Prenatal Exposure to Imidacloprid Affects Cognition and Anxiety-Related Behaviors in Male and Female CD-1 Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Dosing
2.2. Barnes Maze (Spatial Learning)
2.3. T-Maze (Procedural Learning)
2.4. Elevated Plus Maze (Anxiety-like Behavior)
2.5. Light-Dark Transition Test (Anxiety-like Behavior)
2.6. Tail Suspension Test (Depression-like Behavior)
2.7. Y-Maze (Working Memory)
2.8. mRNA Expression of α4 and α7 Receptor Subunits of nAChRs in the Brain
2.9. Statistics
3. Results
3.1. IMI Exposure Impairs Spatial Learning in Both Males and Females
3.2. Both IMI-Exposed Males and Females Displayed Impairments in Procedural Learning
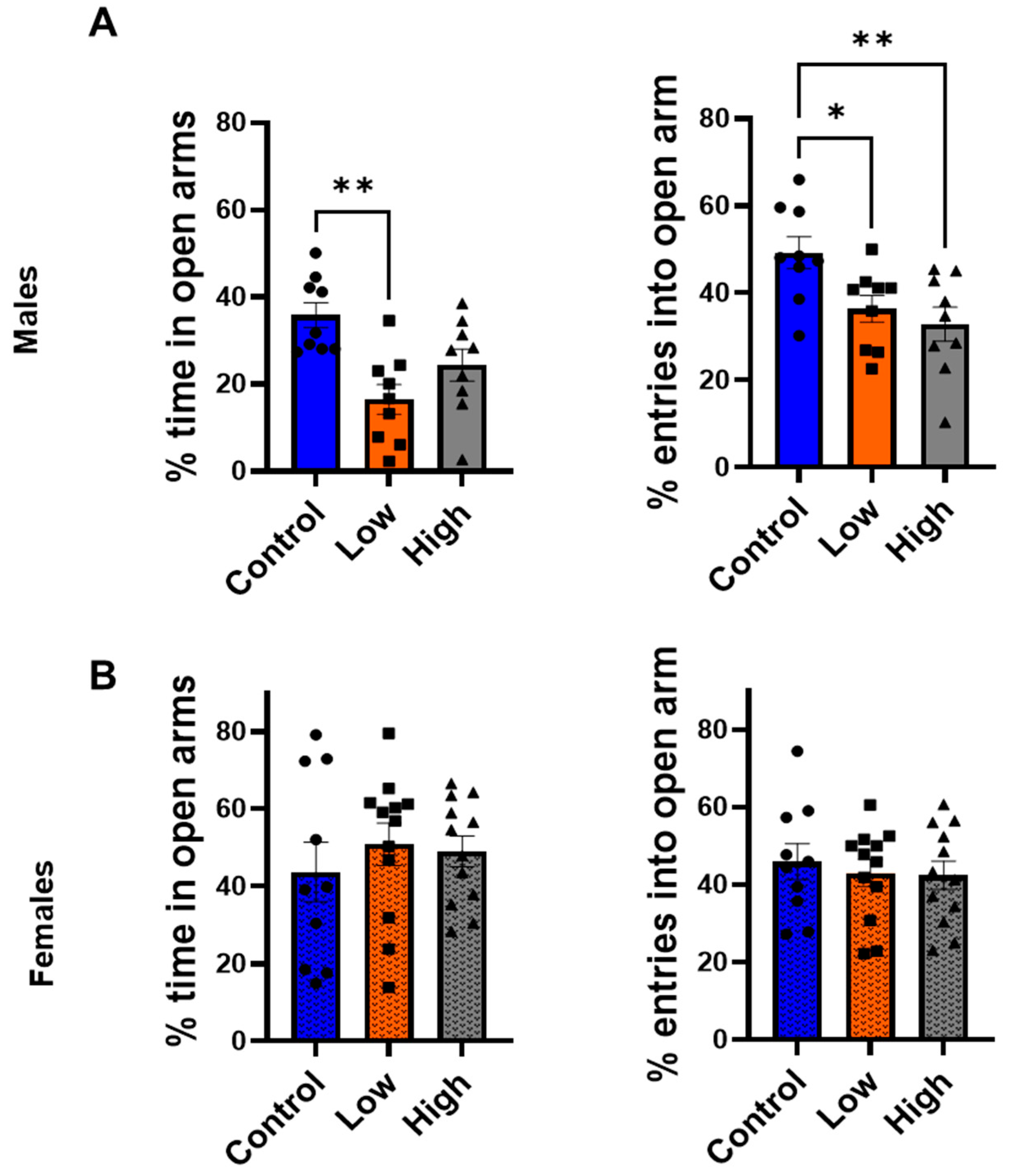
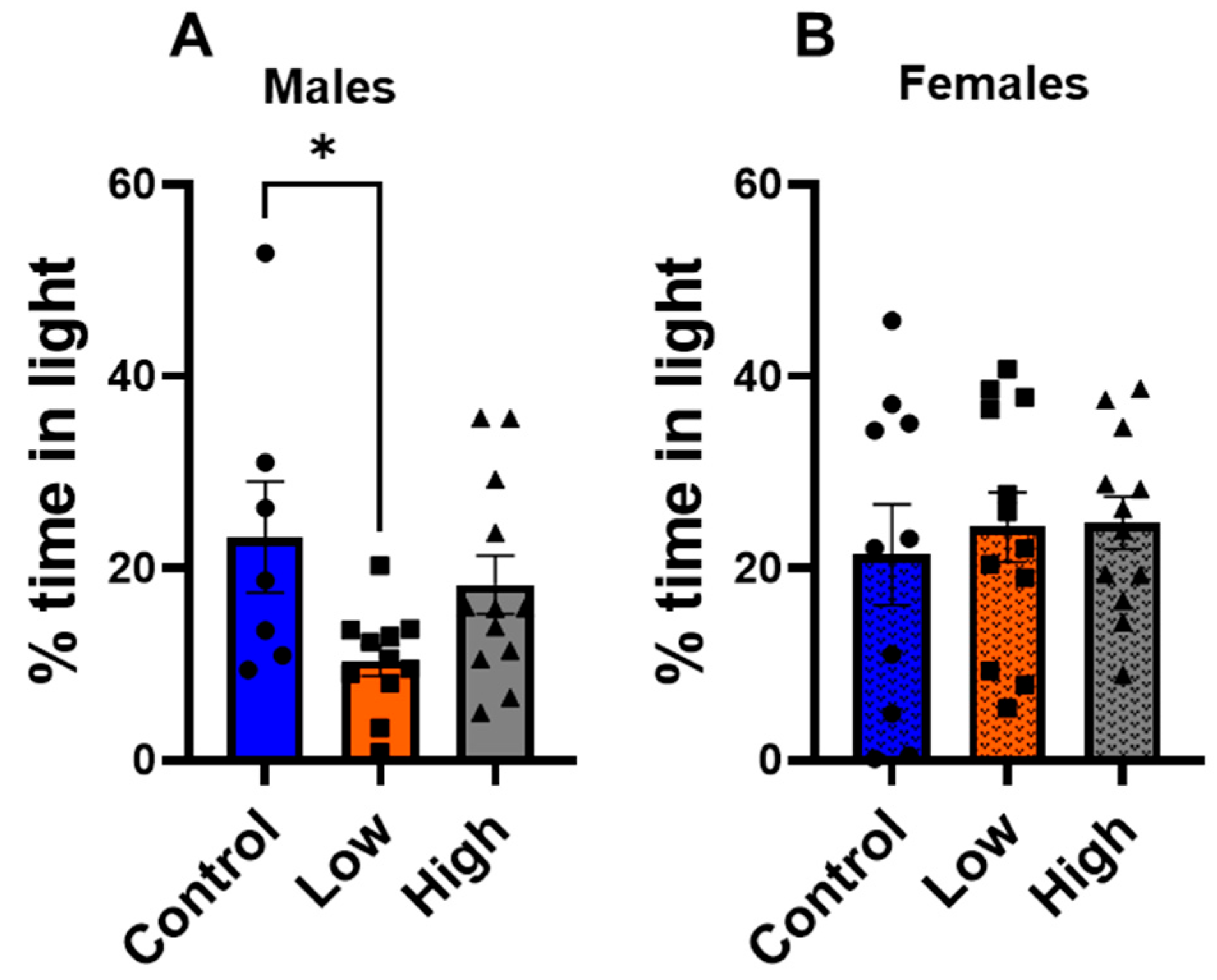
3.3. Prenatal IMI Increased Anxiety-like Behavior in Males but Not Females
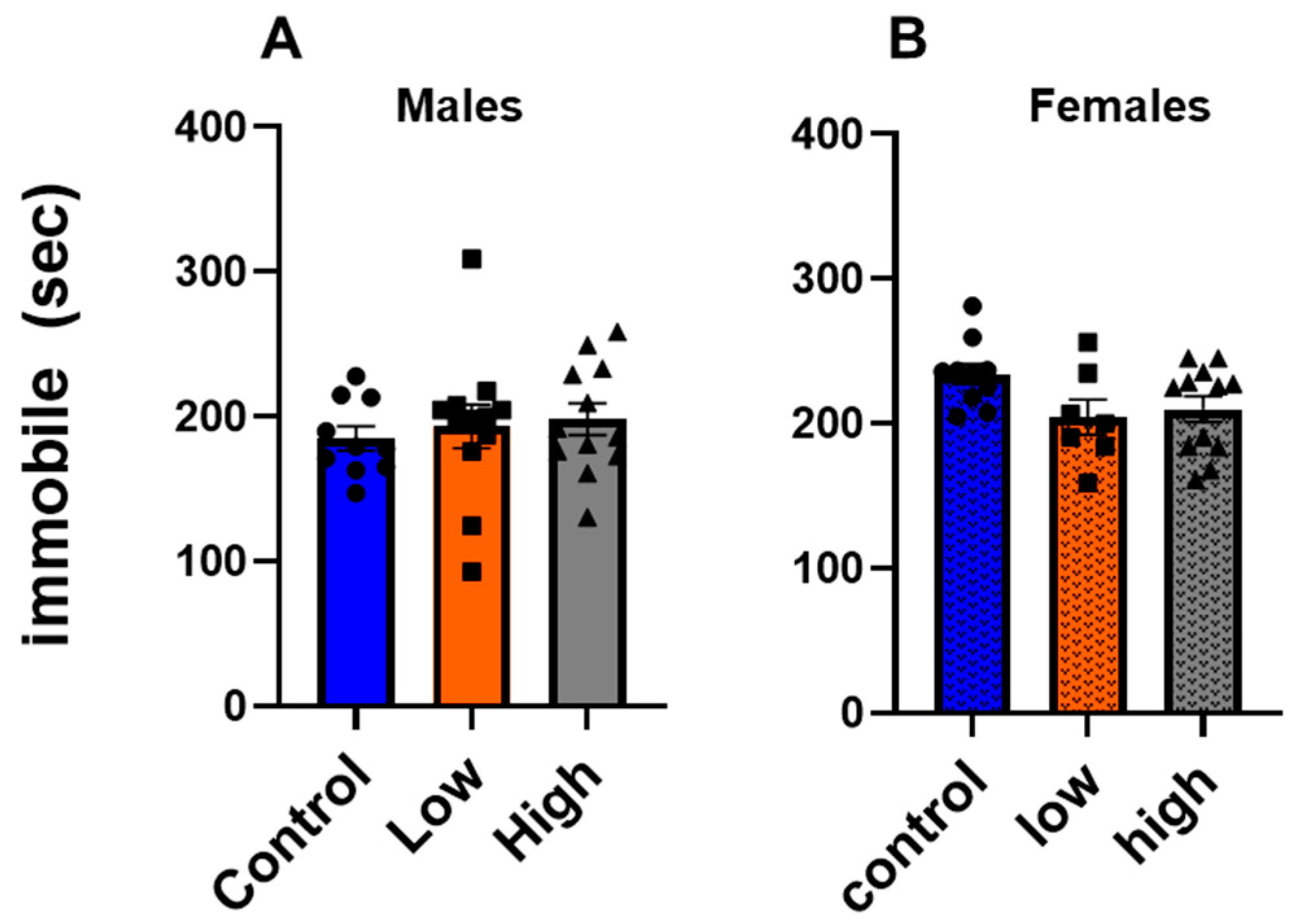
3.4. Prenatal IMI Did Not Affect Depression-like Behavior in Either Sex
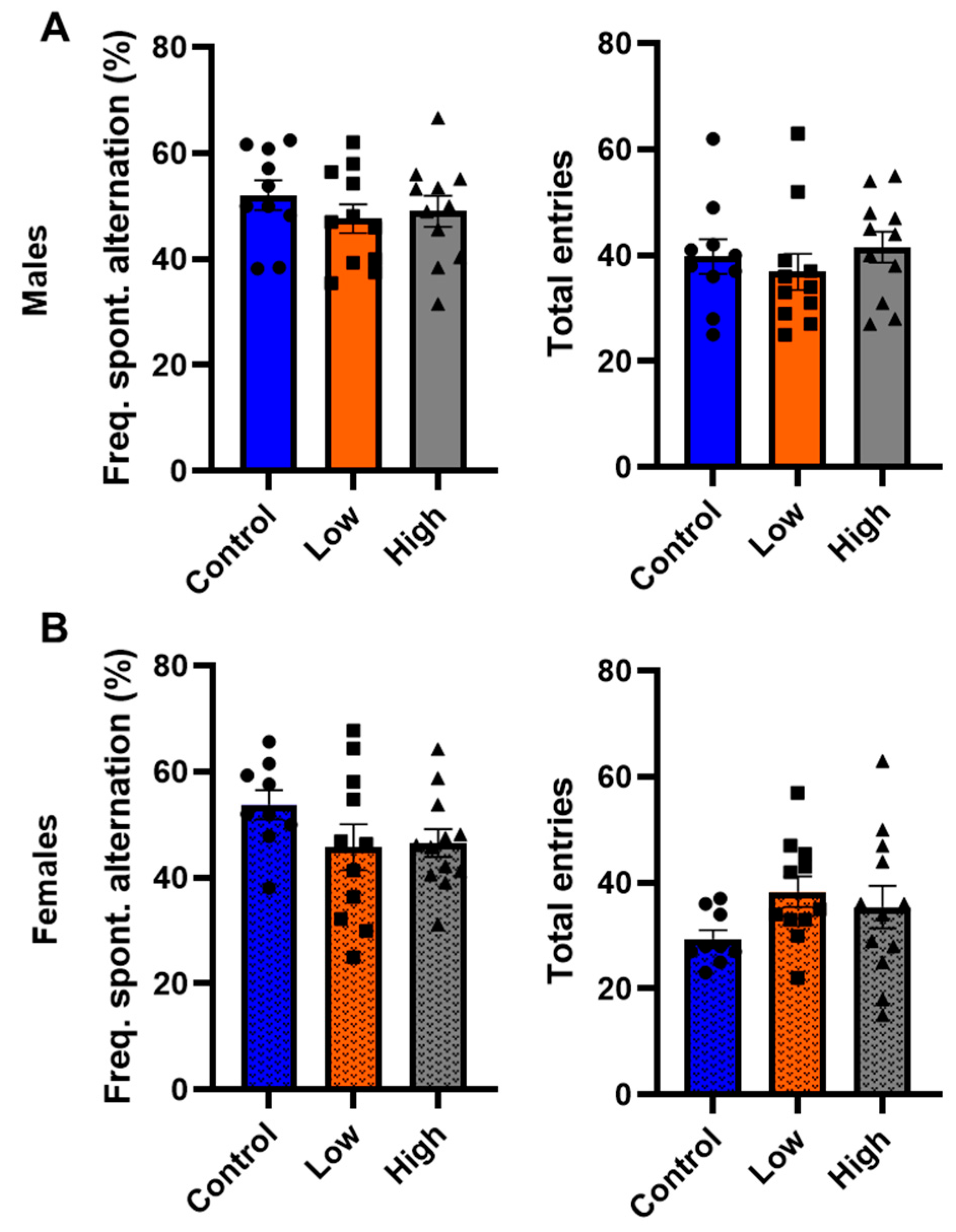
3.5. Prenatal IMI Did Not Influence Working Memory in Either Sex
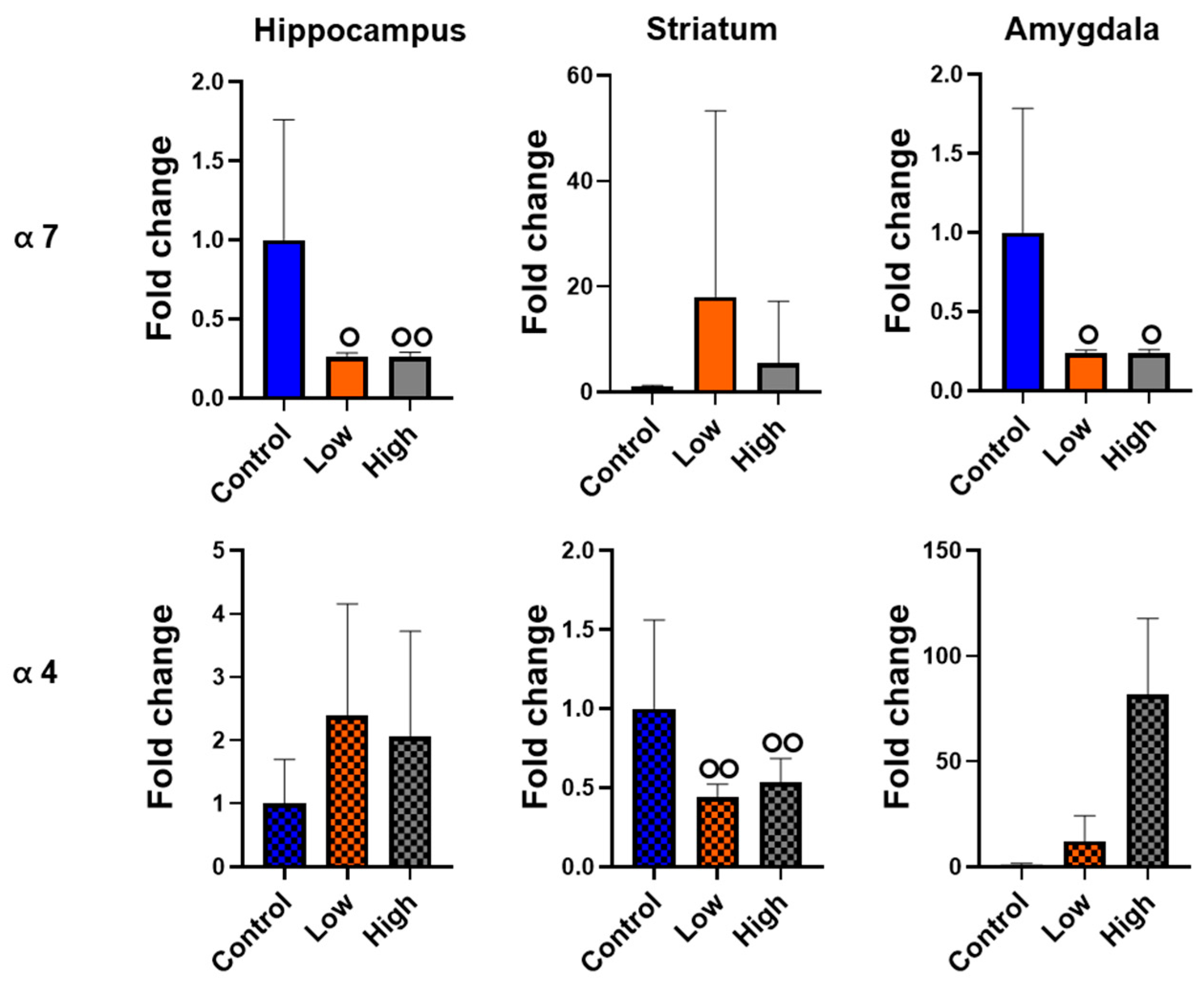
3.6. Prenatal IMI Exposure Altered α7 and α4 nAChR Expression in Males but Not Females
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPM | Elevated Plus Maze |
| IMI | Imidacloprid |
| nAChR | Nicotinic Acetylcholine Receptors |
| NOAEL | No Observable Adverse Effects Level |
| SCM | Sweetened Condensed Milk |
References
- Tomizawa, M.; Casida, J.E. Neonicotinoid insecticide toxicology: Mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 247–268. [Google Scholar] [CrossRef]
- Tan, J.; Galligan, J.J.; Hollingworth, R.M. Agonist actions of neonicotinoids on nicotinic acetylcholine receptors expressed by cockroach neurons. Neurotoxicology 2007, 28, 829–842. [Google Scholar] [CrossRef]
- Yamamoto, I.; Yabuta, G.; Tomizawa, M.; Saito, T.; Miyamoto, T.; Kagabu, S. Molecular Mechanism for Selective Toxicity of Nicotinoids and Neonicotinoids. J. Pestic. Sci. 1995, 20, 33–40. [Google Scholar] [CrossRef]
- Douglas, M.R.; Tooker, J.F. Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in US field crops. Environ. Sci. Technol. 2015, 49, 5088–5097. [Google Scholar] [CrossRef] [PubMed]
- Perkins, R.; Goulson, D. To flea or not to flea: Survey of UK companion animal ectoparasiticide usage and activities affecting pathways to the environment. PeerJ 2023, 11, e15561. [Google Scholar] [CrossRef] [PubMed]
- Covert, S.A.; Shoda, M.E.; Stackpoole, S.M.; Stone, W.W. Pesticide mixtures show potential toxicity to aquatic life in US streams, water years 2013–2017. Sci. Total Environ. 2020, 745, 141285. [Google Scholar] [CrossRef]
- Wan, Y.; Han, Q.; Wang, Y.; He, Z. Five degradates of imidacloprid in source water, treated water, and tap water in Wuhan, central China. Sci. Total Environ. 2020, 741, 140227. [Google Scholar] [CrossRef]
- Hladik, M.L.; Main, A.R.; Goulson, D. Environmental Risks and Challenges Associated with Neonicotinoid Insecticides. Environ. Sci. Technol. 2018, 52, 3329–3335. [Google Scholar] [CrossRef]
