. Methods 2025, 35, 894–918. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yan, M.; Pan, C.; Liu, Z.; Sha, X.; Jiang, C.; Li, L.; Pan, M.; Li, D.; Han, X.; et al. Chronic exposure to polystyrene microplastics induced male reproductive toxicity and decreased testosterone levels via the LH-mediated LHR/cAMP/PKA/StAR pathway. Part. Fibre Toxicol. 2022, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Saha, U.; Kumari, P.; Ghosh, A.; Sinha, A.; Jena, S.; Kirti, A.; Gupta, A.; Choudhury, A.; Simnani, F.Z.; Nandi, A.; et al. Detrimental consequences of micropolymers associated plasticizers on endocrinal disruption. Mater. Today Bio 2024, 27, 101139. [Google Scholar] [CrossRef] [PubMed]
- Najahi, H.; Alessio, N.; Venditti, M.; Oliveri Conti, G.; Ferrante, M.; Di Bernardo, G.; Galderisi, U.; Minucci, S.; Banni, M. Impact of Environmental Microplastic Exposure on Caco-2 Cells: Unraveling Proliferation, Apoptosis, and Autophagy Activation. Int. J. Environ. Res. Public Health 2025, 22, 922. [Google Scholar] [CrossRef] [PubMed]
- Fanghella, F.; Pesce, M.; Franceschelli, S.; Panella, V.; Elsallabi, O.; Lupi, T.; Rizza, B.; Di Battista, M.G.; Bruno, A.; Ballerini, P.; et al. Biological modulation of autophagy by nanoplastics: A current overview. Int. J. Mol. Sci. 2025, 26, 7035. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, J.; Sun, J.; Zhu, Y.; Li, X.; Liu, X.; Zhang, X.; Liu, L.; Li, L.; Yang, J.; et al. Dust Fall Microplastics from a Megacity of China Inhibit Autophagy via the PI3K/Akt/mTOR Pathway. Environ. Health 2025, 3, 469–481. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yu, T.; Li, H.; Sun, Q.; Chen, M.; Lin, Y.; Dai, J.; Wang, W.; Li, Q.; Ju, S. Polystyrene nanoplastic exposure actives ferroptosis by oxidative stress-induced lipid peroxidation in porcine oocytes during maturation. J. Anim. Sci. Biotechnol. 2024, 15, 117. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Han, H.; Yang, H.; Xu, B.; Dai, W.; Liu, L.; He, T.; Du, X.; Pei, X. Nrf2-mediated ferroptosis of spermatogenic cells involved in male reproductive toxicity induced by polystyrene nanoplastics in mice. J. Zhejiang Univ.–Sci. B 2024, 25, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Z.; Wang, H. The role of NCOA4-mediated ferritinophagy in the ferroptosis of hepatocytes: A mechanistic viewpoint. Pathol. Res. Pract. 2025, 270, 155996. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Chen, R.; Wang, M.; Bai, R.; Cui, X.; Liu, Y.; Wu, C.; Chen, C. Perturbation of gut microbiota plays an important role in micro/nanoplastics-induced gut barrier dysfunction. Nanoscale 2021, 13, 8790–8806. [Google Scholar] [CrossRef] [PubMed]
- Bora, S.S.; Gogoi, R.; Sharma, M.R.; Anshu Borah, M.P.; Deka, P.; Bora, J.; Naorem, R.S.; Das, J.; Teli, A.B. Microplastics and human health: Unveiling the gut microbiome disruption and chronic disease risks. Front. Cell. Infect. Microbiol. 2024, 14, 1492759. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Arbab, S.; Tian, Y.; Liu, C.Q.; Chen, Y.; Qijie, L.; Khan, M.I.U.; Hassan, I.U.; Li, K. The gut microbiota-brain axis in neurological disorder. Front. Neurosci. 2023, 17, 1225875. [Google Scholar] [CrossRef] [PubMed]
- Dal Yöntem, F.; Aydoğan Ahbab, M. Mitochondria as a Target of Micro- and Nanoplastic Toxicity; Cambridge Prisms: Plastics; Cambridge University Press: Cambridge, UK, 2024. [Google Scholar] [CrossRef]
- Cortés, C.; Domenech, J.; Salazar, M.; Pastor, S.; Marcos, R.; Hernández, A. Nanoplastics as a potential environmental health factor: Effects of polystyrene nanoparticles on human intestinal epithelial Caco-2 cells. Environ. Sci. Nano 2020, 7, 272–285. [Google Scholar] [CrossRef]
- Jia, R.; Han, J.; Liu, X.; Li, K.; Lai, W.; Bian, L.; Yan, J.; Xi, Z. Exposure to Polypropylene Microplastics via Oral Ingestion Induces Colonic Apoptosis and Intestinal Barrier Damage through Oxidative Stress and Inflammation in Mice. Toxics 2023, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Schwiebs, A.; Solhaug, H.; Stenvik, J.; Nilsen, A.M.; Wagner, M.; Relja, B.; Radeke, H.H. Nanoplastics affect the inflammatory cytokine release by primary human monocytes and dendritic cells. Environ. Int. 2022, 163, 107173. [Google Scholar] [CrossRef] [PubMed]
- Bishop, B.; Webber, W.S.; Atif, S.M.; Ley, A.; Pankratz, K.A.; Kostelecky, R.; Colgan, S.P.; Dinarello, C.A.; Zhang, W.; Li, S. Micro- and nano-plastics induce inflammation and cell death in human cells. Front. Immunol. 2025, 16, 1528502. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, M.; Bao, T.T.; Lan, H. Long-term exposure to polystyrene microplastics triggers premature testicular aging. Part. Fibre Toxicol. 2023, 20, 35. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhou, C.; Xu, W.; Huang, Y.; Wang, W.; Ma, Z.; Huang, J.; Li, J.; Hu, L.; Xue, Y.; et al. The ovarian-related effects of polystyrene nanoplastics on human ovarian granulosa cells and female mice. Ecotoxicol. Environ. Saf. 2023, 257, 114941. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Wang, X.; Chen, W.; Wang, M.; Zhao, J.; Xu, Z.; Wang, R.; Mi, C.; Zheng, Z.; Zhang, H. Exposure to high dose of polystyrene nanoplastics causes trophoblast cell apoptosis and induces miscarriage. Part. Fibre Toxicol. 2024, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, P.; Doyle, D.; Mojarad, N.; Taherkhani, S.; Janzadeh, A.; Honardoost, M.; Gholami, M. Effects of micro- and nanoplastic exposure on macrophages: A review of molecular and cellular mechanisms. Toxicol. Mech. Methods 2025, 35, 823–846. [Google Scholar] [CrossRef] [PubMed]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, C.; Duan, X.; Liang, B.; Genbo Xu, E.; Huang, Z. Micro- and nanoplastics: A new cardiovascular risk factor? Environ. Int. 2023, 171, 107662. [Google Scholar] [CrossRef] [PubMed]
