1. Introduction
Recent decades have seen a substantial increase in human metabolic disorders—including obesity, diabetes, and metabolic syndrome—which now represent a major public health challenge in the United States and globally [
1,
2]. Agricultural practices have evolved in tandem with the widespread application of pesticides. Of these, herbicides are by far the most heavily applied class of pesticides, accounting for ~88% of agricultural herbicide mass during 2013–2017 in the United States, whereas insecticides and fungicides each contributed ~6%. Moreover, by 2016 glyphosate alone comprised ~44% of all herbicide mass applied to crops [
3,
4]. Our prior study demonstrated a significant association between glyphosate exposure, measured in urine, and metabolic syndrome using nationally representative NHANES data, with marked differences observed across racial groups [
5]. These findings suggest that herbicide exposure may be an underappreciated environmental co-factor of metabolic dysfunction. However, given that agricultural herbicide exposures rarely occur in isolation, a comprehensive analysis that includes multiple compounds is warranted to elucidate interactive and mixture effects on metabolic health.
In the present study, we extend our investigation with an ecologic study of herbicide application and its associations with county-level obesity rates by incorporating a panel of thirteen herbicides—glyphosate, herbicide 2,4-D (2,4-D), atrazine, dicamba, trifluralin, acetochlor, dimethenamid-P, glufosinate, metolachlor, metolachlor + metolachlor-S, pendimethalin, paraquat—and one fungicide—chlorothalonil—that are most extensively applied in U.S. agriculture and have been the subject of extensive toxicologic and epidemiologic evaluation due to their widespread application and potential health and environmental impacts [
6]. In addition glyphosate applications have increased dramatically since 1996 (15-fold by 2016) following the development of “Roundup
TM-ready” crops [
7]. Enthusiastic adoption of “Roundup
TM-ready” crops has resulted in increased applications of dicamba and herbicide 2-4D to manage herbicide-resistant weeds. The selection of the fourteen compounds studied here was informed by multiple criteria: (1) their high prevalence in agricultural use in the United States, and documented potential for environmental persistence; (2) emerging toxicological evidence implicating them in endocrine disruption, oxidative stress, and perturbations in glucose and lipid metabolism [
8,
9,
10]; and (3) epidemiological observations that link these exposures to adverse metabolic outcomes [
11]. Importantly, many of these herbicides are applied together in modern cropping systems, potentially leading to synergistic and mixture effects that are not adequately captured by single-pollutant studies.
The biology linking these compounds to metabolic disturbances is supported by several mechanistic pathways. For instance, glyphosate has been implicated in disrupted insulin signaling and altering the gut microbiome—both of which are critical regulators of energy homeostasis and metabolic function [
12,
13,
14,
15]. Similarly, atrazine and 2,4-D have been associated with endocrine disruption, which may interfere with hormonal regulation of adipogenesis and glucose metabolism [
10,
16]. The neurotoxic potential of paraquat [
17,
18] further raises concerns about systemic oxidative stress, a contributor to insulin resistance and beta-cell dysfunction [
19,
20]. In integrating diverse lines of evidence, our study seeks to clarify whether cumulative exposures to herbicides contribute to metabolic disorders at a population level.
After assessing associations between county-level obesity rates and single pollutants using mixed-effects models with a random term for county FIPS, we use a mixture model approach to assess mixture effects, using a county-level random effect to account for location. This approach enables us to accommodate geographic variations in herbicide usage and to control for spatially specific local demographic and socioeconomic factors that may modify exposure–outcome associations. Finally, by stratifying analyses on National Center for Health Statistics (NCHS) urban–rural designation, we aim to reveal potential environmental health disparities that could inform targeted public health interventions.
Mixture effects. Mixture effects occur when multiple environmental pollutants interact in ways that amplify their individual health effects, and may be more common when compounds are in related families [
21,
22]. Pollutant mixtures may have joint effects [
23], even at low doses [
24,
25], and if there are interactions between components the total mixture effect can be amplified [
26]. As pollutant mixture effects are a long-recognized source of health risk, methods to assess the effects of pollutant mixtures, primarily for air pollutant mixtures, were in use as early as 2000 [
27]. Despite some focus on acute herbicide exposure (poisoning), the effects of chronic ambient environmental exposure herbicide mixtures are little studied in view of increasing global application of herbicide mixtures for everything from weed control in cropping to crop desiccation, aquatic or wildland weed control, and urban area management [
28,
29].
In summary, our study addresses critical gaps in the current understanding of environmental risk factors for metabolic disorders. By examining a spectrum of herbicides with documented endocrine and metabolic perturbations, this study provides a nuanced assessment of the complex interplay between agricultural chemical exposures and metabolic health outcomes as a function of exposure setting and magnitude. Such insights are vital for informing regulatory policies and for designing future studies that can elucidate the causal pathways underlying these associations.