- Craddock, H.A.; Huang, D.; Turner, P.C.; Quiros-Alcala, L.; Payne-Sturges, D.C. Trends in neonicotinoid pesticide residues in food and water in the United States, 1999–2015. Environ. Health 2019, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Batikian, C.M.; Lu, A.; Watanabe, K.; Pitt, J.; Gersberg, R.M. Temporal pattern in levels of the neonicotinoid insecticide, imidacloprid, in an urban stream. Chemosphere 2019, 223, 83–90. [Google Scholar] [CrossRef]
- Struger, J.; Grabuski, J.; Cagampan, S.; Sverko, E.; McGoldrick, D.; Marvin, C.H. Factors influencing the occurrence and distribution of neonicotinoid insecticides in surface waters of southern Ontario, Canada. Chemosphere 2017, 169, 516–523. [Google Scholar] [CrossRef]
- Perkins, R.; Barron, L.; Glauser, G.; Whitehead, M.; Woodward, G.; Goulson, D. Down-the-drain pathways for fipronil and imidacloprid applied as spot-on parasiticides to dogs: Estimating aquatic pollution. Sci. Total Environ. 2024, 917, 170175. [Google Scholar] [CrossRef] [PubMed]
- Sadaria, A.M.; Sutton, R.; Moran, K.D.; Teerlink, J.; Brown, J.V.; Halden, R.U. Passage of fiproles and imidacloprid from urban pest control uses through wastewater treatment plants in northern California, USA. Environ. Toxicol. Chem. 2017, 36, 1473–1482. [Google Scholar] [CrossRef]
- Sadaria, A.M.; Supowit, S.D.; Halden, R.U. Mass Balance Assessment for Six Neonicotinoid Insecticides During Conventional Wastewater and Wetland Treatment: Nationwide Reconnaissance in United States Wastewater. Environ. Sci. Technol. 2016, 50, 6199–6206. [Google Scholar] [CrossRef]
- Baker, S.E.; Serafim, A.B.; Morales-Agudelo, P.; Vidal, M.; Calafat, A.M.; Ospina, M. Quantification of DEET and neonicotinoid pesticide biomarkers in human urine by online solid-phase extraction high-performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 669–678. [Google Scholar] [CrossRef]
- Ospina, M.; Wong, L.-Y.; Baker, S.E.; Serafim, A.B.; Morales-Agudelo, P.; Calafat, A.M. Exposure to neonicotinoid insecticides in the U.S. general population: Data from the 2015–2016 national health and nutrition examination survey. Environ. Res. 2019, 176, 108555. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, S. Human exposure to neonicotinoids and the associated health risks: A review. Environ. Int. 2022, 163, 107201. [Google Scholar] [CrossRef]
- Laubscher, B.; Diezi, M.; Renella, R.; Mitchell, E.A.D.; Aebi, A.; Mulot, M.; Glauser, G. Multiple neonicotinoids in children’s cerebro-spinal fluid, plasma, and urine. Environ. Health 2022, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Zhu, H.; Lu, S.; Wang, Y.; Xue, J.; Zhang, T.; Kannan, K.; Sun, H. Infantile Internal and External Exposure to Neonicotinoid Insecticides: A Comparison of Levels across Various Sources. Environ. Sci. Technol. 2023, 57, 5358–5367. [Google Scholar] [CrossRef] [PubMed]
- Botnaru, A.A.; Lupu, A.; Morariu, P.C.; Jitareanu, A.; Nedelcu, A.H.; Morariu, B.A.; Anton, E.; Di Gioia, M.L.; Lupu, V.V.; Dragostin, O.M.; et al. Neurotoxic Effects of Pesticides: Implications for Neurodegenerative and Neurobehavioral Disorders. J. Xenobiot. 2025, 15, 83. [Google Scholar] [CrossRef]
- Sass, J.B.; Donley, N.; Freese, W. Neonicotinoid pesticides: Evidence of developmental neurotoxicity from regulatory rodent studies. Front. Toxicol. 2024, 6, 1438890. [Google Scholar] [CrossRef]