- Nihart, A.J.; Garcia, M.A.; El Hayek, E.; Liu, R.; Olewine, M.; Kingston, J.D.; Castillo, E.F.; Gullapalli, R.R.; Howard, T.; Bleske, B.; et al. Bioaccumulation of microplastics in decedent human brains. Nat. Med. 2025, 31, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Berber, A.A.; Akinci Kenanoğlu, N.; Nur Demi, R.Ş.; Aksoy, H. Genotoxic and cytotoxic effects of polystyrene nanoplastics on human lymphocytes: A comprehensive analysis. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2025, 902, 503850. [Google Scholar] [CrossRef] [PubMed]
- Płuciennik, K.; Sicińska, P.; Misztal, W.; Bukowska, B. Important Factors Affecting Induction of Cell Death, Oxidative Stress and DNA Damage by Nano- and Microplastic Particles In Vitro. Cells 2024, 13, 768. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Roursgaard, M. Exposure to nanoplastic particles and DNA damage in mammalian cells. Mutat. Res. Rev. Mutat. Res. 2023, 792, 108468. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Cheng, X.; Ma, Z.Q.; Wang, H.P.; Zhou, C.; Li, J.; Zhang, D.L.; Hu, L.L.; Cui, Y.F.; Huang, J.; et al. Polystyrene nanoplastics induce apoptosis, autophagy, and steroidogenesis disruption in granulosa cells to reduce oocyte quality and fertility by inhibiting the PI3K/AKT pathway in female mice. J. Nanobiotechnol. 2024, 22, 460. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Kamruzzaman, M.; Rima, U.K. Polystyrene Microplastics-Induced Thyroid Dysfunction in Mice: A Study of Gene Expression, Oxidative Stress, and Histopathological Changes. Vet. Med. Sci. 2025, 11, e70393. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Zhang, J.; Feng, G.; Miao, S.; Lu, R.; Tian, X.; Ye, Y. Antioxidant intervention against microplastic hazards. Antioxidants 2025, 14, 797. [Google Scholar] [CrossRef]
- Aloisi, M.; Poma, A.M.G. Nanoplastics as Gene and Epigenetic Modulators of Endocrine Functions: A Perspective. Int. J. Mol. Sci. 2025, 26, 2071. [Google Scholar] [CrossRef] [PubMed]
- Tyc, H.J.; Kłodnicka, K.; Teresińska, B.; Karpiński, R.; Flieger, J.; Baj, J. Micro- and Nanoplastics as Disruptors of the Endocrine System-A Review of the Threats and Consequences Associated with Plastic Exposure. Int. J. Mol. Sci. 2025, 26, 6156. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Wang, X.; Yang, L.; Zhang, J.; Wang, N.; Xu, F.; Hou, Y.; Zhang, H.; Zhang, L. Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology 2021, 449, 152665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Wang, Y.; Xue, Q.; Yao, J.; Chen, L.; Feng, S.; Shao, J.; Guo, Z.; Zhou, B.; Xie, J. Polystyrene microplastics disrupt kidney organoid development via oxidative stress and Bcl-2/Bax/caspase pathway. Chem. Biol. Interact. 2025, 419, 111642. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, Q.; Yang, N.; Xu, S. Polystyrene-microplastics and DEHP co-exposure induced DNA damage, cell cycle arrest and necroptosis of ovarian granulosa cells in mice by promoting ROS production. Sci. Total Environ. 2023, 871, 161962. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Sun, J.; Li, Z.; Zhang, W.; Liu, Z.; Li, C.; Peng, C.; Cui, G.; Shao, H.; Du, Z. Activation of pyroptosis and ferroptosis is involved in the hepatotoxicity induced by polystyrene microplastics in mice. Chemosphere 2022, 291 Pt 2, 132944. [Google Scholar] [CrossRef] [PubMed]
- Bu, W.; Cui, Y.; Jin, Y.; Wang, X.; Jiang, M.; Huang, R.; Egbobe, J.O.; Zhao, X.; Tang, J. Unmasking the Invisible Threat: Biological Impacts and Mechanisms of Polystyrene Nanoplastics on Cells. Toxics 2024, 12, 908. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, J.; Zhao, T.; Sun, M.; Xu, M.; Che, S.; Pan, Z.; Wu, C.; Shen, L. Polystyrenenanoplastics lead to ferroptosis in the lungs. J. Adv. Res. 2024, 56, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, X.; Xu, M.; Zheng, G.; Chen, J.; Li, S.; Cui, J.; Zhang, S. Implication of ferroptosis in hepatic toxicity upon single or combined exposure to polystyrene microplastics and cadmium. Environ. Pollut. (Barking Essex 1987) 2023, 334, 122250. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhao, Z.; Li, H.; Han, Y.; Li, H.; Cui, C.; Hu, Y.; Zhang, B. Nanoplastics exposure exacerbates Aβ plaque deposition in Alzheimer’s disease mice by inducing microglia pyroptosis. Ecotoxicol. Environ. Saf. 2025, 299, 118379. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, X.; Sun, X.; Huang, L.; Kuang, W.; Ou, J.; Zhang, J.; Zhang, Z.; Li, H.; Tang, H.; et al. Polystyrene microplastics induce anxiety via HRAS derived PERK-NF-κB pathway. Environ. Int. 2024, 185, 108543. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Li, T.; Chen, K.; Deng, X.; Zhang, Q.; Tang, H.; Shi, Z.; Zhu, T.; Zhu, J. PS-NPs Induced Neurotoxic Effects in SHSY-5Y Cells via Autophagy Activation and Mitochondrial Dysfunction. Brain Sci. 2022, 12, 952. [Google Scholar] [CrossRef] [PubMed]
- Demarquoy, J. Microplastics and microbiota: Unraveling the hidden environmental challenge. World J. Gastroenterol. 2024, 30, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xia, J.; Pan, Z.; Yang, J.; Wang, W.; Fu, Z. Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish. Environ. Pollut. (Barking Essex 1987) 2018, 235, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Gorain, B. Mechanistic insight of neurodegeneration due to micro/nano-plastic-induced gut dysbiosis. Arch. Toxicol. 2025, 99, 83–101. [Google Scholar] [CrossRef]
- Peng, Y.; Lu, J.; Fan, L.; Dong, W.; Jiang, M. Simulated gastrointestinal digestion of two different sources of biodegradable microplastics and the influence on gut microbiota. Food Chem. Toxicol. 2024, 185, 114474. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.C.; Li, R.; Lai, K.P.; Zhang, X.X. Biological exposure to microplastics and nanoplastics and plastic additives: Impairment of glycolipid metabolism and adverse effects on metabolic diseases. Environ. Sci. Pollut. Res. Int. 2024, 31, 60778–60791. [Google Scholar] [CrossRef]
- Chi, J.; Patterson, J.S.; Jin, Y.; Kim, K.J.; Lalime, N.; Hawley, D.; Lewis, F.; Li, L.; Wang, X.; Campen, M.J.; et al. Metabolic Reprogramming in Gut Microbiota Exposed to Polystyrene Microplastics. Biomedicines 2025, 13, 446. [Google Scholar] [CrossRef] [PubMed]