4. Discussion
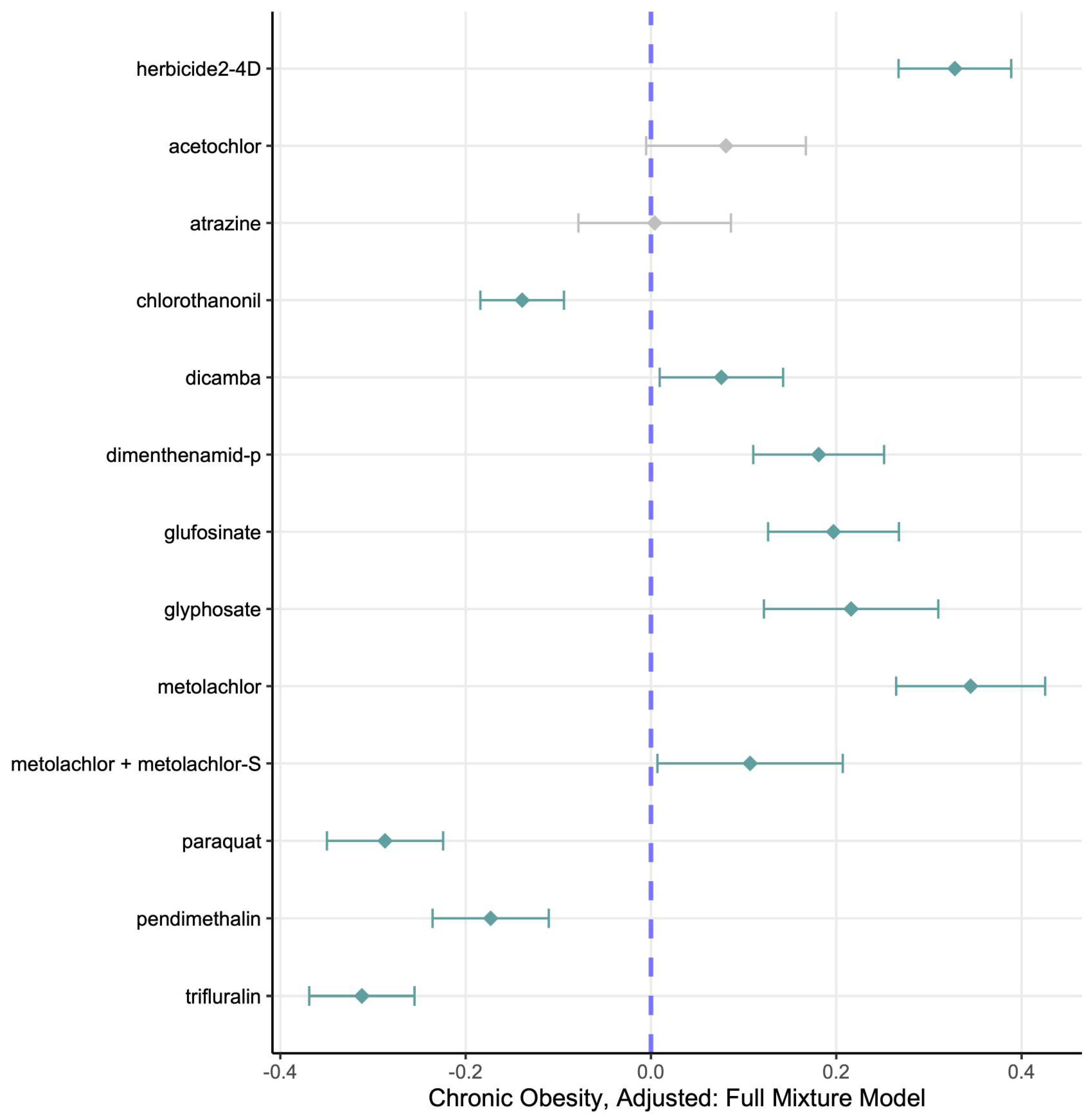
Using freely available public data on county-level obesity and application of the 14 most commonly used herbicides in a five-year period (2013–2018), we have found significant adjusted associations between county-level obesity rates, individual herbicides, and their mixture. We have shown that these associations roughly mirror pesticide applications, and that these are in part, but not fully, associated with rurality and setting.