- Wang, X.; Anadon, A.; Wu, Q.H.; Qiao, F.; Ares, I.; Martinez-Larranaga, M.R.; Yuan, Z.H.; Martinez, M.A. Mechanism of Neonicotinoid Toxicity: Impact on Oxidative Stress and Metabolism. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 471–507. [Google Scholar] [CrossRef]
- Zou, X.; Ebizuka, Y.; Sakamaki, Y.; Shobudani, M.; Tang, Q.; Luo, M.; Kobayashi, M.; Kigata, T.; Shibutani, M. Progressive motor dysfunction and loss of cerebellar Purkinje and granule cells in rat offspring after maternal exposure to imidacloprid. Toxicology 2025, 518, 154246. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Meng, Z.Y.; Tian, S.N.; Teng, M.M.; Yan, J.; Jia, M.; Li, R.S.; Zhou, Z.Q.; Zhu, W.T. Neonicotinoid insecticides exposure cause amino acid metabolism disorders, lipid accumulation and oxidative stress in ICR mice. Chemosphere 2020, 246, 125661. [Google Scholar] [CrossRef]
- Duzguner, V.; Erdogan, S. Acute oxidant and inflammatory effects of imidacloprid on the mammalian central nervous system and liver in rats. Pestic. Biochem. Physiol. 2010, 97, 13–18. [Google Scholar] [CrossRef]
- Kapoor, U.; Srivastava, M.K.; Srivastava, L.P. Toxicological impact of technical imidacloprid on ovarian morphology, hormones and antioxidant enzymes in female rats. Food Chem. Toxicol. 2011, 49, 3086–3089. [Google Scholar] [CrossRef]
- Sardar, A.; David, M.; Jahan, S.; Afsar, T.; Ahmad, A.; Ullah, A.; Almajwal, A.; Shafique, H.; Razak, S. Determination of biochemical and histopathological changes on testicular and epididymis tissues induced by exposure to insecticide Imidacloprid during postnatal development in rats. BMC Pharmacol. Toxicol. 2023, 24, 68. [Google Scholar] [CrossRef] [PubMed]
- Mourikes, V.E.; Santacruz-Marquez, R.; Deviney, A.; Laws, M.J.; Ulanov, A.V.; La Frano, M.R.; Flaws, J.A. Ovarian antral follicles metabolize imidacloprid in vitro. Toxicol. Sci. 2023, 196, 229–237. [Google Scholar] [CrossRef]
- Thompson, D.A.; Lehmler, H.J.; Kolpin, D.W.; Hladik, M.L.; Vargo, J.D.; Schilling, K.E.; LeFevre, G.H.; Peeples, T.L.; Poch, M.C.; LaDuca, L.E.; et al. A critical review on the potential impacts of neonicotinoid insecticide use: Current knowledge of environmental fate, toxicity, and implications for human health. Environ. Sci. Process. Impacts 2020, 22, 1315–1346. [Google Scholar] [CrossRef]
- Bal, R.; Turk, G.; Tuzcu, M.; Yilmaz, O.; Kuloglu, T.; Gundogdu, R.; Gur, S.; Agca, A.; Ulas, M.; Cambay, Z.; et al. Assessment of imidacloprid toxicity on reproductive organ system of adult male rats. J. Environ. Sci. Health Part B 2012, 47, 434–444. [Google Scholar] [CrossRef]
- Mourikes, V.E.; Lee, C.; Oladosu, J.I.; Deviney, A.; Stubblefield, W.; Laws, M.J.; Mahoney, M.; Flaws, J.A. The effects of imidacloprid exposure on the mouse ovary in vivo. Reprod. Toxicol. 2025, 137, 109045. [Google Scholar] [CrossRef]
- Badgujar, P.C.; Jain, S.K.; Singh, A.; Punia, J.S.; Gupta, R.P.; Chandratre, G.A. Immunotoxic effects of imidacloprid following 28 days of oral exposure in BALB/c mice. Environ. Toxicol. Pharmacol. 2013, 35, 408–418. [Google Scholar] [CrossRef]