- Fackelmann, G.; Sommer, S. Microplastics and the gut microbiome: How chronically exposed species may suffer from gut dysbiosis. Mar. Pollut. Bull. 2019, 143, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Elisso, M.C.; Billè, B.; Cappello, T.; Maisano, M. Polystyrene micro- and nanoplastics (PS MNPs): A review of recent advances in the use of -omics in PS MNP toxicity studies on aquatic organisms. Fishes 2024, 9, 98. [Google Scholar] [CrossRef]
- Limonta, G.; Mancia, A.; Benkhalqui, A.; Bertolucci, C.; Abelli, L.; Fossi, M.C.; Panti, C. Microplastics induce transcriptional changes, immune response and behavioral alterations in adult zebrafish. Sci. Rep. 2019, 9, 15775. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, J.; Gao, M.; Wang, T.; Zhang, H. Mechanism of nano-plastics induced inflammation injury in vascular endothelial cells. J. Environ. Sci. 2025, 154, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Capolupo, M.; Sørensen, L.; Jayasena, K.D.R.; Booth, A.M.; Fabbri, E. Chemical composition and ecotoxicity of plastic and car tire rubber leachates to aquatic organisms. Water Res. 2020, 169, 115270. [Google Scholar] [CrossRef] [PubMed]
- Marycleopha, M.; Balarabe, B.Y.; Kumar, S.; Adjama, I. Exploring the impact of microplastics and nanoplastics on macromolecular structure and functions. J. Appl. Toxicol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Covello, C.; Di Vincenzo, F.; Cammarota, G.; Pizzoferrato, M. Micro(nano)plastics and Their Potential Impact on Human Gut Health: A Narrative Review. Curr. Issues Mol. Biol. 2024, 46, 2658–2677. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ladd, D.M.; Kaval, N.; Wang, H.S. Toxicity of long term exposure to low dose polystyrene microplastics and nanoplastics in human iPSC-derived cardiomyocytes. Food Chem. Toxicol. 2025, 202, 115489. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; You, H.; Tang, X.; Su, Y.; Peng, H.; Li, H.; Wei, Z.; Hua, J. Early-life exposure to polypropylene nanoplastics induces neurodevelopmental toxicity in mice and human iPSC-derived cerebral organoids. J. Nanobiotechnol. 2025, 23, 474. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, A.K.A.; Pires, K.S.N.; Cavalcante, I.H.; Cavalcante, I.C.L.; Santos, J.D.; Queiroz, M.I.C.; Leite, A.C.R.; Crispim, A.C.; da Rocha Junior, E.R.; Aquino, T.M.; et al. Polystyrene microplastics exposition on human placental explants induces time-dependent cytotoxicity, oxidative stress and metabolic alterations. Front. Endocrinol. 2024, 15, 1481014. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci. Total Environ. 2019, 684, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Djouina, M.; Loison, S.; Body-Malapel, M. Recent progress in intestinal toxicity of microplastics and nanoplastics: Systematic review of preclinical evidence. Microplastics 2024, 3, 217–233. [Google Scholar] [CrossRef]
- Lee, S.E.; Kim, D.Y.; Jeong, T.S.; Park, Y.S. Micro- and Nano-Plastic-Induced Adverse Health Effects on Lungs and Kidneys Linked to Oxidative Stress and Inflammation. Life 2025, 15, 392. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Goros, R.A.; Dong, Z.; Meng, X.; Li, G.; Chen, W.; Liu, S.; Ma, J.; Zuo, Y.Y. Microplastics and Nanoplastics Impair the Biophysical Function of Pulmonary Surfactant by Forming Heteroaggregates at the Alveolar-Capillary Interface. Environ. Sci. Technol. 2023, 57, 21050–21060. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Huang, J.; Sun, X.; Zhang, W.; Ma, H.; Yin, D.; Wang, Y.; Wang, J.; Yang, C.; Geng, Q. Dissection of the potential mechanism of polystyrene microplastic exposure on cardiomyocytes. Sci. Total Environ. 2025, 973, 179048. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Feng, Y.; Huang, Y.; Wang, B.; Shi, W.; Liang, B.; Li, Z.; Zhang, B.; Du, J.; Xiu, J.; et al. Polystyrene nanoplastics accelerate atherosclerosis: Unraveling the impact on smooth muscle cells through KIF15-mediated migration. Ecotoxicol. Environ. Saf. 2024, 284, 116983. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Begum, M.Y.; Chinglenthoiba, C.; Aran, K.R.; Panda, S.P.; Abomughaid, M.M.; Lakhanpal, S.; Avinash, D.; Jha, N.K.; Gupta, R. Deciphering the Neurotoxic Burden of Micro- and Nanoplastics: From Multi-model Experimental Evidence to Therapeutic Innovation. Mol. Neurobiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Camerano Spelta Rapini, C.; Di Berardino, C.; Peserico, A.; Capacchietti, G.; Barboni, B. Can Mammalian Reproductive Health Withstand Massive Exposure to Polystyrene Micro- and Nanoplastic Derivatives? A Systematic Review. Int. J. Mol. Sci. 2024, 25, 12166. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Q.; Yu, H.; Yang, L.; Sun, Y.; Xu, N.; Wang, N.; Lei, Z.; Hou, J.; Jin, Y.; et al. Polystyrene microplastics induce blood-testis barrier disruption regulated by the MAPK-Nrf2 signaling pathway in rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 47921–47931. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, L.; Liu, J.; Zheng, H.; Wu, X.; Liao, X. The ant that may well destroy a whole dam: A systematic review of the health implication of nanoplastics/microplastics through gut microbiota. Crit. Rev. Food Sci. Nutr. 2025, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Severino, A.; Tohumcu, E.; Tamai, L.; Dargenio, P.; Porcari, S.; Rondinella, D.; Venturini, I.; Maida, M.; Gasbarrini, A.; Cammarota, G.; et al. The microbiome-driven impact of western diet in the development of noncommunicable chronic disorders. Best Pract. Res. Clin. Gastroenterol. 2024, 72, 101923. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Fan, N.; Ma, S.X.; Cheng, X.; Yang, X.; Wang, G. Gut Microbiota Dysbiosis: Pathogenesis, Diseases, Prevention, and Therapy. MedComm 2025, 6, e70168. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lin, G.; Yin, Y.; Wu, Q.; Wang, Y.; Tang, N.; Qi, X. Impact of micro- and nanoplastics on gastrointestinal diseases: Recent advances. Eur. J. Intern. Med. 2025, 139, 106419. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, J.; Fu, R.; Zhang, P.; Chen, H.; Cao, H.; Jiang, Z.; Hong, Y.; Li, Y.; He, C.; et al. Molecular mechanism differences between nanoplastics and microplastics in colon toxicity: Nanoplastics induce ferroptosis-mediated immunogenic cell death, while microplastics cause cell metabolic reprogramming. J. Nanobiotechnol. 2025, 23, 505. [Google Scholar] [CrossRef] [PubMed]
