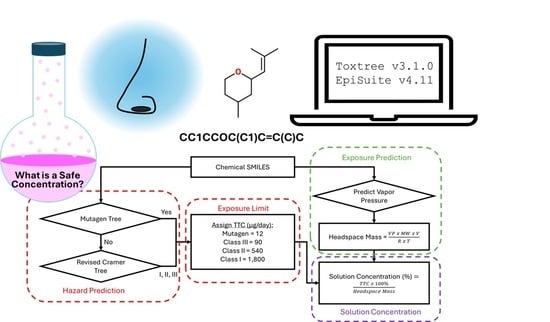
Framework for In Silico Toxicity Screening of Novel Odorants
Abstract
1. Introduction
2. Materials and Methods
2.1. Model Description
2.2. Model Safety Testing
3. Results
3.1. Model Safety Testing
3.2. Solvent Effect
3.3. Case Example, Rose Oxide
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Myatt, G.J.; Ahlberg, E.; Akahori, Y.; Allen, D.; Amberg, A.; Anger, L.T.; Aptula, A.; Auerbach, S.; Beilke, L.; Bellion, P.; et al. In silico toxicology protocols. Regul. Toxicol. Pharmacol. 2018, 96, 1–17. [Google Scholar] [CrossRef]
- Lee, B.K.; Mayhew, E.J.; Sanchez-Lengeling, B.; Wei, J.N.; Qian, W.W.; Little, K.A.; Andres, M.; Nguyen, B.B.; Moloy, T.; Yasonik, J.; et al. A principal odor map unifies diverse tasks in olfactory perception. Science 2023, 381, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Benigni, R.; Bossa, C. Mechanisms of chemical carcinogenicity and mutagenicity: A review with implications for predictive toxicology. Chem. Rev. 2011, 111, 2507–2536. [Google Scholar] [CrossRef] [PubMed]
- Benigni, R.; Bossa, C.; Tcheremenskaia, O. Nongenotoxic carcinogenicity of chemicals: Mechanisms of action and early recognition through a new set of structural alerts. Chem. Rev. 2013, 113, 2940–2957. [Google Scholar] [CrossRef] [PubMed]
- Ideaconsult, Ltd. Toxtree—Toxic Hazard Estimation by Decision Tree Approach. Version 3.1.0. Available online: http://toxtree.sourceforge.net (accessed on 30 September 2025).
- Organisation for Economic Co-Operation and Development (OECD). QSAR Toolbox. Available online: https://www.oecd.org/en/data/tools/oecd-qsar-toolbox.html (accessed on 30 September 2025).
- Istituto di Ricerche Farmacologiche Mario Negri (IRCCS) (Milan, Italy). VEGA QSAR. Available online: https://www.vegahub.eu/download/vega-qsar-download/ (accessed on 30 September 2025).
- Honma, M.; Kitazawa, A.; Cayley, A.; Williams, R.V.; Barber, C.; Hanser, T.; Saiakhov, R.; Chakravarti, S.; Myatt, G.J.; Cross, K.P.; et al. Improvement of quantitative structure-activity relationship (QSAR) tools for predicting Ame’s mutagenicity: Outcomes of the Ames/QSAR International Challenge Project. Mutagenesis 2019, 34, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Van Bossuyt, M.; Van Hoeck, E.; Raitano, G.; Vanhaecke, T.; Benfenati, E.; Mertens, B.; Rogiers, V. Performance of In Silico Models for Mutagenicity Prediction of Food Contact Materials. Toxicol. Sci. 2018, 163, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Cramer, G.M.; Ford, R.A.; Hall, R.L. Estimation of toxic hazard—A decision tree approach. Food Cosmet. Toxicol. 1978, 16, 255–276. [Google Scholar] [CrossRef] [PubMed]
- Revised Cramer Decision Tree. Available online: https://toxtree.sourceforge.net/cramer3.html (accessed on 30 September 2025).
- Kroes, R.; Renwick, A.G.; Cheeseman, M.; Kleiner, J.; Mangelsdorf, I.; Piersma, A.; Schilter, B.; Schlatter, J.; van Schothorst, F.; Vos, J.G.; et al. Structure-based thresholds of toxicological concern (TTC): Guidance for application to substances present at low levels in the diet. Food Chem. Toxicol. 2004, 42, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Kroes, R.; Renwick, A.G.; Feron, V.; Galli, C.L.; Gibney, M.; Greim, H.; Guy, R.H.; Lhuguenot, J.C.; van de Sandt, J.J. Application of the threshold of toxicological concern (TTC) to the safety evaluation of cosmetic ingredients. Food Chem. Toxicol. 2007, 45, 2533–2562. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Schultz, T.; Roberts, D.; Shen, J.; Kromidas, L.; Api, A.M. Comparison of Cramer classification between Toxtree, the OECD QSAR Toolbox and expert judgment. Regul. Toxicol. Pharmacol. 2015, 71, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Joshi, K.; Rose, J.; Laufersweiler, M.; Felter, S.P.; Api, A.M. Bolstering the existing database supporting the non-cancer threshold of toxicological concern values with toxicity data on fragrance-related materials. Regul. Toxicol. Pharmacol 2020, 116, 104718. [Google Scholar] [CrossRef]
- ISO/TS 21726; Biological Evaluation of Medical Devices—Application of the Threshold of Toxicological Concern (TTC) for Assessing Biocompatibility of Medical Device Constituents. International Organization for Standardization (ISO): Geneva, Switzerland, 2019; (E). 12p.
