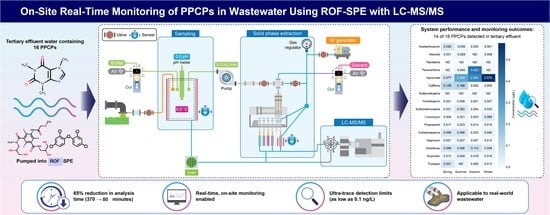
Robotic and On-Flow Solid Phase Extraction Coupled with LC-MS/MS for Simultaneous Determination of 16 PPCPs: Real-Time Monitoring of Wastewater Effluent in Korea
Highlights
- A fully automated robotic on-flow SPE system enables the real-time LC–MS/MS monitoring of 16 PPCPs in wastewater effluent directly on site.
- The system autonomously performs sampling, filtration, pH adjustment, extraction, and direct LC–MS/MS injection on site.
- Long-term field operation (12 months) demonstrated a >70 % reduction in analysis time vs. offline SPE with sub-ng/L detection limits.
- These results show that a fully automated, on-site robotic and on-flow SPE–LC–MS/MS system can continuously monitor trace PPCPs in wastewater with high throughput.
- This advancement signals a paradigm shift toward real-time, autonomous environmental monitoring, facilitating smarter, more efficient water quality management and rapid pollution response.
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
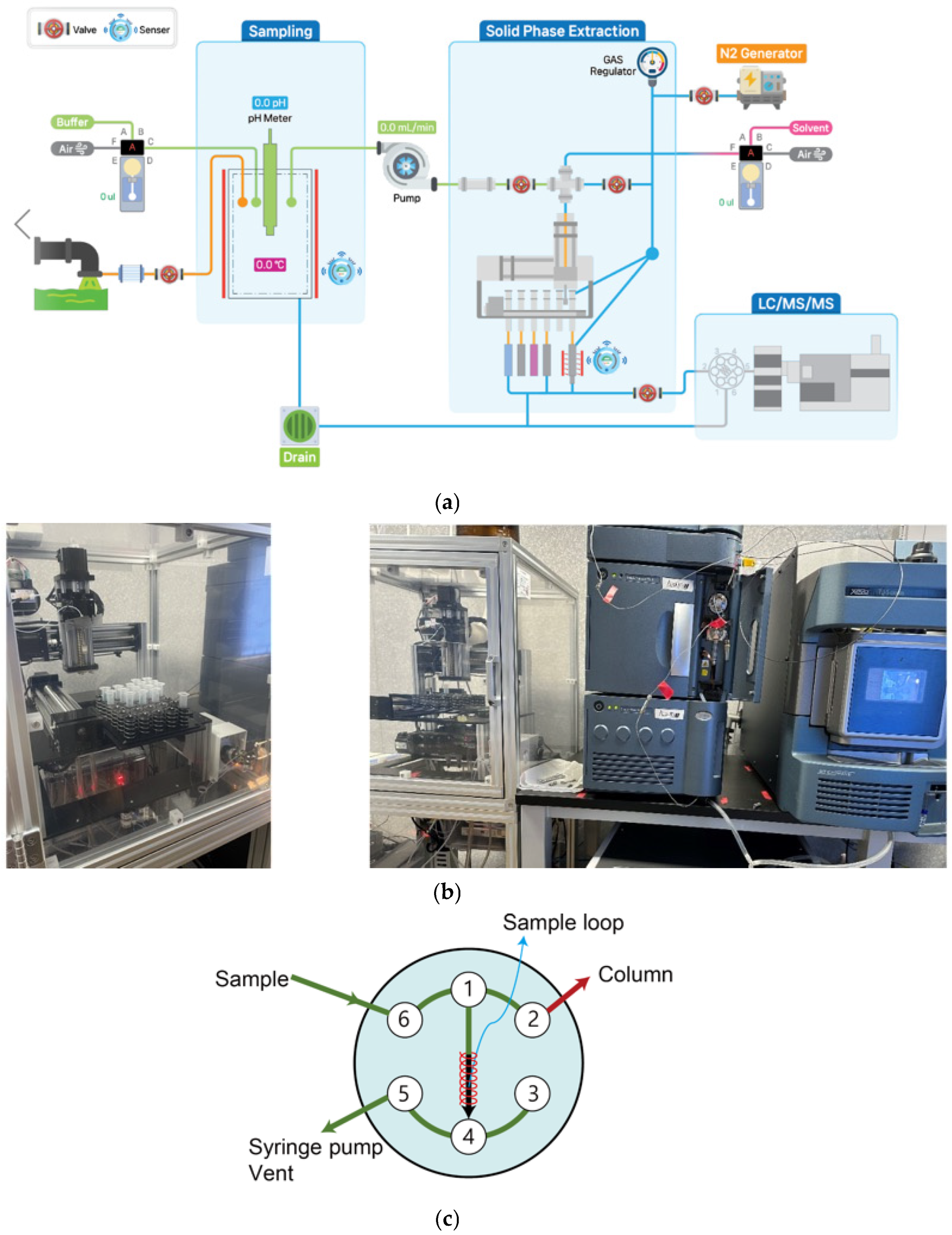
2.2. The Robotic and On-Flow SPE System
2.3. Liquid Chromatography-Tandem Mass Spectrometry
2.4. The Robotic and On-Flow Solid-Phase Extraction Method
2.5. Method Validation
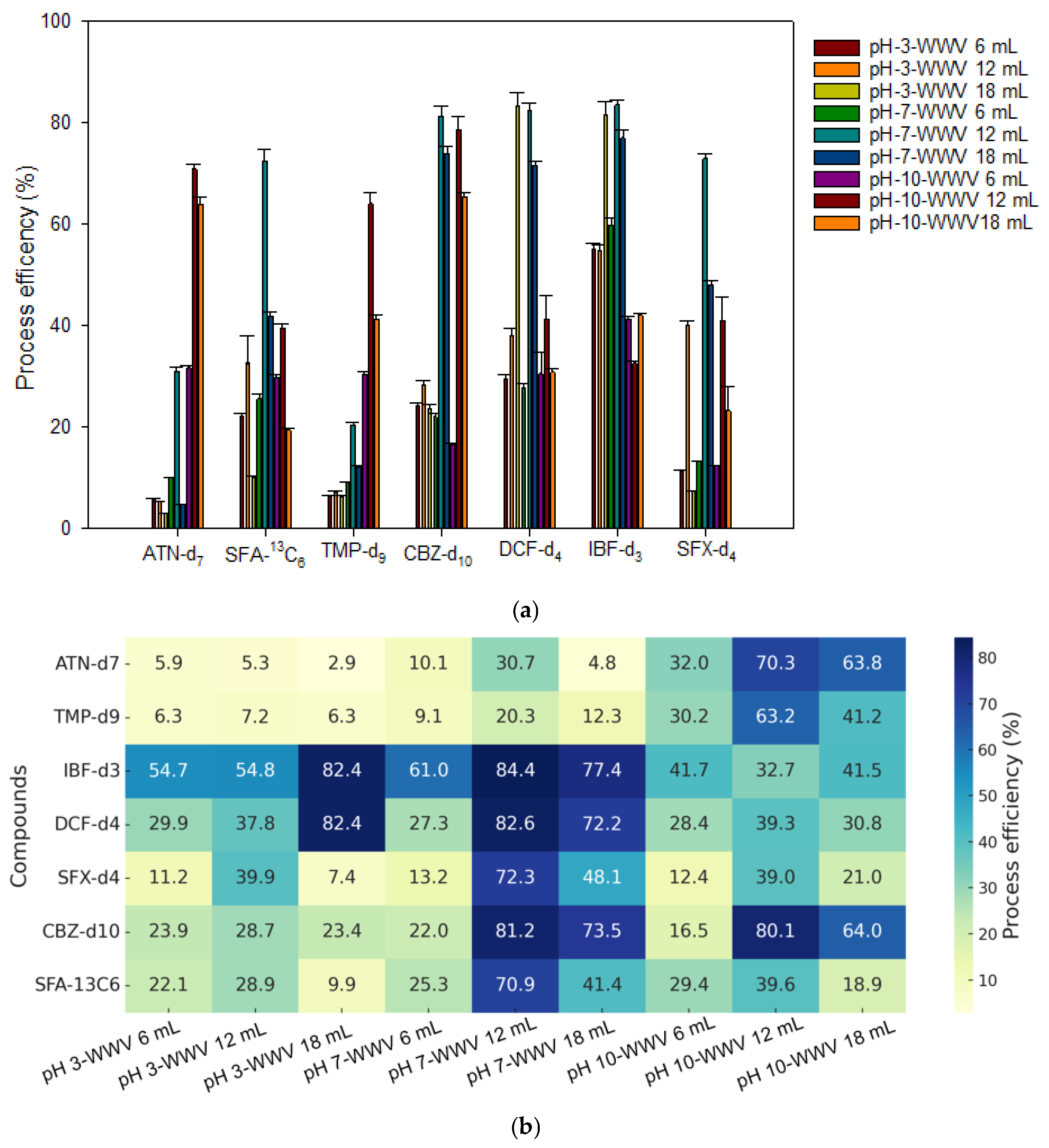
2.6. Matrix Effect and SPE Extraction Recovery
2.7. Water Quality
3. Results
3.1. LC-MS/MS Optimization
3.2. Method Validation
3.3. Matrix Effect
3.4. Optimization of Robotic and On-Flow SPE Method
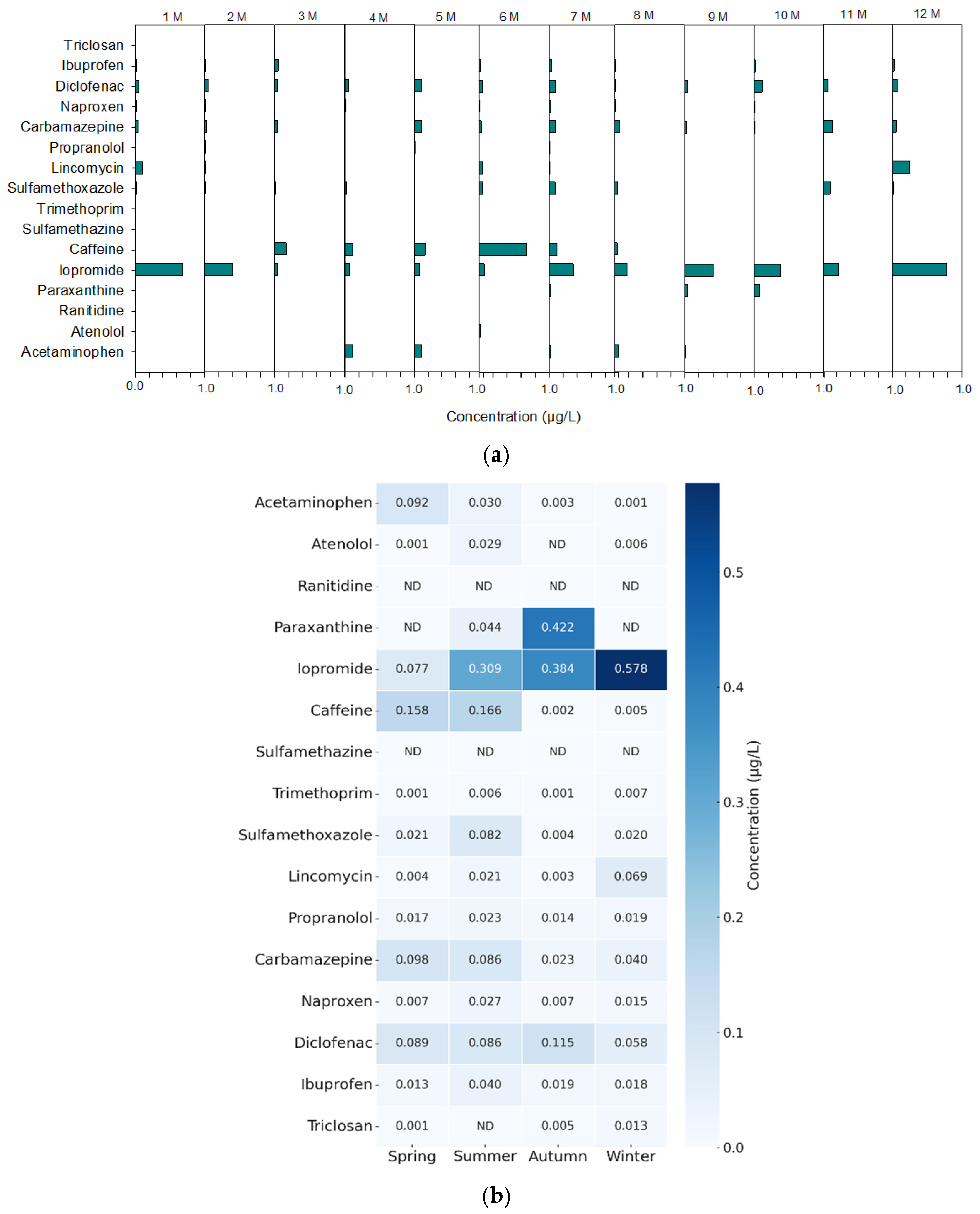
3.5. Analysis of Real Water Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuzmanović, M.; Ginebreda, A.; Petrović, M.; Barceló, D. Risk assessment based prioritization of 200 organic micropollutants in four Iberian rivers. Sci. Total Environ. 2015, 503–504, 289–299. [Google Scholar] [CrossRef]
- Nozaki, K.; Tanoue, R.; Kunisue, T.; Tue, N.M.; Fujii, S.; Sudo, N.; Isobe, T.; Nakayama, K.; Sudaryanto, A.; Subramanian, A.; et al. Pharmaceuticals and personal care products (PPCPs) in surface water and fish from three Asian countries: Species-specific bioaccumulation and potential ecological risks. Sci. Total Environ. 2023, 866, 161258. [Google Scholar] [CrossRef]
- Xin, X.; Huang, G.; Zhang, B. Review of aquatic toxicity of pharmaceuticals and personal care products to algae. J. Hazard. Mater. 2021, 410, 124619. [Google Scholar] [CrossRef]
- Ohoro, C.R.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Distribution and chemical analysis of pharmaceuticals and personal care products (PPCPs) in the environmental systems: A review. Int. J. Environ. Res. Public Health 2019, 16, 3026. [Google Scholar] [CrossRef]
- Alzola-Andres, M.; Cerveny, D.; Domingo-Echaburu, S.; Lekube, X.; Ruiz-Sancho, L.; Brodin, T.; Orive, G.; Lertxundi, U. Pharmaceutical residues in stranded dolphins in the Bay of Biscay. Sci. Total Environ. 2024, 912, 168570. [Google Scholar] [CrossRef] [PubMed]
- Ebele, A.J.; Abdallah, M.A.E.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Ellis, J.B. Assessing sources and impacts of priority PPCP compounds in urban receiving waters. In Proceedings of the 11th International Conference on Urban Drainage, Edinburgh, UK, 31 August–5 September 2008. [Google Scholar]