- Zeyneloglu, C.; Babayev, H.; Ogulur, I.; Ardicli, S.; Pat, Y.; Yazici, D.; Zhao, B.; Chang, L.; Liu, X.; D’Avino, P.; et al. The epithelial barrier theory proposes a comprehensive explanation for the origins of allergic and other chronic noncommunicable diseases. FEBS Lett. 2025. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F.; Adcock, I.M. Multifaceted mechanisms in COPD: Inflammation, immunity, and tissue repair and destruction. Eur. Respir. J. 2008, 31, 1334–1356. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Bao, Q.; Zhang, C.; Zhong, Y.; Deng, M.; Huang, Y.; Ye, Z.; Jing, J. PVC nanoplastics impair cardiac function via lysosomal and mitochondrial dysfunction. Biochem. Biophys. Res. Commun. 2025, 762, 151736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, S.; Ge, Y.; Yin, L.; Pu, Y.; Gu, Z.; Chen, Z.; Liang, G. Unveiling the Heart’s Hidden Enemy: Dynamic Insights into Polystyrene Nanoplastic-Induced Cardiotoxicity Based on Cardiac Organoid-on-a-Chip. ACS Nano 2024, 18, 31569–31585. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Kong, Q.; Hua, J.; Chen, Q.; Wang, D. Cardiotoxicity of polystyrene nanoplastics and associated mechanism of myocardial cell injury in mice. Ecotoxicol. Environ. Saf. 2025, 290, 117712. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Tong, X.; Xue, F.; Qianru, C.; Xinyu, T.; Zhe, L.; Zhikun, B.; Shu, L. Polystyrene nanoplastics exacerbate lipopolysaccharide-induced myocardial fibrosis and autophagy in mice via ROS/TGF-β1/Smad. Toxicology 2022, 480, 153338. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, S.; Liu, Q.; Wei, J.; Jin, Y.; Wang, X.; Zhang, L. Polystyrene microplastics cause cardiac fibrosis by activating Wnt/β-catenin signaling pathway and promoting cardiomyocyte apoptosis in rats. Environ. Pollut. 2020, 265 Pt A, 115025. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yan, L.; Zhang, Y.; Liu, X.; Wei, Y.; Zhao, Y.; Li, K.; Shi, Y.; Liu, H.; Lai, W.; et al. Maternal exposure to nanopolystyrene induces neurotoxicity in offspring through P53-mediated ferritinophagy and ferroptosis in the rat hippocampus. J. Nanobiotechnol. 2024, 22, 651. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Nan, Y.; Xu, L.; Dai, A.; Orteg, R.M.M.; Ma, M.; Zeng, Y.; Li, J. Polystyrene nanoplastics exposure induces cognitive impairment in mice via induction of oxidative stress and ERK/MAPK-mediated neuronal cuproptosis. Part. Fibre Toxicol. 2025, 22, 13. [Google Scholar] [CrossRef] [PubMed]
- Suman, A.; Mahapatra, A.; Gupta, P.; Ray, S.S.; Singh, R.K. Polystyrene microplastics modulated bdnf expression triggering neurotoxicity via apoptotic pathway in zebrafish embryos. Comparative biochemistry and physiology. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2023, 271, 109699. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liang, B.; Li, Z.; Zhong, Y.; Wang, B.; Zhang, B.; Du, J.; Ye, R.; Xian, H.; Min, W.; et al. Polystyrene nanoplastic exposure induces excessive mitophagy by activating AMPK/ULK1 pathway in differentiated SH-SY5Y cells and dopaminergic neurons in vivo. Part. Fibre Toxicol. 2023, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Lafram, A.; Krami, M.; Akarid, K.; Laadraoui, J.; Roky, R. Effects of exposure to micro/nanoplastics of polystyrene on neuronal oxidative stress, neuroinflammation, and anxiety-like behavior in mice: A systematic review. Emerg. Contam. 2024, 11, 100442. [Google Scholar] [CrossRef]
- Budhwar, M.; Mehra, S.; Sharma, M.; Ahsan, A.U.; Chopra, M. Unveiling micro-nanoplastics (MNPs) induced developmental toxicity, transgenerational transport and associated signaling pathways. J. Hazard. Mater. Adv. 2025, 17, 100581. [Google Scholar] [CrossRef]
- He, Y.; Yin, R. The reproductive and transgenerational toxicity of microplastics and nanoplastics: A threat to mammalian fertility in both sexes. J. Appl. Toxicol. 2024, 44, 66–85. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Qin, Q.; Zhang, X.; Ma, J. Characteristics and adsorption behavior of typical microplastics in long-term accelerated weathering simulation. Environ. Sci. Process. Impacts 2024, 26, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Besseling, E.; Shim, W.J. Nanoplastics in the Aquatic Environment. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 325–340. [Google Scholar] [CrossRef]
- Gigault, J.; El Hadri, H.; Nguyen, B.; Grassl, B.; Rowenczyk, L.; Tufenkji, N.; Feng, S.; Wiesner, M. Nanoplastics are neither microplastics nor engineered nanoparticles. Nat. Nanotechnol. 2021, 16, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Pasti, L.; Chenet, T.; Rusanova, P.; Smaoui, S.; Aït-Kaddour, A.; Bono, G. Detection methods of micro and nanoplastics. Adv. Food Nutr. Res. 2023, 103, 175–227. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Kelkar, V.; Kumar, R.; Halden, R.U. Methods and challenges in the detection of microplastics and nanoplastics: A mini-review. Polym. Int. 2022, 71, 4–11. [Google Scholar] [CrossRef]
- Domenech, J.; Marcos, R. Pathways of human exposure to microplastics, and estimation of the total burden. Curr. Opin. Food Sci. 2021, 42, 144–151. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Dietary and Inhalation Exposure to Nano- and Microplastic Particles and Potential Implications for Human Health; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240054608 (accessed on 14 October 2025).