- Namba, K.; Tominaga, T.; Ishihara, Y. Decreases in the number of microglia and neural circuit dysfunction elicited by developmental exposure to neonicotinoid pesticides in mice. Environ. Toxicol. 2024, 39, 3944–3955. [Google Scholar] [CrossRef]
- Loser, D.; Grillberger, K.; Hinojosa, M.G.; Blum, J.; Haufe, Y.; Danker, T.; Johansson, Y.; Möller, C.; Nicke, A.; Bennekou, S.H.; et al. Acute effects of the imidacloprid metabolite desnitro-imidacloprid on human nACh receptors relevant for neuronal signaling. Arch. Toxicol. 2021, 95, 3695–3716. [Google Scholar] [CrossRef]
- Kimura-Kuroda, J.; Komuta, Y.; Kuroda, Y.; Hayashi, M.; Kawano, H. Nicotine-Like Effects of the Neonicotinoid Insecticides Acetamiprid and Imidacloprid on Cerebellar Neurons from Neonatal Rats. PLoS ONE 2012, 7, e32432. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Gould, T.J. Hippocampal nAChRs mediate nicotine withdrawal-related learning deficits. Eur. Neuropsychopharmacol. 2009, 19, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Ji, Z.; Sumikawa, K. Inactivation of alpha7 ACh receptors and activation of non-alpha7 ACh receptors both contribute to long term potentiation induction in the hippocampal CA1 region. Neurosci. Lett. 2000, 286, 134–138. [Google Scholar] [CrossRef]
- Mahai, G.; Wan, Y.; Xia, W.; Wang, A.; Qian, X.; Li, Y.; He, Z.; Li, Y.; Xu, S. Exposure assessment of neonicotinoid insecticides and their metabolites in Chinese women during pregnancy: A longitudinal study. Sci. Total Environ. 2022, 818, 151806. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.P.; Kuiper, J.R.; Bennett, D.H.; Barrett, E.S.; Bastain, T.; Breton, C.V.; Chinthakindi, S.; Dunlop, A.L.; Farzan, S.F.; Herbstman, J.B.; et al. Exposure to Contemporary and Emerging Chemicals in Commerce among Pregnant Women in the United States: The Environmental influences on Child Health Outcome (ECHO) Program. Environ. Sci. Technol. 2022, 56, 6560–6573. [Google Scholar] [CrossRef]
- Pan, C.; Yu, J.; Yao, Q.; Lin, N.; Lu, Z.; Zhang, Y.; Zhao, S.; Wang, Z.; Lei, X.; Tian, Y.; et al. Prenatal neonicotinoid insecticides Exposure, oxidative Stress, and birth outcomes. Environ. Int. 2022, 163, 107180. [Google Scholar] [CrossRef]
- Saito, H.; Furukawa, Y.; Sasaki, T.; Kitajima, S.; Kanno, J.; Tanemura, K. Behavioral effects of adult male mice induced by low-level acetamiprid, imidacloprid, and nicotine exposure in early-life. Front. Neurosci. 2023, 17, 1239808. [Google Scholar] [CrossRef] [PubMed]
- Raja, G.L.; Subhashree, K.D.; Kantayya, K.E. In utero exposure to endocrine disruptors and developmental neurotoxicity: Implications for behavioural and neurological disorders in adult life. Environ. Res. 2022, 203, 111829. [Google Scholar] [CrossRef]
- Gavini, K.; Yang, E.; Parameshwaran, K. Developmental nicotine exposure impairs memory and reduces acetylcholine levels in the hippocampus of mice. Brain Res. Bull. 2021, 176, 1–7. [Google Scholar] [CrossRef]
- Wang, H.; Davila-Garcia, M.I.; Yarl, W.; Gondre-Lewis, M.C. Gestational nicotine exposure regulates expression of AMPA and NMDA receptors and their signaling apparatus in developing and adult rat hippocampus. Neuroscience 2011, 188, 168–181. [Google Scholar] [CrossRef]