- US Environmental Protection Agency. Download EPI Suite™—Estimation Program Interface v4.11. Available online: https://www.epa.gov/tsca-screening-tools/download-epi-suitetm-estimation-program-interface-v411 (accessed on 30 September 2025).
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). ICH Harmonised Guideline: Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk M7(R2). International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH): Geneva, Switzerland, 2023; 32p. [Google Scholar]
- Shin, J.H.; Lee, B.H.; Lee, S.K. Development of QSAR model for subchronic inhalation toxicity using random forest regression method. Bull. Korean Chem. Soc. 2019, 40, 819–825. [Google Scholar] [CrossRef]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). ICH Harmonised Guideline: Impurities: Guideline for Residual Solvents Q3C(R9). (Current Step 4 Version); International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH): Geneva, Switzerland, 2024; 50p. [Google Scholar]
- Organisation for Economic Co-Operation and Development (OECD). OECD Guidelines for the Testing of Chemicals: 90-Day (Subchronic) Inhalation Toxicity Study. OECD/OCDE 413; Organisation for Economic Co-Operation and Development (OECD): Paris, France, 2018; 23p. [Google Scholar]
- European Chemicals Agency (ECHA). REACH Dossier for 2,4,6-Trichloro-1,3,5-Triazine (CAS No. 108-77-0). Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/14952/7/6/3 (accessed on 31 August 2025).
- European Chemicals Agency (ECHA). REACH Dossier for Acetic Anhydride (CAS No. 108-24-7). Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/15314/7/6/3 (accessed on 31 August 2025).
- National Research Council; Committee on Toxicology. Subcommittee on Acute Exposure Guideline Levels. In Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 4; National Academies Press: Washington, DC, USA, 2004; 76p. [Google Scholar]


| Hazard Prediction | TTC (μg/day) |
|---|---|
| Mutagen | 12 1 |
| Cramer Class III | 90 |
| Cramer Class II | 540 |
| Cramer Class I | 1800 |
| Safety Margin Statistics | Solvent | ||
|---|---|---|---|
| Propylene Glycol | Water | Ethanol/Water (50:50) | |
| Geometric Mean (range) | 1124 (0.19–1.78 × 1011) | 2358 (0.35–1.78 × 1011) | 1878 (0.33–1.78 × 1011) |
| <1 (No. chemicals) | 4 | 2 | 3 |
| <10 (No. chemicals) | 13 | 8 | 9 |
| Parameter | Value |
|---|---|
| Chemical Description | |
| Name | 4-methyl-2-(2-methylprop-1-enyl)oxane (rose oxide) |
| CAS No. | 16409-43-1 |
| Molecular Formula | C10H18O |
| SMILES | CC1CCOC(C1)C=C(C)C |
| Toxicology Predictions | |
| Mutagenicity | No alerts |
| Cramer Classification | Intermediate (Class II) |
| Assigned TTC | 540 μg/day |
| Physicochemical Predictions | |
| Molecular Weight | 154.25 g/mol |
| Vapor Pressure (predicted at 25 °C) | 0.657 mmHg |
| Headspace mass (V = 0.1 L) | 546 μg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohar, I.; Hansen, B.C.; Hollowed, D.M.; Mainland, J.D. Framework for In Silico Toxicity Screening of Novel Odorants. Toxics 2025, 13, 902. https://doi.org/10.3390/toxics13100902
Mohar I, Hansen BC, Hollowed DM, Mainland JD. Framework for In Silico Toxicity Screening of Novel Odorants. Toxics. 2025; 13(10):902. https://doi.org/10.3390/toxics13100902
Chicago/Turabian StyleMohar, Isaac, Brad C. Hansen, Destiny M. Hollowed, and Joel D. Mainland. 2025. "Framework for In Silico Toxicity Screening of Novel Odorants" Toxics 13, no. 10: 902. https://doi.org/10.3390/toxics13100902
APA StyleMohar, I., Hansen, B. C., Hollowed, D. M., & Mainland, J. D. (2025). Framework for In Silico Toxicity Screening of Novel Odorants. Toxics, 13(10), 902. https://doi.org/10.3390/toxics13100902