- Zarus, G.M.; Muianga, C.; Hunter, C.M.; Pappas, R.S. A review of data for quantifying human exposures to micro and nanoplastics and potential health risks. Sci. Total Environ. 2021, 756, 144010. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, T.; Casillas, G.; Zarus, G.M.; Barr, D.B. Systematic review of microplastics and nanoplastics in indoor and outdoor air: Identifying a framework and data needs for quantifying human inhalation exposures. J. Expo. Sci. Environ. Epidemiol. 2024, 34, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.; Dierkes, G.; Ternes, T.A.; Völker, C.; Wagner, M. Benchmarking the in Vitro Toxicity and Chemical Composition of Plastic Consumer Products. Environ. Sci. Technol. 2019, 53, 11467–11477. [Google Scholar] [CrossRef] [PubMed]
- Käppler, A.; Windrich, F.; Löder, M.G.; Malanin, M.; Fischer, D.; Labrenz, M.; Eichhorn, K.J.; Voit, B. Identification of microplastics by FTIR and Raman microscopy: A novel silicon filter substrate opens the important spectral range below 1300 cm−1 for FTIR transmission measurements. Anal. Bioanal. Chem. 2015, 407, 6791–6801. [Google Scholar] [CrossRef] [PubMed]
- Primpke, S.; Fischer, M.; Lorenz, C.; Gerdts, G.; Scholz-Böttcher, B.M. Comparison of pyrolysis gas chromatography/mass spectrometry and hyperspectral FTIR imaging spectroscopy for the analysis of microplastics. Anal. Bioanal. Chem. 2020, 412, 8283–8298. [Google Scholar] [CrossRef] [PubMed]
- Rauert, C.; Charlton, N.; Bagley, A.; Dunlop, S.A.; Symeonides, C.; Thomas, K.V. Assessing the Efficacy of Pyrolysis-Gas Chromatography-Mass Spectrometry for Nanoplastic and Microplastic Analysis in Human Blood. Environ. Sci. Technol. 2025, 59, 1984–1994. [Google Scholar] [CrossRef] [PubMed]
- Valido, I.H.; Fuentes-Cebrian, V.; Hernández, A.; Valiente, M.; López-Mesas, M. Validated method for polystyrene nanoplastic separation in aqueous matrices by asymmetric-flow field flow fraction coupled to MALS and UV-Vis detectors. Mikrochimica Acta 2023, 190, 285. [Google Scholar] [CrossRef] [PubMed]
- Loeschner, K.; Vidmar, J.; Hartmann, N.B.; Bienfait, A.M.; Velimirovic, M. Finding the tiny plastic needle in the haystack: How field flow fractionation can help to analyze nanoplastics in food. Anal. Bioanal. Chem. 2023, 415, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Nene, A.; Sadeghzade, S.; Viaroli, S.; Yang, W.; Uchenna, U.P.; Kandwal, A.; Liu, X.; Somani, P.; Galluzzi, M. Recent advances and future technologies in nano-microplastics detection. Environ. Sci. Eur. 2025, 37, 7. [Google Scholar] [CrossRef]
- Wang, L.; Ma, J.Q.; Song, L.J.; Qu, X.P.; Zhang, Y.; Fan, H.M.; Wang, C.; Zheng, L.L.; Gao, G.D.; Qu, Y.; et al. Comprehensive multi-omics, behavioral and morphological analysis of the hazards of nano-plastics in mice with internal carotid artery occlusion. Ecotoxicol. Environ. Saf. 2025, 289, 117711. [Google Scholar] [CrossRef] [PubMed]
- Garrido Gamarro, E.; Costanzo, V. Microplastics in Food Commodities—A Food Safety Review on Human Exposure Through Dietary Sources (Food Safety and Quality Series No. 18); Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Barrowclough, D.; Deere Birkbeck, C. Transforming the Global Plastics Economy: The Role of Economic Policies in the Global Governance of Plastic Pollution. Soc. Sci. 2022, 11, 26. [Google Scholar] [CrossRef]
- Nielsen, M.B.; Clausen, L.P.W.; Cronin, R.; Hansen, S.F.; Oturai, N.G.; Syberg, K. Unfolding the science behind policy initiatives targeting plastic pollution. Microplastics Nanoplastics 2023, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- OECD. Policy Scenarios for Eliminating Plastic Pollution by 2040. Organisation for Economic Co-operation and Development. 2024. Available online: https://www.oecd.org/en/publications/policy-scenarios-for-eliminating-plastic-pollution-by-2040_76400890-en.html (accessed on 14 October 2025).

| Mechanism | Key Features | Representative References |
|---|---|---|
| Oxidative stress and mitochondrial dysfunction | Mitochondrial membrane depolarization, ROS overproduction, ETC complex I/III disruption, ATP depletion, mtDNA damage | [28,29,30] |
| Inflammation | Activation of NF-κB, NLRP3 inflammasome, cytokine release (IL-1β, IL-6, TNF-α), TLR4 signaling, MAPK/PI3K/Akt pathways. | [31,32] |
| Immune activation | PRR engagement (e.g., TLRs), monocyte/dendritic cell skewing, lymphocyte depletion, neutrophilia, altered hematopoiesis and immune response, persistent low-grade inflammation. | [33,34] |
| Genotoxicity | DNA strand breaks, 8-OHdG formation, γ-H2AX, micronuclei, mtDNA leakage, suppression of DNA repair genes (OGG1, XRCC1, PARP1) | [35,36,37] |
| Endocrine disruption | Hormonal imbalance (estrogen, androgen, thyroid axes), receptor interference, altered gene expression, epigenetic changes, HPG axis disruption | [38,39,40] |
| Apoptosis | Intrinsic mitochondrial pathway activation, cytochrome c release, caspase-9/-3 activation, Bcl-2/Bax imbalance | [30,41] |
| Autophagy disruption | Impaired autophagic flux, LC3B/Beclin1 upregulation, p62 accumulation, lysosomal dysfunction, mTOR inhibition | [42,43] |
| Ferroptosis | Iron overload, GPX4 suppression, lipid ROS, ACSL4 and TfRC upregulation, ferritinophagy (NCOA4), glutathione depletion | [44,45,46] |
| Microbiome disruption | Gut dysbiosis, reduced microbial diversity, loss of beneficial bacteria, increased intestinal permeability, altered microbial metabolism, gut–brain axis disruption | [47,48,49] |
| Model | Particle Type and Size | Detection Method | Tested Concentration | Endpoints Measured | Major Findings | Limitations | Study (Ref.) |
|---|---|---|---|---|---|---|---|
| Human neuroblastoma SH-SY5Y cells | Polystyrene NPs (PS-NPs, 50 nm) | Not reported | 20, 50, 100, 200, and 500 mg/L (for 24 h) | Cell viability, LDH release, ROS, Ca2+, apoptosis, mitochondrial function, autophagy markers | PS-NPs induced oxidative stress, mitochondrial dysfunction, apoptosis (via caspase pathway), and autophagy activation; NAC mitigated effects | Single cell line, high concentrations, short exposure | [79] |