- Li, J.; Bo, L.; Zhang, P.; Gao, Q.; Li, L.; Tang, J.; Wu, C.; Li, D.; Xiao, J.; Chen, J.; et al. Exposure to nicotine during pregnancy and altered learning and memory in the rat offspring. Nicotine Tob. Res. 2015, 17, 661–666. [Google Scholar] [CrossRef]
- Gold, A.B.; Keller, A.B.; Perry, D.C. Prenatal exposure of rats to nicotine causes persistent alterations of nicotinic cholinergic receptors. Brain Res. 2009, 1250, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Abou-Donia, M.B.; Goldstein, L.B.; Bullman, S.; Tu, T.; Khan, W.A.; Dechkovskaia, A.M.; Abdel-Rahman, A.A. Imidacloprid Induces Neurobehavioral Deficits and Increases Expression of Glial Fibrillary Acidic Protein in the Motor Cortex and Hippocampus in Offspring Rats Following in Utero Exposure. J. Toxicol. Environ. Health Part A 2008, 71, 119–130. [Google Scholar] [CrossRef]
- Bhaskar, R.; Mishra, A.K.; Mohanty, B. Neonatal Exposure to Endocrine Disrupting Chemicals Impairs Learning Behaviour by Disrupting Hippocampal Organization in Male Swiss Albino Mice. Basic Clin. Pharmacol. Toxicol. 2017, 121, 44–52. [Google Scholar] [CrossRef]
- Hirano, T.; Yanai, S.; Takada, T.; Yoneda, N.; Omotehara, T.; Kubota, N.; Minami, K.; Yamamoto, A.; Mantani, Y.; Yokoyama, T.; et al. NOAEL-dose of a neonicotinoid pesticide, clothianidin, acutely induce anxiety-related behavior with human-audible vocalizations in male mice in a novel environment. Toxicol. Lett. 2018, 282, 57–63. [Google Scholar] [CrossRef]
- Hirai, A.; Sugio, S.; Nimako, C.; Nakayama, S.M.M.; Kato, K.; Takahashi, K.; Arizono, K.; Hirano, T.; Hoshi, N.; Fujioka, K.; et al. Ca2+ imaging with two-photon microscopy to detect the disruption of brain function in mice administered neonicotinoid insecticides. Sci. Rep. 2022, 12, 5114. [Google Scholar] [CrossRef] [PubMed]
- Shoda, A.; Murata, M.; Kimura, M.; Hara, Y.; Yonoichi, S.; Ishida, Y.; Mantani, Y.; Yokoyama, T.; Hirano, T.; Ikenaka, Y.; et al. Transgenerational effects of developmental neurotoxicity induced by exposure to a no-observed-adverse-effect level (NOAEL) of neonicotinoid pesticide clothianidin. J. Vet. Med. Sci. 2023, 85, 1023–1029. [Google Scholar] [CrossRef]
- Kubo, S.; Hirano, T.; Miyata, Y.; Ohno, S.; Onaru, K.; Ikenaka, Y.; Nakayama, S.M.M.; Ishizuka, M.; Mantani, Y.; Yokoyama, T.; et al. Sex-specific behavioral effects of acute exposure to the neonicotinoid clothianidin in mice. Toxicol. Appl. Pharmacol. 2022, 456, 116283. [Google Scholar] [CrossRef]
- Sano, K.; Isobe, T.; Yang, J.; Win-Shwe, T.T.; Yoshikane, M.; Nakayama, S.F.; Kawashima, T.; Suzuki, G.; Hashimoto, S.; Nohara, K.; et al. In utero and Lactational Exposure to Acetamiprid Induces Abnormalities in Socio-Sexual and Anxiety-Related Behaviors of Male Mice. Front. Neurosci. 2016, 10, 228. [Google Scholar] [CrossRef]
- Kaku, K.; Sasaki, T.; Hara, K.; Tanemura, K. A single dose of clothianidin exposure induces varying sex-specific behavioral changes in adulthood depending on the developmental stage of its administration. J. Toxicol. Sci. 2024, 49, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Song, S.; Bai, X.; He, Y.; Zhang, B.; Gui, M.; Kannan, K.; Lu, S.; Huang, Y.; Sun, H. A nationwide survey of urinary concentrations of neonicotinoid insecticides in China. Environ. Int. 2019, 132, 105114. [Google Scholar] [CrossRef]