- Harish, H.; Jegatheesan, V. A review of sources, worldwide legislative measures and the factors influencing the treatment technologies for contaminants of emerging concern (CECs). Curr. Pollut. Rep. 2025, 11, 44. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Y.; Chen, Y.; Yao, B.; Chen, X.; Yu, Y.; Yang, J.; Zhou, Y. Pharmaceuticals and personal care products (PPCPs) in the aquatic environment: Biotoxicity, determination and electrochemical treatment. J. Clean. Prod. 2023, 388, 135923. [Google Scholar] [CrossRef]
- Doepker, C.; Lieberman, H.R.; Smith, A.P.; Peck, J.D.; El-Sohemy, A.; Welsh, B.T. Caffeine: Friend or foe? Annu. Rev. Food Sci. Technol. 2016, 7, 117–137. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Li, Y.; Liu, H.; Lin, C.S.K.; Sun, J.; Wang, Q. Unveiling the occurrence and ecological risks of triclosan in surface water through meta-analysis. Environ. Pollut. 2024, 361, 124901. [Google Scholar] [CrossRef]
- Taggart, M.A.; Cuthbert, R.; Das, D.; Sashikumar, C.; Pain, D.J.; Green, R.E.; Feltrer, Y.; Shultz, S.; Cunningham, A.A.; Meharg, A.A. Diclofenac disposition in Indian cow and goat with reference to Gyps vulture population declines. Environ. Pollut. 2007, 147, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Jallouli, N.; Elghniji, K.; Hentati, O.; Ribeiro, A.R.; Silva, A.M.T.; Ksibi, M. UV and solar photo-degradation of naproxen: TiO2 catalyst effect, reaction kinetics, products identification and toxicity assessment. J. Hazard. Mater. 2016, 304, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Lajin, B.; Schweighofer, N.; Goessler, W.; Obermayer-Pietsch, B. The determination of the paraxanthine/caffeine ratio as a metabolic biomarker for CYP1A2 activity in various human matrices by UHPLC-ESI-MS/MS. Talanta 2021, 234, 122658. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Song, S.; Chen, X.; Shi, Y.; Cui, H.; Liu, Y.; Yang, S. Source-specific ecological risks and critical source identification of PPCPs in surface water: Comparing urban and rural areas. Sci. Total Environ. 2023, 854, 158792. [Google Scholar] [CrossRef]
- Kumar, R.; Sarmah, A.K.; Padhye, L.P. Fate of pharmaceuticals and personal care products in a wastewater treatment plant with parallel secondary wastewater treatment train. J. Environ. Manag. 2019, 233, 649–659. [Google Scholar] [CrossRef]
- Agunbiade, F.O.; Moodley, B. Pharmaceuticals as emerging organic contaminants in Umgeni River water system, KwaZulu-Natal, South Africa. Environ. Monit. Assess. 2014, 186, 7273–7291. [Google Scholar] [CrossRef]
- Carmona, E.; Andreu, V.; Picó, Y. Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: From waste to drinking water. Sci. Total Environ. 2014, 484, 53–63. [Google Scholar] [CrossRef]
- Deo, R.P. Pharmaceuticals in the surface water of the USA: A review. Curr. Environ. Health Rep. 2014, 1, 113–122. [Google Scholar] [CrossRef]
- Liu, W.R.; Zhao, J.L.; Liu, Y.S.; Chen, Z.F.; Yang, Y.Y.; Zhang, Q.Q.; Ying, G.G. Biocides in the Yangtze River of China: Spatiotemporal distribution, mass load and risk assessment. Environ. Pollut. 2015, 200, 53–63. [Google Scholar] [CrossRef]