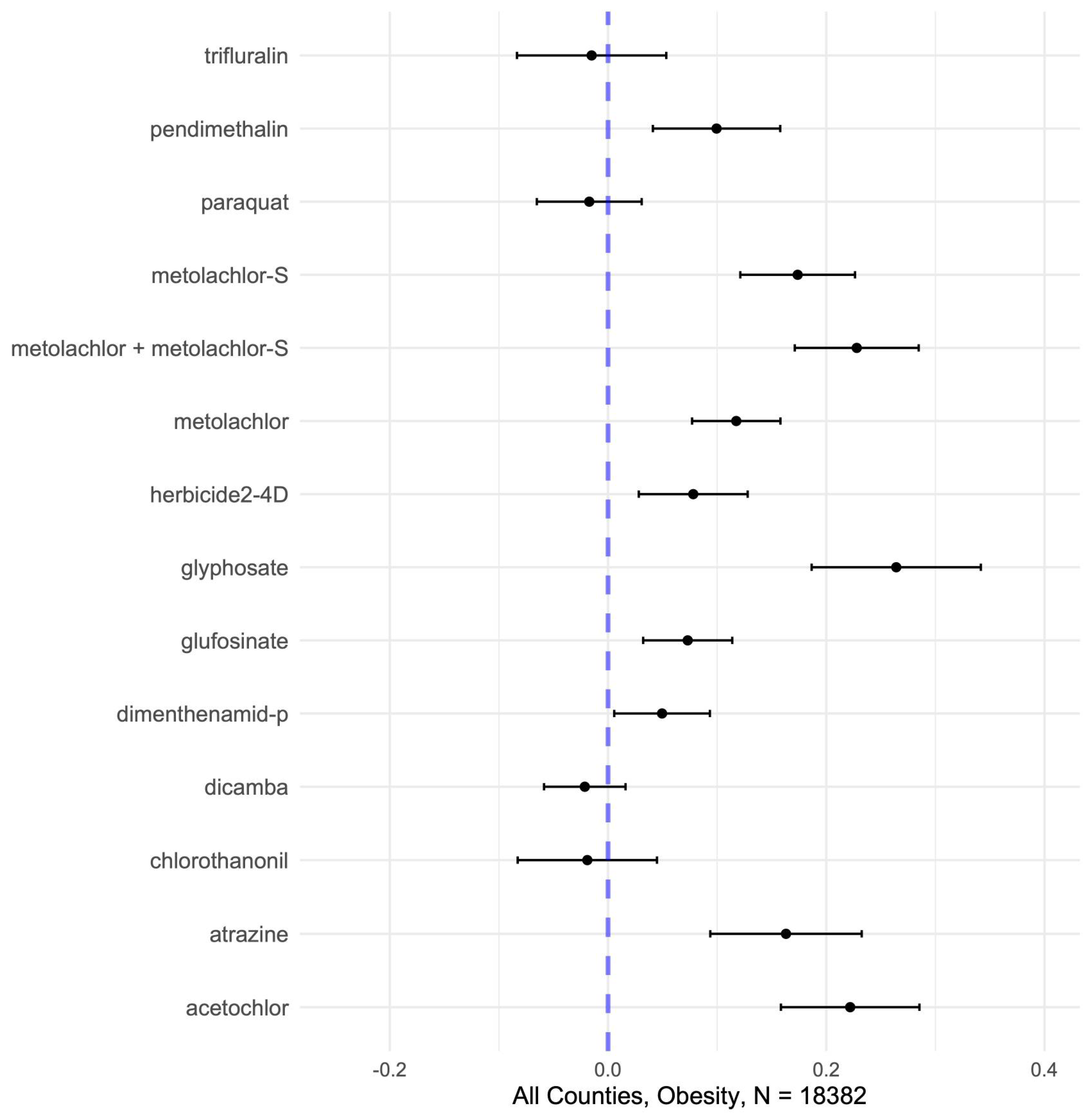
Our analysis demonstrates that U.S. counties with greater herbicide use, including glyphosate and several other common agents, tend to exhibit higher obesity rates, consistent with prior evidence that herbicide exposure contributes to adverse metabolic outcomes [
48,
49]. We observed significant positive associations from single-pollutant models between county-level obesity prevalence and the application of multiple herbicides (10 of the 14 analyzed in standardized continuous data, 12 of 14 in quantile categorized), most notably glyphosate, acetochlor, metolachlor -S, metolachlor (including its S-isomer), atrazine, metolachlor, pendimethalin, 2,4-D, glufosinate, and dimenthenamid-P (
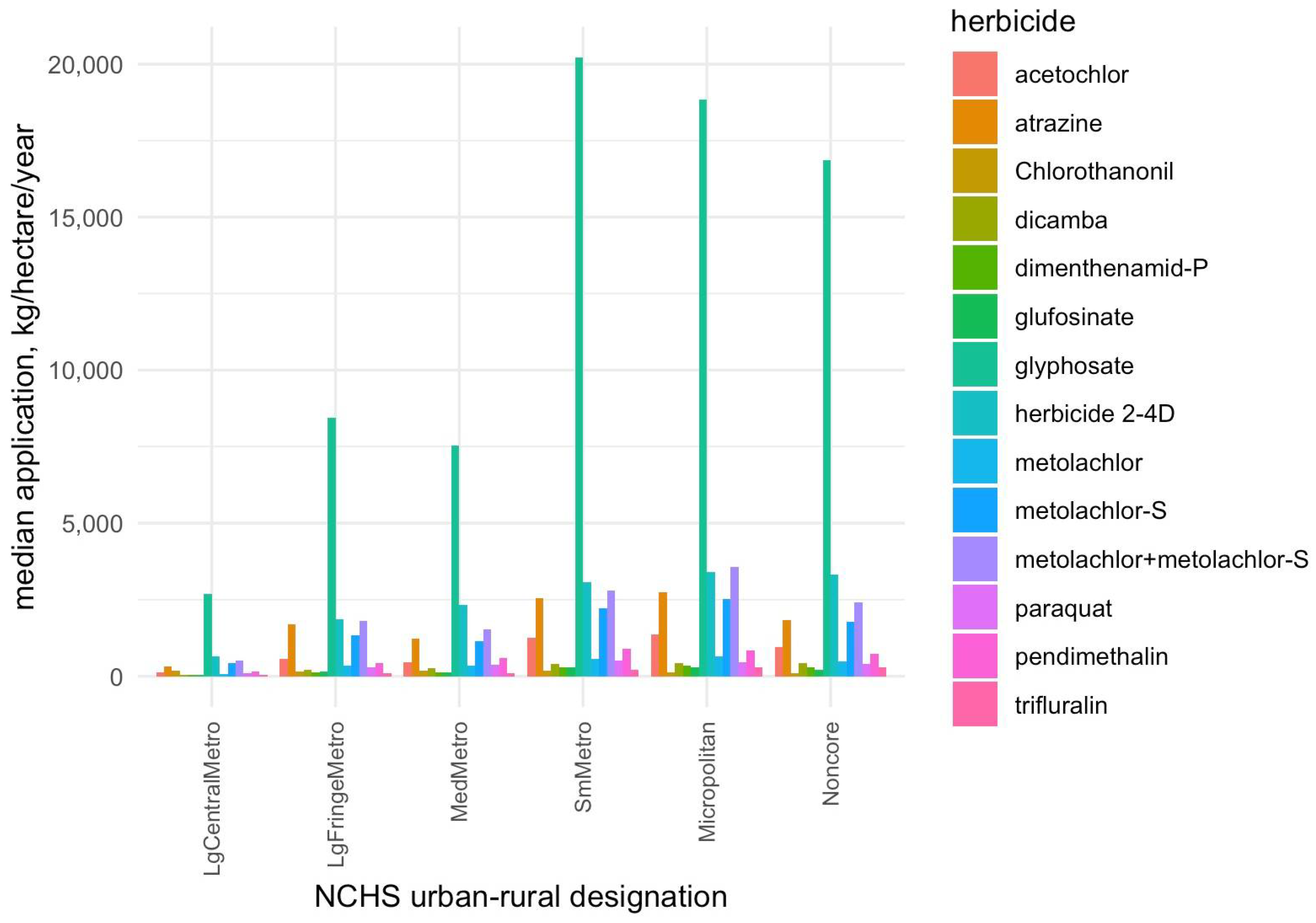
Figure 1), as well as some evidence of a nonlinear dose–response relationship. Glyphosate, as the most heavily used herbicide (
Figure 4), showed the strongest individual associations with obesity, followed by 2,4-D and atrazine, consistent with human studies linking these herbicides to metabolic syndrome, of which obesity is a key component [
5,
50,
51,
52,
53,
54]. Experimental studies provide plausible biological mechanisms by which these herbicides may influence metabolic outcomes like obesity. Glyphosate has been shown to alter gut microbiota composition, increase oxidative stress, and up-regulate expression of the NF-κB (Nuclear Factor kappa-light-chain-enhancer of activated B cells), all of which can impair insulin signaling and promote adipogenesis [
14,
55,
56]. Atrazine and 2,4-D have been linked to endocrine disruption through interference with estrogenic and thyroid pathways, activation of PPARγ—a transcription factor central to fat storage and adipocyte differentiation—and mitochondrial dysfunction, leading to insulin resistance [
50,
57]. These mechanistic insights support the plausibility that chronic exposure to herbicide mixtures could dysregulate energy balance, lipid metabolism, and glucose homeostasis, thereby contributing to obesity risk.
Not all compounds had significant linear independent effects in continuous data (e.g., dicamba, paraquat, trifluralin, and chlorothalonil showed no positive single-pollutant association with obesity in continuous data). However, most showed partial positive effects (usually the highest levels, Q4 and Q5 relative to Q1) in quintile categorized data, highlighting that the health impacts of herbicides are not uniform across compounds, and that slight nonlinearities may mask significant associations when examined in continuous data. This heterogeneity likely reflects different toxicological mechanisms and exposure patterns which may influence metabolic outcomes in subtle and indirect ways.