- Godbole, A.M.; Moonie, S.; Coughenour, C.; Zhang, C.; Chen, A.; Vuong, A.M. Exploratory analysis of the associations between neonicotinoids and measures of adiposity among US adults: NHANES 2015–2016. Chemosphere 2022, 300, 134450. [Google Scholar] [CrossRef]
- Arfat, Y.; Mahmood, N.; Tahir, M.U.; Rashid, M.; Anjum, S.; Zhao, F.; Li, D.J.; Sun, Y.L.; Hu, L.; Zhihao, C.; et al. Effect of imidacloprid on hepatotoxicity and nephrotoxicity in male albino mice. Toxicol. Rep. 2014, 1, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, U.; Srivastava, M.K.; Trivedi, P.; Garg, V.; Srivastava, L.P. Disposition and acute toxicity of imidacloprid in female rats after single exposure. Food Chem. Toxicol. 2014, 68, 190–195. [Google Scholar] [CrossRef]
- Passoni, A.; Mariani, A.; Comolli, D.; Fanelli, R.; Davoli, E.; De Paola, M.; Bagnati, R. An integrated approach, based on mass spectrometry, for the assessment of imidacloprid metabolism and penetration into mouse brain and fetus after oral treatment. Toxicology 2021, 462, 152935. [Google Scholar] [CrossRef] [PubMed]
- Gawel, K.; Gibula, E.; Marszalek-Grabska, M.; Filarowska, J.; Kotlinska, J.H. Assessment of spatial learning and memory in the Barnes maze task in rodents—Methodological consideration. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 1–18. [Google Scholar] [CrossRef]
- Deacon, R.M.J.; Rawlins, J.N.P. T-maze alternation in the rodent. Nat. Protoc. 2006, 1, 7–12. [Google Scholar] [CrossRef]
- Murithi, C.K.; Kabaru, J.M.; Patel, N.B. Catha edulis Forsk (khat) reduces spontaneous and rewarded alternation in female mice. IBRO Rep. 2020, 9, 270–275. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The Tail Suspension Test. J. Vis. Exp. 2011, 59, e3769. [Google Scholar] [CrossRef] [PubMed]
- Mourikes, V.E.; Santacruz-Marquez, R.; Deviney, A.; Neff, A.; Laws, M.J.; Flaws, J.A. Neonicotinoids differentially modulate nicotinic acetylcholine receptors in immature and antral follicles in the mouse ovary. Biol. Reprod. 2024, 111, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size—Or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef]
- Cohen, J. A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T. Reproductive and neurobehavioral effects of clothianidin administered to mice in the diet. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2012, 95, 151–159. [Google Scholar] [CrossRef]
- Tanaka, T. Effects of maternal clothianidin exposure on behavioral development in F(1) generation mice. Toxicol. Ind. Health 2012, 28, 697–707. [Google Scholar] [CrossRef]
- Shamsi, M.; Soodi, M.; Shahbazi, S.; Omidi, A. Effect of Acetamiprid on spatial memory and hippocampal glutamatergic system. Environ. Sci. Pollut. Res. 2021, 28, 27933–27941. [Google Scholar] [CrossRef]
- Zou, X.; Tang, Q.; Ojiro, R.; Ozawa, S.; Shobudani, M.; Sakamaki, Y.; Ebizuka, Y.; Jin, M.; Yoshida, T.; Shibutani, M. Increased spontaneous activity and progressive suppression of adult neurogenesis in the hippocampus of rat offspring after maternal exposure to imidacloprid. Chem.-Biol. Interact. 2024, 399, 111145. [Google Scholar] [CrossRef]