| Human hepatocellular carcinoma (HepG2) cells | PS-NPs, 21.5 ± 2.7 nm | Transmission electron microscopy (TEM) | 6.25, 12.5, 25, 50 μg/mL (24 h) | Viability, ATP, mitochondrial membrane potential) (MMP), ROS, mitochondrial fission proteins, apoptosis markers | Concentration-dependent cytotoxicity; mitochondrial damage via ROS and DRP1-dependent fission; apoptosis | Tumor-derived cell line, short-term exposure | [30] |
| Human colon adenocarcinoma (Caco-2) cells | PS-NPs, 0.04 to 0.09 μm (fluorescent & non-fluorescent) | TEM | 25, 50, 100, 125, 150, 175 and 200 μg/mL (for 24/48 h) | Cellular uptake, Cytotoxicity, ROS, genotoxicity (MN, Comet assay), DNA oxidative damage, stress-related gene expression | PS-MNPLs internalized; induced oxidative stress and genotoxicity | No concentration-response data; limited mechanistic insight | [51] |
| Primary human monocytes & monocyte-derived dendritic cells | PS, PMMA, PVC NPs (50, 100, 310 nm; irregular vs. spherical) | Attenuated total reflection Fourier-transform infrared spectroscopy (ATR-FTIR), py-GC–MS, | 30–300 particles/cell | Cytokine release (inflammation) (IL-6, IL-10, TNF, IL-12p70, IL-23) | PVC and irregular particles caused strongest cytokine responses; shape- and polymer-dependent effects | Donor variability, limited mechanistic data | [53] |
| Various human cells (THP-1 cells, PBMCs -peripheral blood mononuclear cells-, whole blood, T84 intestinal epithelial cells) | PP, PE, PS, PET MNPs (commercial and environmental water sources, mixed origins) | FTIR spectroscopy, SEM (scanning electron microscopy), XRD (X-ray diffraction), confocal imaging | 100 ng/mL to 1 mg/mL | IL-1β, IL-6 secretion, cell death, morphology | PET-rich MNPs caused strongest inflammation and cell death; authentic environmental particles highly reactive | Heterogeneous MNPs mixture; limited mechanistic resolution | [54] |
| Human ovarian granulosa-like tumor (KGN) cells | PS-NPs, fluorescent (20 nm) | TEM, SEM | 100 μg/mL (for 48 h) | Proliferation, apoptosis, ROS, Hippo signaling (MST1, LATS1, YAP1) | NPs internalized; induced ROS, apoptosis, Hippo pathway dysregulation; salidroside attenuated effects | Cancer-derived line, high exposure concentration | [56] |
| Normal human hepatic (L02) & lung (BEAS-2B) cells | PS-NPs, 80 nm | Fluorescent labeling and TEM, metabolomics | 0.006, 0.0125, 0.03125, 0.0625, 0.125, or 0.25 mg/mL (for 24 h) | Mitochondrial function, ROS, respiration, metabolomic changes | NPs internalized; mitochondrial dysfunction and oxidative stress, especially in L02 cells | Short exposure; polymer unspecified | [35] |
| Human peripheral lymphocytes | PS-NPs, 50 nm (45–68 nm) | TEM, SEM | 0.001, 0.01, 0.1, 1, 10, 100 μg/mL (for 24–48 h) | Cytotoxicity, genotoxicity (DNA damage) | Significant DNA damage and mitotic inhibition at most concentrations | No mechanistic data beyond genotoxicity | [62] |
| Human iPSC-derived kidney organoids | PS-MPs, various (1 μm) | TEM, FTIR | 1.25, 2.5, 5, 10, 20 μg/mL (for 48 h) | Differentiation, apoptosis, mitochondrial function, signaling pathways | PS-MPs impaired nephron development, induced mitochondrial apoptosis via Bcl-2/Bax/caspase pathway | Developmental model only; no chronic exposure | [71] |
| Human iPSC-derived cardiomyocytes (hiPSC-CMs) | PS MNPs (1 μm and 0.05 μm) | SCIOS Dual-Beam SEM, Raman microscopy (image and spectra) | 0.1, 1, 10 and 1000 μg/L (for 7, 10 15 and 20 days) | Cell viability, contractility, Ca2+ transients, mitochondrial function (MMP, mitochondrial ROS) | Dose-dependent reduction in contractility, ROS increase, mitochondrial dysfunction | Only PS tested; long-term but low-dose limited | [93] |
| Human cerebral organoids | PP-NPs (100 nm) | TEM, Laser Granulometer (LS230) | 0, 10, 25, and 50 µg/mL (for 30 days) | Growth, neuronal differentiation, gene expression | Impaired neurogenesis and differentiation; altered neuroactive ligand–receptor pathways | No particle quantification in organoids; limited dose metrics | [94] |
| Human placental chorionic villi explants | PS-MPs, 5 μm | Raman spectroscopy (XploRA Raman spectrometry) 1H NMR, biochemical assays | 1, 10, 100 μg/mL (for 24 h) | Cytotoxicity, oxidative stress, antioxidant activity, metabolomics | Dose-dependent cytotoxicity and oxidative stress; disrupted antioxidant balance and metabolism | Ex vivo explant model; acute exposure | [95] |
| Human PBMCs, HMC-1, RAW 264.7 (murine) | PP microplastics, 20–200 μm | SEM, field emission scanning electron microscope (FE-SEM) | 0, 500 μg/L. 10, 50, 100, 500, and 1000 μ g/mL (for RAW cells) (for 48 h) | Cytotoxicity, cytokine secretion, ROS, proliferation | Small PP particles at high doses induced cytokine/histamine release; immune activation | Large particle size; heterogeneous preparation | [96] |
| Porcine aortic endothelial cells (AOC) | Polystyrene NPs (PS-NPs, 100 nm) | Fluorescence co-localization | 5, 25 and 75 µg/mL (for 48 h) | Cell growth, ROS, antioxidant defense, VEGF production, metabolic activity | NPs internalized by AOC; increased VEGF, ROS, and metabolic activity; disrupted redox status | Polymer type and concentration not specified; limited mechanistic insight | [26] |
| Rat ovarian granulosa cells (GCs) from Wistar rats | PS-MPs, 0.5 μm | SEM, FTIR | 1, 5, 25 μg/mL (for 90 days) | ROS, apoptosis, anti-Müllerian hormone (AMH), fibrosis markers | PS-MPs entered GCs, caused ROS, apoptosis, fibrosis via Wnt/β-catenin pathway; NAC reversed effects | Rat origin; in vitro data linked to in vivo exposure | [70] |
| Murine macrophage Raw 264.7 cells | Mixed microplastics (MPs, various polymers from dust fall, from 178.54 to 726.27 μm) | Py-GC-MS, Western blot Field-emission scanning electron microscopy (FESEM) | 100 μg/g of the dust fall MP (DF-O)/MP samples (for 24 h) | Cytotoxicity, autophagy, apoptosis signaling, cytokines | MPs inhibited autophagy (↓LC3B, p-Akt/mTOR) and induced apoptosis (↑Bax/Bcl-2, Caspase-3) | Complex real-world MP mixture; unclear concentration | [43] |