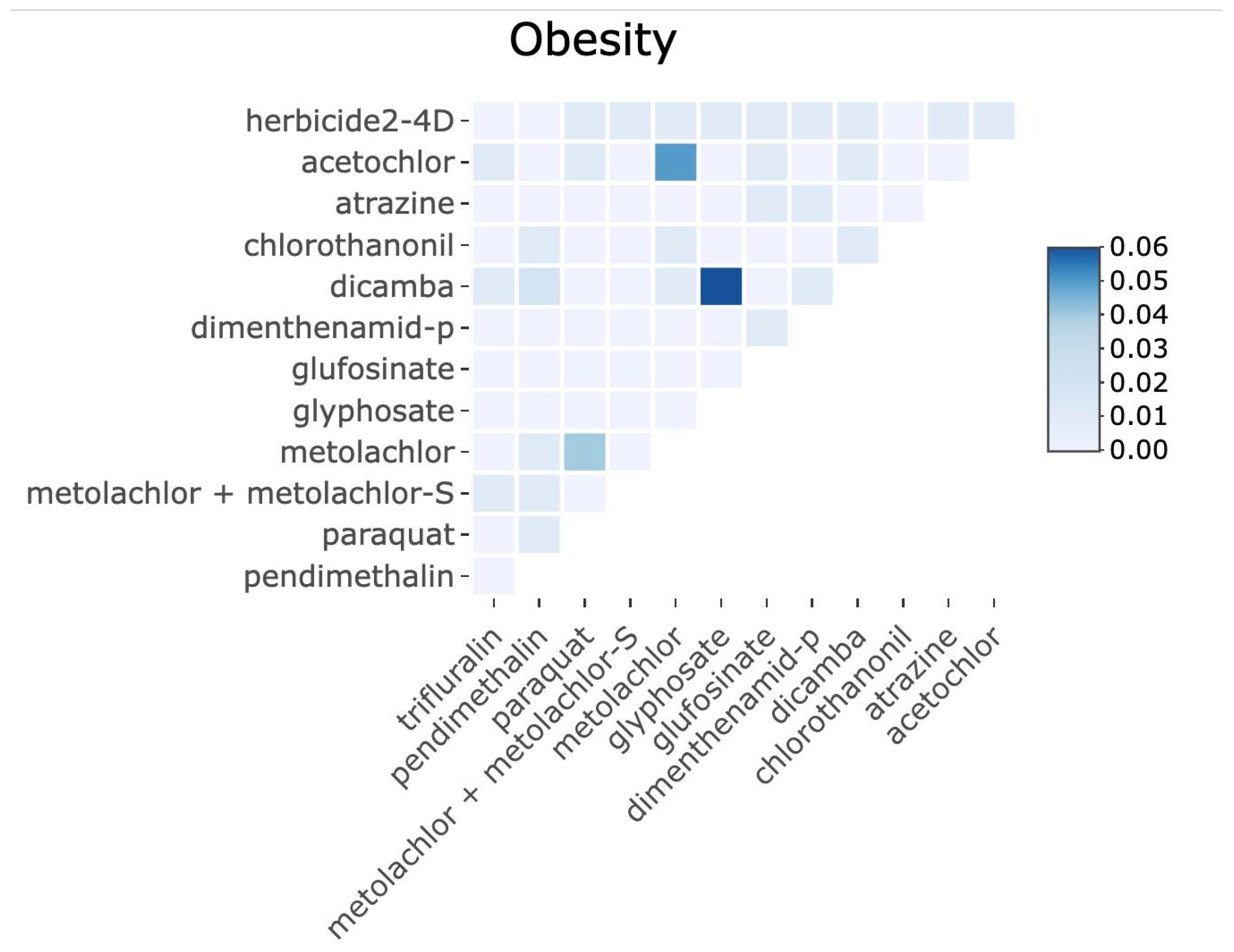
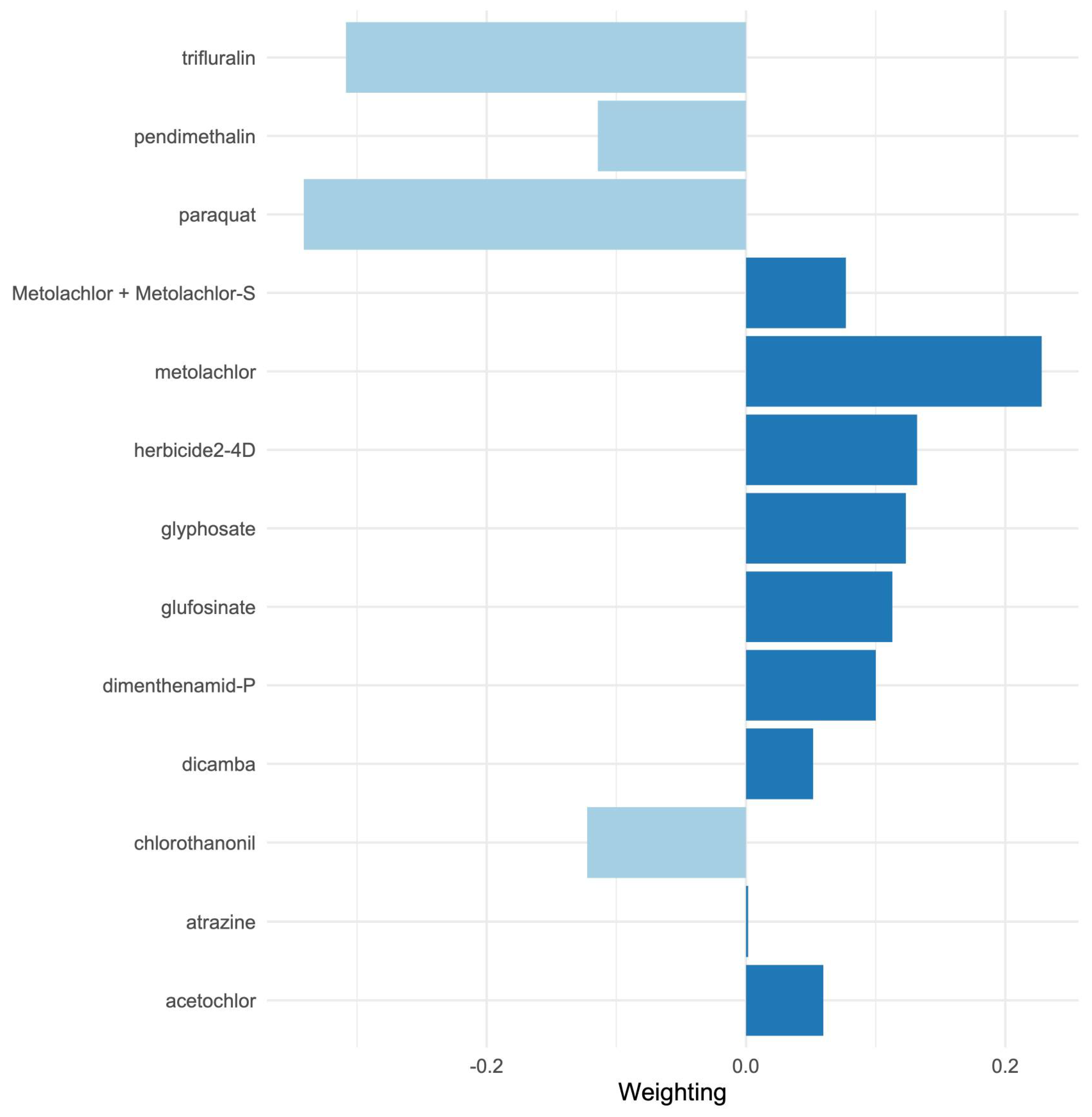
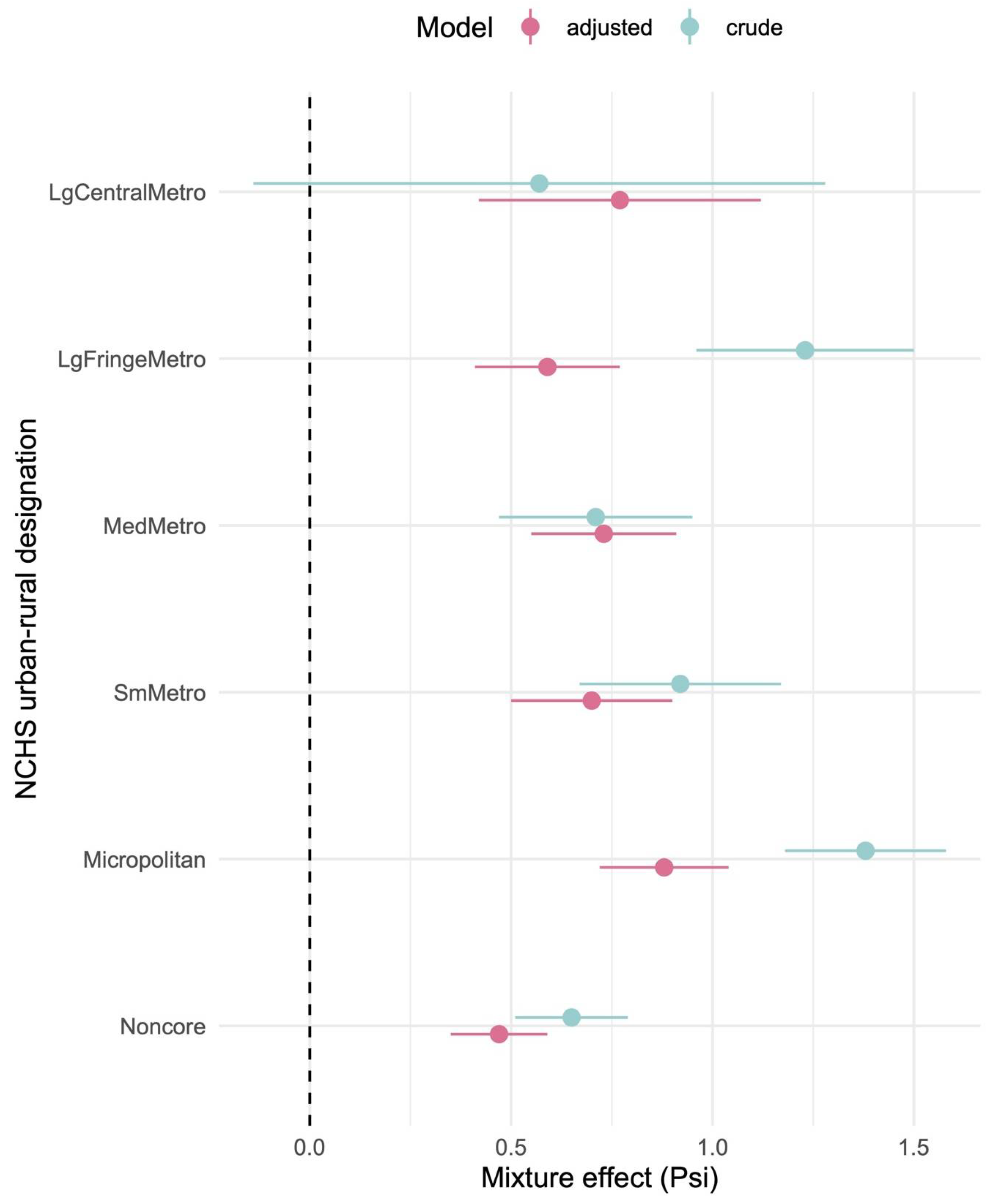
Importantly, our mixture modeling analysis suggests that combined exposures to herbicides may better explain population obesity patterns than individual chemicals alone. Using quantile-based g-computation, we found that a one-quantile increase in the overall herbicide mixture was associated with a significant increase in county obesity rates, even after adjusting for socioeconomic covariates. In the adjusted mixture model, herbicides such as 2,4-D, glyphosate, metolachlor, glyphosate, dimethenamid-P, glufosinate, metolachlor (including its S-isomer), dicamba, acetochlor, and atrazine showed the largest positive weight contributions to this mixture effect, whereas others (paraquat, trifluralin, pendimethalin, and chlorothalonil) contributed negative weights. Notably, some herbicides that showed null or modest single-pollutant associations (e.g., paraquat) exhibited negative contributions in the mixture context, possibly reflecting complex interactions (see
Appendix A,
Figure A3) or spatial usage patterns that are not fully accounted for in the mixture model (for instance, areas with high paraquat use may have lower use of other obesogenic herbicides, diluting the overall mixture effect). On the other hand, glyphosate—while strongly associated on its own—did not singularly dominate the mixture effect, as several co-occurring herbicides also exerted substantial influence. These results underscore that glyphosate is not uniquely responsible for the observed obesity link; rather, it is one significant part of a total herbicide mixture effect. In practical terms, evaluating herbicides in isolation may bias risk estimates, whereas accounting for concurrent exposure mixtures provides a more realistic assessment of environmental influences on obesity. Our study is among the first ecological analyses to examine herbicide mixtures in relation to metabolic health, and it provides novel evidence that the cumulative effects of multiple agrichemicals are relevant to population obesity rates.
4.1. Contextualizing Rural–Urban Disparities
A notable finding from our study is the heightened impact of herbicide exposure in more rural areas. herbicide usage tends to be highest in counties of intermediate-to-high rurality (e.g., small metropolitan and micropolitan areas) and lowest in major urban centers (