- Mudgal, R.; Sharma, S.; Singh, S.; Ravichandiran, V. The neuroprotective effect of ascorbic acid against imidacloprid-induced neurotoxicity and the role of HO-1 in mice. Front. Neurol. 2023, 14, 1130575. [Google Scholar] [CrossRef]
- Manion, M.T.C.; Glasper, E.R.; Wang, K.H. A sex difference in mouse dopaminergic projections from the midbrain to basolateral amygdala. Biol. Sex Differ. 2022, 13, 75. [Google Scholar] [CrossRef]
- Palotai, M.; Bagosi, Z.; Jaszberenyi, M.; Csabafi, K.; Dochnal, R.; Manczinger, M.; Telegdy, G.; Szabo, G. Ghrelin and nicotine stimulate equally the dopamine release in the rat amygdala. Neurochem. Res. 2013, 38, 1989–1995. [Google Scholar] [CrossRef]
- Moen, J.K.; Lee, A.M. Sex Differences in the Nicotinic Acetylcholine Receptor System of Rodents: Impacts on Nicotine and Alcohol Reward Behaviors. Front. Neurosci. 2021, 15, 745783. [Google Scholar] [CrossRef] [PubMed]
- Mitsushima, D.; Takase, K.; Funabashi, T.; Kimura, F. Gonadal Steroids Maintain 24 h Acetylcholine Release in the Hippocampus: Organizational and Activational Effects in Behaving Rats. J. Neurosci. 2009, 29, 3808–3815. [Google Scholar] [CrossRef] [PubMed]
- Takase, K.; Kimura, F.; Yagami, T.; Mitsushima, D. Sex-specific 24-h acetylcholine release profile in the medial prefrontal cortex: Simultaneous measurement of spontaneous locomotor activity in behaving rats. Neuroscience 2009, 159, 7–15. [Google Scholar] [CrossRef]
- Knight, P.; Chellian, R.; Wilson, R.; Behnood-Rod, A.; Panunzio, S.; Bruijnzeel, A.W. Sex differences in the elevated plus-maze test and large open field test in adult Wistar rats. Pharmacol. Biochem. Behav. 2021, 204, 173168. [Google Scholar] [CrossRef] [PubMed]
- Puinean, A.M.; Foster, S.P.; Oliphant, L.; Denholm, I.; Field, L.M.; Millar, N.S.; Williamson, M.S.; Bass, C. Amplification of a Cytochrome P450 Gene Is Associated with Resistance to Neonicotinoid Insecticides in the Aphid Myzus persicae. PLoS Genet. 2010, 6, e1000999. [Google Scholar] [CrossRef]
- Zoh, M.G.; Gaude, T.; Prud’homme, S.M.; Riaz, M.A.; David, J.P.; Reynaud, S. Molecular bases of P450-mediated resistance to the neonicotinoid insecticide imidacloprid in the mosquito. Aquat. Toxicol. 2021, 236, 105860. [Google Scholar] [CrossRef]
- Højland, D.H.; Vagn Jensen, K.M.; Kristensen, M. A comparative study of P450 gene expression in field and laboratory Musca domestica L. strains. Pest Manag. Sci. 2014, 70, 1237–1242. [Google Scholar] [CrossRef]
- Gerges, S.H.; El-Kadi, A.O.S. Sexual Dimorphism in the Expression of Cytochrome P450 Enzymes in Rat Heart, Liver, Kidney, Lung, Brain, and Small Intestine. Drug Metab. Dispos. 2023, 51, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Hill, C.E.; Myers, J.P.; Vandenberg, L.N. Nonmonotonic Dose-Response Curves Occur in Dose Ranges That Are Relevant to Regulatory Decision-Making. Dose-Response 2018, 16, 1559325818798282. [Google Scholar] [CrossRef] [PubMed]
- Hirai, A.; Yamazaki, R.; Kobayashi, A.; Kimura, T.; Nomiyama, K.; Shimma, S.; Nakayama, S.M.M.; Ishizuka, M.; Ikenaka, Y. Detection of Changes in Monoamine Neurotransmitters by the Neonicotinoid Pesticide Imidacloprid Using Mass Spectrometry. Toxics 2022, 10, 696. [Google Scholar] [CrossRef]