- De Jongh, C.M.; Kooij, P.J.F.; de Voogt, P.; ter Laak, T.L. Screening and human health risk assessment of pharmaceuticals and their transformation products in Dutch surface waters and drinking water. Sci. Total Environ. 2012, 427–428, 70–77. [Google Scholar] [CrossRef]
- Pai, C.W.; Leong, D.; Chen, C.Y.; Wang, G.S. Occurrences of pharmaceuticals and personal care products in the drinking water of Taiwan and their removal in conventional water treatment processes. Chemosphere 2020, 256, 127002. [Google Scholar] [CrossRef]
- Papagiannaki, D.; Morgillo, S.; Bocina, G.; Calza, P.; Binetti, R. Occurrence and human health risk assessment of pharmaceuticals and hormones in drinking water sources in the metropolitan area of Turin in Italy. Toxics 2021, 9, 88. [Google Scholar] [CrossRef]
- Vulliet, E.; Cren-Olivé, C.; Grenier-Loustalot, M.F. Occurrence of pharmaceuticals and hormones in drinking water treated from surface waters. Environ. Chem. Lett. 2011, 9, 103–114. [Google Scholar] [CrossRef]
- Schultz, M.M.; Furlong, E.T.; Kolpin, D.W.; Werner, S.L.; Schoenfuss, H.L.; Barber, L.B.; Blazer, V.S.; Norris, D.O.; Vajda, A.M. Antidepressant pharmaceuticals in two U.S. effluent-impacted streams: Occurrence and fate in water and sediment, and selective uptake in fish neural tissue. Environ. Sci. Technol. 2010, 44, 1918–1925. [Google Scholar] [CrossRef]
- Wu, J.L.; Liu, Z.H.; Ma, Q.G.; Dai, L.; Dang, Z. Occurrence, removal and risk evaluation of ibuprofen and acetaminophen in municipal wastewater treatment plants: A critical review. Sci. Total Environ. 2023, 891, 164600. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.; Durán-Álvarez, J.C.; León Estrada, K.; Chávez, A.; Cisneros, B.J. Accumulation and leaching potential of some pharmaceuticals and potential endocrine disruptors in soils irrigated with wastewater in the Tula Valley, Mexico. Chemosphere 2010, 81, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wang, B.; Lu, S.; Zhang, Y.; Yin, L.; Huang, J.; Deng, S.; Wang, Y.; Yu, G. Characterization of pharmaceutically active compounds in Dongting Lake, China: Occurrence, chiral profiling and environmental risk. Sci. Total Environ. 2016, 557–558, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, R.; Huang, B.; Lin, C.; Wang, Y.; Pan, X. Distribution and bioaccumulation of steroidal and phenolic endocrine disrupting chemicals in wild fish species from Dianchi Lake, China. Environ. Pollut. 2011, 159, 2815–2822. [Google Scholar] [CrossRef]
- Yuan, S.F.; Liu, Z.H.; Huang, R.P.; Yin, H.; Dang, Z. Occurrence, fate, and mass balance of selected pharmaceutical and personal care products (PPCPs) in an urbanized river. Sci. Total Environ. 2020, 727, 138706. [Google Scholar] [CrossRef]
- Huang, C.L.; Ma, H.W.; Yu, C.P. Substance flow analysis and assessment of environmental exposure potential for triclosan in mainland China. Sci. Total Environ. 2014, 499, 265–275. [Google Scholar] [CrossRef]