Figure 4). Consistently, we observed that the overall herbicide mixture effect on obesity was more pronounced in more rural counties: the estimated obesity increase per mixture quartile was smallest in large metropolitan areas and grew larger moving toward small metro and micropolitan counties. Interestingly, mixture effects in the most remote rural counties (“non-core” areas) did not follow a strictly linear trend—they showed the highest baseline obesity rates but a somewhat lower mixture effect estimate—suggesting that beyond a certain point, additional exposures may yield diminishing returns, or that these communities may face saturated obesity risk from many non-measured contributors. Overall, however, the pattern indicates that communities with intensive agriculture (often rural) may experience a double burden: greater chemical exposure alongside underlying vulnerabilities such as higher poverty, limited health care access, and other lifestyle risk factors [
58]. These contextual factors can amplify the health impact of environmental exposures. Indeed, rural populations often have fewer resources to mitigate or treat chronic conditions, which could exacerbate the observed associations. Our findings therefore support calls for region-specific public health interventions and regulatory approaches. In practice, this could mean prioritizing cumulative risk assessments for heavily agricultural rural regions and tailoring obesity prevention programs to address both lifestyle and environmental factors in these communities. By recognizing that rurality can be a proxy for both elevated exposure and increased susceptibility, public health officials can better target efforts to reduce herbicide exposure and bolster health care support in high-risk counties. Recent work has underscored how socioeconomic and infrastructural disparities can modify the health impacts of environmental exposures [
58].
4.2. Confounding by Smoking and Diet
We adjusted for county-level adult smoking prevalence because rural smoking remains higher than urban smoking in the U.S. after adjustment for socio-demographics, showing substantially higher smoking in rural/upstate counties even where poverty is high [
59,
60]. We adjusted for diet proxies, specifically food insecurity and educational attainment, because both are well-established county-level predictors of diet quality, and because counties with higher food insecurity and lower education consistently show poorer diet quality, including lower fiber intake, higher added sugar intake, and worse overall Healthy Eating Index scores [