| Gut bacterial cultures (E. coli, L. rhamnosus, Erysipelotrichales) from C57BL/6 mouse fecal samples | PS-MPs, 1 μm | Not reported | 0, 10, 20, 50, 100, 500 μg/mL (for 24 h) | Growth rate, metabolomics, microbiota composition | MPs reduced bacterial growth, altered metabolism (sugar/sulfur pathways) | Bacterial model; relevance to human microbiota indirect. No particle characterization | [85] |
| Organ/System | Key Mechanisms | Functional Outcomes | Representative References |
|---|---|---|---|
| Gastrointestinal | Oxidative stress, ER stress, mitochondrial dysfunction, barrier disruption, NLRP3 inflammasome activation, microbiota dysbiosis | Intestinal permeability, endotoxemia, inflammation, altered microbial metabolism | [31,92,97] |
| Respiratory | Oxidative stress, ferroptosis, mitochondrial damage, ER stress, autophagy impairment, NLRP3 inflammasome activation, barrier dysfunction | Chronic inflammation, lung fibrosis, epithelial remodeling | [75,98,99] |
| Cardiovascular | Mitochondrial ROS, NF-κB and TGF-β1/Smad activation, ER stress, autophagy inhibition, calcium signaling disruption, endothelial injury, Wnt/β-catenin signaling | Myocardial fibrosis, arrhythmias, endothelial dysfunction, atherosclerosis | [93,100,101] |
| Nervous System | BBB disruption, oxidative stress, mitochondrial dysfunction, neuroinflammation (via NF-κB), microglial activation, synaptic gene downregulation, impaired neuroplasticity | Neuroinflammation, cognitive impairment, anxiety, memory loss, impaired neurodevelopment | [11,94,102] |
| Reproductive System | Oxidative stress, apoptosis, ferroptosis, necroptosis, hormone synthesis disruption, placental barrier dysfunction, epigenetic alteration | Infertility, placental damage, impaired sperm quality, ovarian dysfunction, gametogenesis failure, altered hormone signaling transgenerational effects | [72,95,103,104] |
| Biological Matrix/Model | Particle Type and Size | Detection Method | Endpoints Measured | Major Findings | Limitations | Study (Ref.) |
|---|---|---|---|---|---|---|
| Human lung tissue (autopsy samples) | Polymeric particles (<5.5 µm) and fibers (8.1–16.8 µm); mainly polyethylene, polypropylene | Microscopy and polymer characterization | Presence and morphology of MNPs | Microplastics (MPs) found in human lungs, indicating inhalation exposure; particle heterogeneity may relate to respiratory effects | Small sample size; no health outcome data; contamination control challenges | [1] |
| Human whole blood (22 volunteers) | ≥700 nm; polyethylene terephthalate, polyethylene, styrene polymers, PMMA | Double-shot pyrolysis-GC/MS | Quantification of polymeric particles | First evidence of plastic particles in human blood; suggests systemic uptake | Small sample; no temporal exposure data; unknown biological fate | [2] |
| Human placenta (6 donors) | 5–10 µm; polypropylene and pigmented microplastics | Raman microspectroscopy | Presence, morphology, chemical composition | MPs found in maternal, fetal, and membrane sides of placenta; evidence of translocation potential | Limited sample size; contamination risk; no exposure correlation | [3] |
| Human feces (8 volunteers, Europe & Asia) | 50–500 µm; 9 polymer types including polypropylene, PET | FTIR microspectroscopy | Presence and abundance of MPs | All samples positive for MPs; ingestion likely from food, water, and air | Very small cohort; single sample per person; no link to exposure route | [4] |
| Human kidney, liver, and brain (postmortem tissues) | Polyethylene-dominant MNPs, nanoscale shard-like fragments | Pyrolysis-GC/MS, ATR-FTIR, electron microscopy with EDS | Tissue concentrations, morphology, composition | Confirmed MNP presence in deep tissues; higher polyethylene in brain; possible link with dementia | Cross-sectional design; postmortem contamination control; mechanistic uncertainty | [46] |
| Species/Model | Particle Type and Size | Dose and Route | Duration | Organ/System Studied | Endpoints Measured | Major Findings | Limitations | Study (Ref.) |
|---|---|---|---|---|---|---|---|---|
| Mouse (C57BL/6) | Polystyrene nanoplastics (PS-NPs), 100 nm | Intraperitoneal injection (5 μg/g); with/without LPS | Every other day for 2 weeks | Intestine (duodenum) | Duodenal structure, oxidative stress (ROS), NF-κB/NLRP3 activation, inflammatory cytokines, tight junction proteins | PS-NPs aggravated LPS-induced duodenal inflammation and permeability via ROS-driven NF-κB/NLRP3 activation; QNZ mitigated effects | Lack of full dose/duration details; limited to duodenal outcomes | [31] |
| Mouse (C57BL/6) | Polypropylene microplastics (PP-MPs), 8 and 70 μm | Oral gavage, 0.1–10 mg/mL 1, 10, 100 mg/kg/day | 28 days | Colon | Histopathology, redox balance, cytokines, tight junctions, apoptosis markers | PP-MPs caused oxidative stress, inflammation, apoptosis, barrier disruption via TLR4/NF-κB activation | No systemic toxicity assessment; no recovery or chronic phase | [52] |
| Female mouse (Balb/c) | Polystyrene nanoplastics (PS-NPs), 15 and 38 nm | Oral exposure, 1 mg/day | 5 weeks | Ovary | Fertility rate, ovarian histology, apoptosis, ROS, Hippo signaling proteins | PS-NPs accumulated in ovaries, disrupted granulosa cells via ROS/Hippo signaling, reducing fertility; salidroside mitigated effects | No long-term reproductive outcomes measured | [56] |
| Pregnant mouse C57BL/6 (miscarriage model) | Polystyrene nanoplastics (PS-NPs), 50 nm | Oral, 50–100 mg/kg | GD 5 to GD18 | Placenta and trophoblast | Oxidative stress, apoptosis, Bcl-2/caspase pathway, miscarriage incidence | PS-NPs induced miscarriage via mitochondrial apoptosis signaling; Bcl-2 overexpression mitigated effects | Human relevance inferred; unclear environmental exposure relevance | [57] |