- Sim, W.J.; Lee, J.W.; Oh, J.E. Occurrence and fate of pharmaceuticals in wastewater treatment plants and rivers in Korea. Environ. Pollut. 2010, 158, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Son, D.J.; Kim, C.S.; Lee, J.H.; Yoon, J.K.; Lee, S.H.; Jeong, D.H. Occurrence assessment of pharmaceuticals in various sewage treatment plants and effluent-receiving streams in Korea. Water 2023, 15, 3897. [Google Scholar] [CrossRef]
- Harbi, K.; Makridis, P.; Koukoumis, C.; Papadionysiou, M.; Vgenis, T.; Kornaros, M.; Ntaikou, I.; Giokas, S.; Dailianis, S. Evaluation of a battery of marine species-based bioassays against raw and treated municipal wastewaters. J. Hazard. Mater. 2017, 321, 537–546. [Google Scholar] [CrossRef]
- Yu, X.; Sui, Q.; Lyu, S.; Zhao, W.; Liu, J.; Cai, Z.; Yu, G.; Barcelo, D. Municipal solid waste landfills: An underestimated source of pharmaceutical and personal care products in the water environment. Environ. Sci. Technol. 2020, 54, 9757–9768. [Google Scholar] [CrossRef]
- Guedes-Alonso, R.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. An on-line solid phase extraction method coupled with UHPLC-MS/MS for the determination of steroid hormone compounds in treated water samples from wastewater treatment plants. Anal. Methods 2015, 7, 5996–6005. [Google Scholar] [CrossRef]
- Kubo, T.; Kuroda, K.; Tominaga, Y.; Naito, T.; Sueyoshi, K.; Hosoya, K.; Otsuka, K. Effective determination of a pharmaceutical, sulpiride, in river water using online solid-phase extraction coupled with high-performance liquid chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014, 89, 111–117. [Google Scholar] [CrossRef]
- Qu, L.; Fan, Y.; Wang, W.; Ma, K.; Yin, Z. Development, validation and clinical application of an online-SPE-LC-HRMS/MS for simultaneous quantification of phenobarbital, phenytoin, carbamazepine, and its active metabolite carbamazepine 10,11-epoxide. Talanta 2016, 158, 77–88. [Google Scholar] [CrossRef]
- Shao, B.; Chen, D.; Zhang, J.; Wu, Y.; Sun, C. Determination of 76 pharmaceutical drugs by liquid chromatography–tandem mass spectrometry in slaughterhouse wastewater. J. Chromatogr. A 2009, 1216, 8312–8318. [Google Scholar] [CrossRef]
- Xao, X.; Jinfeng, G.; Hu, Q.; Ma, J.; Yuan, D.; Fu, X.; Qi, Y.; Volmer, D.A. An advanced LC–MS/MS protocol for simultaneous detection of pharmaceuticals and personal care products in the environment. Rapid Commun. Mass Spectrom. 2022, 37, e9397. [Google Scholar] [CrossRef]
- Belay, M.H.; Precht, U.; Mortensen, P.; Marengo, E.; Robotti, E. A fully automated online SPE-LC-MS/MS method for the determination of 10 pharmaceuticals in wastewater samples. Toxics 2022, 10, 103. [Google Scholar] [CrossRef]
- Chen, L.; Yan, X.; Zhou, X.; Peng, P.; Sun, Q.; Zhao, F. Advances in the on-line solid-phase extraction-liquid chromatography-mass spectrometry analysis of emerging organic contaminants. TrAC Trends Anal. Chem. 2023, 160, 116976. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, J.; Zhong, Q.; Yu, D.; Yao, J.; Huang, T.; Zhu, M.; Zhou, T. A fully automatic cross-used solid-phase extraction online coupled with ultra-high performance liquid chromatography–tandem mass spectrometry system for the trace analysis of multi-class pharmaceuticals in water samples. J. Pharm. Biomed. Anal. 2019, 174, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Marasco Junior, C.A.; da Silva, B.F.; Lamarca, R.S. Automated method to determine pharmaceutical compounds in wastewater using on-line solid-phase extraction coupled to LC-MS/MS. Anal. Bioanal. Chem. 2021, 413, 5147–5160. [Google Scholar] [CrossRef] [PubMed]