41,
61,
62]. Furthermore, although we could not directly account for food consumption patterns, national studies confirm that rural populations tend to consume ultra-processed diets, with less fiber and more added sugar, which could contribute to higher obesity prevalence independent of herbicide exposure [
63,
64]. Therefore, our adjustment of selected dietary proxies help reduce confounding by diet, which may be systematically poorer in rural areas, although cannot fully address the lack of direct county-level measures of diet and physical activity. Other exposures common in agricultural settings, including the use of other types of pesticides (e.g., insecticides, rodenticides, etc.), air pollution, and heavy metals, may also confound or interact with herbicide–obesity associations, and their omission represents an additional limitation.
4.3. New Dimensions in Exposure Assessment
Our study contributes methodologically by leveraging publicly available county-level herbicide application data to approximate population exposures. This ecological approach enabled us to probe potential exposure–response relationships on a national scale. We observed, for example, that counties with the highest herbicide application levels tended to have some of the highest obesity prevalence, with obesity rates plateauing around ~31% in the most agriculturally intensive areas. Although based on observational correlations, this pattern raises the hypothesis of threshold effects whereby metabolic health risks may accelerate once herbicide use (and by inference, community exposure) exceeds a certain level. Our analysis, which captured geographic variability and multiple chemicals simultaneously, provides a more comprehensive real-world exposure scenario than single-chemical studies. By accounting for dozens of U.S. states and a spectrum of herbicide compounds, we capture the complex environmental conditions under which human populations actually live. Furthermore, our ecological approach adds a new dimension to exposure science by marrying large-scale data with mixture modeling to yield insights that are not apparent when studying one chemical or one location at a time. This integrated exposure assessment is a significant step forward in bridging the gap between epidemiological observations and mechanistic toxicology.