- Nunes-Freitas, A.L.; Ribeiro-Carvalho, A.; Lima, C.S.; Dutra-Tavares, A.C.; Manhaes, A.C.; Lisboa, P.C.; Oliveira, E.; Gaspar de Moura, E.; Filgueiras, C.C.; Abreu-Villaca, Y. Nicotine exposure during the third trimester equivalent of human gestation: Time course of effects on the central cholinergic system of rats. Toxicol. Sci. 2011, 123, 144–154. [Google Scholar] [CrossRef]
- Pang, X.; Liu, L.; Ngolab, J.; Zhao-Shea, R.; McIntosh, J.M.; Gardner, P.D.; Tapper, A.R. Habenula cholinergic neurons regulate anxiety during nicotine withdrawal via nicotinic acetylcholine receptors. Neuropharmacology 2016, 107, 294–304. [Google Scholar] [CrossRef]
- Kutlu, M.G.; Gould, T.J. Nicotine modulation of fear memories and anxiety: Implications for learning and anxiety disorders. Biochem. Pharmacol. 2015, 97, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a Neuromodulator: Cholinergic Signaling Shapes Nervous System Function and Behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef]
- Ho, T.N.T.; Abraham, N.; Lewis, R.J. Structure-Function of Neuronal Nicotinic Acetylcholine Receptor Inhibitors Derived from Natural Toxins. Front. Neurosci. 2020, 14, 609005. [Google Scholar] [CrossRef]
- Torres-Altoro, M.I.; Mathur, B.N.; Drerup, J.M.; Thomas, R.; Lovinger, D.M.; O’Callaghan, J.P.; Bibb, J.A. Organophosphates dysregulate dopamine signaling, glutamatergic neurotransmission, and induce neuronal injury markers in striatum. J. Neurochem. 2011, 119, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Wu, C.H.; Chan, S.H.; Chang, A.Y. Muscarinic receptor-independent activation of cyclic adenosine monophosphate-dependent protein kinase in rostral ventrolateral medulla underlies the sympathoexcitatory phase of cardiovascular responses during mevinphos intoxication in the rat. Shock 2007, 27, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Montes-Grajales, D.; Olivero-Verbel, J. Structure-based Identification of Endocrine Disrupting Pesticides Targeting Breast Cancer Proteins. Toxicology 2020, 439, 152459. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Quito, J.; Poteat, T.; Mourikes, V.E.; Flaws, J.A.; Mahoney, M.M. Prenatal Exposure to Imidacloprid Affects Cognition and Anxiety-Related Behaviors in Male and Female CD-1 Mice. Toxics 2025, 13, 918. https://doi.org/10.3390/toxics13110918
Lee C, Quito J, Poteat T, Mourikes VE, Flaws JA, Mahoney MM. Prenatal Exposure to Imidacloprid Affects Cognition and Anxiety-Related Behaviors in Male and Female CD-1 Mice. Toxics. 2025; 13(11):918. https://doi.org/10.3390/toxics13110918
Chicago/Turabian StyleLee, Colin, Jessica Quito, Truman Poteat, Vasiliki E. Mourikes, Jodi A. Flaws, and Megan M. Mahoney. 2025. "Prenatal Exposure to Imidacloprid Affects Cognition and Anxiety-Related Behaviors in Male and Female CD-1 Mice" Toxics 13, no. 11: 918. https://doi.org/10.3390/toxics13110918
APA StyleLee, C., Quito, J., Poteat, T., Mourikes, V. E., Flaws, J. A., & Mahoney, M. M. (2025). Prenatal Exposure to Imidacloprid Affects Cognition and Anxiety-Related Behaviors in Male and Female CD-1 Mice. Toxics, 13(11), 918. https://doi.org/10.3390/toxics13110918