- Senta, I.; Rodríguez-Mozaz, S.; Corominas, L.; Covaci, A.; Petrovic, M. Applicability of an on-line solid-phase extraction liquid chromatography–tandem mass spectrometry for the wastewater-based assessment of human exposure to chemicals from personal care and household products. Sci. Total Environ. 2022, 845, 157309. [Google Scholar] [CrossRef]
- Medina, D.A.V.; Maciel, E.V.S.; Lanças, E.M. Modern automated sample preparation for the determination of organic compounds: A review on robotic and on-flow systems. TrAC Trends Anal. Chem. 2023, 166, 117171. [Google Scholar] [CrossRef]
- De Alwis, H.; Heller, D.H. Multiclass, multiresidue method for the detection of antibiotic residues in distillers grains by liquid chromatography and ion trap tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 3046–3084. [Google Scholar] [CrossRef]
- Paíga, P.; Santos, L.H.M.L.M.; Delerue-Matos, C. Development of a multi-residue method for the determination of human and veterinary pharmaceuticals and some of their metabolites in aqueous environmental matrices by SPE-UHPLC-MS/MS. J. Pharm. Biomed. Anal. 2017, 135, 75–86. [Google Scholar] [CrossRef]
- Nefau, T.; Karolak, S.; Castillo, L.; Boireau, V.; Levi, Y. Presence of illicit drugs and metabolites in influents and effluents of 25 sewage water treatment plants and map of drug consumption in France. Sci. Total Environ. 2013, 461–462, 712–722. [Google Scholar] [CrossRef]
- Senta, I.; Krizman, I.; Ahel, M.; Terzic, S. Multiresidual analysis of emerging amphetamine-like psychoactive substances in wastewater and river water. J. Chromatogr. A 2015, 1425, 204–212. [Google Scholar] [CrossRef]
- Sadutto, D.; Picó, Y. Sample preparation to determine pharmaceutical and personal care products in an all-water matrix: Solid phase extraction. Molecules 2020, 25, 5204. [Google Scholar] [CrossRef]
- Furey, A.; Moriarty, M.; Bane, V.; Kinsella, B.; Lehane, M. Ion suppression: A critical review on causes, evaluation, prevention and applications. Talanta 2013, 115, 104–122. [Google Scholar] [CrossRef]
- Senekowitsch, S.; Freitag, T.; Dubinski, D.; Freiman, T.M.; Maletzki, C.; Hinz, B. Validation of an LC-MS/MS method for the simultaneous intracellular quantification of the CDK4/6 inhibitor abemaciclib and the EZH2 inhibitors GSK126 and tazemetostat. Pharmaceutics 2025, 17, 433. [Google Scholar] [CrossRef]
- Raposo, F.; Barceló, D. Challenges and strategies of matrix effects using chromatography-mass spectrometry: An overview from research versus regulatory viewpoints. Trends Anal. Chem. 2021, 134, 116068. [Google Scholar] [CrossRef]
- Boras, J.A.; Vaqué, D.; Maynou, F.; Sà, E.L.; Weinbauer, M.G.; Sala, M.M. Factors shaping bacterial phylogenetic and functional diversity in coastal waters of the NW Mediterranean Sea. Estuar. Coast. Shelf Sci. 2015, 154, 102–110. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Johnson, M.S.; Yoshimura, C. Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous–TiO2 composites: A critical review of recent literature. Water Res. 2018, 142, 26–45. [Google Scholar] [CrossRef]