| Female mouse (Kun Ming, KM) | Polystyrene nanoplastics (PS-NPs), 25 nm | Oral, 50 mg/kg (chronic exposure) | 42 days | Ovary (granulosa cells, oocytes) | RNA-seq, PI3K-AKT, autophagy, apoptosis, oocyte quality | PS-NPs deactivated PI3K-AKT, triggered granulosa cell autophagy/apoptosis, reducing oocyte quality; estradiol reversed effects | No dose–response analysis; no fertility rate data | [65] |
| Male BALB/c mouse | Polystyrene microplastics (PS-MPs), 0.5, 4, 10 μm | Drinking water, 100 and 1000 μg/L | 180 days | Testis | Hormones (T, LH, FSH), sperm quality, histology, StAR/LHR pathway | Chronic PS-MP exposure reduced testosterone and sperm quality via LHR/cAMP/PKA/StAR suppression | Only male model; environmental relevance of dose uncertain | [39] |
| Male Swiss albino mouse | Polystyrene microplastics (PS-MPs), 5 μm | Oral gavage, 0.1 and 0.2 mg | 28 days | Thyroid | Hormones (TSH, T3, T4), oxidative stress, TSHR & TPO expression, histopathology | PS-MPs disrupted thyroid hormone balance and follicular structure via oxidative stress and gene downregulation | Only two doses; lacks systemic endocrine profiling | [66] |
| Female Wistar rat | Polystyrene microplastics (PS-MPs), 0.5 μm | Oral, 0.015, 0.15, 1.5 mg/day | 90 days | Ovary | Follicle count, AMH, fibrosis markers, Wnt/β-catenin, oxidative stress | PS-MPs induced fibrosis via Wnt/β-catenin and ROS-driven granulosa apoptosis, reducing ovarian reserve | Lacks fertility outcome measures; limited to one particle type | [70] |
| Female mouse | Polystyrene microplastics (PS-MPs, 5–10 μm), 100 mg/L + DEHP 200 mg/kg | Oral (single and co-exposure), 100 mg/L | 35 days | Ovary (granulosa cells) | ROS, DNA damage, Hippo & CNR1/CRBN/YY1/CYP2E1 signaling, necroptosis | Co-exposure with DEHP caused oxidative stress-mediated DNA damage, necroptosis, ovarian injury; inhibitors (AM251, DAS) reversed toxicity | Co-exposure model limits attribution of effects to MPs alone | [72] |
| Adult male zebrafish | Polystyrene microplastics (PS-MPs), 0.5 and 50 μm | Waterborne, 100 and 1000 μg/L | 14 days | Gut microbiota | Mucus production, microbial diversity, cytokines (IL-1α, IL-1β, IFN) | PS-MPs altered gut microbiota, increased inflammation and mucus production | Aquatic model limits mammalian relevance | [81] |
| Male C57BL/6 mouse | Polystyrene nanoplastics (PS-NPs), unspecified nm | Oral, 30, 60, 100 mg/L | 42 days | Heart | Echocardiography, blood pressure, fibrosis, TNF-α/NF-κB, P38/MAPK | PS-NPs caused ventricular dilation, fibrosis, oxidative stress, and cardiac dysfunction | Only male mice; lacks recovery or reversibility data | [114] |
| Male C57BL/6 Mouse | Polystyrene nanoplastics (PS-NPs), 100 nm + LPS | i.p., 5 μg/g | Every other day for 2 weeks | Heart | ROS, fibrosis markers, autophagy (AMPK/mTOR/ULK1), TGF-β/Smad | PS-NPs aggravated LPS-induced myocardial fibrosis and autophagy via ROS/TGF-β1/Smad | Lack of chronic exposure data; mechanism inferred from acute effects | [115] |
| Male Wistar rat | Polystyrene microplastics (PS-MPs), 0.5 μm | Oral, 0.5, 5, 50 mg/L | 90 days | Heart | Serum CK-MB, troponin I, histology, Wnt/β-catenin | PS-MPs induced oxidative stress, myocardial apoptosis, fibrosis via Wnt/β-catenin | Limited mechanistic validation; male-only study | [116] |
| Male C57BL/6J mouse | Polystyrene nanoplastics (PS-NPs), 50 nm | Oral, 250 mg/kg/day | 28 days | Brain (dopaminergic neurons) | Mitochondrial function, mitophagy (AMPK/ULK1), behavior, motor tests | PS-NPs caused PD-like neurodegeneration via excessive mitophagy; melatonin mitigated effects | High dose; short duration; only male model | [120] |
| Male Wistar rat | Polystyrene microplastics (PS-MPs), 500 nm | Oral, 0.015, 0.15, 1.5 mg/day | 90 days | Testis | Sperm parameters, blood-testis barrier (BTB) integrity, oxidative stress, p38 MAPK/Nrf2 pathway | PS-MPs impaired spermatogenesis and BTB via MAPK-Nrf2-mediated oxidative stress | No recovery data; species differences untested | [104] |
| Exposure Route | Estimated Human Exposure Range (Environmental, Real-World) | Typical Experimental Doses (Mechanistic Studies) | Notes on Relevance |
|---|---|---|---|
| Ingestion | ~103–105 particles/day (≈μg–mg/day, depending on size/density) 1 | 106–109 particles/mL in vitro media; 106–108 particles/kg/day in vivo | Many in vitro doses exceed environmental estimates by several orders of magnitude; high doses often used to elicit measurable mechanistic effects. |
| Inhalation | ~102–104 particles/day (higher indoors; infants may have greater exposure) 1,2 | 105–107 particles/mL (cell culture); acute in vivo exposures equivalent to >106 particles/kg/day | Indoor air exposures generally higher than outdoor; experimental doses typically far above environmental levels. |
| Dermal | Quantitative estimates scarce; likely low under normal conditions 1 | Limited studies; often high-dose topical or subcutaneous administration | Dermal uptake under environmental conditions remains poorly characterized; experimental designs may not reflect real-world exposure. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, A.F.; Lacasaña, M.; Tsatsakis, A.M.; Docea, A.O. Cellular and Molecular Mechanisms of Micro- and Nanoplastics Driving Adverse Human Health Effects. Toxics 2025, 13, 921. https://doi.org/10.3390/toxics13110921
Hernández AF, Lacasaña M, Tsatsakis AM, Docea AO. Cellular and Molecular Mechanisms of Micro- and Nanoplastics Driving Adverse Human Health Effects. Toxics. 2025; 13(11):921. https://doi.org/10.3390/toxics13110921
Chicago/Turabian StyleHernández, Antonio F., Marina Lacasaña, Aristidis M. Tsatsakis, and Anca Oana Docea. 2025. "Cellular and Molecular Mechanisms of Micro- and Nanoplastics Driving Adverse Human Health Effects" Toxics 13, no. 11: 921. https://doi.org/10.3390/toxics13110921
APA StyleHernández, A. F., Lacasaña, M., Tsatsakis, A. M., & Docea, A. O. (2025). Cellular and Molecular Mechanisms of Micro- and Nanoplastics Driving Adverse Human Health Effects. Toxics, 13(11), 921. https://doi.org/10.3390/toxics13110921