4.4. Strengths, Limitations, and Future Directions
Strengths of our study include that we analyzed a large, nationally representative dataset covering six years (2013–2018) and over 3000 counties, which provides ample statistical power and broad generalizability. We integrated high-quality data from federal sources—including USGS agricultural herbicide use estimates and CDC-modeled county obesity prevalence—and adjusted for a range of demographic and socioeconomic covariates to reduce confounding. To address missing data, we employed multiple imputation methods customized to the dataset, maximizing the use of available information and limiting bias from incomplete records. Notably, we applied a rigorous mixture modeling approach (quantile g-computation) to estimate the joint effect of 13 herbicides on obesity. This method, developed specifically for epidemiologic mixtures, allowed us to calculate an overall effect estimate (Psi) for the herbicide blend while yielding weights for each component. The quantile-based approach is robust to distributional extremes and multicollinearity, enhancing our confidence that the observed mixture effect is not an artifact of one highly prevalent chemical. Taken together, the study design and analytical techniques provide a robust triangulation of evidence at the population level, complementing prior individual-level study.
Our study has several limitations. First, the study is ecologic and analysis was performed on data that is aggregated at county level, thus results cannot be interpreted as causal effects at the individual level, and indeed, spatiotemporal aggregation may obscure finer-scale relationships. However, a gold-standard randomized controlled trial (RCT) for these exposures and outcomes would establish causality at an individual level, but would be unethical. And, while a longitudinal study would strengthen causality, one of this size would be prohibitively expensive and, if observational, would not establish causality on its own. Thus, while it has stated limitations, our study leverages publicly available datasets to explore associations between a ubiquitous set of exposures and a chronic health outcome on national scales. Our study is based on an assumption that county-level herbicide application is correlated with local human exposure, and results may be influenced by unmeasured county-level factors such as diet, physical activity, or other correlated exposures. We address this by including county-level random effects, adjusting for rurality, and controlling for multiple socio-demographic variables. Aggregation at the county level may introduce a modifiable area unit problem. Our herbicide use metric is a surrogate for human exposure and does not account for individual behaviors or chemical drift/dynamics, which may lead to non-differential exposure misclassification and bias estimates toward the null. County obesity prevalence was obtained from a modeling method (BRFSS small-area estimates) that has its own uncertainty. However, by including year as a fixed effect and county random effects we attempted to account for differences associated with time and spatially associated variability. Fourth, while quantile g-computation is a powerful tool for mixtures, it computes additive effects of increasing all exposures by one quantile and de facto assumes linearity unless nonlinear terms are explicitly incorporated into a model. We found minimal evidence of pairwise interactions among herbicides in supplementary analyses, suggesting the additivity assumption was reasonable. Still, very high correlation between certain herbicides (e.g., metolachlor and metolachlor-S) required us to drop one variable to avoid collinearity, highlighting a general challenge in multi-pollutant studies. Our current dataset (county-level CDC obesity data with additional county-level covariates from the ACS) comprises population rates by county and thus does not allow stratification by sex, and does not include occupational proportions. Therefore, we were unable to stratify by sex or by rural occupational status, other than including rate of unemployment by county. Future studies with individual-level data could address these important sources of heterogeneity.
Finally, our focus was on obesity as outcome; we did not examine other metabolic outcomes (such as diabetes, hypercholesterolemia, or hypertension rates) in this analysis. It remains possible that herbicide mixtures impact various metabolic health indicators differently, an aspect a future study will explore. In view of these limitations, our study should be considered exploratory.
We recommend several directions for further research. Controlled longitudinal studies—for example, following birth cohorts or agricultural communities over time—are needed to test the temporality of herbicide exposure and obesity onset, which our ecologic design cannot establish. Incorporating individual-level exposure data (e.g., urinary or blood biomarkers of herbicides) would greatly strengthen causal inference by reducing exposure misclassification and allowing dose–response relationship assessment. Indeed, one recent longitudinal study of young adults (the CHAMACOS cohort) reported that cumulative glyphosate exposure was associated with elevated metabolic syndrome risk, illustrating the value of detailed exposure tracking [
51]. Future studies might also consider experimental and mechanistic investigations, such as animal or cellular models of herbicide mixtures, to unravel how these chemicals might jointly disrupt metabolic regulation. For instance, do low-dose combinations of glyphosate, 2,4-D, and atrazine induce greater adipogenesis or insulin resistance in vivo than each compound alone? Questions like this remain unanswered, and toxicological research could elucidate potential synergistic or antagonistic interactions at the molecular level. Additionally, examining spatial patterns using finer geographic resolutions (e.g., census tract data, as in recent environmental determinant studies) could help identify localized “hotspots” of metabolic disease tied to herbicide use, thereby refining intervention targets.