- Sadutto, D.; Andreu, V.; Ilo, T.; Akkanen, J.; Picó, Y. Pharmaceuticals and personal care products in a Mediterranean coastal wetland: Impact of anthropogenic and spatial factors and environmental risk assessment. Environ. Pollut. 2021, 271, 116353. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, T.; Zeng, Q.; Pei, J.; Li, Q.; Lin, K.; Sun, Q. Application of osmotic pump coupled solid phase extraction samplers for the on-site sampling and enrichment of pharmaceuticals and personal care products in wastewater treatment plants. Environ. Technol. Innov. 2024, 36, 103869. [Google Scholar] [CrossRef]
- Li, W.L.; Zhang, Z.F.; Sparham, C.; Li, Y.F. Validation of sampling techniques and SPE-UPLC/MS/MS for home and personal care chemicals in the Songhua Catchment, Northeast China. Sci. Total Environ. 2020, 707, 136038. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Q.; Zhang, H.; Wang, Z.; Wu, A.; Luo, Y.; Zhou, Q. For the occurrence of PPCPs from source to tap: A novel approach modified in terms of sample preservation and SPE cartridge to monitor PPCPs in our water supply. Anal. Chim. Acta 2024, 1308, 342662. [Google Scholar] [CrossRef]
- Nannou, C.; Efthymiou, C.; Boti, V.; Albanis, T. Unveiling the pharmaceutical footprint in freshwater and seawater: HRMS method optimization, validation, and uncertainty estimation. Microchem. J. 2025, 210, 112933. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Zioris, I.; Danis, T.; Bikiaris, D.; Lambropoulou, D. Comprehensive investigation of a wide range of pharmaceuticals and personal care products in urban and hospital wastewaters in Greece. Sci. Total Environ. 2019, 694, 133565. [Google Scholar] [CrossRef]
- Folorunsho, O.; Bogush, A.; Kourtchev, I. Occurrence of emerging and persistent organic pollutants in the rivers Cam, Ouse and Thames, UK. Sci. Total Environ. 2025, 962, 178436. [Google Scholar] [CrossRef]
- Löher, F.; Palma, W.-U.; Schaffer, M.; Olsson, O. Concentrations and sources of methylxanthines in a Northern German river system. Sci. Total Environ. 2021, 775, 145898. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Hu, A.; Rashid, A.; Ashfaq, M.; Wang, Y.; Wang, H.; Luo, H.; Yu, C.P.; Sun, Q. Monitoring, mass balance and fate of pharmaceuticals and personal care products in seven wastewater treatment plants in Xiamen City, China. J. Hazard. Mater. 2018, 354, 81–90. [Google Scholar] [CrossRef]


| Compounds | Abbreviation | pKa | Log Kow | Pv (mmHg) | MW | Molecular Formula |
|---|---|---|---|---|---|---|
| Acetaminophen | ACP | 9.38 | 0.46 | 7 × 10−6 | 151.17 | C8H9NO2 |
| Atenolol | ATN | 9.6 | 0.16 | 2.924 × 10−10 | 266.34 | C14H22N2O3 |
| Ranitidine | RNT | 8.2 | 0.27 | 1.2 × 10−7 | 314.41 | C13H22N4O3S |
| Paraxanthine | PRX | 0.9 | −0.07 | 7.6 × 10−5 | 180.16 | C7H8N4O2 |
| Iopromide | IPM | 8.4 | −3.1 | 1.4 × 10−20 | 791.1 | C18H24I3N3O8 |
| Caffeine | CFI | 14 | −0.07 | 2.5 × 10−3 | 194.19 | C8H10N4O2 |
| Sulfamethazine | SFA | 7.4 | 0.14 | 5.2 × 10−8 | 264.30 | C11H12N4O2S |
| Trimethoprim | TMP | 7.12 | 0.91 | 9.88 × 10−9 | 290.32 | C14H18N4O3 |
| Sulfamethoxazole | SFX | 6.0 | 0.89 | 6.93 × 10−8 | 253.28 | C10H11N3O3S |
| Lincomycin | LCM | 7.6 | 0.29 | 2.7 × 10−20 | 406.54 | C18H34N2O6S |
| Propranolol | PPN | 9.5 | 3.48 | 1.7 × 10−5 | 259.80 | C16H21NO2 |
| Carbamazepine | CBZ | 7 | 2.47 | 1.84 × 10−7 | 236.27 | C15H12N2O |
| Naproxen | NPX | 4.15 | 3.18 | 1.892 × 10−6 | 230.27 | C14H14O3 |
| Diclofenac | DCF | 4.14 | 4.51 | 6.14 × 10−8 | 296.16 | C14H10Cl2NO2 |
| Ibuprofen | IBF | 4.91 | 3.97 | 1.162 × 10−11 | 206.23 | C13H18O2 |
| Triclosan | TCS | 7.9 | 4.76 | 4.0 × 10−6 | 444.44 | C22H24N2O8 |
| Item | Value |
|---|---|
| pH | 6.8 ± 0.2 |
| Turbidity (NTU) | 0.54 ± 0.34 |
| TDS (mg/L) | 325 ± 105 |
| TOC (mg/L) | 5.2 ± 1.7 |
| UV254 (cm−1) | 0.088 ± 0.020 |
| BOD5 (mg/L) | 1.8 ± 0.5 |
| T-N (mg/L) | 14.12 ± 3.40 |
| T-P (mg/L) | 0.169 ± 0.080 |
| Compounds | Precursor Ion (m/z) | Product Ion (m/z) | RT (min) | Collision Energy (eV) |
|---|---|---|---|---|
| Acetaminophen | 152 [M + H]+ | 65 | 2.28 | 10 |
| Atenolol | 267 [M + H]+ | 116 | 2.04 | 30 |
| Ranitidine | 315 [M + H]+ | 176 | 2.05 | 30 |
| Paraxanthine | 180 [M + H]+ | 149 | 2.58 | 24 |
| Iopromide | 791 [M + H]+ | 774 | 2.45 | 50 |
| Caffeine | 195 [M + H]+ | 83 | 3.17 | 20 |
| Sulfamethazine | 279 [M + H]+ | 124 | 3.22 | 30 |
| Trimethoprim | 291 [M + H]+ | 123 | 2.87 | 20 |
| Sulfamethoxazole | 254 [M + H]+ | 92 | 3.61 | 10 |
| Lincomycin | 407 [M + H]+ | 126 | 2.89 | 30 |
| Propranolol | 206 [M + H]+ | 116 | 5.19 | 20 |
| Carbamazepine | 237 [M + H]+ | 179 | 6.31 | 20 |
| Naproxen | 231 [M − H]− | 141 | 7.41 | 10 |
| Diclofenac | 296 [M − H]− | 213 | 8.49 | 5 |
| Ibuprofen | 205 [M − H]− | 161 | 8.66 | 20 |
| Triclosan | 288 [M − H]− | 35 | 2.87 | 20 |
| Acetaminophen-d4 | 156 [M + H]+ | 114 | 2.34 | 10 |
| Atenolol-d7 | 247 [M + H]+ | 145 | 2.07 | 30 |
| Ranitidine-d6 | 343 [M + H]+ | 207 | 2.03 | 20 |
| Paraxanthine-d3 | 183 [M + H]+ | 124 | 2.52 | 30 |
| Iopromide-d3 | 792 [M + H]+ | 603 | 2.32 | 50 |
| Caffeine-d9 | 207 [M + H]+ | 150 | 3.12 | 20 |
| Trimethoprim-d9 | 300 [M − H]+ | 123 | 2.83 | 10 |
| Sulfamethoxazole-d4 | 259 [M − H]+ | 97 | 3.57 | 10 |
| Lincomycin-d3 | 408 [M − H]+ | 126 | 2.88 | 30 |
| Propranolol-d7 | 268 [M + H]+ | 116 | 5.17 | 20 |
| Carbamazepine-d10 | 247 [M − H]+ | 201 | 6.24 | 30 |
| Naproxen-d4 | 233 [M − H]− | 189 | 7.47 | 10 |
| Diclofenac-d4 | 298 [M − H]− | 254 | 8.42 | 10 |
| Ibuprofen-d3 | 208 [M − H]− | 164 | 8.59 | 5 |
| Triclosan-d3 | 290 [M − H]− | 35 | 2.88 | 20 |
| Sulfamethazine-13C6 | 162 [M − H]+ | 98 | 3.58 | 30 |
| Compounds | IS | Linear Range (μg/L) | MDL (ng/L) | MQL (ng/L) | RSD (%) (n = 5) | Accuracy (%) |
|---|---|---|---|---|---|---|
| Acetaminophen | Acetaminophen-d4 | 0.01–100 | 2.8 | 8.9 | 7.4 | 85.3 |
| Atenolol | Atenolol-d7 | 0.05–100 | 5.6 | 20.7 | 11.2 | 85.5 |
| Ranitidine | Ranitidine-d6 | 0.05–100 | 7.8 | 25.5 | 4.4 | 87.7 |
| Paraxanthine | Paraxanthine-d3 | 0.5–100 | 103.4 | 328.9 | 3.3 | 99.5 |
| Iopromide | Iopromide-d3 | 1–100 | 4.5 | 59.4 | 5.7 | 95.8 |
| Caffeine | Caffeine-d9 | 0.05–100 | 7.2 | 24.1 | 9.3 | 86.3 |
| Sulfamethazine | Sulfamethazine-13C6 | 0.01–100 | 4.7 | 3.8 | 8.9 | 95.4 |
| Trimethoprim | Trimethoprim-d9 | 0.05–100 | 3.4 | 11.4 | 15.4 | 95.7 |
| Sulfamethoxazole | Sulfamethoxazole-d4 | 0.01–100 | 3.4 | 3.6 | 5.0 | 104.9 |
| Lincomycin | Lincomycin-d3 | 0.02–100 | 4.4 | 11.4 | 9.4 | 85.8 |
| Propranolol | Propranolol-d7 | 0.01–100 | 3.4 | 3.6 | 12.0 | 92.9 |
| Carbamazepine | Carbamazepine-d10 | 0.05–100 | 3.4 | 11.4 | 8.1 | 88.2 |
| Naproxen | Naproxen-d3 | 0.02–100 | 2.5 | 8.4 | 6.5 | 100.6 |
| Diclofenac | Diclofenac-d4 | 0.02–100 | 2.5 | 8.4 | 8.5 | 95.1 |
| Ibuprofen | Ibuprofen-d3 | 0.1–100 | 16.2 | 54.0 | 6.5 | 97.6 |
| Triclosan | Triclosan-d3 | 0.05–100 | 7.8 | 25.9 | 5.4 | 86.4 |
| Compounds | Matrix Effect | |||||
|---|---|---|---|---|---|---|
| 1 (μg/L) | 5 (μg/L) | 20 (μg/L) | ||||
| Mean (%) | RSD (%) | Mean (%) | RSD (%) | Mean (%) | RSD (%) | |
| Acetaminophen | 82.4 | 9.2 | 84.9 | 7.4 | 103.5 | 5.2 |
| Atenolol | 103.1 | 14.1 | 106.3 | 12.1 | 112.7 | 9.8 |
| Ranitidine | 87.2 | 9.2 | 89.9 | 9.4 | 114.6 | 5.4 |
| Paraxanthine | 69.5 | 5.4 | 73.2 | 3.4 | 87.5 | 3.4 |
| Iopromide | 110.7 | 6.7 | 114.1 | 5.7 | 88.6 | 5 |
| Caffeine | 109.5 | 6.36 | 112.9 | 5.6 | 112.8 | 5.6 |
| Sulfamethazine | 89.3 | 6.7 | 92.1 | 6 | 113.8 | 5.8 |
| Trimethoprim | 97.4 | 5.5 | 104.4 | 4.5 | 112.9 | 3.5 |
| Sulfamethoxazole | 75 | 8.9 | 78.9 | 5.6 | 84.7 | 5.1 |
| Lincomycin | 85.9 | 7.5 | 86.5 | 6.9 | 104.6 | 5.3 |
| Propranolol | 132.6 | 6.4 | 136.5 | 5.4 | 113.7 | 4.2 |
| Carbamazepine | 92.1 | 6.9 | 95.1 | 6.2 | 110.5 | 3.2 |
| Naproxen | 93.7 | 6.8 | 96.6 | 6.5 | 102.8 | 3.2 |
| Diclofenac | 121.3 | 8.3 | 120.7 | 6.3 | 112.3 | 3.3 |
| Ibuprofen | 71.8 | 10.4 | 75.6 | 8.4 | 80.4 | 6.9 |
| Triclosan | 58.8 | 8.9 | 61.9 | 5.4 | 97.9 | 6.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, S.-H.; Kye, H.; Lee, J.; Kim, E.; Koo, J.-W.; Park, J.; Shin, Y.; Lee, J.; Hwang, T.-M. Robotic and On-Flow Solid Phase Extraction Coupled with LC-MS/MS for Simultaneous Determination of 16 PPCPs: Real-Time Monitoring of Wastewater Effluent in Korea. Toxics 2025, 13, 899. https://doi.org/10.3390/toxics13100899
Nam S-H, Kye H, Lee J, Kim E, Koo J-W, Park J, Shin Y, Lee J, Hwang T-M. Robotic and On-Flow Solid Phase Extraction Coupled with LC-MS/MS for Simultaneous Determination of 16 PPCPs: Real-Time Monitoring of Wastewater Effluent in Korea. Toxics. 2025; 13(10):899. https://doi.org/10.3390/toxics13100899
Chicago/Turabian StyleNam, Sook-Hyun, Homin Kye, Juwon Lee, Eunju Kim, Jae-Wuk Koo, Jeongbeen Park, Yonghyun Shin, Jonggul Lee, and Tae-Mun Hwang. 2025. "Robotic and On-Flow Solid Phase Extraction Coupled with LC-MS/MS for Simultaneous Determination of 16 PPCPs: Real-Time Monitoring of Wastewater Effluent in Korea" Toxics 13, no. 10: 899. https://doi.org/10.3390/toxics13100899
APA StyleNam, S.-H., Kye, H., Lee, J., Kim, E., Koo, J.-W., Park, J., Shin, Y., Lee, J., & Hwang, T.-M. (2025). Robotic and On-Flow Solid Phase Extraction Coupled with LC-MS/MS for Simultaneous Determination of 16 PPCPs: Real-Time Monitoring of Wastewater Effluent in Korea. Toxics, 13(10), 899. https://doi.org/10.3390/toxics13100899